Abstract
In mammalians, serotonin (5-HT) has critical roles in the central nervous system (CNS), including mood stability, pain tolerance, or sleep patterns. However, the vast majority of serotonin is produced by intestinal enterochromaffin cells of the gastrointestinal tract and circulating blood platelets, also acting outside of the CNS. Serotonin effects are mediated through its interaction with 5-HT receptors (5-HTRs), a superfamily with a repertoire of at least fourteen well-characterized members. 5-HT7 receptors are the last 5-HTR member to be identified, with well-defined functions in the nervous, gastrointestinal, and vascular systems. The effects of serotonin on the immune response are less well understood. Mast cells are known to produce serotonin, while T cells, dendritic cells, monocytes, macrophages and microglia express 5-HT7 receptor. Here, we review the known roles of 5-HT7 receptors in the immune system, as well as their potential therapeutic implication in inflammatory and immune-mediated disorders.
Keywords: 5-HT7 receptors; Signaling pathway; 5-HT7 effect; 5-HT7 distribution; Inflammation; Dendritic cell; Microglia, macrophages; Lymphocytes
Introduction
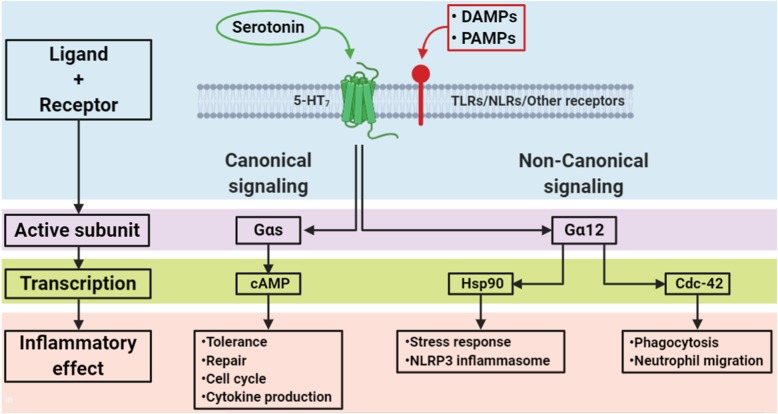
Serotonin (5-hydroxytryptamine [5-HT]), a monoamine neurotransmitter discovered over seven decades ago as a vasoconstricting agent (Rapport et al. 1948), has critical -and well defined- roles in the central nervous system (CNS), including regulation of mood stability, pain tolerance, or sleep patterns to name a few. Serotonin receptors are expressed throughout the immune system (Herr et al. 2017; Ahern 2011). 5-HT7 receptor is a member of the family of serotonin receptors, originally cloned in 1993 (Bard et al. 1993; Lovenberg et al. 1993; Ruat et al. 1993) a little more than a decade after the first receptor, 5-HT1 receptor, was (Peroutka and Snyder 1981). Like other serotonin receptors, 5-HT7 receptors are members of the G protein-coupled receptor superfamily. Their activation leads to the initiation of two well-characterized signaling pathways: the canonical signaling occurs through Gαs, while a non-canonical pathway signals through Gα12 (Guseva et al. 2014b). Different 5-HT7 receptor isoforms have been described (5-HT7a, 5-HT7b, 5-HT7d, all expressed in humans and rats, as well as 5-HT7c, expressed only in rats) differing only on the carboxy terminus length, nonetheless, no relevant functional differences have been observed between them (Liu et al. 2001).
Distribution of 5-HT7 receptors
5-HT7 receptors are expressed mainly in two compartments: the CNS (Hedlund and Sutcliffe 2004) and the gastrointestinal (GI) tract (Yaakob et al. 2015), although they are also expressed in other tissues including immune cells (see below). In the CNS, the receptor is broadly expressed in the spinal cord, suprachiasmatic nucleus of the hypothalamus, antedorsal thalamus, globus pallidus, prefrontal cortex, trigeminal nucleus caudalis, raphe nuclei area, amygdala and hippocampus, particularly in pyramidal cells of cornu ammonis (CA)1 and CA3, where they are expressed in both, neurons and glial cells, including microglia, the CNS-specific phagocytic cell (Chapin and Andrade 2001; Dogrul and Seyrek 2006; Gill et al. 2002; Horisawa et al. 2013; Thomas and Hagan 2004; Tokarski et al. 2003; Russo et al. 2005; Hedlund and Sutcliffe 2004; Lovenberg et al. 1993).
5-HT7 receptors are found on smooth muscle cells in several arteries, including the aorta, cerebral, coronary, and pulmonary arteries, where their primary known role is to induce vasodilation (Jasper et al. 1997; Nilsson et al. 1999; Jähnichen et al. 2005; Chang Chien et al. 2015; Terrón and Falcón-Neri 1999). In the GI tract, 5-HT7 receptors are expressed not only in gut-associated neurons, but also in enterocyte-like and immune cells in lymphatic tissues scattered all along the gut (Iceta et al. 2009; Kim et al. 2013; Guseva et al. 2014b), including monocytes, lymphocytes and dendritic cells (DCs) (León-Ponte et al. 2007; Wu et al. 2019), where it may play a crucial role in inflammation signaling (Urbina et al. 2014; Soga et al. 2007; Holst et al. 2015). Also, 5-HT7 receptors are found in neutrophils, but the net effect of 5-HT7 receptor signaling and modulation on neutrophil function is yet to be defined (Rapalli et al. 2016). 5-HT7 receptors have also been identified in hepatic stellate cells and hepatocytes (Ruddell et al. 2006; Svejda et al. 2013).
Of direct relevance to the present review, 5-HT7 receptors are also present in immune tissues, including the spleen and thymus; peripheral blood (Stefulj et al. 2000); DCs and other bone marrow-derived mononuclear cells (Shen et al. 1993; Vanhoenacker et al. 2000; Idzko et al. 2004).
Methods
We performed a comprehensive search of English language literature to identify all original research, and review articles regarding 5-HT7 receptors, signaling pathways and the effects on the immune system; PubMed database since 1993 was used. We used the following Medical Subject Headings (MeSH) and main keywords for searches: 5-HT7, LP-211, LP-44, LP-21, AS-19, SB 269970, 5-HT7 physiology, 5-HT7 receptor mechanism of action, 5-HT7 receptor signaling pathway, 5-HT7 receptor effect, 5-HT7 receptor distribution, inflammation, dendritic cell, microglia, macrophages, and lymphocytes. We also reviewed the reference lists of the articles identified during the search. The authors independently reviewed the selected articles.
Signaling pathways
There are at least two separate signaling pathways downstream of 5-HT7 receptors (Fig. 1). The activation of the canonical signaling pathway leads to the phosphorylation of different adenylyl cyclases (AC), specially AC1 and AC8 (Baker et al. 1998). The increased activity of AC results in an increased production of cyclic adenosine monophosphate (cAMP), activation of protein kinase type A (PKA) and subsequently the phosphorylation of different target proteins like extracellular signal-regulated kinase (ERK) and Protein kinase B (also known as Akt) (Errico et al. 2001; Johnson-Farley et al. 2005). Signaling through the non-canonical pathway leads to activation of Gα12, whose downstream activity is mainly exerted by the Rho family of small guanosine triphosphate (GTP)-ases (Rho, Rac, cell division control protein 42 [Cdc-42]) (Guseva et al. 2014b).
Fig. 1.
Signaling pathways downstream of 5-HT7 receptors
Interestingly, 5-HT7 receptors may interact with other members of the 5-HT family of receptors. For instance, there is a well-characterized interaction between 5HT7 and 5-HT1A receptors. 5-HT7 receptors can form heterodimers with 5-HT1A receptors, resulting in a reduction in the activity of 5-HT7 receptor (Renner et al. 2005). Moreover, 5-HT1A also inhibits the same signaling cascade as 5-HT7 receptor-mediated Gs (Zhou et al. 2019). Although to our knowledge no study has demonstrated a biological effect of this crosstalk in the immune system, this interaction could potentially explain the neutral effect of serotonin or SSRI administration.
In neuroblastoma cells, activation of 5-HT7 receptors induces the formation of filopodia via a Cdc-42-mediated pathway (Kvachnina 2005); in cultured hippocampal neurons, promotes the formation of dendritic spines and accelerates synaptogenesis; moreover, in cultured striatal and cortical neurons, activation of Gα12 leads to pronounced neurite growth via the activation of cyclin-dependent kinase 5 (Cdk5) and ERK (Speranza et al. 2013). All that suggests that 5-HT7 receptor signaling is critical for synaptogenesis and cell-cell communication which may also occur between non-neuronal cells, including immune ones as will be discussed below.
Effects of 5-HT7 receptor signaling in immune cells
Dendritic cells
5-HT7 receptors are highly expressed in mature, but not immature DCs (Idzko et al. 2004; Holst et al. 2015). 5-HT7 receptor activation induces DCs to release interleukin (IL)-1β and IL-8, while reducing the secretion of IL-12 and tumor necrosis factor (TNF)-α (Idzko et al. 2004). As observed in neurons, 5-HT7 receptor signaling in mature DCs also induces process branching and elongation via Cdc-42. While DCs do not show chemotactic response to 5-HT7 receptors, the non-selective high-affinity agonist 5-carboxamidotryptamine enhances velocity and distance of the chemotactic response to chemokine ligand (CCL)19 (Holst et al. 2015). The above mentioned data is summarized in Table 1.
Table 1.
Effect of 5-HT7 receptor signaling on different immune cells and inflammatory conditions
| Cell Type | 5-HT7 Effect | |
| Dendritic cells |  |
●Induces secretion of IL-1β and IL-8; reduces secretion of IL-12 and TNF-α ●Induces process branching and elongation |
| Monocytes, Macrophagues, Microglia |  |
●Pro- and anti-inflammatory ○Anti-apoptotic ○Increase in TNF-α, IL-6, Bcl-6, NF-kB ○AS-19 (agonist) decreases IL-12, TNF-α, and type 1 interferons; enhances production of TGF-β1 ○SB-269970 (antagonist) increases TNF-α and IL-12 |
| Lymphocyte |  |
●Concanavalin A, reserpine, and physical restrain increased expression of 5-HT7 ●Increase in proliferation rate, expression of CD25 |
| Disease Model | 5-HT7 Effect | |
| Inflammatory Bowel Disease |  |
●5-HT7 expression increased in DSS-induced colitis ●5-HT7 blockade/ablation results in increased severity of acute and chronic colitis ●5-HT7 agonists have anti-inflammatory effect |
| Lung Injury |  |
●5-HT7 antagonists decrease lung fluid content, TNF-α, IL-6, oxidative stress in bleomycin-induced lung injury ●5-HT7 antagonists reduce collagen deposition, expression of TGF-β1 and procollagen type Ӏ |
| Central nervous system inflammation |  |
●LP-211 (agonist) reduces neurotoxic effect of β-amyloid in a model of Alzheimer disease ●AS-19 (agonist) reduces pro-apoptotic effect of streptozotocin |
| Sepsis |  |
●In LPS-induced sepsis, 5-HT7 mRNA increases in parallel to TNF-α, IL-1β, NF-κB ●LP-44 (agonist) attenuates cell injury and reduces iNOS and TNF-α ●In a CLP-induced sepsis, AS19 increases survival; reduces tissue injury, inflammatory cytokines, lung NF-κB |
| Liver Injury |  |
●5-HT7 signaling induced during chronic liver injury ○Reduced ALT and AST levels ○Increased superoxide dismutase ○Reduced TNF-α, IL-6, TGF-β1 |
| Soft tissue inflamation |  |
●In carrageenan-induced paw inflammation, 5-HT7 agonists reduce cyclooxygenase mRNA expression; decrease oxidative stress, serum cytokine levels |
Monocytes and macrophages
Experimental evidence has shown an in vitro effect of 5-HT7 receptors on these cells, but the net effect is still incompletely understood. Monocytes treated with serotonin, or methiothepin maleate (a non-specific 5-HT1/6/7 receptor agonist), exhibit an inflammatory and anti-apoptotic polarization, including upregulation of TNF-α and IL-6, as well as upregulation of the transcription factors B-cell lymphoma 2 (Bcl-2), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB); and inhibition of caspase-3. Moreover, treating monocytes with serotonin resulted in increased expression of the costimulatory molecules cluster of differentiation (CD) 40, CD80, and CD86, but not of MHC class II molecules (Soga et al. 2007). In contrast, the selective 5-HT7 receptor antagonist SB 269970 reverts the anti-inflammatory effect of serotonin on dextran sodium sulfate (DSS)-stimulated M2 macrophages, increasing the production of TNF-α and IL-12, while also interfering with polarization (de las Casas-Engel et al. 2013).
In human and murine macrophages, it has been recently shown that serotonin (as well as AS19, a selective 5-HT7 receptor agonist) decrease inflammatory priming, in part by reducing the production of IL-12, TNF-α, and type 1 interferons, as well as enhancing the production of transforming growth factor β 1 (TGF-β1). Moreover, 5-HT7 receptor signaling promotes pro-fibrotic gene signature in a 5-HT7 and PKA-dependent manner (Domínguez-Soto et al. 2017).
A role for 5-HT7 receptors has been experimentally observed in murine models of skin fibrosis and DSS-induced colitis. In the former, macrophage infiltration and collagen deposition are blunted by genetically or chemically interfering with 5-HT7 receptor signaling (Domínguez-Soto et al. 2017). Oral administration of DSS results in a well-characterized model of gastrointestinal inflammation. Interestingly, DSS also results in increased expression of 5-HT7 receptors in a subset of anti-inflammatory myeloid (CD11b+CD68+) cells, suggesting that myeloid expression of 5-HT7 receptors may also –under specific insults- attenuate the inflammatory response (Guseva et al. 2014a).
Microglia
These, CNS-specific phagocytic mononuclear cells (Wolf et al. 2017), are produced in the yolk sac and migrate during early CNS development, before the blood brain barrier is formed. In adult life, microglia are involved in a number of homeostatic functions, including neurogenesis, synaptogenesis and synapsis remodeling, as well as neuronal apoptosis and removal (Li and Barres 2018). Microglia also actively survey the CNS for preserved molecular patterns suggestive of infection (pathogen-associated molecular patterns [PAMPs]) and tissue injury (damage-associated molecular patterns [DAMPs]) (Sankowski et al. 2015; Salter and Stevens 2017). Adult microglia express several serotonin receptors, including 5-HT2a, 5-HT2b, 5-HT5a, and 5-HT7 receptors (Krabbe et al. 2012). Microglia express at least two splice variants of the 5-HT7 receptor: 5-HT7(a/b). In these cells, the administration of serotonin, as well as 5-carboxamidotryptamine (5-CT) induces an inflammatory priming and IL-6 production, indicating that these receptors may play a role in CNS inflammation and repair (Mahé et al. 2005).
T cells
Lymphocytes express functional serotonin receptors (Cedeño et al. 2005; Müller et al. 2009). However, information on the role of 5-HT7 receptors in lymphoid cells is scant. However, preliminary evidence indicates that lymphocytes obtained from rats exposed to either concanavalin A, reserpine, or physical restraint have an increase in number of 5-HT7 receptor-positive lymphocytes, as well as increased expression of 5-HT7 mRNA (Urbina et al. 2014). Naïve splenic T cells express 5-HT7 receptors; their ex vivo exposure to serotonin leads to a rapid 5-HT7 receptor-dependent phosphorylation of ERK 1/2, increased proliferation rate, and increased expression of CD25; that response is abrogated by the 5-HT7 receptor antagonist SB 269970 (León-Ponte et al. 2007). Together, this suggests that 5-HT7 receptors play a role in T cell responses to inflammatory stimuli.
Neutrophils
While neutrophil migration can be regulated through the effect of serotonin in other receptors, current evidence suggest that 5-HT7 receptor has no role on neutrophil recruitment (Rapalli et al. 2016).
Hepatocyte response to injury
The above mentioned data is summarized in Table 1. Serotonin has been observed to play a role in liver remodeling in response to inflammatory injury, but the mechanism is incompletely understood. Current evidence suggests that hepatocyte proliferation may be regulated by 5-HT receptors at a number of levels. An in vitro study on rat hepatocytes showed that 5-HT7 receptor activation by serotonin dose-dependently increases cAMP and PKA signaling, whereas the pharmacological blockade by SB 269970 (a highly specific antagonist) reverted this effect, an observation with potential implications for extra-hepatic tumor seeding to the liver (Svejda et al. 2013).
Organ and disease specific effects of 5-HT7
Sepsis
In lipopolysaccharide (LPS)-induced sepsis, lung expression of 5-HT7 receptors increases in parallel to the increased expression of TNF-α, IL-1β, and NF-κB. Moreover, in that model, activating 5-HT7 receptors with LP44 attenuates LPS-induced cell injury, reducing the levels of inducible nitric oxide synthase (iNOS) and TNF-α in a dose-dependent manner (Ayaz et al. 2017).
Cecal ligation and puncture (CLP) is a well validated model of severe poli-microbial sepsis that results in acute and chronic inflammation (Valdés-Ferrer 2014; Buras et al. 2005; Valdés-Ferrer et al. 2013). Administration of a selective 5-HT7 receptor agonist (AS19) in a rat model of CLP-induced sepsis results in increased survival, decreased tissue injury, a reduction in circulating inflammatory cytokines (IL-1β, IL-6 and TNF-α), an increase in antioxidant mediators (superoxide dismutase and glutathione), and a reduction in lung NF-κB (Cadirci et al. 2013). The above mentioned data is summarized in Table 1.
Inflammatory bowel disease
5-HT7 receptors are expressed in enteric neurons and CD11c+ DCs in the colon; as mentioned above, 5-HT7 receptor expression is significantly increased after the induction of colitis by DSS (Domínguez-Soto et al. 2017). In that model, the blockade or genetic ablation of 5-HT7 receptors results in increased severity of acute and chronic colitis; in contrast, 5-HT7 receptor agonists result in an anti-inflammatory effect (Guseva et al. 2014a; Kim et al. 2013). However, pharmacological blockade of 5-HT7 receptors has no effect in 2, 4, 6 trinitrobence sulfonic acid-induced colitis (Rapalli et al. 2016), suggesting that the inflammatory effect of 5-HT7 receptors is model-specific. More studies of the downstream-signaling mediators are needed to further understand the therapeutic potential of 5-HT7 receptors in experimental inflammatory bowel disease.
Lung inflammation
Bleomycin induces experimental pulmonary fibrosis (Adamson and Bowden 1974). During the acute inflammatory phase, 5-HT7 receptor antagonists decrease lung fluid content, inflammatory cytokines (TNF-α, IL-6) and oxidative stress burden. In the chronic fibrogenic phase, 5-HT7 receptor antagonism reduces collagen deposition, and mRNA expression of TGF-β1 and procollagen type Ӏ (Tawfik and Makary 2017). In contrast, in CLP-induced sepsis, 5-HT7 receptor agonists reduce pro-inflammatory mediators and increase survival (Cadirci et al. 2013). Altogether, the current evidence is ambivalent regarding the usefulness of pharmacologically interfering with 5-HT7 receptor signaling in experimental lung injury.
Liver disease
To our knowledge only one study has focused on the effect of 5-HT7 receptor signaling during chronic liver injury induced by carbon tetrachloride. There, 5-HT7 receptor agonists reduced alanine transaminase (ALT) and aspartate transaminase (AST) levels; increased the level of superoxide dismutase; and decreased the levels of TNF-α, IL-6 and TGF-β1. By the same token, 5-HT7 receptor antagonism increases cytokines levels. In their histopathological analysis, the carbon tetrachloride group showed severe vacuolar degeneration, necrosis, irregular walls of the vena centralis and lytic areas. In contrast, the administration of the 5-HT7 receptor agonist LP-44 partially rescued animals from liver damage. This suggests that 5-HT7 receptors may be a potential therapeutic target for chronic liver inflammation (Polat et al. 2017).
Alzheimer disease (AD)
Immune activation in response to inflammatory insults is a key mediator of neurodegeneration in AD, depression, as well as other CNS disorders (Baganz and Blakely 2013; Strasser et al. 2016). Microglia engulfs and degrades β-amyloid, leading to an excessive release of inflammatory cytokines that further propagate inflammatory damage (Holmes and Butchart 2011). Also, β-amyloid binds to receptors for advances glycation end products (RAGE), resulting in further microglia activation (Querfurth and Laferla 2018). In an AD animal model, the intracerebroventricular (ICV) administration of LP-211 (a 5-HT7 receptor specific agonist) inhibited the neurotoxic effect of β-amyloid in hippocampus (Quintero-Villegas et al. 2018). In a rat model of stroptozotocin-induced AD, ICV administration of the 5-HT7 receptor-selective agonist AS19 rescued neuronal apoptosis and synaptic dysfunction (Hashemi-Firouzi et al. 2017). Altogether, available preliminary evidence derived from animal models indicates that pharmacological manipulation of the 5-HT7 receptor may have a niche in the treatment of AD.
Soft-tissue inflammation
The above mentioned data is summarized in Table 1. In a carrageenan-induced paw inflammation model, 5-HT7 receptor agonists reduced cyclooxygenase mRNA expression, decreased oxidative stress and serum cytokine levels (Albayrak et al. 2013).
Conclusions
5-HT7 receptors are widely expressed in a vast repertoire of immune and non-immune cells. The receptor has diverse -and even discrepant- roles in the immune response, probably a reflection of at least two clearly defined signaling pathways: in dendritic cells it induces the secretion of IL-1 and IL-6; in monocytes, for instance, it may either be pro- or anti-inflammatory; while in lymphocytes it increases the proliferation rate, suggesting a proinflammatory pattern.
Regarding an organ-specific effect, in CNS, soft tissue, and liver inflammatory injury, the net effect of 5-HT7 receptors is anti-inflammatory (reducing cell death, inflammatory cytokine release, and oxidative stress). In models of severe sepsis, 5-HT7 receptor agonists have resulted in a net anti-inflammatory effect. In contrast, bleomycin-induced lung injury is the only experimental model where 5-HT7 receptors seems to be pro-inflammatory. In experimental murine inflammatory bowel disease, the effect of 5-HT7 receptors has shown contradictory results.
While our understanding of the connection between 5-HT7 receptors and inflammation is still limited, significant advances have emerged in the past two decades. Nonetheless, current evidence suggests that pharmacological interventions targeting 5-HT7 receptors may be potentially useful for treating inflammatory conditions.
Acknowledgements
The figures on this manuscript were done using biorender.com software.
Abbreviations
- 5-HT
Serotonin
- 5-HTRs
5-HT receptor family
- AC
Adenylyl cyclase
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- Bcl-2
B-cell lymphoma 2 transcription factor
- CA
Cornu ammonis region of the hippocampus
- cAMP
Cyclic adenosine monophosphate
- CCL
Chemokine ligand
- CD
Cluster of differentiation
- Cdc-42
Cell division control protein 42
- Cdk5
Cyclin-dependent kinase 5
- CLP
Cecal ligation and puncture
- CNS
Central nervous system
- DAMPs
Damage-associated molecular patterns
- DCs
Dendritic cells
- DSS
Dextran sodium sulfate
- ERK
Extracellular signal-regulated kinase
- GI
Gastrointestinal
- GTP
Guanosine triphosphate
- ICV
Intracerebroventricular
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- mRNA
Messenger RNA
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PAMPs
Pathogen-associated molecular patterns
- PKA
Protein kinase type A
- TGF-β1
Transforming growth factor beta 1
- TNF
Tumor necrosis factor
Authors’ contributions
All authors read and approved the final manuscript.
Funding
This manuscript was supported by a grant (FOSISS-289788 to SIVF) from Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico.
Availability of data and materials
Not applicable (other than the referenced publications).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11:29–33. doi: 10.1016/j.coph.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayrak A, Halici Z, Cadirci E, Polat B, Karakus E, Bayir Y, Unal D, Atasoy M, Dogrul A. Inflammation and peripheral 5-HT7 receptors: the role of 5-HT7 receptors in carrageenan induced inflammation in rats. Eur J Pharmacol. 2013;715:270–279. doi: 10.1016/j.ejphar.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Ayaz G, Halici Z, Albayrak A, Karakus E, Cadirci E. Evaluation of 5-HT7 receptor trafficking on in Vivo and in vitro model of lipopolysaccharide (LPS)-induced inflammatory cell injury in rats and LPS-treated A549 cells. Biochem Genet. 2017;55:34–47. doi: 10.1007/s10528-016-9769-2. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LP, Nielsen MD, Impey S, Metcalf MA, Poser SW, Chan G, Obrietan K, Hamblin MW, Storm DR. Stimulation of type 1 and type 8 Ca 2+ /Calmodulin-sensitive adenylyl Cyclases by the G s -coupled 5-Hydroxytryptamine subtype 5-HT 7A receptor. J Biol Chem. 1998;273:17469–17476. doi: 10.1074/jbc.273.28.17469. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to Adenylate Cyclase. J Biochem Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Cadirci E, Halici Z, Bayir Y, Albayrak A, Karakus E, Polat B, Unal D, Atamanalp SS, Aksak S, Gundogdu C. Peripheral 5-HT7 receptors as a new target for prevention of lung injury and mortality in septic rats. Immunobiology. 2013;218:1271–1283. doi: 10.1016/j.imbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Cedeño N, Urbina M, Obregón F, Lima L. Characterization of serotonin transporter in blood lymphocytes of rats. Modulation by in vivo administration of mitogens. J Neuroimmunol. 2005;159:31–40. doi: 10.1016/j.jneuroim.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Chang Chien CC, Hsin LW, Su MJ. Activation of serotonin 5-HT7 receptor induces coronary flow increase in isolated rat heart. Eur J Pharmacol. 2015;748:68–75. doi: 10.1016/j.ejphar.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h) J Pharmacol Exp Ther. 2001;297:403–409. [PubMed] [Google Scholar]
- de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A, Samaniego R, Loza M, Corcuera MT, Gómez-Aguado F, Bustos M, Sánchez-Mateos P, Corbí AL. Serotonin skews human macrophage polarization through HTR 2B and HTR 7. J Immunol. 2013;190:2301–2310. doi: 10.4049/jimmunol.1201133. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT 7 , but not 5-HT 1A and 5-HT 2 receptors in the spinal cord. Br J Pharmacol. 2006;149:498–505. doi: 10.1038/sj.bjp.0706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Soto Á, Usategui A, Las Casas-Engel MD, Simón-Fuentes M, Nieto C, Cuevas VD, Vega MA, Luis Pablos J, Corbí ÁL. Serotonin drives the acquisition of a profibrotic and anti-inflammatory gene profile through the 5-HT7R-PKA signaling axis. Sci Rep. 2017;7:1–15. doi: 10.1038/s41598-017-15348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico M, Crozier RA, Plummer MR, Cowen DS. 5-HT7 receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001;102:361–367. doi: 10.1016/S0306-4522(00)00460-7. [DOI] [PubMed] [Google Scholar]
- Gill CH, Soffin EM, Hagan JJ, Davies CH. 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology. 2002;42:82–92. doi: 10.1016/S0028-3908(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, Buettner M, Bleich A, Pabst O, Bachmann O, Ponimaskin EG. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516–1529. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- Guseva D, Wirth A, Ponimaskin E. Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front Behav Neurosci. 2014;8:1–8. doi: 10.3389/fnbeh.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi-Firouzi N, Komaki A, Soleimani Asl S, Shahidi S. The effects of the 5-HT7 receptor on hippocampal long-term potentiation and apoptosis in a rat model of Alzheimer’s disease. Brain Res Bull. 2017;135:85–91. doi: 10.1016/j.brainresbull.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Hedlund P, Sutcliffe J. Functional, molecular and pharmacological advances in 5-HT receptor research. Trends Pharmacol Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:1–11. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Clive, Butchart Joe. Systemic inflammation and Alzheimer's disease. Biochemical Society Transactions. 2011;39(4):898–901. doi: 10.1042/BST0390898. [DOI] [PubMed] [Google Scholar]
- Holst K, Guseva D, Schindler S, Sixt M, Braun A, Chopra H, Pabst O, Ponimaskin E. The serotonin receptor 5-HT7R regulates the morphology and migratory properties of dendritic cells. J Cell Sci. 2015;128:2866–2880. doi: 10.1242/jcs.167999. [DOI] [PubMed] [Google Scholar]
- Horisawa T, Ishiyama T, Ono M, Ishibashi T, Taiji M. Binding of lurasidone, a novel antipsychotic, to rat 5-HT7 receptor: analysis by [3H]SB-269970 autoradiography. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;40:132–137. doi: 10.1016/j.pnpbp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Iceta R, Mesonero JE, Aramayona JJ, Alcalde AI. Expression of 5-HT1A and 5-HT7 receptors in caco-2 cells and their role in the regulation of serotonin transporter activity. J Physiol Pharmacol. 2009;60:157–164. [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M, Fiebich B, Herouy Y, Elsner P, Norgauer J, Ferrari D. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- Jähnichen S, Glusa E, Pertz HH. Evidence for 5-HT2B and 5-HT7 receptor-mediated relaxation in pulmonary arteries of weaned pigs. Naunyn Schmiedeberg's Arch Pharmacol. 2005;371:89–98. doi: 10.1007/s00210-004-1006-6. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Kosaka A, Z.P. To. Chang DJ, Eglen RM. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT 7 receptor (h5-HT 7(b) ) Br J Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley NN, Kertesy SB, Dubyak GR, Cowen DS. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J Neurochem. 2005;92:72–82. doi: 10.1111/j.1471-4159.2004.02832.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Bridle BW, Ghia J-E, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB, Khan WI. Targeted Inhibition of Serotonin Type 7 (5-HT 7 ) Receptor Function Modulates Immune Responses and Reduces the Severity of Intestinal Inflammation. J Immunol. 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HWGM, Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun. 2012;26:419–428. doi: 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Kvachnina E. 5-HT7 receptor is coupled to G subunits of Heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Ponte M, Ahern GP, O’Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Liu H, Irving HR, Coupar IM. Expression patterns of 5-HT7 receptor isoforms in the rat digestive tract. Life Sci. 2001;69:2467–2475. doi: 10.1016/S0024-3205(01)01318-2. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-L. [DOI] [PubMed] [Google Scholar]
- Mahé C, Loetscher E, Dev KK, Bobirnac I, Otten U, Schoeffter P. Serotonin 5-HT7 receptors coupled to induction of interleukin-6 in human microglial MC-3 cells. Neuropharmacology. 2005;49:40–47. doi: 10.1016/j.neuropharm.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Müller T, Dürk T, Blumenthal B, Grimm M, Cicko S, Panther E, Sorichter S, Herouy Y, Di Virgilio F, Ferrari D, Norgauer J, Idzko M. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4:1–8. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Longmore J, Shaw D, Pantev E, Bard JA, Branchek T, Edvinsson L. Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol. 1999;372:49–56. doi: 10.1016/S0014-2999(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Two distinct serotonin receptors: regional variations in receptor binding in mammalian brain. Brain Res. 1981;208:339–347. doi: 10.1016/0006-8993(81)90562-X. [DOI] [PubMed] [Google Scholar]
- Polat B, Halici Z, Cadirci E, Karakus E, Bayir Y, Albayrak A, Unal D. Liver 5-HT7 receptors: a novel regulator target of fibrosis and inflammation-induced chronic liver injury in vivo and in vitro. Int Immunopharmacol. 2017;43:227–235. doi: 10.1016/j.intimp.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, Laferla FM. Alzheimer’s disease. 2018. pp. 329–344. [DOI] [PubMed] [Google Scholar]
- Quintero-Villegas A, Álvarez-Manzo HS, Bernal-Mondragón C, Guevara-Guzmám R, Valenzuela-Almada MOA. SciFed journal of Alzheimer’s and dementia. SF J Alzh Dement. 2018;1:1–10. [Google Scholar]
- Rapalli A, Bertoni S, Arcaro V, Saccani F, Grandi A, Vivo V, Cantoni AM, Barocelli E. Dual role of endogenous serotonin in 2,4,6-Trinitrobenzene sulfonic acid-induced colitis. Front Pharmacol. 2016;7. 10.3389/fphar.2016.00068. [DOI] [PMC free article] [PubMed]
- Rapport MM, Green AA, Page IH. Partial purification of the vasoconstrictor in beef serum. J Biol Chem. 1948;174:735–741. [PubMed] [Google Scholar]
- Renner U., Zeug A., Woehler A., Niebert M., Dityatev A., Dityateva G., Gorinski N., Guseva D., Abdel-Galil D., Frohlich M., Doring F., Wischmeyer E., Richter D. W., Neher E., Ponimaskin E. G. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. Journal of Cell Science. 2012;125(10):2486–2499. doi: 10.1242/jcs.101337. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci U S A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861–876. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Pellitteri R, Monaco S, Romeo R, Stanzani S. “In vitro” postnatal expression of 5-HT7 receptors in the rat hypothalamus: an immunohistochemical analysis. Dev Brain Res. 2005;154:211–216. doi: 10.1016/j.devbrainres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- Sankowski R, Mader S, Valdés-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:1–20. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- Speranza L, Chambery A, Di Domenico M, Crispino M, Severino V, Volpicelli F, Leopoldo M, Bellenchi GC, di Porzio U, Perrone-Capano C. The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology. 2013;67:155–167. doi: 10.1016/j.neuropharm.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun. 2000;14:219–224. doi: 10.1006/brbi.1999.0579. [DOI] [PubMed] [Google Scholar]
- Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- Svejda B, Kidd M, Timberlake A, Harry K, Kazberouk A, Schimmack S, Lawrence B, Pfragner R, Modlin IM. Serotonin and the 5-HT7 receptor: the link between hepatocytes, IGF-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013;104:844–855. doi: 10.1111/cas.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik MK, Makary S. 5-HT7 receptor antagonism (SB-269970) attenuates bleomycin-induced pulmonary fibrosis in rats via downregulating oxidative burden and inflammatory cascades and ameliorating collagen deposition: comparison to terguride. Eur J Pharmacol. 2017;814:114–123. doi: 10.1016/j.ejphar.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Terrón JA, Falcón-Neri A. Pharmacological evidence for the 5-HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br J Pharmacol. 1999;127:609–616. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Hagan J. 5-HT7 Receptors. Curr Drug Target -CNS Neurol Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. 2003;993:230–234. doi: 10.1016/j.brainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Urbina M, Arroyo R, Lima L. 5-HT7 receptors and tryptophan hydroxylase in Lymphoctytes of rats: mitogen activation, physical restraint or treatment with reserpine. Neuroimmunomodulation. 2014;21:240–249. doi: 10.1159/000357148. [DOI] [PubMed] [Google Scholar]
- Valdés-Ferrer SI. The challenges of long-term sepsis survivors: when surviving is just the beginning. Rev Investig Clin. 2014;66:439–449. [PubMed] [Google Scholar]
- Valdés‐Ferrer S. I., Rosas‐Ballina M., Olofsson P. S., Lu B., Dancho M. E., Ochani M., Li J. H., Scheinerman J. A., Katz D. A., Levine Y. A., Hudson L. K., Yang H., Pavlov V. A., Roth J., Blanc L., Antoine D. J., Chavan S. S., Andersson U., Diamond B., Tracey K. J. Expression of Concern: HMGB 1 mediates splenomegaly and expansion of splenic CD 11b+ L y‐6 C high inflammatory monocytes in murine sepsis survivors. Journal of Internal Medicine. 2013;274(4):381–390. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE. 5-HT 7 receptors: current knowledge and future prospects. Trends Pharmacol Sci. 2000;21:70–77. doi: 10.1016/S0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HWGM, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: the role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Yaakob NS, Chinkwo KA, Chetty N, Coupar IM, Irving HR. Distribution of 5-HT 3 , 5-HT 4 , and 5-HT 7 Receptors Along the Human Colon. J Neurogastroenterol Motil. 2015;21:361–369. doi: 10.5056/jnm14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang R, Zhang S, Wu J, Sun X. Activation of 5-HT1A receptors promotes retinal ganglion cell function by inhibiting the cAMP-PKA pathway to modulate presynaptic GABA release in chronic glaucoma. J Neurosci. 2019;39:1484–1504. doi: 10.1523/JNEUROSCI.1685-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable (other than the referenced publications).