Abstract
Background
Atherosclerosis is a chronic and multifactorial disease, and it is the main reason of coronary heart disease, cerebral infarction, and peripheral vascular disease, which leads to the formation of lesions in arterial blood vessels. Our study aimed to explore the protective effect and its underlying mechanism of atorvastatin (ATV) on oxidized low-density lipoprotein (ox-LDL)-induced atherosclerosis.
Material/Methods
Human umbilical vascular endothelial cells (HUVECs) were cultured and pretreated with ox-LDL to establish an in vitro atherosclerotic cell model. Cell Counting Kit-8 (CCK-8) assay, TUNEL staining, and Transwell assay were used to detect the cell activity, apoptosis, and migration in HUVECs. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot were applied to measure the mRNA and protein expressions of adhesion-related genes in HUVECs.
Results
Pretreated with 100 mg/L ox-LDL resulted in a 57.23% decrease of cell viability and 81.09% increase of apoptotic injury in HUVECs compare to the control. Meanwhile, ox-LDL pretreatment increased the cell migration and the expression of miR-26a-5p in HUVECs. ATV treatment could effectively reverse the cellular damage induced by ox-LDL, decrease the release of adhesion-related molecules, and downregulate the expression of miR-26a-5p by 44.79% in HUVECs. Moreover, phosphatase and tensin homolog (PTEN) was demonstrated to be the target gene of miR-26a-5p.
Conclusions
Our results highlight that ATV protects against ox-LDL-induced downregulation of cell viability, upregulation of cell apoptosis, migration, as well as the release of adhesion-related molecules in HUVECs through the miR-26a-5p/PTEN axis. This study provides new insights into the underlying mechanism of ATV therapeutic potential in atherosclerosis, and also provides a new strategy for the treatment of atherosclerosis.
MeSH Keywords: Atherosclerosis; Human Umbilical Vein Endothelial Cells; Receptors, Oxidized LDL
Background
Atherosclerosis is a chronic, complicated cardiovascular disease, which is caused by various factors, including lipid deposition, inflammation, and oxidative stress [1]. In recent years, atherosclerosis has become a common and frequently occurring disease that seriously endangers human health worldwide [2]. Factors contributing to atherosclerosis might include hypertension, hyperlipidemia, heavy smoking, as well as diabetes, obesity, and some genetic factors [3]. However, the pathogenesis of atherosclerosis is complex, and it has not been fully illuminated.
Three theories over the past half century about the formation and pathogenesis of atherosclerosis have been: “lipid infiltration theory”, “thrombosis theory”, and “response to injury” [4]. During the development of atherosclerosis, abnormal lipid metabolism is the main factor related to endothelial and smooth muscle injuries. More and more studies have focused on expanding the understanding of oxidized low-density lipoprotein (ox-LDL) on the pathogenesis of atherosclerosis [5,6]. Previous studies have confirmed that the formation of ox-LDL is an important stage in the occurrence and development of early atherosclerosis. Therefore, ox-LDL has been considered an important diagnostic biomarker for atherosclerosis [7]. Ox-LDL participates in the process of atherosclerosis through various mechanisms, for example, ox-LDL pre-treatment could induce the downregulation of cell viability in human umbilical cord blood endothelial cells (HUCBECs) [8,9]. Moreover, cells can be activated by ox-LDL to secrete chemical factors, cytokines, and inflammatory factors, which are involved in the formation and development of early atherosclerosis. Therefore, ox-LDL has been widely used to establish an in vitro model of atherosclerosis.
Atorvastatin (ATV) is a commonly used drug for primary hypercholesterolemia. ATV plays a therapeutic role in hypercholesterolemia by reducing the increased total cholesterol (TC), LDL cholesterol (LDL-c), Apolipoprotein B-100 (Apo B), and triglyceride (TG) [10]. At present, more and more studies have focused on the pharmacological effects of ATV in cardiovascular system. Previous studies have shown that ATV may play an anti-atherosclerosis role by regulating inflammation, oxidative stress and lipid metabolism [11]. However, the specific potential mechanism of ATV for atherosclerosis remains to be further explored.
Therefore, the aim of our study was to establish an atherosclerosis model in vitro by ox-LDL pretreatment on human umbilical vascular endothelial cells (HUVECs), then to observe the function of ATV in ox-LDL-induced HUVECs, and finally to clarify the underlying protective mechanism of ATV in ox-LDL-induced HUVECs.
Material and Methods
Cell culture and treatment
HUVECs were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured in RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, USA) and 1% endothelial cell growth supplement at 37°C in a humidified atmosphere containing 5% CO2 for 24 hours. HUVECs were treated with different concentrations of ox-LDL (AngYuBio, China) for 24 hours to establish the in vitro model of atherosclerosis. ATV (Sigma-Aldrich, USA) was dissolved in DMSO (Sigma-Aldrich, USA).
Cell viability assay
The cell viability was detected with Cell Counting Kit-8 (CCK-8) (Beyotime, China). Briefly, HUVECs were seeded in 96-well plates at a density of 5×103 cells per well. Then, the cells were pretreated with different concentrations of ox-LDL or ATV for 24 hours. After removing the culture medium, HUVECs were washed with phosphate buffer saline (PBS) 3 times, and 10 μL of CCK-8 solution was added to each well. Then, the cells were incubated for 2 hours at 37°C. The cell viability was assessed by the absorbance and detected at 450 nm using a microplate reader (Thermo, USA). The experiment was repeated 3 times.
Apoptosis assay
The apoptosis of HUVECs was detected by the terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay, which was performed by using an Apoptosis Kit (Roche, Switzerland). Briefly, HUVECs were seeded in 6-well plates (5×105 cells per well) for 24 hours, and then ox-LDL was added to the medium with or without ATV treatment. After 24 hours, the cells were washed 3 times with PBS, and fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 20 minutes. Then, the cells were washed with PBS 3 times. Next, the TUNEL reaction mixture was added dropwise, reacted for 1 hour at 37°C, and rinsed 3 times with PBS. Then, DAPI (Sigma-Aldrich, USA) was added, and plates were rinsed 3 times with PBS. Finally, the cells were observed and pictured under a fluorescence microscope (Olympus, Japan).
Transwell migration assay
HUVECs with 200 μL non-serum RPMI-1640 medium were loaded onto the upper chamber of a 24-well Transwell plate (2×104 cells per well) (Corning, USA), and precoated with Matrigel on the upper side of the chamber. The lower chamber was filled with 600 μL RPMI-1640 medium and 10% FBS. Following incubation for 24 hours, the cells were treated with ox-LDL and/or ATV for 24-hour migration. Then, the cells that had not migrated on the upper chamber were scraped gently with a cotton swab. The migrated cells to lower surface of the membrane were fixed by 95% ethyl alcohol for 20 minutes, and then stained with 0.1% crystal violet for 15 minutes, then the cells were washed 3 times with PBS. Cells were counted under an optical microscope and photographed using a digital camera (Olympus, Japan).
Cell transfection
HUVECs were seeded in 6-well plates (5×105 cells per well) for 24 hours, and then the cells were transfected with the negative control (NC), miR-26a-5p mimic, and miR-26a-5p inhibitor (GenePharma, China). Following mixing of Lipofectamine®2000 (ThermoFisher Scientific, USA) with the NC, miR-26a-5p mimic, or miR-26a-5p inhibitor, the mixture was added to the cells and incubated for 24 hours. Then, the cells were collected for the following experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from HUVECs by using TRIzol (Life Technologies Corporation, USA). Then, the reverse RNA was conducted by PrimeScript Reverse Transcriptase (Takara, China). The reaction conditions were as follows: 42°C for 2 minutes, 95°C for 5 seconds and 37°C for 15 minutes. The cDNA was subsequently obtained and stored at 4°C for further use. Then 1 μg of RNA samples were selected for quantitative polymerase chain reaction (qPCR), and the obtained cDNA was analyzed 3 times using SYBR green PCR master mix (Fermentas Life Sciences, USA). qRT-PCR was performed by using an ABI Prism 7500 Fast Real-time PCR instrument (Applied Biosystems, USA). The amplification process was carried out as follows: pre-denaturation at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 45 seconds. The internal control for microRNAs was U6, and the internal control for MCP-1, ICAM-1, and phosphatase and tensin homolog (PTEN) was β-actin. The 2−ΔΔCt method was applied for the analysis of qRT-PCR results. Each experiment was repeated 3 times.
Western blot
HUVECs were collected and added with RIPA buffer (Beyotime, China) to extract the total protein. The concentrations of total protein were determined by using BCA method with a BCA Protein Assay Kit (KeyGEN Biotech, China). Proteins were then separated by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). Next, the PVDF membrane was blocked in 5% nonfat milk (Becton Dickinson, USA) for 30 minutes at room temperature; followed by an overnight incubation at 4°C with primary antibodies, including cleaved caspase-3 (ab2302, Abcam, USA), PTEN (ab192396, Abcam, USA), and β-actin (ab8226, Abcam, USA). After the overnight incubation, PVDF membrane was washed for 3 times with TBST, and then the membrane was incubated with the secondary antibody for 2 hours at room temperature. The protein bands were quantified by using the ImageJ software, and the intensity of the protein bands was analyzed by the Quantity One analysis system (BioRad, USA). β-Actin was used as the internal control.
Statistics
Data are expressed as mean±standard error of the mean (SEM). Comparisons between 2 different groups were determined by using Student’s paired t-test. Multiple comparisons for 3 or more groups were performed by applying the one-way analysis of variance (ANOVA). GraphPad Prism 6 software (GraphPad Software, USA) was conducted for the further analyses. Statistically significant differences were considered by P<0.05 with a 95% confidence interval.
Results
ATV ameliorates ox-LDL-induced apoptosis and migration of HUVECs
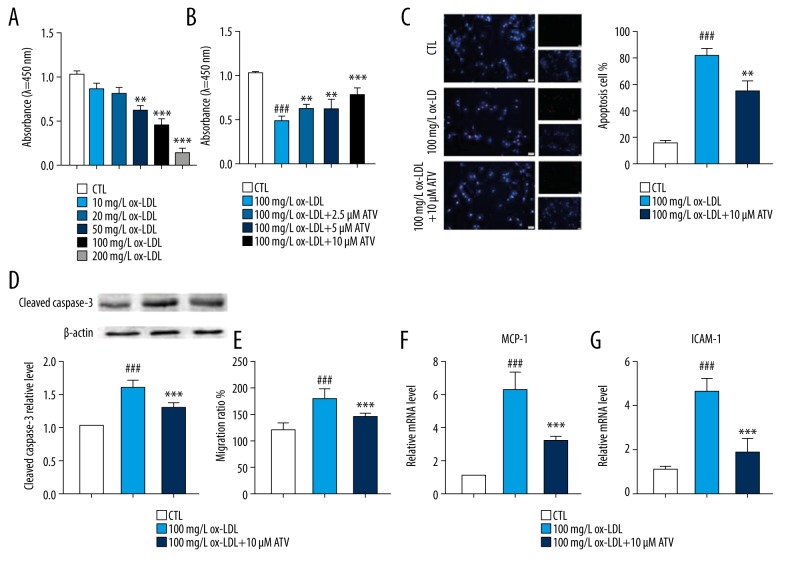
CCK-8 assay showed that the cell viability of HUVECs was inhibited by ox-LDL pre-treatment in a dose-dependent manner, and the cell viability downregulated closely to 50% when the HUVECs were stimulated by 100 mg/L of ox-LDL for 24 hours (Figure 1A). Therefore, the pre-treatment with 100 mg/L of ox-LDL for 24 hours was used as the optimal pre-treatment condition in the subsequent experiments. Meanwhile, CCK-8 assay also showed that different concentrations of ATV treatment could upregulate the cell viability of HUVECs in a dose-dependent manner, and 10 μM ATV pre-treatment for 24 hours significantly increased the cell viability of HUVECs (Figure 1B). Therefore, 10 μM ATV was selected as an optimal pre-treatment condition in the subsequent experiments. Then, the protective function of ATV on ox-LDL-induced apoptosis and migration of HUVECs was further investigated. TUNEL staining indicated that the numbers of apoptotic cells were significantly increased in 100 mg/L ox-LDL, while significantly decreased by 10 μM ATV conditioning (Figure 1C). At the same time, western blot showed that 10 μM ATV treatment could significantly decrease the expression of cleaved caspase-3, which is a representative apoptotic-related protein (Figure 1D). Moreover, the expressions of MCP-1 and ICAM-1, which represented the migration of HUVECs, were significantly upregulated by ox-LDL pretreatment and downregulated by ATV treatment (Figures 1E–1G).
Figure 1.
ATV ameliorates ox-LDL-induced apoptosis and migration of HUVECs. (A) CCK-8 assay was applied to analyze the inhibitory effects of ox-LDL (10, 20, 50, 100, and 200 mg/L) pre-treatment for 24 hours on HUVECs; n=6. ** P<0.01, *** P<0.001 compared to the CTL group. (B) The cell viability of HUVECs treated by various concentrations of ATV for 24 hours; n=6. ### P<0.001 compared to the CTL group. ** P<0.01, *** P<0.001 compared to the ox-LDL group. (C) TUNEL staining was applied to detect the apoptosis of HUVECs treated by 10 μM ATV for 24 hours. (D) Western blot was used to detect the expression of cleaved caspase-3 of HUVECs treated by 10 μM ATV for 24 hours. (E) Relative migration ratio, the mRNA levels of (F) MCP-1 and (G) ICAM-1 of HUVECs after being treated with 10 μM ATV and 100 mg/L ox-LDL for 24 hours. ### P<0.001 compared to the CTL group. ** P<0.01, *** P<0.001 compared to the ox-LDL group. ATV – atorvastatin; ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vascular endothelial cells; CCK-8 – Cell Counting Kit-8; CTL, control.
The expressions of miR-26a-5p, miR-29b, miR-214, and miR-363-3p were downregulated by ATV treatment
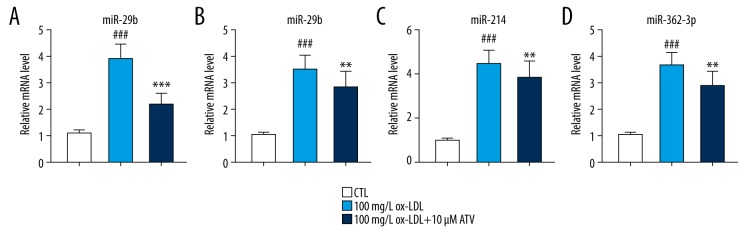
Previous studies showed that the expressions of some pro-inflammatory miRNAs, such as miR-26a-5p, miR-29b, miR-214, and miR-363-3p, can be upregulated in the ox-LDL-induced cells or ApoE knockout mice [12–14]. Then, we detected the expressions of these miRNAs so that we can draw a conclusion that whether the protective effect of ATV or the damage effect of ox-LDL on HUVECs was in a miRNA-dependent pathway. qRT-PCR assay showed that the expressions of miR-26a-5p, miR-29b, miR-214, and miR-363-3p were significantly increased by ox-LDL pre-treatment on HUVECs. While, ATV treatment could partially reverse the increased expressions of these miRNAs induced by ox-LDL pre-treatment (Figure 2). Among these miRNAs, the expression changes of miR-26a-5p was the most remarkable, and therefore, miR-26a-5p had been chosen for the following experiments.
Figure 2.
(A–D) The expressions of miR-26a-5p, miR-29b, miR-214, and miR-363-3p were downregulated by ATV treatment. RNA levels of miR-26a-5p, miR-29b, miR-214, and miR-363-3p in HUVECs after being treated with 100 mg/L ox-LDL and 10 μM ATV for 24 hours. ### P<0.001 compared to the CTL group. ** P<0.01, *** P<0.001 compared to the ox-LDL group. ATV – atorvastatin; ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vascular endothelial cells; CTL – control.
ATV inhibits ox-LDL-induced apoptosis and migration of HUVECs by downregulating the expression of miR-26a-5p
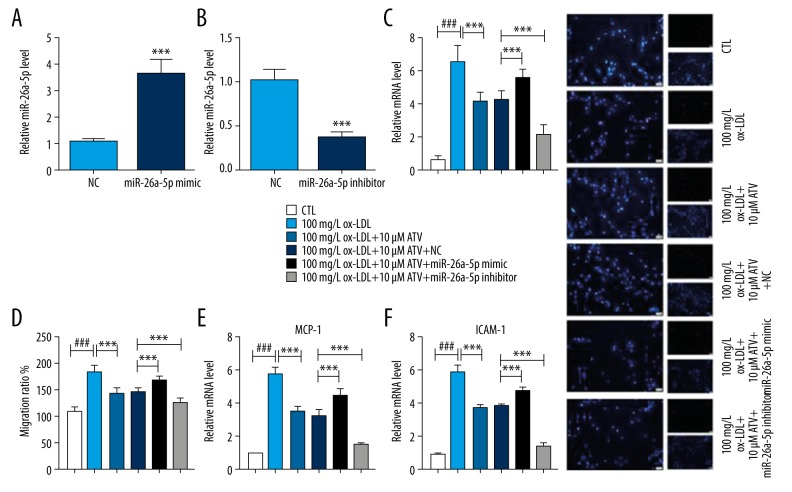
In order to investigate the role of miR-26a-5p in ox-LDL-induced HUVECs, we transfected miR-26a-5p mimic, miR-26a-5p inhibitor, and their corresponding negative control into HUVECs. We found that the expression of miR-26a-5p was remarkably increased by miR-26a-5p mimic transfection, and the expression of miR-26a-5p was significantly decreased by miR-26a-5p inhibitor transfection (Figure 3A, 3B). TUNEL staining showed that the inhibition of apoptosis induced by ATV in ox-LDL-induced HUVECs was attenuated by miR-26a-5p mimic, while it was enhanced by miR-26a-5p inhibitor (Figure 3C). Besides, the migration inhibition induced by ATV was attenuated by miR-26a-5p mimic and enhanced by miR-26a-5p inhibitor (Figure 3D). qRT-PCR also showed that the downregulations of MCP-1 and ICAM-1 after ATV treatment was decreased by miR-26a-5p inhibitor transfection and increased by miR-26a-5p mimic transfection (Figure 3E, 3F).
Figure 3.
ATV inhibits ox-LDL-induced apoptosis and migration of HUVECs by downregulating the expression of miR-26a-5p. (A, B) qRT-PCR was applied to detect the expression of miR-26a-5p in HUVECs after transfection with (A) miR-26a-5p mimic, (B) miR-26a-5p inhibitor and negative control (NC). (C) TUNEL staining was used to observe the percentage of apoptotic cells. (D–F) After being treated by ATV and ox-LDL for 24 hours, HUVECs were transfected with miR-26a-5p mimic, miR-26a-5p inhibitor, or NC to detect the (D) relative migration ratio, mRNA levels of (E) MCP-1 and (F) ICAM-1. Scale bar: 20 μm. ### P<0.001 compared to the CTL group. *** P<0.001 compared to indicated group. ATV – atorvastatin; ox-LDL – oxidized low-density lipoprotein; HUVECs – human umbilical vascular endothelial cells; qRT-PCR – quantitative real-time polymerase chain reaction; CTL – control.
MiR-26a-5p inhibits ox-LDL-induced migration and the release of adhesion-related molecules by regulating PTEN
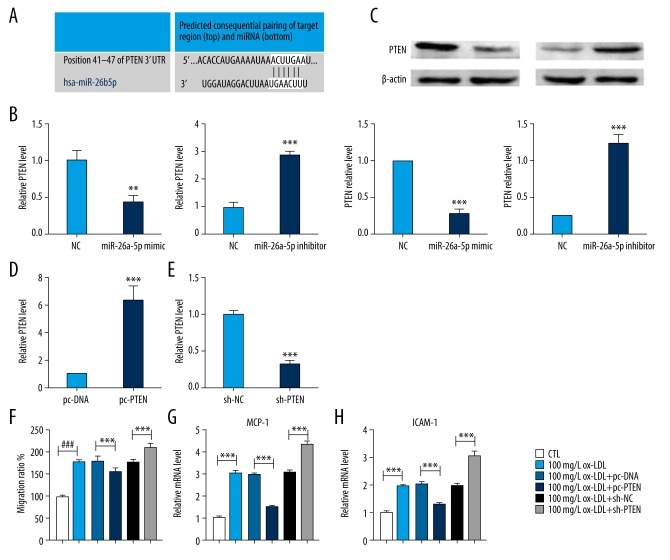
Finally, we found that there was a targeting relationship between miR-26a-5p and PTEN by bioinformatics prediction on the website of Targetscan database (http://www.targetscan.org) (Figure 4A). Therefore, to estimate the function of PTEN and miR-26a-5p in the occurrence and development of atherosclerosis, we measured the expression changes of PTEN in response to the changes of miR-26a-5p. qRT-PCR showed that the expression of PTEN was inhibited by miR-26a-5p mimic transfection, while increased by miR-26a-5p inhibitor transfection (Figure 4B, 4C). We also transfected the vector or shRNA of PTEN to evaluate the directly effect of PTEN in ox-LDL-induced HUVECs. qRT-PCR also showed that transfected with pc-PTEN could significantly increase the expression of PTEN, and the expression of PTEN could also be decrease by sh-PTEN transfection. Moreover, we found that the overexpression of PTEN could significantly downregulate the migration ratio of ox-LDL-induced HUVECs and decrease the expressions of MCP-1 and ICAM-1. Besides, the inhibition of PTEN could significantly upregulate the migration ratio of ox-LDL-induced HUVECs and increase the expressions of MCP-1 and ICAM-1 (Figures 4F–4H).
Figure 4.
MiR-26a-5p inhibits ox-LDL-induced migration and the release of adhesion-related molecules by regulating PTEN. (A) Predicted consequential pairing of PTEN and miR-26a-5p. (B) qRT-PCR was applied to detect the expression of PTEN in HUVECs after transfection with miR-26a-5p mimic, miR-26a-5p inhibitor and NC; n=6. ** P<0.01, *** P<0.001 compared to negative control (NC). (C) Western blot was applied to detect the expression of PTEN in HUVECs after transfection with miR-26a-5p mimic, miR-26a-5p inhibitor, and NC; n=6. *** P<0.001 compared to NC. (D, E) qRT-PCR was applied to detect the expression of PTEN in ox-LDL-induced HUVECs after being transfected with pc-DNA, pc-PTEN, sh-NC, and sh-PTEN. (F) Relative migration ratio. (G, H) qRT-PCR was applied to detect the expression of MCP-1 and ICAM-1 in ox-LDL-induced HUVECs after being transfected with pc-DNA, pc-PTEN, sh-NC, and sh-PTEN; n=4. *** P<0.001 compared to the indicated group. ox-LDL – oxidized low-density lipoprotein; PTEN – phosphatase and tensin homolog; HUVECs – human umbilical vascular endothelial cells; qRT-PCR – quantitative real-time polymerase chain reaction; CTL – control.
Discussion
Atherosclerosis is a systemic inflammatory disease, and it has been the major cause of life-threatening events including cardiovascular disease and stroke [15]. It is well known that the maintenance of normal structure and physiological function of vascular endothelial cells plays a crucial role in the early occurrence and development of atherosclerosis. In this study, we observed the effects of ATV on ox-LDL-induced damage of HUVECs, and we estimate the potential application of ATV in atherosclerosis. Our results demonstrated that ATV treatment could prevent HUVECs against ox-LDL-induced inhibition of cell viability, and the increase of apoptosis, migration and the release of MCP-1 and ICAM-1. Besides, miR-26a-5p could modulate the cell cycle progression of ox-LDL-induced HUVECs at least in part through PTEN. Our results suggested that ATV regulates HUVECs cell viability and migration through the miR-26a-5p/PTEN axis, clarifying a new mechanism of ATV on protecting against atherosclerosis.
Vascular endothelial cell (VECs) injury is the most important cause of atherosclerosis, and atherosclerosis is characterized by the proliferation and migration of VECs and inflammatory lesions. Previous studies have shown that ox-LDL could facilitate the migration and proliferation of HUVECs in the patient with atherosclerosis [16–18]. High concentrations of ox-LDL can induce the apoptosis of different cells through various ways, including promoting the production of ROS, activating caspase signaling pathway, and inducing the expression changes of apoptosis-related gene [19,20]. In this study, we found that 10–200 mg/L ox-LDL treatment could significantly decrease the cell viability in a dose-dependent manner. Especially, 100 mg/L ox-LDL treatment could significantly increase the numbers of apoptotic cells, as well as activate the expression of cleaved caspase-3.
ATV is a commonly used drug in the treatment of hypercholesterolemia, and the anti-atherosclerosis effect of ATV has been widely researched. In this study, we examined the effect of ATV on ox-LDL-induced atherosclerotic cell model. Our results indicated that 10 μM ATV pre-treatment could significantly reverse the ox-LDL-induced downregulation of cell viability in HUVECs. Li et al., demonstrated that ox-LDL treatment could inhibit the proliferation and migration of VSMCs, and at the same time increased the production of inflammatory cytokines and the expression of monocyte chemoattractant protein-1 (MCP-1) in a dose-dependent manner [21]. Wang et al., confirmed that ox-LDL treatment could promote the apoptosis and vascular inflammation, and migration of VSMCs [22]. Then, we next explored the influence of ATV on the migration ability and inflammatory reaction of HUVECs. Our results demonstrated that ox-LDL treatment could promote the migration of HUVECs, which can be reversed by ATV pre-treatment. The initiation and development of atherosclerosis are closely associated with the inflammation at the lesion site. The dysfunctional endothelium secretes adhesion molecules (e.g., MCP-1 and ICAM-1), which recruit inflammatory factors and trigger inflammation in the atherosclerotic lesion [23]. Next, we detect the expression of MCP-1 and ICAM-1 in ox-LDL-induced HUVECs with or without ATV treatment. Our results showed that ox-LDL could increase the overproductions of MCP-1 and ICAM-1, which were two adhesion-related molecules, and these results further demonstrated that ox-LDL could play a migration-promoting role in HUVECs. While this migration-promoting function can be reversed by ATV pre-treatment, indicating the potential usage of ATV in atherosclerosis.
MicroRNAs (miRNAs) are endogenous noncoding RNAs that regulate the expression of human genes and play important roles in various pathological and physiological processes [24,25]. Previous studies have shown that the specific expression of certain miRNAs has been identified as a key regulator of the cardiovascular system and increasing evidence has implicated some miRNAs can become necessary regulators of atherosclerosis by targeting important factors or key pathways [25]. Atherosclerosis is an inflammatory related disease, with expression changes of multiple of miRNAs(26]. Among these microRNAs (miRNAs), miR-26a-5p, miR-29b, miR-214 and miR-363-3p have been emerged as critical regulators in inflammation-mediated processes [12,13,27,28]. The present study showed that the expressions of miR-26a-5p, miR-29b, miR-214 and miR-363-3p were all increased in ox-LDL-treated HUVECs, and decreased by ATV pre-treatment. Our results demonstrated that the protective effects of ATV against ox-LDL-induced injury in HUVECs were attenuated by miR-26a-5p mimic transfection, while ameliorated by miR-26a-5p inhibitor transfection. These results suggested that ATV might play a protective role in ox-LDL-induced damage in HUVECs by downregulating the expression of miR-26a-5p. The phosphatase and tensin homolog (PTEN) gene has emerged as a well-known tumor suppressor gene. It is a crucial downregulator of the PI3K/AKT/mTOR pathway, which regulates the cell cycle, proliferation, migration and apoptosis [29–31]. However, the function of PTEN in atherosclerosis has rarely been reported. Through the bioinformatics analysis we found that PTEN was the downstream target of miR-26a-5p. Therefore, we detected the expression of PTEN in miR-26a-5p mimic or miR-26a-5p inhibitor treated HUVECs. Results showed that PTEN was increased by miR-26a-5p inhibitor, while decreased by miR-26a-5p mimic, indicating PTEN was one of the downstream genes of miR-26a-5p. Additionally, our results demonstrated that the overexpression of PTEN could facilitate ATV to maintain inhibition effect on migration and adhesion in ox-LDL-induced HUVECs.
Conclusions
Our results confirmed that ATV prevents HUVECs against ox-LDL-induced downregulation of cell viability, and upregulation of apoptosis, migration and the release of adhesion-related molecules by targeting miR-26a-5p/PTEN axis, providing us a new target and strategy for the treatment of atherosclerosis.
Footnotes
Source of support: This project was supported by the Natural Science Foundation of Heilongjiang Province (Grant LH2019H085)
Conflict of interests
None.
References
- 1.Prasher D, Greenway SC, Singh RB. The impact of epigenetics on cardiovascular disease. Biochem Cell Biol. :2019. doi: 10.1139/bcb-2019-0045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Ray S, Sawhney JPS, Das MK, et al. Adaptation of 2016 European Society of Cardiology/European Atherosclerosis Society guideline for lipid management to Indian patients – A consensus document. Indian Heart J. 2018;70(5):736–44. doi: 10.1016/j.ihj.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phrommintikul A, Krittayaphong R, Wongcharoen W, et al. Management of atherosclerosis risk factors for patients at high cardiovascular risk in real-world practice: A multicentre study. Singapore Med J. 2017;58(9):535–42. doi: 10.11622/smedj.2017044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao BC, Meng LB, Hao ML, et al. Chronic stress: A critical risk factor for atherosclerosis. J Int Med Res. 2019;47(4):1429–40. doi: 10.1177/0300060519826820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao S, Zhao D, Wang M, et al. Association between circulating oxidized ldl and atherosclerotic cardiovascular disease: A meta-analysis of observational studies. Can J Cardiol. 2017;33(12):1624–32. doi: 10.1016/j.cjca.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Di X, Tang X, Di X. Montelukast inhibits oxidized low-density lipoproteins (ox-LDL) induced vascular endothelial attachment: an implication for the treatment of atherosclerosis. Biochem Biophys Res Commun. 2017;486(1):58–62. doi: 10.1016/j.bbrc.2017.02.125. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang X, Li R, Maimaitijiang A, et al. miR-221-3p inhibits oxidized low-density lipoprotein induced oxidative stress and apoptosis via targeting a disintegrin and metalloprotease-22. J Cell Biochem. 2019;120(4):6304–14. doi: 10.1002/jcb.27917. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Yang C, Shi J, Gao T. Ox-LDL-induced lncRNA MALAT1 promotes autophagy in human umbilical vein endothelial cells by sponging miR-216a-5p and regulating Beclin-1 expression. Eur J Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.04.019. 172338. [DOI] [PubMed] [Google Scholar]
- 9.Fu C, Yin D, Nie H, Sun D. Notoginsenoside R1 protects HUVEC against oxidized low-density lipoprotein (Ox-LDL)-induced atherogenic response via down-regulating miR-132. Cell Physiol Biochem. 2018;51(4):1739–50. doi: 10.1159/000495677. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, Gao X, Jia Y, et al. Atorvastatin decreased circulating RANTES levels in impaired glucose tolerance patients with hypercholesterolemia: an interventional study. Diabetes Ther. 2017;8(2):309–19. doi: 10.1007/s13300-017-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WL, Yan WJ, Sun B, Zou ZP. Synergistic effects of atorvastatin and rosiglitazone on endothelium protection in rats with dyslipidemia. Lipids Health Dis. 2014;13:168. doi: 10.1186/1476-511X-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong X, Zhang L, Li Y, et al. Kaempferol alleviates ox-LDL-induced apoptosis by upregulation of miR-26a-5p via inhibiting TLR4/NF-kappaB pathway in human endothelial cells. Biomed Pharmacother. 2018;108:1783–89. doi: 10.1016/j.biopha.2018.09.175. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Wang F, Yu P, et al. Inhibition of miR-29b suppresses MAPK signaling pathway through targeting SPRY1 in atherosclerosis. Vascul Pharmacol. 2018;102:29–36. doi: 10.1016/j.vph.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Gonsalves CS, Li C, Mpollo MS, et al. Erythropoietin-mediated expression of placenta growth factor is regulated via activation of hypoxia-inducible factor-1alpha and post-transcriptionally by miR-214 in sickle cell disease. Biochem J. 2015;468(3):409–23. doi: 10.1042/BJ20141138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YH, Chen JW, Lin TH, et al. A performance guide for major risk factors control in patients with atherosclerotic cardiovascular disease in Taiwan. J Formos Med Assoc. :2019. doi: 10.1016/j.jfma.2019.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Wang Y, Ma J, et al. MicroRNA-20a participates in the aerobic exercise-based prevention of coronary artery disease by targeting PTEN. Biomed Pharmacother. 2017;95:756–63. doi: 10.1016/j.biopha.2017.08.086. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Li SF, Chen H, Song JX. MiR-106b-5p inhibits tumor necrosis factor-alpha-induced apoptosis by targeting phosphatase and tensin homolog deleted on chromosome 10 in vascular endothelial cells. Chin Med J (Engl) 2016;129(12):1406–12. doi: 10.4103/0366-6999.183414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Zhou M, Qin G, et al. MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress. Biochem Biophys Res Commun. 2014;446(4):952–58. doi: 10.1016/j.bbrc.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J, Cui R, Chen CH, Du J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148(5):2085–94. doi: 10.1210/en.2006-1709. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Cheng G, Yang X, et al. Tanshinol suppresses endothelial cells apoptosis in mice with atherosclerosis via lncRNA TUG1 upregulating the expression of miR-26a. Am J Transl Res. 2016;8(7):2981–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Zhi W, Liu F, et al. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp Cell Res. 2017;353(1):26–34. doi: 10.1016/j.yexcr.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Ge Z, Cheng Z, Zhao Z. Tanshinone IIA suppresses the progression of atherosclerosis by inhibiting the apoptosis of vascular smooth muscle cells and the proliferation and migration of macrophages induced by ox-LDL. Biol Open. 2017;6(4):489–95. doi: 10.1242/bio.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Jiang LL, Maimaitirexiati XM, et al. Irbesartan attenuates TNF-alpha-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-kappaB pathway in HUVECs. Eur Rev Med Pharmacol Sci. 2015;19(17):3295–302. [PubMed] [Google Scholar]
- 24.Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can J Cardiol. 2017;33(3):313–24. doi: 10.1016/j.cjca.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schober A, Weber C. Mechanisms of MicroRNAs in atherosclerosis. Annu Rev Pathol. 2016;11:583–616. doi: 10.1146/annurev-pathol-012615-044135. [DOI] [PubMed] [Google Scholar]
- 26.Natarelli L, Schober A. MicroRNAs and the response to injury in atherosclerosis. Hamostaseologie. 2015;35(2):142–50. doi: 10.5482/HAMO-14-10-0051. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Ma BD, Liu S, et al. Long noncoding RNA LINC00341 promotes the vascular smooth muscle cells proliferation and migration via miR-214/FOXO4 feedback loop. Am J Transl Res. 2019;11(3):1835–42. [PMC free article] [PubMed] [Google Scholar]
- 28.Kastle M, Bartel S, Geillinger-Kastle K, et al. MicroRNA cluster 106a~363 is involved in T helper 17 cell differentiation. Immunology. 2017;152(3):402–13. doi: 10.1111/imm.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui C, Li S, Wu D. Znhit1 inhibits breast cancer by up-regulating PTEN to deactivate the PI3K/Akt/mTOR pathway. Life Sci. 2019;224:204–11. doi: 10.1016/j.lfs.2019.03.067. [DOI] [PubMed] [Google Scholar]
- 30.Yuan J, Su Z, Gu W, et al. MiR-19b and miR-20a suppress apoptosis, promote proliferation and induce tumorigenicity of multiple myeloma cells by targeting PTEN. Cancer Biomark. 2019;24(3):279–89. doi: 10.3233/CBM-182182. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Liu H, Jiang M, et al. Targeting microRNA-21 suppresses gastric cancer cell proliferation and migration via PTEN/Akt signaling axis. Cell Transplant. 2019;28(3):306–17. doi: 10.1177/0963689719825573. [DOI] [PMC free article] [PubMed] [Google Scholar]