Abstract
Therapies targeting cell-surface antigens in acute myeloid leukemia (AML) have been tested over the past 20 years with limited improvement in overall survival. Recent advances in the understanding of AML pathogenesis support therapeutic targeting of leukemia stem cells as the most promising avenue toward a cure. In this review, we provide an overview of the evolving leukemia stem cell (LSC) model, including evidence of the cell of origin, cellular and molecular disease architecture, and source of relapse in AML. In addition, we explore limitations of current targeted strategies utilized in AML and describe the various immunophenotypic antigens that have been proposed as LSC-directed therapeutic targets. We draw lessons from current approaches as well as from the (pre)-LSC model to suggest criteria that immunophenotypic targets should meet for more specific and effective elimination of disease-initiating clones, highlighting in detail a few targets that we suggest fit these criteria most completely.
Leukemias are hematologic malignancies originating in the bone marrow (BM) that lead to abnormal expansion of white blood cells in an acute or chronic manner. Acute myeloid leukemia (AML) represents around one-third of leukemias and about 10,000 deaths occur yearly in the United States as a result of this disease (Noone et al. 2018). AML is characterized by blocked differentiation and clonal overproliferation of myeloid hematopoietic precursor cells, leading to features of BM failure, and if not treated, results in death within months. Although chemotherapeutic treatments (most commonly cytarabine [ara-C] and anthracyclines) can eliminate detectable leukemic cells in the BM and blood and successfully lead the majority of patients into remission, most patients still fatally relapse (Ferrara and Schiffer 2013; Döhner et al. 2015), necessitating the discovery of novel approaches for treating AML.
Findings throughout the past 25+ years provide evidence that AML and the closely related “pre-leukemic” myelodysplastic syndromes (MDS) arise from primitive hematopoietic cells that harbor mutations and epigenetic aberrations, initially leading to formation and/or expansion of pre-leukemic cells and ultimately to transformed leukemic stem cells (LSCs) capable of regenerating and maintaining the disease. Experimentally, LSCs are most commonly functionally defined by their ability to initiate and maintain AML in mice. Sequencing studies have provided evidence that these disease-initiating cells are not eliminated by chemotherapy and eventually prompt MDS and AML relapse. Therefore, to achieve permanent cures of AML and MDS and prevent relapse, elimination of LSC is essential. The eradication of pre-LSC (hematopoietic stem cells [HSCs] primed for transformation while retaining the ability to contribute to multilineage differentiation) is also likely necessary. In this review, we will summarize the evolving LSC model and analyze current and prospective strategies for treating AML by targeting LSC populations via aberrantly expressed surface antigens.
THE LEUKEMIA STEM CELL MODEL
Significant evidence has been gathered supporting the existence of LSCs capable of initiating, maintaining, and regenerating AML (for review, see Passegue et al. 2003; Pandolfi et al. 2013; Corces et al. 2017; Thomas and Majeti 2017). To validate this model, several areas have been examined, including (1) the cell of origin for AML, (2) functional heterogeneity in the organization of AML, and (3) contribution of LSCs to relapse.
The Cell of Origin for AML
Initial Evidence from Cytogenetics Studies
Cytogenetic examination of human AML cells provided the first evidence of a stem cell origin in this cancer. The coupling of karyotyping with colony-forming potential assays in primary human AML samples identified cytogenetically abnormal cells that were capable of long-term culture and/or multilineage differentiation, suggesting the presence of these aberrations in stem cells (Hogge et al. 1987; Tamura et al. 1993). In addition, cytogenetic analysis together with immunophenotyping uncovered identical aberrations in bilineage leukemias, supporting the origin of these leukemias as an immature cell with both myeloid and lymphoid potential (Sun et al. 1991; Carbonell et al. 1996; Hyakuna et al. 1998). Finally, cytogenetics coupled with fluorescence-activated cell sorting (FACS) detected chromosomal rearrangements in immunophenotypically defined HSCs and hematopoietic progenitor cells (CD34+CD38− and CD34+CD38+ cells, respectively) in primary MDS and AML samples at diagnosis (Haase et al. 1995, 1997; Feuring-Buske et al. 1999; Nilsson et al. 2000, 2002; Barreyro et al. 2012; Will et al. 2012; Elias et al. 2014; Woll et al. 2014; Shastri et al. 2017) and in the relapse setting (Engel et al. 1999; Diez-Martin et al. 2000).
Early Evidence from Mouse Modeling of Pre-Leukemia
Modeling of frequent molecular changes in MDS/AML in murine models provided important early conceptual insight into pre-leukemic stages at the hematopoietic stem/progenitor level and their functional relevance for leukemia development and maintenance. These studies include reduction in expression of the transcription factor PU.1 (Steidl et al. 2006; Will et al. 2015), overexpression of the MLL-AF9 and CBFβ–SMMHC fusion oncogenes (Kuo et al. 2006; Somervaille and Cleary 2006; Pulikkan and Castilla 2018), and introduction of CEBPα mutations (Kirstetter et al. 2008; Bereshchenko et al. 2009), all resulting in distinct pre-leukemic phases characterized by myeloid-biased HSC, blocked differentiation, and accumulation of immature myeloid or myelomonocytic cells in the BM and blood. AML development occurred in these mice with a latency of 2–6 months, which indicated for the first time that a distinct pre-leukemic phase with definable cell-intrinsic properties exists, and that stem and immature progenitor cells play a key role in this process. In addition, it was found that some pre-leukemic molecular alterations, such as down-regulation of the transcription factors PU.1 and JunB, remain functionally critical for fully transformed LSC at later stages, which indicated that targeting pre-leukemic/founding aberrations may represent a promising therapeutic avenue (Steidl et al. 2006).
Evidence from Gene Expression Studies
Further support for the HSC origin of AML in humans was initially collected through studies of gene expression in purified cell populations. Gene expression analysis of phenotypic short-term HSCs (Lin–CD34+CD38–CD90low) from AML patients identified dysregulated expression of genes in this population such as the JunB and PU.1 transcription factors (Steidl et al. 2006), and the epigenetic regulator SATB1 (Steidl et al. 2007). More broadly, microarray transcriptional profiling studies of immunophenotypically defined HSCs from MDS and AML versus normal BM showed that MDS/AML HSCs exhibit gene expression profiles resembling normal HSCs (Nilsson et al. 2007; Gentles et al. 2010; Eppert et al. 2011), but that multiple specific genes and pathways are dysregulated at the stem cell level such as those involved in apoptosis, adherens junctions, interleukin (IL), Jak-STAT, and interferon (IFN) signaling (Nilsson et al. 2007; Majeti et al. 2009a; Barreyro et al. 2012; Will et al. 2012), suggesting involvement of HSCs in human AML pathogenesis.
Evidence from Sequencing Studies Using Single-Nucleotide Variants as Markers of Subclonality
A logical argument for the stem cell origin of leukemia is that the number of mutations and therefore time required for cellular transformation must occur in a long-lived cell with high proliferative potential, as mutations would not have sufficient time to accumulate in progenitor and more differentiated cells because of their transient nature. Single-colony and single-cell sequencing technologies have allowed for direct evidence of this phenomenon. A cell-surface immunophenotype has been identified (Lin− CD34+CD38−CD99−TIM-3−) that distinguishes functionally normal residual HSCs from AML patient samples (as defined by transplantation experiments) (Jan et al. 2011). FACS and targeted sequencing of these normal HSCs from AML patient samples showed that genetic lesions serially accumulate in HSCs, as multiple clones existed in the HSC pool containing the partial spectrum of all mutations seen in AML cells from that patient (Jan et al. 2012). Likewise, whole-exome sequencing on paired diagnosis/remission/relapse AML samples identified that CD34+ progenitors as well as mature cells harbor some of the mutations from the original leukemia at the time of remission (Corces-Zimmerman et al. 2014). The persistence of these mutations in remission indicates that they originate from a stem cell that survived chemotherapy.
An HSC origin is further supported by the detection of the same DNMT3A mutations in both the dominant leukemic clone and in normal mature blood cells such as T cells (Shlush et al. 2014). The presence of identical mutations in AML and T cells strongly suggests that the cell of origin in AML is long-lived and has multilineage differentiation potential. In addition, in at least two documented occasions, transplantations performed with donor BM retrospectively determined to harbor a DNMT3A mutation resulted in AML in the recipient with a DNMT3A-mutant leukemic clone (Yasuda et al. 2014; Hahn et al. 2015). These anecdotal findings suggest an HSC origin as these cells are responsible for long-term repopulation of the immune system.
Single-cell sequencing of CD34+CD38− IL1RAP+/CD123+/CD45RA+ BM cells from longitudinal MDS and secondary AML samples from individual patients revealed that MDS and AML arise from different but ancestrally related clones, further supporting a stem cell origin for these diseases (Chen et al. 2019). Importantly, the stem cell origin theory has been tested prospectively as well. It was recently shown that a succession of stages ultimately leading to a serially transplantable leukemia could be modeled by creating induced pluripotent stem cells from primary MDS and AML samples with increasing numbers of driver mutations (Kotini et al. 2017).
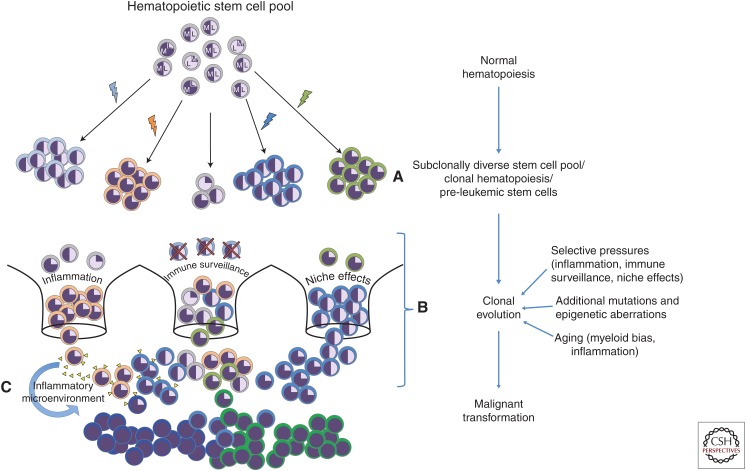
It has been estimated that HSCs acquire between 1 and 2 exonic mutations per decade, and while most of these do not manifest in clonal expansion or disease, several studies have found that at least 5%–10% of individuals over age 65 have disproportionate expansion of single hematopoietic clones, with the percentage further increasing with age. Detection of mutations associated with myeloid malignancies at a variant allele frequency (VAF) of 2% or greater in the peripheral blood currently defines this phenomenon, termed clonal hematopoiesis (CH) (Genovese et al. 2014; Jaiswal et al. 2014; Xie et al. 2014; Shlush 2018). CH mutations found in BM HSCs and hematopoietic progenitors further hints that CH represents a premalignant event preceding myeloid malignancies (Fig. 1A; Arends et al. 2018).
Figure 1.
Selective pressure model of acute myeloid leukemia (AML). A proposed model showing transformation of the hematopoietic stem cell (HSC) pool from normal to malignant. “Normal” HSCs are shown in gray. Myeloid (M) and lymphoid (L) biased stem cells are present, as well as unbiased HSCs (represented as equal proportion M and L). Different aberrations such as mutations (colored lightning bolts) are associated with certain HSC subclones that can be detected, for example, by sequencing (clonal hematopoiesis) (A). These aberrations can occur in a lineage-biased stem cell or may confer a lineage bias. Selective pressures such as inflammation, immune surveillance, and niche effects may give certain HSC clones an advantage over others (i.e., inflammation gives the clone with an orange mutation a selective advantage over normal HSCs). Clonal evolution is also potentially further driven by additional mutations and aging-associated phenomena such as chronic inflammation and myeloid bias (B). For example, an inflammatory microenvironment created by pre-leukemic clones can promote selective expansion of mutated clones over normal HSCs (C). Together, these factors can eventually lead to malignant transformation and achievement of clonal dominance of particular stem cell subclones, and consecutive uncontrolled proliferation of leukemic bulk clones (shown as royal blue and bright green cells).
The nature of the transition from CH to myeloid malignancies is the subject of intense investigation (for review, see Bowman et al. 2018). One study estimated that any detectable CH is associated with an 11-fold greater risk of hematologic cancer and this risk increases to almost 50-fold with a greater clone size (VAF of 10% or greater) (Jaiswal et al. 2014; Steensma et al. 2015; Corces et al. 2017). However, the overall risk for hematologic malignancy in individuals with CH has been estimated to be only 4% (Jaiswal et al. 2014), indicating that further clonal evolution is required for cancer development. Two independent longitudinal analyses detected AML-relevant mutations in whole blood samples several years before AML diagnosis and found that higher mutational burden correlated with increased rates of AML progression (Abelson et al. 2018; Desai et al. 2018). While these studies were not done in BM or in stem-cell-enriched populations, the presence of these mutations years before diagnosis indicates their occurrence in long-lived cells and represents indirect evidence for the accumulation of mutations in HSCs or expansion of HSCs with existing mutations to a detectable limit. It would also be informative, albeit logistically challenging, to perform single-HSC sequencing on these samples to identify co-occurring mutations and their association with development and time-to-development of AML.
Disruption of Hematopoietic Stem Cell Function and Integrity as an Early Step in the Pathogenesis of Myeloid Malignancies
In the BM niche, HSCs are generally maintained in a dormant state with infrequent cell divisions to limit the opportunity for mutations to arise from the byproducts of ATP production (reactive oxygen species [ROS]) or errors during genome replication (Ito et al. 2006; Pietras et al. 2011). Studies have estimated that human HSCs enter the cell cycle on average every 40 weeks (Catlin et al. 2011), implying that there is tight regulation on maintaining HSCs in a noncycling “quiescent” state the vast majority of the time. Accordingly, it has been shown that DNA damage accumulates in HSCs with age, and cell-cycle-associated replication stress contributes to functional impairment in aging HSCs, including reduced proliferative and regenerative capacity (Rossi et al. 2008; Flach et al. 2014).
Although HSCs are usually kept dormant, they must be poised to undergo cell division when an injury or infection cannot be controlled locally. In this case, increased proinflammatory cytokines and Toll-like receptor ligands directly and/or indirectly stimulate hematopoietic stem and progenitor cells (HSPCs) to proliferate and differentiate to regain homeostasis and meet the blood and immune system demands (King and Goodell 2011; Takizawa et al. 2012; Pietras 2017). Different combinations of cytokines and ligands stimulate particular subsets of HSCs to differentiate along specific lineages. For instance, in response to viral infections, IFN-γ is released, promotes differentiation of myeloid-biased HSCs (Matatall et al. 2014) and leads to expression of IL-6 by the BM niche. IL-6 then acts specifically on the multipotent progenitor (MPP) population to regulate hematopoietic output, driving myeloid output at the expense of lymphoid production (Reynaud et al. 2011; Mirantes et al. 2014). Direct cytokine-mediated activation of HSPCs has been demonstrated to be an efficient alternative to cytokine release by mature cells and subsequent paracrine stimulation of HSPCs for rapid myeloid recovery (Zhao et al. 2014).
Although proinflammatory signals can recover homeostasis in acute settings, chronic exposure has been shown to lead to HSC exhaustion and BM failure. For instance, IL-1 exposure in mice resulted in an HSC pool with limited ability to differentiate into nonmyeloid lineages and reduced self-renewal potential as assessed by competitive transplantation (Pietras et al. 2016). Chronic IFN-γ compromised long-term HSC reconstitution capacity after transplantation (Baldridge et al. 2010) and chronic tumor necrosis factor α (TNF-α) has also been shown to inhibit HSC growth and survival, reducing reconstitution ability of human CD34+CD38− cells (Baldridge et al. 2011). These drastic effects of chronic inflammation are likely lessened in the endogenous, nontransplant setting where chronic inflammatory signaling and the resultant cytokine microenvironment have been postulated to instead cause continual disruption of HSC quiescence, frequent cell cycling, and an increased probability of accumulating DNA replication-associated mutations (Cohen et al. 1991; Pietras 2017). This idea is supported by studies showing that injection of mice with the double-stranded RNA mimic pI:pC, which mimics viral infection, induces HSCs to exit from quiescence in an IFN-α-receptor-dependent manner and accumulate DNA damage (Pietras et al. 2014; Walter et al. 2015). Similar effects were seen upon chronic LPS exposure (Esplin et al. 2011; Takizawa et al. 2017). In line with this, large epidemiological studies demonstrated that a prior history of infectious disease led to a 1.3-fold increased risk of AML and MDS and a prior history of autoimmune disease was associated with 1.7- and 2.1-fold increased risks for AML and MDS, respectively (Anderson et al. 2009; Kristinsson et al. 2011).
For a malignancy to develop, a mutated HSC conceivably must still retain its ability to sustain itself. Aside from triggering cell cycling and, consequently, mutations in HSCs, chronic inflammation can also act as a selective force and promote expansion of HSCs that are resistant to the detrimental effects of a proinflammatory environment (Fig. 1B). This phenomenon was elegantly shown in a mouse model of pre-leukemic myeloproliferation with variable expressivity. Specifically, upon genetic knockout of Tet2, the amount of myeloproliferation seen was positively associated with the presence of bacterial dissemination (potentially because of Tet2−/−-related intestinal defects), and the presence of bacteria or of TLR agonists were sufficient to cause this myeloproliferation through increased IL-6 secretion. Further, Tet2−/− HSPCs were specifically sensitive to IL-6, in part because of up-regulation of the IL-6 receptor compared to Tet2+/+ HSCs, providing an example of mutated HSCs acquiring properties that give them a competitive advantage in a proinflammatory environment (Meisel et al. 2018). Likewise, in the human setting, HSCs from patients with myeloproliferative neoplasms harboring activating JAK2 mutations were found to be resistant to suppressive growth signaling from chronic TNF-α stimulation and were clonally selected in this environment (Fleischman et al. 2011; Takizawa et al. 2012; Craver et al. 2018).
Contrastingly, pre-leukemic or CH mutations may foster an inflammatory environment. For instance, although only described in mature hematopoietic cells, Tet2 was shown to repress expression of inflammatory cytokines including IL-1β and IL-6. Loss of function mutations in Tet2 therefore derepressed these signals and contributed to an inflammatory environment, which in the leukemia setting could promote disease progression (Zhang et al. 2015; Fuster et al. 2017; Jaiswal et al. 2017). CH is also associated with increased risk of diseases with inflammatory components, including coronary heart disease and various cancers (Coombs et al. 2017; Jaiswal et al. 2017); thus, a positive feedback loop between an inflammatory microenvironment and the development of and selection for aberrant HSCs may be a causative factor in AML development (Fig. 1C).
The question remains why certain mutations, such as those providing a proliferative advantage, would necessarily result in myeloid malignancies. One theory for this is the changing capacity and characteristics of HSCs as they age. It is well described in human and mouse hematopoiesis that deficiencies in the adaptive immune system occur with aging. This is accompanied by myeloid skewing, specifically demonstrated by increased myeloid repopulation potential in HSC transplantation experiments from young versus old mice and young versus old human BM specimens (Rossi et al. 2005; Rundberg Nilsson et al. 2016; Yamamoto et al. 2018). Accordingly, the majority of pediatric leukemias are lymphoid, whereas adult leukemias are most frequently myeloid, even when initiated by the same oncogene, for instance t(9;22) (MLL-AF9), suggesting an alteration in the potential of HSCs with age (Meyer et al. 2013). This increased propensity toward myeloid differentiation provides insight into the phenomenon of age-related myeloid malignancies (Fig. 1B), yet the cell-intrinsic mechanisms for this occurrence must still be investigated further. Perhaps an age-related inflammatory environment could provide a link to explain this propensity for myeloid disease.
Functional Heterogeneity in the Organization of AML
The first description of the heterogeneous composition of AML was provided by transplantation of subpopulations of cells from leukemic BM specimens into immunodeficient mice. Whereas CD34+CD38− cells were capable of initiating disease in recipient animals, CD34− cells were not, providing the first indication that all leukemia cells are not created equal, and that a hierarchy exists within a cancer cell population (Lapidot et al. 1994; Bonnet and Dick 1997). These studies were validated in vitro by long-term proliferation and colony output assays of immunophenotypic subpopulations from AML samples (Sutherland et al. 1996). Higher-resolution experiments were done by using lentiviral transduction to mark single cells from AML specimens and performing serial transplantations. These experiments led to the identification of a more complex hierarchy, with subpopulations of cells that were capable of short-term or long-term repopulation, and/or whose repopulation only reached a detectable limit after secondary or tertiary transplantation (indicating initial quiescence). Tracking clones via a lentiviral integration site identified that quiescent long-term leukemia-initiating cells (LICs) gave rise to long-term LICs, which gave rise to short-term LICs, eventually generating bulk tumor cells that lose the ability to self-renew, but are also unable to differentiate, leading to their uncontrolled accumulation (Hope et al. 2004). Of note, expanded studies with a higher number of primary AML specimens have identified that the leukemia-initiating population is not limited to the CD34+CD38− immunophenotype and in fact the CD34+CD38+ and CD34− populations are also in some cases capable of leukemia initiation (Martelli et al. 2010; Taussig et al. 2010; Goardon et al. 2011; Sarry et al. 2011; Quek et al. 2016). Although these studies have provided pivotal insight into the heterogeneity that exists in AML, the use of xenotransplantation into mice to determine human tumor heterogeneity may select for different functional properties or may underestimate (or overestimate) the actual LSC frequency. These methodological caveats should be kept in mind in our interpretations with regard to the precise architecture of human AML.
Contribution of LSCs to Relapse
LICs have been shown to exhibit stem cell characteristics that contribute to chemotherapy resistance—including quiescence and drug efflux potential (Terpstra et al. 1996; Costello et al. 2000). Indeed, human AML xenograft studies in mice found that certain cell-cycle-quiescent leukemia cells localize in the BM endosteal region, remain after chemotherapy, and can transplant AML to secondary recipients (Ishikawa et al. 2007; Saito et al. 2010). Similarly, sequencing studies have demonstrated that HSCs harboring leukemia-associated mutations persist after standard chemotherapy in AML patients (Miyamoto et al. 2000; Ding et al. 2012; Welch et al. 2012; Corces-Zimmerman et al. 2014). However, in light of evidence that nonleukemic clones expand following induction chemotherapy (Wong et al. 2016), distinguishing CH with a high VAF from incomplete elimination of leukemic clones will be crucial for elucidating the nature of relapse. Considering that it is currently not possible to detect whether chemotherapy has eliminated every leukemic cell because of sensitivity limitations in minimal residual disease (MRD) detection (Hourigan et al. 2017; Hollein et al. 2018), whether relapse is being driven by residual LSCs (dominant or minor subclones), by chemotherapy-induced mutations that lead to drug resistance, or by chemotherapy-induced clonal evolution of pre-LSCs, is not entirely clear (Jan and Majeti 2013), but current evidence supports the former (Corces et al. 2017; Shlush et al. 2017). Specifically, sequencing of paired diagnosis and relapse samples has demonstrated several cases of minor subclones at diagnosis that present as the dominant subclone at relapse (Parkin et al. 2013; Corces-Zimmerman et al. 2014; Shlush et al. 2017). Because of detection limits of sequencing, even in studies where new subclones appeared at relapse (Ding et al. 2012; Corces-Zimmerman et al. 2014), it cannot be ruled out that these clones were present in low frequency at diagnosis and expanded after chemotherapy.
Until more sensitive techniques are available, it may not become entirely clear what the nature of relapse is. Despite these limitations, the suspected stem cell origin of AML relapse suggests that eradication of both malignant and premalignant HSPC, but not necessarily HSCs in a CH stage, holds promise for achieving lasting cures. Thus, an ideal therapeutic target should be present not only on LSC-enriched populations at diagnosis, but also in the relapse setting, and with expression during complete remission being predictive of relapse.
PRESENT AND FUTURE STRATEGIES FOR TARGETING LSCs IN AML
Associated Limitations of Current Targeted Approaches in AML
In AML, heterogeneity in the cellular compartment of origin and the varied mutational spectrum across patients have challenged the identification of commonly dysregulated pathways that are relevant in the initiation and maintenance of the disease (Sarry et al. 2011; Papaemmanuil et al. 2016). Sequencing studies have determined that AML is genetically complex, with patients typically having multiple driver mutations, and despite there being overlap in these mutations, no single genetic aberration is found in more than 35% of patients (Papaemmanuil et al. 2016). Thus, a treatment designed to eliminate cells carrying a particular mutation will likely only be relevant in a minority of patients. Further, individual patients have high intratumor heterogeneity; any detected mutation is usually only present in a subset of tumor cells and focusing a therapeutic strategy on a particular mutation may leave many subclones (especially smaller ones) unaccounted for (Ding et al. 2012; Welch et al. 2012; Parkin et al. 2013; Chen et al. 2019). For this reason, several groups have sought to identify common characteristics of leukemia cells across AMLs with different genetic profiles—most notably cell-surface protein markers—that might be exploited for therapeutic targeting in most patients (Jordan et al. 2000b; Majeti et al. 2009b). Three of the most commonly sought-after targets in AML cells are CD33, FLT3, and CD123, but clinically, toxicities caused by lack of specificity of expression and resistance mechanisms are presenting considerable challenges.
CD33
Currently, the only approved immunotherapy in AML is gemtuzumab ozogamicin, an anti-CD33 antibody conjugated to a cytotoxic derivative of calicheamicin (Sievers et al. 1999, 2001), but this treatment has been on and off the market because of hematologic and nonhematologic toxicities (Baron and Wang 2018). In 2017, it was reapproved for use at lower doses in combination with chemotherapy and as a single agent in patients not eligible for intensive chemotherapy or with relapsed/refractory AML (Amadori et al. 2010; Castaigne et al. 2012). Whereas CD33 is often expressed on LSC-enriched populations (Griffin et al. 1984; Hauswirth et al. 2007; Sadovnik et al. 2017), hematopoietic toxicity of anti-CD33 treatments relate to its expression on normal hematopoietic cells, including HSCs (Taussig et al. 2005; Kikushige et al. 2010; Sadovnik et al. 2017; Haubner et al. 2019). Several creative approaches are being tested to circumvent this toxicity. For instance, a bispecific antibody targeting CD47 and CD33 was developed and reported to be effective in an AML xenograft model (Boyd-Kirkup et al. 2017). Another approach under development is the use of CRISPR-Cas9 technology to delete CD33 in HSCs and subsequent use of anti-CD33 targeting approaches such as CAR T cells to eliminate leukemic cells (Kim et al. 2018).
FLT3
FLT3, or CD135, is a highly sought-after therapeutic target in AML because of the substantial fraction of patients harboring activating mutations in this gene (up to 35%) (Gilliland and Griffin 2002; Patel et al. 2012; Papaemmanuil et al. 2016). In-frame length polymorphisms (resulting from internal tandem duplications) in the juxtamembrane domain of FLT3 (FLT3-ITD) were discovered in AML patients in 1996, and led to a constitutively active kinase by destabilizing the inhibitory functions of this domain (Chan 2011). FLT3 mutations are demonstrated to be driving mutations in leukemogenesis (Lee et al. 2005; Schessl et al. 2005; Kim et al. 2008; Mallardo et al. 2013), but detection of minor leukemic subclones with FLT3-ITD mutations indicate it is preferentially a late event (Corces-Zimmerman et al. 2014; Shouval et al. 2014). Targeted sequencing of single-LSC-derived colonies has resolved the likely order of acquisition of mutations in several patients, concluding that FLT3 mutations occur late, as they never occur as the sole aberration but only co-occur with other leukemia-relevant mutations (Jan et al. 2012). Meanwhile, sequencing of residual normal HSC-derived colonies showed that FLT3 mutations never occur in these cells (Jan et al. 2012; Corces-Zimmerman et al. 2014), and FLT3 mutations are not found in CH (Abelson et al. 2018; Desai et al. 2018), suggesting they are not early, or pre-leukemic events. In addition, FLT3 mutations are often gained or lost between diagnosis and relapse, further evidence that they are not a part of the founding AML clone (Krönke et al. 2013; Corces-Zimmerman et al. 2014; Shlush et al. 2014; Shouval et al. 2014).
Over 60 clinical trials have been conducted to test FLT3 inhibitors alone or in combination therapy in AML. Currently, only midostaurin has FDA approval for treatment of AML patients with FLT3-ITD mutations in combination with standard induction therapy, as it modestly improved event-free and overall survival in clinical trials (Stone et al. 2017). Although most FLT3-ITD subclones are sensitive to tyrosine kinase inhibitor (TKI) treatment, particularly circulating leukemic cells, BM leukemic cells are not effectively eliminated and MRD often contains FLT3-ITD+ cells. Studies of samples from patients who relapsed while under treatment with the TKI quizartinib have uncovered resistance mechanisms. These include suboptimal pharmacokinetics and pharmacodynamics of TKIs, acquisition of additional mutations in FLT3 (commonly in the kinase domain) leading to decreased inhibitor efficacy (Alvarado et al. 2014; Ghiaur and Levis 2017; Smith et al. 2017), and activation of compensatory signaling pathways, including stromal-derived fibroblast growth factor and CXCL12, which lead to activation of mitogen-activating protein kinases (MAPKs) and Bcl2 family survival pathways (Yang et al. 2014; Traer et al. 2016; Ghiaur and Levis 2017; Smith et al. 2017). In addition, high FLT3 ligand levels in the BM that occur during chemotherapy have been proposed to stimulate FLT3-ITD+ cells through their remaining FLT3 wild-type (WT) copy, facilitating cell survival amid quizartinib treatment, which preferentially targets the mutated receptor (Sato et al. 2011; Yang et al. 2014; Chen et al. 2016; Taylor et al. 2017).
Elimination of MRD is not apparent under the use of FLT3 inhibitors because of one or many of these mechanisms, and the findings that FLT3 mutations are not early events in leukemogenesis may be the driving reason behind their limited sustained efficacy. Whereas proposed immunotherapeutic approaches (Reiter et al. 2018) may prevent some mechanisms of resistance by eliminating any FLT3-expressing leukemic cells, expression on normal HSCs (Sadovnik et al. 2017) will likely prevent progression of this tactic to wider use in the treatment and cure of AML.
CD123
Several studies have reported high expression of CD123 (IL-3Rα) on CD34+CD38− populations from AML samples (Jordan et al. 2000a; Munoz et al. 2001; Graf et al. 2004; Yalcintepe et al. 2006; Sadovnik et al. 2017). The expression of CD123 on normal HSPC is somewhat conflicting. Most analyses of CD123 protein expression by flow cytometry in normal human hematopoietic cells have reported that CD123 is expressed on a low–mid proportion of normal BM CD34+ cells, attributed to expression on myeloid progenitors (common myeloid and granulocyte-macrophage progenitors [CMPs and GMPs]), but with no expression on CD34+CD38− cells (Sato et al. 1993; Jordan et al. 2000a; Munoz et al. 2001; Graf et al. 2004). However, other studies have detected expression in HSCs (Taussig et al. 2005; van Rhenen et al. 2007a; Sadovnik et al. 2017; Haubner et al. 2019) and when side population (SP) stem cells from normal BM were analyzed, CD123 was detected in up to 82% of SP cells with a median of 27% (Moshaver et al. 2008). CD123 was also highly expressed in CD34+CD38− cells in regenerating BM, which may be particularly relevant for patients undergoing chemotherapy (van Rhenen et al. 2007a).
CD123 targeting by monoclonal antibodies has been modestly effective in preclinical models, at least in part via inhibition of IL-3 signaling and Fc-mediated recruitment and cell killing by innate immune effector cells (Jin et al. 2009). Several CD123-directed agents have been tested in phase I/II clinical trials, with results awaited (NCT02113982, NCT02152956, NCT02715011 (suspended), NCT02730312, NCT02848248 (terminated). The CD123 monoclonal antibody talacotuzumab reached a phase III clinical trial to be tested in combination with decitabine in patients with AML not eligible for intensive chemotherapy, but was discontinued (NCT02472145), and another phase II clinical trial with talacotuzumab as a monotherapy (NCT02992860) was terminated because of risk to patients. Toxicity to normal HSPC may limit whether CD123-directed therapies will progress to clinical approval (Thomas and Majeti 2017), particularly given the importance of IL-3 for hematopoietic cell viability and proliferation (Barreda et al. 2004).
Considerations for Prospective LSC Targets
Advances in the LSC model imply conditions that therapeutic targets in AML should meet. For instance, studying the cell of origin and architecture of AML indicate that a Lin−CD34+CD38−CD90− immunophenotype is most characteristic of LICs, thus proteins expressed on this population in AML samples have therapeutic promise. Further, studies of the subclonal composition of relapse suggest that the timing in which a target is expressed in the transformation process should be of high importance. Toxicities associated with current targeted therapies reinforce the importance of minimal expression on normal tissues and hint at the benefits and drawbacks associated with different classes of biopharmaceutical agents. These and other considerations are detailed below.
Immunophenotype
Several proteins expressed on immunophenotypically defined LSCs have been discovered by transcriptional profiling and subsequent validation of protein overexpression in HSC-enriched populations (CD34+CD38− cells) from AML versus normal BM samples. Tables 1 and 2 list these targets, expression on LSC-enriched populations and in normal tissues, described functions in AML, and exploration of targeting strategies. The immunophenotypic populations profiled in many studies are somewhat heterogeneous, containing only a fraction of true LSCs; thus, there have been efforts to improve immunophenotypic enrichment of LSCs. Studies coupling immunophenotyping of AML samples and xenotransplantation have identified that LSC populations in over 90% of cases bear a Lin−CD34+CD38−CD90− immunophenotype (Goardon et al. 2011; Thomas and Majeti 2017). Ideally, a therapeutic target for AML should be highly expressed in this compartment to increase accuracy and reduce targeting irrelevant populations that may contain many normal clones. TIM-3, IL1RAP, and CD96 have been specifically shown to be up-regulated in this population in AML specimens (Jan et al. 2011; Barreyro et al. 2012). Aside from intratumor heterogeneity, contamination with some normal HSPC has not been fully avoided by most profiling approaches. Most studies have hence analyzed mixed populations of leukemic and normal stem cells, leaving unexplored whether expression is fully specific to leukemic cells or to some extent reflects a systemic feature of (otherwise normal) HSPC in a leukemic microenvironment. Transplantation studies combining index FACS sorting and single-clone sequencing can potentially improve this by identifying which surface markers are in fact marking stem cells that are part of the leukemic clone(s) (Jan et al. 2011), keeping in mind that xenograft models leave room for “false negatives” such as LSCs or relevant pre-leukemic clones that do not engraft into immunodeficient mice for various reasons.
Table 1.
Antigens expressed on LSC-enriched populations with limited specificity or functionality
| Gene | Evidence as a leukemic stem marker | Described function in AML? | Investigation of targeting strategies in AML | Expression in normal tissuesa |
|---|---|---|---|---|
| CD9 | Protein expressed on AML CD34+CD38− cells in 75%–90% of patients, expressed on normal BM CD34+CD38− cells in 50%–75% of healthy donors (Sadovnik et al. 2017) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 29% of patients, expression persists at relapse (Coustan-Smith et al. 2018) Protein expression correlated with failure of induction therapy (Wu et al. 2016) |
Not described | N/A | Ubiquitous |
| IL2RA (CD25) | Protein up-regulation on CD34+CD38− AML versus normal BM or CB cells in ∼25% of samples; no expression on normal CD34+CD38−CD133+ BM cells (Saito et al. 2010; de Boer et al. 2018) Protein expressed on AML CD34+CD38− cells in 50%–75% of patients, expressed on normal BM CD34+CD38− cells in 15%–50% of healthy donors (Herrmann et al. 2014; Sadovnik et al. 2017) Protein expression correlates with treatment failure (Terwijn et al. 2009; Cerny et al. 2013; Allan et al. 2018) |
Not described | Phase I clinical trial: NCT02588092 (Flynn et al. 2016; Madhumathi et al. 2017) | Hematopoietic (some HSCs, activated T cells, activated B cells, basophils) (Vincenti et al. 1998; Brisslert et al. 2006; Saito et al. 2010) |
| FCGR2A (CD32) | Protein up-regulation on CD34+CD38− AML versus normal BM or CB cells in ∼34% of samples; expression on <2% of normal CD34+CD38−CD133+ BM cells (Saito et al. 2010) Protein expression distinguishes leukemic versus normal CD34+CD38− cells (Ho et al. 2016) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 31% of patients; expression persists at relapse (Coustan-Smith et al. 2018) |
Not described | N/A | Hematopoietic (macrophages, B cells) |
| CD44 | Protein expression on CD34+CD38− AML cells in all patients analyzed (Florian et al. 2006; Sadovnik et al. 2017) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 35% of patients; expression persists at relapse (Coustan-Smith et al. 2018) Some protein expression on normal BM CD34+CD38− and CD34+CD38+ cells (Reuss-Borst et al. 1992; Liesveld et al. 1994; Jin et al. 2006; Liu and Jiang 2006; Sadovnik et al. 2017) |
Homing, engraftment, proliferation, and cell survival signaling (Jin et al. 2006; Hertweck et al. 2011) | Phase I clinical trial: NCT01641250 (Song et al. 2004; Jin et al. 2006; Quéré et al. 2011) | Ubiquitous (Liu and Jiang 2006) |
| CD47 | Significant protein up-regulation on AML lymphoid− CD34+CD38−CD90− cells versus normal BM HSC (lymphoid−CD34+CD38−CD90+) and MPP (lymphoid−CD34+CD38−CD90−CD45RA−); some protein expression on normal BM HSC and MPP (Majeti et al. 2009b) Protein expressed on AML CD34+CD38− cells in >90% of patients, expressed on normal BM CD34+CD38− cells in >90% of healthy donors (Sadovnik et al. 2017) Expressed on 100% of normal BM CD34+ cells (de Boer et al. 2018) No significant protein up-regulation on CD34+CD19− CD13+/CD33+ AML versus normal BM cells (Coustan-Smith et al. 2018) |
CD47-SIRPα interaction inhibits phagocytosis by macrophages (Jaiswal et al. 2009) | Phase I clinical trials: NCT02641002 (terminated), NCT02663518, NCT02678338, NCT03248479 (Majeti et al. 2009b; for review, see Murata et al. 2018) | Ubiquitous (Majeti et al. 2009b) |
| CD52 (Campath-1) | Protein up-regulation on CD45+CD34+CD38− MDS and AML cells versus AML complete remission samples (Blatt et al. 2014) Protein expressed on AML CD34+CD38− cells in 50%–75% of patients, expressed on normal BM CD34+CD38− cells in 50%–75% of healthy donors (Sadovnik et al. 2017) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 35% of patients; expression persists at relapse (Coustan-Smith et al. 2018) Some protein expression on normal BM CD34+CD38− cells (Olweus et al. 1994) |
Not described | Saito et al. 2011; Blatt et al. 2014 | Not described |
| CD93 | Protein up-regulation on CD34+CD38− AML versus normal BM or CB in ∼7% of samples (Saito et al. 2010) Specific protein expression on CD34+CD38− MLL-rearranged AML cells versus non-MLL-rearranged AML cells; absence of protein expression on CD34+CD38− CB cells; increased leukemia initiating frequency in CD34+CD38−CD93+ versus CD34+CD38−CD93− AML (MLL-AF9) cells (Iwasaki et al. 2015) Protein expressed on AML CD34+CD38− cells in 75%–90% of patients, expressed on normal BM CD34+CD38− cells in 50%–75% of healthy donors (Sadovnik et al. 2017) Protein expression on 99% of CB Lin−CD34+CD38− cells (Danet et al. 2002) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 15% of patients, expression persists at relapse (Coustan-Smith et al. 2018) |
Not described | Iwasaki et al. 2015 | Hematopoietic (platelets, neutrophils, monocytes, macrophages, some B-cell precursors, some dendritic and NK cells, HSCs); microglia; endothelial cells (Fonseca et al. 2001; Danet et al. 2002; McGreal et al. 2002) |
| CD96 (tactile) | Significant protein up-regulation on CD34+CD38− AML versus normal BM cells (Gramatzki et al. 1998; Kikushige et al. 2010; Du et al. 2015) Significant protein up-regulation on CD34+CD38− AML cells (specifically CD90−) compared to normal Lin−CD34+ CD38− BM cells in 2/3 of AML samples examined; >85% of CD34+CD38−CD96+ AML cells did not express lineage markers; increased leukemia initiating frequency in CD34+ CD38−CD96+ versus CD34+CD38−CD96− AML cells (Hosen et al. 2007) Expressed on 5%–20% of normal BM CD34+CD38− cells (Hosen et al. 2007) Protein expressed on AML CD34+CD38− cells in 15%–50% of patients, expressed on normal BM CD34+CD38− cells in <15% of healthy donors (Sadovnik et al. 2017) Protein up-regulation on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 49% of patients; expression persists at relapse (Coustan-Smith et al. 2018) Complete remission rate lower in patients with higher percentage of CD34+CD38−CD96+ cells (Du et al. 2015); high expression associated with poorer overall survival (Jiang et al. 2017) |
Potential inhibitory role in immune function (for review, see Georgiev et al. 2018) | Mohseni Nodehi et al. 2012; Kellner et al. 2013 | Hematopoietic (T cells, NK cells, some HSCs); epithelial (mucosal, vascular) (Perna et al. 2017) |
| CD99 | Protein expression distinguishes functionally normal from leukemia-initiating Lin−CD34+CD38− cells in AML samples (Jan et al. 2012) CD99−CD34+CD38− AML cells lack leukemia-relevant mutations and have multilineage potential (Chung et al. 2017) Expressed on 100% of normal BM CD34+ cells (de Boer et al. 2018) Protein overexpression on CD34+CD19−CD13+/CD33+ AML versus normal BM cells in 26% of patients (Coustan-Smith et al. 2018) |
Not described | Chung et al. 2017 | Ubiquitous |
| ADGRG1 (GPR56) | Protein highly expressed on AML cells that positively engrafted in immunodeficient mice; protein expressed on healthy CD34+ cells (Pabst et al. 2016) Ectopic expression of Gpr56 significantly accelerated HOXA9-induced leukemogenesis in mice (Daria et al. 2016) |
Not described | Daria et al. 2016; Saha et al. 2018 | Hematopoietic (cytotoxic T cells, NK cells); epithelial (lung, GI tract, epidermal cells, reproductive and endocrine tissues); hepatocytes; kidney cells |
AML, Acute myeloid leukemia; BM, bone marrow; CB, cord blood; GI, gastrointestinal; HSC, hematopoietic stem cell; MPP, multipotent progenitor; NK, natural killer.
aExpression data was gathered from Human Protein Atlas (Uhlen et al. 2015), Proteomics Database (Kim et al. 2014), and Human Proteome Map (Wilhelm et al. 2014), as well as the noted references.
Table 2.
Functional LSC targets with no expression on normal/functional HSC
| Gene | Evidence as a leukemic stem marker | Described function in AML? | Investigation of targeting strategies | Expression in normal tissuesa |
|---|---|---|---|---|
| HAVCR2 (CD366, TIM-3) | Significant protein up-regulation on CD34+CD38− AML versus normal BM cells; no expression on normal HSC (Kikushige et al. 2010; Haubner et al. 2019) Protein up-regulation on CD34+CD19−CD13+/ CD33+ AML versus normal BM cells in 41% of patients; expression persists at relapse (Coustan-Smith et al. 2018) Significantly higher expression on lymphoid− CD34+CD38−CD90− AML versus lymphoid−CD34+CD38−CD90+ normal BM cells; lymphoid−CD34+TIM-3+ AML cells gave rise to leukemia in immunodeficient mice, whereas lymphoid−CD34+TIM-3− did not; expression on ∼30% of normal BM lymphoid−CD34+CD38− cells (Jan et al. 2011) TIM-3 expression marks functional LSCs and distinguishes leukemic versus normal CD34+CD38− cells (Ho et al. 2016) |
Galactin-9-mediated signal transduction leading to MCL-1 expression and cell survival (Kikushige et al. 2015) | Kikushige et al. 2010 | Hematopoietic (monocytes, some NK cells, GMPs) (Haubner et al. 2019) |
| CLEC12A (CD371, CLL-1) | Protein expression on CD34+CD38− AML cells; absence of protein expression on normal BM, regenerating BM, and mobilized peripheral blood CD34+CD38− cells (Bakker et al. 2004; van Rhenen et al. 2007a,b; Kikushige et al. 2010; Sadovnik et al. 2017; Bill et al. 2018; de Boer et al. 2018; Haubner et al. 2019) FSClowSSClowCD38low side population cells from AML patients were 2%–100% positive for CLL-1 and cytogenetically abnormal, FSClowSSClowCD38low side population cells from normal BM were 0%–4% positive for CLL-1 (Moshaver et al. 2008) CD34+CD38−CLL-1+ cells gave rise to leukemia in immunodeficient mice, protein expression persists in remission and relapse (van Rhenen et al. 2007b) |
Potential negative regulation of immune system (Han et al. 2004; Marshall et al. 2004; Chen et al. 2006; Gagné et al. 2013; Neumann et al. 2014) | Phase I clinical trial: NCT03038230 (Zhao et al. 2010; Lu et al. 2014; Laborda et al. 2017; Leong et al. 2017; Tashiro et al. 2017; Jiang et al. 2018; Wang et al. 2018; Zheng et al. 2019; for review, see Morsink et al. 2019) | Hematopoietic (myeloid) (Bakker et al. 2004; Perna et al. 2017; Haubner et al. 2019) |
| IL1RAP | Significant protein overexpression on AML versus normal Lin−CD34+CD38− BM cells; IL1RAP protein is absent from normal Lin−CD34+CD38− cells; cytogenetically abnormal cells were contained in the IL1RAP+ fraction of AML specimens (Barreyro et al. 2012) Increased IL1RAP expression on AML CD34+CD38− cells versus normal BM CD34+CD38− cells (Askmyr et al. 2013) IL1RAP protein expression distinguishes leukemic versus normal CD34+CD38− cells (Ho et al. 2016) Protein expressed on CD34+CD38− AML cells in 50%–75% of all AML samples and absent from normal CD34+CD38− cells (Jaras et al. 2010; Sadovnik et al. 2017; de Boer et al. 2018) Protein expression persists in relapse (Ho et al. 2016; de Boer et al. 2018) |
Facilitates IL-1 and receptor tyrosine kinase signaling (Ågerstam et al. 2015; Mitchell et al. 2018) | Askmyr et al. 2013; Ågerstam et al. 2015; Mitchell et al. 2018 | Hematopoietic (monocytes/macrophages, lowly expressed on myeloid progenitors, lymphocytes, NK cells, and mast cells) (Jaras et al. 2010); epithelial (lowly expressed in GI tract, reproductive, and endocrine tissues) (Ågerstam et al. 2015); lowly expressed on skin fibroblasts and keratinocytes |
AML, Acute myeloid leukemia; BM, bone marrow; HSC, hematopoietic stem cell; CB, cord blood; GI, gastrointestinal; MPP, multipotent progenitor; NK, natural killer; GMP, granulocyte-macrophage progenitor; IL, interleukin.
aExpression data was gathered from Human Protein Atlas (Uhlen et al. 2015), Proteomics Database (Kim et al. 2014), and Human Proteome Map (Wilhelm et al. 2014), as well as the noted references.
Expression on Normal HSC and Other Tissues
As demonstrated by toxicities associated with CD33- and CD123-directed therapies, the expression (albeit often lower) of LSC antigens on normal HSC complicates the therapeutic efficacy of many otherwise potent targets. Therefore, studies have sought to identify markers of LSCs that truly distinguish them from normal HSCs to minimize off-tumor, on-target toxicity. Some LSC antigens found to be absent from normal BM HSC include CD32, CLL-1, and IL1RAP (see Tables 1 and 2 for relevant references), so developing therapies against these proteins may result in reduced toxicity. Although some studies have detected its expression on normal HSC, TIM-3 expression has been demonstrated to distinguish residual, normal HSCs from LSCs in AML patient specimens using xenotransplantation (Jan et al. 2011), providing evidence of a strong therapeutic window for this target as well. Minimal expression of therapeutic targets on normal HSC and hematopoietic progenitors is particularly important for immunotherapeutic approaches as cells with any expression of the target may be eliminated. Along these lines, an ideal therapeutic target should be minimally expressed in normal tissues, particularly those that are vital.
Timing in AML Pathogenesis
A relationship between the order of mutations in AML and whether these mutations predict relapse has begun to be established. As DNMT3A, TET2, and ASXL1 mutations (which often occur early and are associated with CH) detected in MRD were found not to be predictive of relapse while mutations in NPM1 were predictive (Jongen-Lavrencic et al. 2018), mutations acquired after the initial CH stage in leukemogenesis may be better markers of MRD than CH markers. Likewise, immunophenotypic markers expressed after the CH stage may be more efficient and less toxic therapeutic targets than early markers whose expression may occur on nondiseased, clonal hematopoietic cells. One indication that aberrant expression of a target commonly occurs in this timeframe is if expression predicts relapse when detected at the complete remission/MRD stage; this has been shown to be the case for CLL-1 (van Rhenen et al. 2007b). It has been proposed that FLT3 mutations may be useful in MRD diagnosis because of their presence only on frank, leukemic clones (Corces et al. 2017); however, the limited efficacy of FLT3 targeting in AML suggests that very late hits in the leukemic progression may not be ideal targets either. Therefore, a target that is not commonly expressed on cells only harboring CH-associated mutations but that is expressed in later pre-leukemic stages may be most efficient at eliminating malignant and relevant premalignant stem cells while sparing normal hematopoietic clones. Whereas the order of genetic hits in AML has been elucidated by various model systems and methods, future studies should monitor the timing of aberrant cell-surface protein expression on LSCs throughout the process of leukemic transformation. For instance, the expression of potential LSC targets could be measured by flow cytometry in serial samples collected from patients progressing from CH to AML, and in cells harboring progressive numbers of leukemia-relevant driver mutations.
Target Functionality
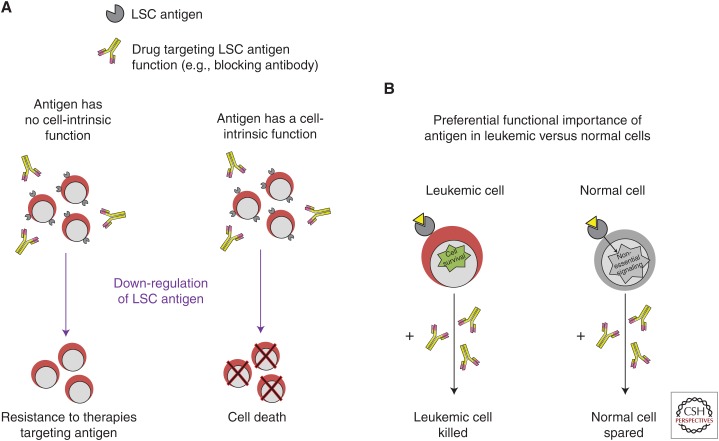
Aside from differential expression on leukemic versus normal HSCs, functionality is also prudent for an LSC target. Whereas, in principle, certain targeted therapies such as immunotherapeutic approaches do not depend on a target having a cancer cell-intrinsic function to be effective, without functionality, down-regulation or mutation of the target, could readily lead to resistance to any type of therapeutic agent (Fig. 2A). Functionality also provides an opportunity for a larger therapeutic window in the case that a therapeutic target is expressed in normal HSPC or other tissues; if a leukemic cell is more dependent on the target for cell survival than a normal cell, a targeting strategy that inhibits the target's function could be preferentially cytotoxic to the leukemic cells while sparing normal cells (Fig. 2B). CD44, CD47, TIM-3, CLL-1, and IL1RAP have been found to play a functional role in AML cells, while the roles of many other LSC targets have yet to be explored (Tables 1 and 2).
Figure 2.
Importance of target functionality for therapeutic targeting. (A) If a therapeutic target does not have a cell-intrinsic function in leukemic cells, leukemic cells may be able to escape elimination by the drug through down-regulating the target. (B) If a target provides essential signaling in leukemic cells but is dispensable for normal cells, even normal cells expressing the target could be spared by a drug that inhibits the target's function (but would likely be eliminated with an immunotherapeutic approach or antibody–drug conjugate).
Targeting Strategy
Target function and relative expression in leukemic and normal cells are important factors for determining which therapeutic modality will create a proper balance between elimination of leukemic cells and sparing of normal cells. A direct targeting approach (such as a blocking antibody or small molecule inhibitor) may reduce on-target off-tumor toxicity in the presence of lower levels of antigen on normal HSPC than on LSCs or preferential dependence of leukemic versus normal cells on the antigen for cell survival. For instance, if a normal cell expresses the target but is not reliant on it for its function, this cell could be spared by a direct targeting approach but would be killed by an immunotherapeutic approach or antibody–drug conjugate (Fig. 2B). In addition, while immunotherapeutic approaches (such as CAR T cells, bispecific T-cell engager antibodies, and antibodies capable of Fc-mediated recruitment) in other hematologic malignancies have shown effectiveness in eliminating cells with even minimal expression of the relevant target (Cheson and Leonard 2008; June and Sadelain 2018), patients with AML often have aberrant immune responses, thus, antileukemic immune activation may not be effective (Le Dieu et al. 2009). It may therefore be important to explore AML therapies with mechanisms of action dominated by direct targeting and functional inhibition for this reason as well.
Of the LSC therapeutic targets that have been proposed, three targets that best fit the criteria described above are TIM-3, CLL-1, and IL1RAP.
TIM-3
TIM-3 protein was found to be significantly up-regulated on lymphoid−CD34+CD38−CD90− AML versus lymphoid−CD34+CD38−CD90+ normal BM cells (Jan et al. 2011), and more broadly in CD34+CD38− (Kikushige et al. 2010; Ho et al. 2016) and myeloid CD34+ cells (Coustan-Smith et al. 2018) in AML versus normal BM samples. TIM-3 has been shown to identify CD34+ AML cells with leukemia initiating activity upon xenotransplantation (Kikushige et al. 2010; Jan et al. 2011; Ho et al. 2016).
There is conflicting data on the expression of TIM-3 in normal HSCs. One study found expression on ∼30% of normal BM lymphoid− CD34+CD38− cells, but further showed that within this population, TIM-3− cells contributed to significantly increased multilineage engraftment in immunocompromised mice than TIM-3+ cells, leading to the conclusion that functional HSCs are primarily TIM-3− (Jan et al. 2011). However, other studies found no TIM-3 expression on normal BM CD34+CD38− cells (Kikushige et al. 2010; Haubner et al. 2019). TIM-3 expression is restricted to the hematopoietic system, within which TIM-3 is only expressed on monocytes and a fraction of natural killer cells and GMPs (Kikushige et al. 2010; Haubner et al. 2019).
A TIM-3-directed monoclonal antibody reduced engraftment of primary AML cells in immunodeficient mice and minimized AML engraftment upon secondary transplantation, indicating LSCs were targeted. Normal CD34+ cord blood cells were not affected in similar xenograft/antibody experiments. The authors attributed the effects on AML cells to antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (Kikushige et al. 2010).
TIM-3 has been described to be a receptor for a molecule secreted by AML cells, galactin-9 (Gal-9). Kikushige and colleagues demonstrated that TIM-3 activation by Gal-9 led to activation of Akt, ERK, and NF-κB, and to nuclear translocation of β-catenin in primary AML cells. They also showed that Gal-9 is functionally important for LSCs as antibody-mediated neutralization of Gal-9 reduced engraftment of CD34+ AML cells in xenograft models (including the CD34+ CD38− population) but did not affect engraftment of normal human cord blood cells (Kikushige et al. 2015). Finally, because expression of TIM-3 persists at relapse in most patients, these cells are likely part of a therapy-refractory clone (Ho et al. 2016; Coustan-Smith et al. 2018).
CLL-1
Expression of CLL-1 has been reported on CD34+CD38− as well as FSClowSSClowCD38low SP AML cells by several groups; meanwhile, expression is absent on these populations in normal BM, on regenerating BM CD34+CD38− cells, and on mobilized peripheral blood CD34+CD38− cells (Bakker et al. 2004; van Rhenen et al. 2007a,b; Moshaver et al. 2008; Kikushige et al. 2010; Sadovnik et al. 2017; Bill et al. 2018; de Boer et al. 2018; Haubner et al. 2019). Further, CLL-1+ AML cells were found to be cytogenetically abnormal (Moshaver et al. 2008) and CD34+CD38−CLL-1+ AML cells gave rise to leukemia in immunodeficient mice (van Rhenen et al. 2007b).
CLL-1 expression has only been detected in the hematopoietic system (Bakker et al. 2004), where it is expressed by some normal myeloid cells, including monocytes, macrophages, dendritic cells, granulocytes, and myeloid precursors (primarily GMPs, but also on a smaller percentage on CMPs and megakaryocyte–erythrocyte progenitors) (Bakker et al. 2004; Perna et al. 2017; Bill et al. 2018; de Boer et al. 2018; Haubner et al. 2019). No significant increase in CLL-1 expression was found in immunophenotypic myeloid progenitors in AML versus normal samples (Coustan-Smith et al. 2018; de Boer et al. 2018) and expression of CLL-1 is lower in LSCs compared to bulk AML cells and normal mature myeloid cells (de Boer et al. 2018). These may be important caveats for therapeutic targeting of this protein.
Several CLL-1-directed therapies have been developed that lead to cytotoxicity in AML cell lines and primary AML cells, as well as in AML cell line xenografts, while sparing normal HSCs (for a review, see Morsink et al. 2019). These include monoclonal antibodies (Zhao et al. 2010), antibody–drug conjugates (Jiang et al. 2018; Zheng et al. 2019), CLL-1/CD3 bispecific antibodies (Lu et al. 2014; Leong et al. 2017), and anti-CLL-1 CAR T cells (Laborda et al. 2017; Tashiro et al. 2017; Wang et al. 2018). A CLL-1/CD3 bispecific antibody (MCLA-117) is being tested in a phase I clinical trial in patients with AML (NCT03038230), as is a compound CLL-1/CD33 CAR T-cell therapy (Liu et al. 2018).
Given the high expression of CLL-1 in monocytes and granulocytes, CLL-1-directed therapies may have toxicity issues. This is evidenced by studies showing that only bispecific antibodies with low (vs. high) affinity for T cells were tolerated in preclinical trials in monkeys (Leong et al. 2017), and CLL-1 antibody–drug conjugates and CAR T cells were cytotoxic to mature normal myeloid cells (Tashiro et al. 2017; Zheng et al. 2019). CAR T approaches against this target must incorporate a suicide system for safety reasons (Tashiro et al. 2017). It will be important to test the relative functional relevance of CLL-1 in these normal cell types versus AML cells to determine whether a strategy that blocks the function of CLL-1 instead of recruiting the immune system might limit toxicity. In this light, studies on CLL-1 (Clec12a) knockout mice provide some encouragement. Although the number of Clecl12a−/− mice born is lower than Mendelian ratios, once born, Clecl12a−/− mice have no major abnormalities, a normal life span, and no signs of disease. These mice show normal cellularity in hematopoietic organs and no changes in frequency of mature hematopoietic cells compared to WT mice (Neumann et al. 2014). These studies suggest a broadly nonessential role of CLL-1.
Although a specific role for CLL-1 has not been studied in leukemia or cancer in general, studies in normal hematopoietic cells indicate it plays a role in negative regulation of the immune system. For instance, CLL-1 was found to physically associate with the signaling phosphatases SHP-1 and SHP-2, inhibiting activation of immune cells through a cytoplasmic inhibitory motif (Han et al. 2004; Marshall et al. 2004). CLL-1 was shown to be involved in Toll-like receptor and T-cell activation pathways (such as CD40L) through regulating cytokine production (Chen et al. 2006). It was also found that CLL-1 dampens immune responses triggered by damage-associated molecular patterns (Gagné et al. 2013; Neumann et al. 2014). Together, these studies suggest that CLL-1 normally inhibits some inflammatory processes, and suggest the role of CLL-1 in leukemia could be to modulate immune responses as an immune-evasion mechanism.
Lastly, CLL-1 expression persists in remission and relapse in most patients, implying CLL-1 is part of a chemotherapy-resistant clone (van Rhenen et al. 2007b; Coustan-Smith et al. 2018; Haubner et al. 2019). Specifically, CLL-1 expression on CD34+CD38− cells at complete remission was associated with relapse in several patients and more strongly correlated with relapse than did standard MRD detection by leukemia-associated (immune) phenotype expression. Furthermore, lasting remissions occurred in patients with low or absent CLL-1 expression in the stem cell population at complete remission (van Rhenen et al. 2007b).
IL1RAP
IL1RAP gene expression was specifically shown to be significantly up-regulated in AML Lin−CD34+CD38−CD90− cells (a population highly enriched for LSCs; Goardon et al. 2011) compared to this population in age-matched healthy BM samples (Barreyro et al. 2012). Multiple additional studies have identified IL1RAP as a target for LSC-directed therapy in AML based on its surface expression in HSC-enriched populations from AML specimens (Askmyr et al. 2013; Bonardi et al. 2013; Ho et al. 2016; Sadovnik et al. 2017).
IL1RAP protein expression is absent from normal HSCs (Jaras et al. 2010; Barreyro et al. 2012; Ho et al. 2016; Sadovnik et al. 2017; de Boer et al. 2018), but is present in mature cells of the immune system, primarily monocytes and macrophages, and to a lesser extent lymphocytes, natural killer cells, and mast cells. It is lowly expressed on some myeloid progenitors as well (Jaras et al. 2010; Barreyro et al. 2012; de Boer et al. 2018). It is known that the canonical ligand for the IL1RAP/IL-1 receptor complex, IL-1β, acts on epithelial cells, fibroblasts, keratinocytes, endothelial cells, muscle cells, and neurons (Striz 2017); thus, IL1RAP may be expressed in these tissues, at least in the inflammatory setting. Protein array data confirms low IL1RAP expression in some of these tissues (Kim et al. 2014; Wilhelm et al. 2014; Ågerstam et al. 2015; Uhlen et al. 2015). Toxicity in these cell types would be important to monitor if IL1RAP-directed therapies move forward. However, given that total body Il1rap−/− mice do not have overt abnormalities, and Il1rap−/− BM is capable of complete and long-term (>20 wk) reconstitution of lymphoid and myeloid hematopoietic cells (Mitchell et al. 2018), on-target, off-tumor toxicity may be limited.
Several groups have targeted IL1RAP in AML using different strategies. Some have focused on the utility of IL1RAP as a surface label and target for immune effector cells in AML. For instance, an IL1RAP-directed monoclonal antibody significantly reduced leukemic burden in BM and spleens of mice transplanted with MLL-AF9 and activated NRAS cotransduced cord blood cells as well as mice transplanted with primary AML cells, with effects attributed to natural killer cell ADCC activity (Ågerstam et al. 2015). These studies have led to testing of an IL1RAP-directed antibody in a phase I clinical trial (CANFOUR trial) where dose escalation will first be tested in patients with solid malignant tumors (NCT03267316). Jiang and colleagues showed in a xenograft of the AML cell line EOL-1 that several different IL1RAP-directed monoclonal antibodies completely eliminated AML engraftment in BM and blood. These antibodies induced CDC of primary AML blasts when incubated with cells and complement in vitro (Jiang et al. 2016). Additionally, an IL1RAP/CD3 bispecific antibody is in development, and both in vivo and in vitro effects on the MV4;11 AML cell line were presented at the 2017 American Society of Hematology meeting (Meng et al. 2017).
The functional importance of IL1RAP in leukemic cells has also been tested. shRNA-mediated knockdown of IL1RAP in primary AML cells led to reduced clonogenic growth and in AML cell lines led to reduced engraftment in immunodeficient mice. In addition, IL1RAP-directed antibodies increased apoptosis of primary AML cells in the absence of immune effector cells but spared normal hematopoietic cells. These IL1RAP-directed antibodies nearly eliminated leukemic cells in the BM in AML cell line xenografts. Finally, Il1rap loss impaired LSC function/frequency in MLL-AF9-driven AML. These studies imply that IL1RAP is functionally important for growth and survival of AML cells (Mitchell et al. 2018).
Specific functions of IL1RAP in AML cells have been recently explored. IL1RAP antibodies inhibited IL-1-induced AML cell growth (Ågerstam et al. 2015) and signaling (Mitchell et al. 2018) in vitro. In MLL-AF9 AML, shRNA-mediated knockdown of IL1RAP led to reduced degradation of the WT MLL protein, which normally competes with the MLL-AF9 fusion; thus, IL1RAP targeting reduced MLL-AF9 occupancy and aberrant transcription (Liang et al. 2017). Whereas the canonical role of IL1RAP is to facilitate signaling through the IL-1 pathway, several lines of evidence suggest that IL1RAP has alternative or at least additional mechanisms in AML. FLT3 WT and FLT3 mutant AML cells were found to be differentially sensitive to IL1RAP antibody-mediated inhibition, which led to studies demonstrating that IL1RAP modulates FLT3 ligand-mediated signaling and that growth and these two proteins physically interact. This relationship was also demonstrated for the receptor tyrosine kinase c-KIT (Mitchell et al. 2018). Thus, AML cells may use up-regulation of IL1RAP as a way to amplify multiple signaling pathways involved in cell survival and proliferation.
High IL1RAP expression in AML correlates with poor prognosis (Barreyro et al. 2012; de Boer et al. 2018), and IL1RAP expression persists at relapse (de Boer et al. 2018). IL1RAP expression may be associated with the acquisition of certain mutations, as it was found to mark genetically distinct AML subclones such as those with NRAS mutations (de Boer et al. 2018). In monosomy 7 AML patient samples, the clone size of IL1RAP+ AML HSPCs included and exceeded that of cells bearing the chromosomal aberration, suggesting IL1RAP overexpression preceded loss of chromosome 7, potentially marking a pre-leukemic cell population. The timing of IL1RAP overexpression in leukemic transformation should be further explored in future studies.
CONCLUDING REMARKS
For AML patients over the age of 65, which represent the majority (Noone et al. 2018), the 5-year overall survival is currently only estimated to be ∼14%, and has improved marginally in the last 45 years (Mokdad et al. 2017; Lancet 2018). In recent years, therapeutic advances leading to improved patient outcomes in AML are a result of the optimization of existing drugs rather than the use of novel therapeutics (Fernandez et al. 2009; Lancet et al. 2017; Wei and Tiong 2017). Clearly, improved and more specific treatments are necessary in this disease, yet a universal antigen that fits all criteria for an ideal LSC target has been challenging to find.
Because of their lack of expression on normal HSC and minimal expression/essentiality on other normal tissues, their functional importance in AML cells, and persistent expression at relapse, we would propose TIM-3, CLL-1, and IL1RAP as the most promising current therapeutic targets to safely eliminate LSCs. Recent findings appear to favor the development of direct targeting strategies against these targets to reduce toxicities and minimize potential resistance mechanisms (Fig. 2). In addition, while CD25 only marks LSCs in a fraction of patients, it appears to be a strong predictor of relapse and may represent another potent drug target in a smaller population of patients.
Although targeting a common immunophenotypic antigen at the stem/progenitor cell level is a potential means to overcoming the daunting genetic complexity, inter- and intrapatient heterogeneity of AML, therapies may still need to be tailored to distinct molecular subtypes of AML, and, accordingly, more studies will be required to relate mutational profiles to cell-surface expression of putative LSC antigens in larger cohorts of patients. Targeting several antigens to eliminate leukemic cells that persist at remission may be necessary (Haubner et al. 2019). Overall, advances in our understanding of AML pathogenesis provide insight into the improvement of LSC-targeted therapies in AML: continued development of the LSC model, including further understanding of the progression from CH to pre-leukemia and AML, hold promise in informing better targets and therapeutic agents for the treatment and cure of AML.
Footnotes
Editors: Michael G. Kharas, Ross L. Levine, and Ari M. Melnick
Additional Perspectives on Leukemia and Lymphoma: Molecular and Therapeutic Insights available at www.perspectivesinmedicine.org
REFERENCES
- Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L, Sundaravadanam Y, et al. 2018. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559: 400–404. 10.1038/s41586-018-0317-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, von Palffy S, Högberg C, Rissler M, Wunderlich M, Juliusson G, et al. 2015. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci 112: 10786–10791. 10.1073/pnas.1422749112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan JN, Roboz GJ, Askin G, Ritchie E, Scandura J, Christos P, Hassane DC, Guzman ML. 2018. CD25 expression and outcomes in older patients with acute myelogenous leukemia treated with plerixafor and decitabine. Leuk Lymphoma 59: 821–828. 10.1080/10428194.2017.1352089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado Y, Kantarjian HM, Luthra R, Ravandi F, Borthakur G, Garcia-Manero G, Konopleva M, Estrov Z, Andreeff M, Cortes JE. 2014. Treatment with FLT3 inhibitor in patients with FLT3-mutated acute myeloid leukemia is associated with development of secondary FLT3-tyrosine kinase domain mutations. Cancer 120: 2142–2149. 10.1002/cncr.28705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori S, Suciu S, Selleslag D, Stasi R, Alimena G, Baila L, Rizzoli V, Borlenghi E, Gaidano G, Magro D, et al. 2010. Randomized trial of two schedules of low-dose gemtuzumab ozogamicin as induction monotherapy for newly diagnosed acute myeloid leukaemia in older patients not considered candidates for intensive chemotherapy. A phase II study of the EORTC and GIMEMA leukaemia groups (AML-19). Br J Haematol 149: 376–382. 10.1111/j.1365-2141.2010.08095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. 2009. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer 100: 822–828. 10.1038/sj.bjc.6604935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends CM, Galan-Sousa J, Hoyer K, Chan W, Jäger M, Yoshida K, Seemann R, Noerenberg D, Waldhueter N, Fleischer-Notter H, et al. 2018. Hematopoietic lineage distribution and evolutionary dynamics of clonal hematopoiesis. Leukemia 32: 1908–1919. 10.1038/s41375-018-0047-7 [DOI] [PubMed] [Google Scholar]
- Askmyr M, Agerstam H, Hansen N, Gordon S, Arvanitakis A, Rissler M, Juliusson G, Richter J, Jaras M, Fioretos T. 2013. Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood 121: 3709–3713. 10.1182/blood-2012-09-458935 [DOI] [PubMed] [Google Scholar]
- Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N, Geuijen CA, et al. 2004. C-type lectin-like molecule-1: A novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res 64: 8443–8450. 10.1158/0008-5472.CAN-04-1659 [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. 2010. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465: 793–797. 10.1038/nature09135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Goodell MA. 2011. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol 32: 57–65. 10.1016/j.it.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J, Wang ES. 2018. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev Clin Pharmacol 11: 549–559. 10.1080/17512433.2018.1478725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreda DR, Hanington PC, Belosevic M. 2004. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol 28: 509–554. 10.1016/j.dci.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, et al. 2012. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood 120: 1290–1298. 10.1182/blood-2012-01-404699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko O, Mancini E, Moore S, Bilbao D, Månsson R, Luc S, Grover A, Jacobsen SE, Bryder D, Nerlov C. 2009. Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPα mutant AML. Cancer Cell 16: 390–400. 10.1016/j.ccr.2009.09.036 [DOI] [PubMed] [Google Scholar]
- Bill M, van Kooten Niekerk PB, Woll PS, Laine Herborg L, Stidsholt Roug A, Hokland P, Nederby L. 2018. Mapping the CLEC12A expression on myeloid progenitors in normal bone marrow; implications for understanding CLEC12A-related cancer stem cell biology. J Cell Mol Med 22: 2311–2318. 10.1111/jcmm.13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt K, Herrmann H, Hoermann G, Willmann M, Cerny-Reiterer S, Sadovnik I, Herndlhofer S, Streubel B, Rabitsch W, Sperr WR, et al. 2014. Identification of campath-1 (CD52) as novel drug target in neoplastic stem cells in 5q-patients with MDS and AML. Clin Cancer Res 20: 3589–3602. 10.1158/1078-0432.CCR-13-2811 [DOI] [PubMed] [Google Scholar]
- Bonardi F, Fusetti F, Deelen P, van Gosliga D, Vellenga E, Schuringa JJ. 2013. A proteomics and transcriptomics approach to identify leukemic stem cell (LSC) markers. Mol Cell Proteomics 12: 626–637. 10.1074/mcp.M112.021931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737. 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- Bowman RL, Busque L, Levine RL. 2018. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 22: 157–170. 10.1016/j.stem.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kirkup J, Thakkar D, Brauer P, Zhou J, Chng WJ, Ingram PJ. 2017. HMBD004, a novel anti-CD47xCD33 bispecific antibody displays potent anti-tumor effects in pre-clinical models of AML. Blood 130: 1378–1378. [Google Scholar]
- Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. 2006. Phenotypic and functional characterization of human CD25+ B cells. Immunology 117: 548–557. 10.1111/j.1365-2567.2006.02331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell F, Swansbury J, Min T, Matutes E, Farahat N, Buccheri V, Morilla R, Secker-Walker L, Catovsky D. 1996. Cytogenetic findings in acute biphenotypic leukaemia. Leukemia 10: 1283–1287. [PubMed] [Google Scholar]
- Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, et al. 2012. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 379: 1508–1516. 10.1016/S0140-6736(12)60485-1 [DOI] [PubMed] [Google Scholar]
- Catlin SN, Busque L, Gale RE, Guttorp P, Abkowitz JL. 2011. The replication rate of human hematopoietic stem cells in vivo. Blood 117: 4460–4466. 10.1182/blood-2010-08-303537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny J, Yu H, Ramanathan M, Raffel GD, Walsh WV, Fortier N, Shanahan L, O'Rourke E, Bednarik J, Barton B, et al. 2013. Expression of CD25 independently predicts early treatment failure of acute myeloid leukaemia (AML). Br J Haematol 160: 262–266. 10.1111/bjh.12109 [DOI] [PubMed] [Google Scholar]
- Chan PM. 2011. Differential signaling of Flt3 activating mutations in acute myeloid leukemia: A working model. Protein Cell 2: 108–115. 10.1007/s13238-011-1020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Floyd H, Olson NE, Magaletti D, Li C, Draves K, Clark EA. 2006. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 107: 1459–1467. 10.1182/blood-2005-08-3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ishikawa Y, Akashi A, Naoe T, Kiyoi H. 2016. Co-expression of wild-type FLT3 attenuates the inhibitory effect of FLT3 inhibitor on FLT3 mutated leukemia cells. Oncotarget 7: 47018–47032. 10.18632/oncotarget.10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kao YR, Sun D, Todorova TI, Reynolds D, Narayanagari SR, Montagna C, Will B, Verma A, Steidl AU. 2019. Myelodysplastic syndrome progression to acute myeloid leukemia at the stem cell level. Nat Med 25: 103–110. 10.1038/s41591-018-0267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Leonard JP. 2008. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med 359: 613–626. 10.1056/NEJMra0708875 [DOI] [PubMed] [Google Scholar]
- Chung SS, Eng WS, Hu W, Khalaj M, Garrett-Bakelman FE, Tavakkoli M, Levine RL, Carroll M, Klimek VM, Melnick AM, et al. 2017. CD99 is a therapeutic target on disease stem cells in myeloid malignancies. Sci Transl Med 9: eaaj2025 10.1126/scitranslmed.aaj2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Purtilo DT, Ellwein LB. 1991. Ideas in pathology. Pivotal role of increased cell proliferation in human carcinogenesis. Mod Pathol 4: 371–382. [PubMed] [Google Scholar]
- Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, et al. 2017. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21: 374–382.e4. 10.1016/j.stem.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Chang HY, Majeti R. 2017. Preleukemic hematopoietic stem cells in human acute myeloid leukemia. Front Oncol 7: 263 10.3389/fonc.2017.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. 2014. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci 111: 2548–2553. 10.1073/pnas.1324297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, Olive D. 2000. Human acute myeloid eukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res 60: 4403–4411. [PubMed] [Google Scholar]
- Coustan-Smith E, Song G, Shurtleff S, Yeoh AE, Chng WJ, Chen SP, Rubnitz JE, Pui CH, Downing JR, Campana D. 2018. Universal monitoring of minimal residual disease in acute myeloid leukemia. JCI Insight 3: 98561 10.1172/jci.insight.98561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver BM, El Alaoui K, Scherber RM, Fleischman AG. 2018. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers (Basel) 10: E104 10.3390/cancers10040104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA. 2002. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci 99: 10441–10445. 10.1073/pnas.162104799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daria D, Kirsten N, Muranyi A, Mulaw M, Ihme S, Kechter A, Hollnagel M, Bullinger L, Döhner K, Döhner H, et al. 2016. GPR56 contributes to the development of acute myeloid leukemia in mice. Leukemia 30: 1734–1741. 10.1038/leu.2016.76 [DOI] [PubMed] [Google Scholar]
- de Boer B, Prick J, Pruis MG, Keane P, Imperato MR, Jaques J, Brouwers-Vos AZ, Hogeling SM, Woolthuis CM, Nijk MT, et al. 2018. Prospective isolation and characterization of genetically and functionally distinct AML subclones. Cancer Cell 34: 674–689.e8. 10.1016/j.ccell.2018.08.014 [DOI] [PubMed] [Google Scholar]
- Desai P, Mencia-Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, Samuel M, Ritchie EK, Guzman ML, Ballman KV, et al. 2018. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med 24: 1015–1023. 10.1038/s41591-018-0081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Martin JL, Buno I, Llamas P, Gosalvez J, Lopez-Fernandez C, Polo N, Regidor C. 2000. Fluorescence in situ hybridization evaluation of minimal residual disease on stem-cell harvests. Cancer Detect Prev 24: 169–172. [PubMed] [Google Scholar]
- Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, et al. 2012. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481: 506–510. 10.1038/nature10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhner H, Weisdorf DJ, Bloomfield CD. 2015. Acute myeloid leukemia. N Engl J Med 373: 1136–1152. 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- Du W, Hu Y, Lu C, Li J, Liu W, He Y, Wang P, Cheng C, Hu YU, Huang S, et al. 2015. Cluster of differentiation 96 as a leukemia stem cell-specific marker and a factor for prognosis evaluation in leukemia. Mol Clin Oncol 3: 833–838. 10.3892/mco.2015.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias HK, Schinke C, Bhattacharyya S, Will B, Verma A, Steidl U. 2014. Stem cell origin of myelodysplastic syndromes. Oncogene 33: 5139–5150. 10.1038/onc.2013.520 [DOI] [PubMed] [Google Scholar]
- Engel H, Drach J, Keyhani A, Jiang S, Van NT, Kimmel M, Sanchez-Williams G, Goodacre A, Andreeff M. 1999. Quantitation of minimal residual disease in acute myelogenous leukemia and myelodysplastic syndromes in complete remission by molecular cytogenetics of progenitor cells. Leukemia 13: 568–577. 10.1038/sj.leu.2401359 [DOI] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. 2011. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med 17: 1086–1093. 10.1038/nm.2415 [DOI] [PubMed] [Google Scholar]
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. 2011. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol 186: 5367–5375. 10.4049/jimmunol.1003438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, et al. 2009. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 361: 1249–1259. 10.1056/NEJMoa0904544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Schiffer CA. 2013. Acute myeloid leukaemia in adults. Lancet 381: 484–495. 10.1016/S0140-6736(12)61727-9 [DOI] [PubMed] [Google Scholar]
- Feuring-Buske M, Haase D, Buske C, Hiddemann W, Wörmann B. 1999. Clonal chromosomal abnormalities in the stem cell compartment of patients with acute myeloid leukemia in morphological complete remission. Leukemia 13: 386–392. 10.1038/sj.leu.2401300 [DOI] [PubMed] [Google Scholar]
- Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, et al. 2014. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512: 198–202. 10.1038/nature13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S, Vasudevan KB, LaTocha DH, Yang F, Press RD, et al. 2011. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 118: 6392–6398. 10.1182/blood-2011-04-348144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian S, Sonneck K, Hauswirth AW, Krauth MT, Schernthaner GH, Sperr WR, Valent P. 2006. Detection of molecular targets on the surface of CD34+/CD38− stem cells in various myeloid malignancies. Leuk Lymphoma 47: 207–222. 10.1080/10428190500272507 [DOI] [PubMed] [Google Scholar]
- Flynn MJ, Zammarchi F, Tyrer PC, Akarca AU, Janghra N, Britten CE, Havenith CE, Levy JN, Tiberghien A, Masterson LA, et al. 2016. ADCT-301, a pyrrolobenzodiazepine (PBD) dimer-containing antibody-drug conjugate (ADC) targeting CD25-expressing hematological malignancies. Mol Cancer Ther 15: 2709–2721. 10.1158/1535-7163.MCT-16-0233 [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Carpenter PM, Park M, Palmarini G, Nelson EL, Tenner AJ. 2001. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J Leukoc Biol 70: 793–800. [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. 2017. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355: 842–847. 10.1126/science.aag1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné V, Marois L, Levesque JM, Galarneau H, Lahoud MH, Caminschi I, Naccache PH, Tessier P, Fernandes MJ. 2013. Modulation of monosodium urate crystal-induced responses in neutrophils by the myeloid inhibitory C-type lectin-like receptor: Potential therapeutic implications. Arthritis Res Ther 15: R73 10.1186/ar4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. 2014. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371: 2477–2487. 10.1056/NEJMoa1409405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. 2010. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA 304: 2706–2715. 10.1001/jama.2010.1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev H, Ravens I, Papadogianni G, Bernhardt G. 2018. Coming of age: CD96 emerges as modulator of immune responses. Front Immunol 9: 1072 10.3389/fimmu.2018.01072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiaur G, Levis M. 2017. Mechanisms of resistance to FLT3 inhibitors and the role of the bone marrow microenvironment. Hematol Oncol Clin North Am 31: 681–692. 10.1016/j.hoc.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland DG, Griffin JD. 2002. The roles of FLT3 in hematopoiesis and leukemia. Blood 100: 1532–1542. 10.1182/blood-2002-02-0492 [DOI] [PubMed] [Google Scholar]
- Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, et al. 2011. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 19: 138–152. 10.1016/j.ccr.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. 2004. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): Implications for future therapeutical strategies. Eur J Haematol 72: 89–106. 10.1046/j.0902-4441.2003.00184.x [DOI] [PubMed] [Google Scholar]
- Gramatzki M, Ludwig WD, Burger R, Moos P, Rohwer P, Grunert C, Sendler A, Kalden JR, Andreesen R, Henschke F, et al. 1998. Antibodies TC-12 (“unique”) and TH-111 (CD96) characterize T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid leukemia. Exp Hematol 26: 1209–1214. [PubMed] [Google Scholar]
- Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. 1984. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res 8: 521–534. 10.1016/0145-2126(84)90001-8 [DOI] [PubMed] [Google Scholar]
- Haase D, Feuring-Buske M, Konemann S, Fonatsch C, Troff C, Verbeek W, Pekrun A, Hiddemann W, Wormann B. 1995. Evidence for malignant transformation in acute myeloid leukemia at the level of early hematopoietic stem cells by cytogenetic analysis of CD34+ subpopulations. Blood 86: 2906–2912. [PubMed] [Google Scholar]
- Haase D, Feuring-Buske M, Schäfer C, Schoch C, Troff C, Gahn B, Hiddemann W, Wormann B. 1997. Cytogenetic analysis of CD34+ subpopulations in AML and MDS characterized by the expression of CD38 and CD117. Leukemia 11: 674–679. 10.1038/sj.leu.2400638 [DOI] [PubMed] [Google Scholar]
- Hahn CN, Ross DM, Feng J, Beligaswatte A, Hiwase DK, Parker WT, Ho M, Zawitkowski M, Ambler KL, Cheetham GD, et al. 2015. A tale of two siblings: Two cases of AML arising from a single pre-leukemic DNMT3A mutant clone. Leukemia 29: 2101–2104. 10.1038/leu.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhang M, Li N, Chen T, Zhang Y, Wan T, Cao X. 2004. KLRL1, a novel killer cell lectinlike receptor, inhibits natural killer cell cytotoxicity. Blood 104: 2858–2866. 10.1182/blood-2004-03-0878 [DOI] [PubMed] [Google Scholar]
- Haubner S, Perna F, Köhnke T, Schmidt C, Berman S, Augsberger C, Schnorfeil FM, Krupka C, Lichtenegger FS, Liu X, et al. 2019. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia 33: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, Schernthaner GH, Wacheck V, Selzer E, Sperr WR, et al. 2007. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest 37: 73–82. 10.1111/j.1365-2362.2007.01746.x [DOI] [PubMed] [Google Scholar]
- Herrmann H, Sadovnik I, Cerny-Reiterer S, Rulicke T, Stefanzl G, Willmann M, Hoermann G, Bilban M, Blatt K, Herndlhofer S, et al. 2014. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 123: 3951–3962. 10.1182/blood-2013-10-536078 [DOI] [PubMed] [Google Scholar]
- Hertweck MK, Erdfelder F, Kreuzer KA. 2011. CD44 in hematological neoplasias. Ann Hematol 90: 493–508. 10.1007/s00277-011-1161-z [DOI] [PubMed] [Google Scholar]
- Ho TC, LaMere M, Stevens BM, Ashton JM, Myers JR, O'Dwyer KM, Liesveld JL, Mendler JH, Guzman M, Morrissette JD, et al. 2016. Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood 128: 1671–1678. 10.1182/blood-2016-02-695312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge DE, Shannon KM, Kalousek DK, Schonberg S, Schaffner V, Zoger S, Eaves CJ, Eaves AC. 1987. Juvenile monosomy 7 syndrome: Evidence that the disease originates in a pluripotent hemopoietic stem cell. Leuk Res 11: 705–709. 10.1016/0145-2126(87)90006-3 [DOI] [PubMed] [Google Scholar]
- Hollein A, Jeromin S, Meggendorfer M, Fasan A, Nadarajah N, Kern W, Haferlach C, Haferlach T. 2018. Minimal residual disease (MRD) monitoring and mutational landscape in AML with RUNX1-RUNX1T1: A study on 134 patients. Leukemia 32: 2270–2274. 10.1038/s41375-018-0086-0 [DOI] [PubMed] [Google Scholar]
- Hope KJ, Jin L, Dick JE. 2004. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5: 738–743. 10.1038/ni1080 [DOI] [PubMed] [Google Scholar]
- Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL. 2007. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci 104: 11008–11013. 10.1073/pnas.0704271104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. 2017. Measurable residual disease testing in acute myeloid leukaemia. Leukemia 31: 1482–1490. 10.1038/leu.2017.113 [DOI] [PubMed] [Google Scholar]
- Hyakuna N, Naritomi K, Ito E. 1998. Retrospective analysis of clonality and detection of residual disease in myeloid leukemia by FISH on long-term stored bone marrow smears. Acta Paediatr Jpn 40: 318–323. 10.1111/j.1442-200X.1998.tb01939.x [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. 2007. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25: 1315–1321. 10.1038/nbt1350 [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. 2006. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451. 10.1038/nm1388 [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Liedtke M, Gentles AJ, Cleary ML. 2015. CD93 marks a non-quiescent human leukemia stem cell population and is required for development of MLL-rearranged acute myeloid leukemia. Cell Stem Cell 17: 412–421. 10.1016/j.stem.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138: 271–285. 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. 2014. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371: 2488–2498. 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. 2017. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 377: 111–121. 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Majeti R. 2013. Clonal evolution of acute leukemia genomes. Oncogene 32: 135–140. 10.1038/onc.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Chao MP, Cha AC, Alizadeh AA, Gentles AJ, Weissman IL, Majeti R. 2011. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci 108: 5009–5014. 10.1073/pnas.1100551108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, Majeti R. 2012. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4: 149ra118 10.1126/scitranslmed.3004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaras M, Johnels P, Hansen N, Agerstam H, Tsapogas P, Rissler M, Lassen C, Olofsson T, Bjerrum OW, Richter J, et al. 2010. Isolation and killing of candidate chronic myeloid leukemia stem cells by antibody targeting of IL-1 receptor accessory protein. Proc Natl Acad Sci 107: 16280–16285. 10.1073/pnas.1004408107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Liu BY, Huang J, Lu J, Sharmili R, Mishra M, Zhao X, Lin J, Hsi ED, Junutula JR. 2016. Targeting acute myeloid leukemia via anti-IL1RAP antibodies. In Proceedings of the 107th Annual Meeting of the American Association for Cancer Research American Association for Cancer Research, New Orleans, LA. [Google Scholar]
- Jiang Y, Xu P, Yao D, Chen X, Dai H. 2017. CD33, CD96 and death associated protein kinase (DAPK) expression are associated with the survival rate and/or response to the chemotherapy in the patients with acute myeloid leukemia (AML). Med Sci Monit 23: 1725–1732. 10.12659/MSM.900305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YP, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, Mishra M, Huang J, Dao-Pick T, Roy S, et al. 2018. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv 2: 1738–1749. 10.1182/bloodadvances.2018020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. 2006. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12: 1167–1174. 10.1038/nm1483 [DOI] [PubMed] [Google Scholar]
- Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, et al. 2009. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 5: 31–42. 10.1016/j.stem.2009.04.018 [DOI] [PubMed] [Google Scholar]
- Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, Erpelinck-Verschueren CAJ, Gradowska PL, Meijer R, Cloos J, et al. 2018. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 378: 1189–1199. 10.1056/NEJMoa1716863 [DOI] [PubMed] [Google Scholar]
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al. 2000a. The interleukin-3 receptor α chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14: 1777–1784. 10.1038/sj.leu.2401903 [DOI] [PubMed] [Google Scholar]
- Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al. 2000b. The interleukin-3 receptor α chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14: 1777–1784. 10.1038/sj.leu.2401903 [DOI] [PubMed] [Google Scholar]
- June CH, Sadelain M. 2018. Chimeric antigen receptor therapy. N Engl J Med 379: 64–73. 10.1056/NEJMra1706169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner C, Peipp M, Schub N, Humpe A, Gramatzki M. 2013. Targeting CD96 for antibody based elimination of leukemic stem cells in AML: A new strategy in stem cell transplantation. Blood 122: 3972. [Google Scholar]
- Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, et al. 2010. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 7: 708–717. 10.1016/j.stem.2010.11.014 [DOI] [PubMed] [Google Scholar]
- Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, Niiro H, Yurino A, Miyawaki K, Takenaka K, et al. 2015. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell 17: 341–352. 10.1016/j.stem.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Kim HG, Kojima K, Swindle CS, Cotta CV, Huo Y, Reddy V, Klug CA. 2008. FLT3-ITD cooperates with inv(16) to promote progression to acute myeloid leukemia. Blood 111: 1567–1574. 10.1182/blood-2006-06-030312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, et al. 2014. A draft map of the human proteome. Nature 509: 575–581. 10.1038/nature13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, Aljanahi AA, Schreeder D, Klichinsky M, Shestova O, et al. 2018. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell 173: 1439–1453.e19. 10.1016/j.cell.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA. 2011. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11: 685–692. 10.1038/nri3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Schuster MB, Bereshchenko O, Moore S, Dvinge H, Kurz E, Theilgaard-Monch K, Mansson R, Pedersen TA, Pabst T, et al. 2008. Modeling of C/EBPα mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell 13: 299–310. 10.1016/j.ccr.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Kotini AG, Chang CJ, Chow A, Yuan H, Ho TC, Wang T, Vora S, Solovyov A, Husser C, Olszewska M, et al. 2017. Stage-specific human induced pluripotent stem cells map the progression of myeloid transformation to transplantable leukemia. Cell Stem Cell 20: 315–328.e7. 10.1016/j.stem.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson SY, Björkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. 2011. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol 29: 2897–2903. 10.1200/JCO.2011.34.8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke J, Bullinger L, Teleanu V, Tschürtz F, Gaidzik VI, Kühn MWM, Rücker FG, Holzmann K, Paschka P, Kapp-Schwörer S, et al. 2013. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia. Blood 122: 100–108. 10.1182/blood-2013-01-479188 [DOI] [PubMed] [Google Scholar]
- Kuo YH, Landrette SF, Heilman SA, Perrat PN, Garrett L, Liu PP, Le Beau MM, Kogan SC, Castilla LH. 2006. Cbfβ-SMMHC induces distinct abnormal myeloid progenitors able to develop acute myeloid leukemia. Cancer Cell 9: 57–68. 10.1016/j.ccr.2005.12.014 [DOI] [PubMed] [Google Scholar]
- Laborda E, Mazagova M, Shao S, Wang X, Quirino H, Woods AK, Hampton EN, Rodgers DT, Kim CH, Schultz PG, et al. 2017. Development of a chimeric antigen receptor targeting C-type lectin-like molecule-1 for human acute myeloid leukemia. Int J Mol Sci 18: E2259 10.3390/ijms18112259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet JE. 2018. Is the overall survival for older adults with AML finally improving? Best Pract Res Clin Haematol 31: 387–390. 10.1016/j.beha.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Lancet JE, Rizzieri D, Schiller GJ, Stuart RK, Kolitz JE, Solomon SR, Newell LF, Erba HP, Uy GL, Ryan R, et al. 2017. Overall survival (OS) with CPX-351 versus 7+3 in older adults with newly diagnosed, therapy-related acute myeloid leukemia (tAML): Subgroup analysis of a phase III study. J Clin Oncol 35: 7035–7035. 10.1200/JCO.2017.35.15_suppl.7035 [DOI] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648. 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, Lee AM, Lister TA, Gribben JG. 2009. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood 114: 3909–3916. 10.1182/blood-2009-02-206946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Williams IR, Anastasiadou E, Boulton CL, Joseph SW, Amaral SM, Curley DP, Duclos N, Huntly BJ, Fabbro D, et al. 2005. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene 24: 7882–7892. 10.1038/sj.onc.1208933 [DOI] [PubMed] [Google Scholar]
- Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, Wong A, Tam L, Newman R, Vuillemenot BR, et al. 2017. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood 129: 609–618. 10.1182/blood-2016-08-735365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Volk AG, Haug JS, Marshall SA, Woodfin AR, Bartom ET, Gilmore JM, Florens L, Washburn MP, Sullivan KD, et al. 2017. Therapeutic targeting of MLL degradation pathways in MLL-rearranged leukemia. Cell 168: 59–72.e13. 10.1016/j.cell.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesveld JL, Dipersio JF, Abboud CN. 1994. Integrins and adhesive receptors in normal and leukemic CD34+ progenitor cells: Potential regulatory checkpoints for cellular traffic. Leuk Lymphoma 14: 19–28. 10.3109/10428199409049647 [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang G. 2006. CD44 and hematologic malignancies. Cell Mol Immunol 3: 359–365. [PubMed] [Google Scholar]
- Liu F, Pinz K, Ma Y, Wada M, Chen K, Ma G, Su Y, Zhang S, He G, Ma Y. 2018. First-in-human CLL1-CD33 compound CAR T cells as a two-pronged approach for the treatment of refractory acute myeloid leukemia. In European Hematology Association Congress 23, Stockholm, Sweden. [Google Scholar]
- Lu H, Zhou Q, Deshmukh V, Phull H, Ma J, Tardif V, Naik RR, Bouvard C, Zhang Y, Choi S, et al. 2014. Targeting human C-type lectin-like molecule-1 (CLL1) with a bispecific antibody for immunotherapy of acute myeloid leukemia. Angew Chem Int Ed Engl 53: 9841–9845. 10.1002/anie.201405353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhumathi J, Sridevi S, Verma RS. 2017. CD25 targeted therapy of chemotherapy resistant leukemic stem cells using DR5 specific TRAIL peptide. Stem Cell Res 19: 65–75. 10.1016/j.scr.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Majeti R, Becker MW, Tian Q, Lee TL, Yan X, Liu R, Chiang JH, Hood L, Clarke MF, Weissman IL. 2009a. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci 106: 3396–3401. 10.1073/pnas.0900089106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL. 2009b. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138: 286–299. 10.1016/j.cell.2009.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardo M, Caronno A, Pruneri G, Raviele PR, Viale A, Pelicci PG, Colombo E. 2013. NPMc+ and FLT3_ITD mutations cooperate in inducing acute leukaemia in a novel mouse model. Leukemia 27: 2248–2251. 10.1038/leu.2013.114 [DOI] [PubMed] [Google Scholar]
- Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. 2004. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem 279: 14792–14802. 10.1074/jbc.M313127200 [DOI] [PubMed] [Google Scholar]
- Martelli MP, Pettirossi V, Thiede C, Bonifacio E, Mezzasoma F, Cecchini D, Pacini R, Tabarrini A, Ciurnelli R, Gionfriddo I, et al. 2010. CD34+ cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice. Blood 116: 3907–3922. 10.1182/blood-2009-08-238899 [DOI] [PubMed] [Google Scholar]
- Matatall KA, Shen CC, Challen GA, King KY. 2014. Type II interferon promotes differentiation of myeloid-biased hematopoietic stem cells. Stem Cells 32: 3023–3030. 10.1002/stem.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. 2002. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J Immunol 168: 5222–5232. 10.4049/jimmunol.168.10.5222 [DOI] [PubMed] [Google Scholar]
- Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, et al. 2018. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 557: 580–584. 10.1038/s41586-018-0125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Del Real M, Wei G, Hernandez R, Marcucci E, Lin A, Mcdonald T, Zhao D, Wu H, Carlesso N, et al. 2017. Anti-IL1RAP/CD3 bispecific Antibody (BsAb) is a promising novel and effective therapy for acute myeloid leukemia (AML). Blood 130: 1361–1361. [Google Scholar]
- Meyer C, Hofmann J, Burmeister T, Groger D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A, Villarese P, Macintyre E, et al. 2013. The MLL recombinome of acute leukemias in 2013. Leukemia 27: 2165–2176. 10.1038/leu.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirantes C, Passegué E, Pietras EM. 2014. Pro-inflammatory cytokines: Emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res 329: 248–254. 10.1016/j.yexcr.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Barreyro L, Todorova TI, Taylor SJ, Antony-Debré I, Narayanagari SR, Carvajal LA, Leite J, Piperdi Z, Pendurti G, et al. 2018. IL1RAP potentiates multiple oncogenic signaling pathways in AML. J Exp Med 215: 1709–1727. 10.1084/jem.20180147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Weissman IL, Akashi K. 2000. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci 97: 7521–7526. 10.1073/pnas.97.13.7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni Nodehi S, Repp R, Kellner C, Bräutigam J, Staudinger M, Schub N, Peipp M, Gramatzki M, Humpe A. 2012. Enhanced ADCC activity of affinity maturated and Fc-engineered mini-antibodies directed against the AML stem cell antigen CD96. PLoS ONE 7: e42426 10.1371/journal.pone.0042426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi-Villa A, Morozoff C, Charara R, Allen C, Naghavi M, Murray CJ. 2017. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA 317: 388–406. 10.1001/jama.2016.20324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink LM, Walter RB, Ossenkoppele GJ. 2019. Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev 34: 26–33. 10.1016/j.blre.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Moshaver B, van Rhenen A, Kelder A, van der Pol M, Terwijn M, Bachas C, Westra AH, Ossenkoppele GJ, Zweegman S, Schuurhuis GJ. 2008. Identification of a small subpopulation of candidate leukemia-initiating cells in the side population of patients with acute myeloid leukemia. Stem Cells 26: 3059–3067. 10.1634/stemcells.2007-0861 [DOI] [PubMed] [Google Scholar]
- Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, Brunet S, Sierra J. 2001. Interleukin-3 receptor α chain (CD123) is widely expressed in hematologic malignancies. Haematologica 86: 1261–1269. [PubMed] [Google Scholar]
- Murata Y, Saito Y, Kotani T, Matozaki T. 2018. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci 109: 2349–2357. 10.1111/cas.13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Castiñeiras-Vilariño M, Höckendorf U, Hannesschläger N, Lemeer S, Kupka D, Meyermann S, Lech M, Anders HJ, Kuster B, et al. 2014. Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity 40: 389–399. 10.1016/j.immuni.2013.12.015 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Astrand-Grundstrom I, Arvidsson I, Jacobsson B, Hellstrom-Lindberg E, Hast R, Jacobsen SE. 2000. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: Evidence for involvement at the hematopoietic stem cell level. Blood 96: 2012–2021. [PubMed] [Google Scholar]
- Nilsson L, Astrand-Grundstrom I, Anderson K, Arvidsson I, Hokland P, Bryder D, Kjeldsen L, Johansson B, Hellstrom-Lindberg E, Hast R, et al. 2002. Involvement and functional impairment of the CD34+CD38-Thy–1+ hematopoietic stem cell pool in myelodysplastic syndromes with trisomy 8. Blood 100: 259–267. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Eden P, Olsson E, Mansson R, Astrand-Grundstrom I, Strombeck B, Theilgaard-Monch K, Anderson K, Hast R, Hellstrom-Lindberg E, et al. 2007. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood 110: 3005–3014. 10.1182/blood-2007-03-079368 [DOI] [PubMed] [Google Scholar]
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A YM, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. 2018. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute, Bethesda, MD: https://seer.cancer.gov/csr/1975_2016 [Google Scholar]
- Olweus J, Lund-Johansen F, Terstappen LW. 1994. Expression of cell surface markers during differentiation of CD34+, CD38−/lo fetal and adult bone marrow cells. Immunomethods 5: 179–188. 10.1006/immu.1994.1054 [DOI] [PubMed] [Google Scholar]
- Pabst C, Bergeron A, Lavallee VP, Yeh J, Gendron P, Norddahl GL, Krosl J, Boivin I, Deneault E, Simard J, et al. 2016. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood 127: 2018–2027. 10.1182/blood-2015-11-683649 [DOI] [PubMed] [Google Scholar]
- Pandolfi A, Barreyro L, Steidl U. 2013. Concise review: Preleukemic stem cells: Molecular biology and clinical implications of the precursors to leukemia stem cells. Stem Cells Transl Med 2: 143–150. 10.5966/sctm.2012-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, et al. 2016. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374: 2209–2221. 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin B, Ouillette P, Li Y, Keller J, Lam C, Roulston D, Li C, Shedden K, Malek SN. 2013. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood 121: 369–377. 10.1182/blood-2012-04-427039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL. 2003. Normal and leukemic hematopoiesis: Are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci 100: 11842–11849. 10.1073/pnas.2034201100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, et al. 2012. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 366: 1079–1089. 10.1056/NEJMoa1112304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, Hendrickson RC, Brennan CW, Sadelain M. 2017. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell 32: 506–519.e5. 10.1016/j.ccell.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM. 2017. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 130: 1693–1698. 10.1182/blood-2017-06-780882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Warr MR, Passegué E. 2011. Cell cycle regulation in hematopoietic stem cells. J Cell Biol 195: 709–720. 10.1083/jcb.201102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Lakshminarasimhan R, Techner JM, Fong S, Flach J, Binnewies M, Passegué E. 2014. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J Exp Med 211: 245–262. 10.1084/jem.20131043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, et al. 2016. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol 18: 607–618. 10.1038/ncb3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulikkan JA, Castilla LH. 2018. Preleukemia and leukemia-initiating cell activity in inv(16) acute myeloid leukemia. Front Oncol 8: 129 10.3389/fonc.2018.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek L, Otto GW, Garnett C, Lhermitte L, Karamitros D, Stoilova B, Lau IJ, Doondeea J, Usukhbayar B, Kennedy A, et al. 2016. Genetically distinct leukemic stem cells in human CD34− acute myeloid leukemia are arrested at a hemopoietic precursor-like stage. J Exp Med 213: 1513–1535. 10.1084/jem.20151775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quéré R, Andradottir S, Brun AC, Zubarev RA, Karlsson G, Olsson K, Magnusson M, Cammenga J, Karlsson S. 2011. High levels of the adhesion molecule CD44 on leukemic cells generate acute myeloid leukemia relapse after withdrawal of the initial transforming event. Leukemia 25: 515–526. 10.1038/leu.2010.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter K, Polzer H, Krupka C, Maiser A, Vick B, Rothenberg-Thurley M, Metzeler KH, Dorfel D, Salih HR, Jung G, et al. 2018. Tyrosine kinase inhibition increases the cell surface localization of FLT3-ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia 32: 313–322. 10.1038/leu.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss-Borst MA, Buhring HJ, Klein G, Müller CA. 1992. Adhesion molecules on CD34+ hematopoietic cells in normal human bone marrow and leukemia. Ann Hematol 65: 169–174. 10.1007/BF01703110 [DOI] [PubMed] [Google Scholar]
- Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegué E. 2011. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell 20: 661–673. 10.1016/j.ccr.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. 2005. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci 102: 9194–9199. 10.1073/pnas.0503280102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. 2008. Stems cells and the pathways to aging and cancer. Cell 132: 681–696. 10.1016/j.cell.2008.01.036 [DOI] [PubMed] [Google Scholar]
- Rundberg Nilsson A, Soneji S, Adolfsson S, Bryder D, Pronk CJ. 2016. Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLoS ONE 11: e0158369 10.1371/journal.pone.0158369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovnik I, Herrmann H, Eisenwort G, Blatt K, Hoermann G, Mueller N, Sperr WR, Valent P. 2017. Expression of CD25 on leukemic stem cells in BCR-ABL1+ CML: Potential diagnostic value and functional implications. Exp Hematol 51: 17–24. 10.1016/j.exphem.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha HR, Kaneda-Nakashima K, Shimosaki S, Suekane A, Sarkar B, Saito Y, Ogoh H, Nakahata S, Inoue K, Watanabe T, et al. 2018. Suppression of GPR56 expression by pyrrole-imidazole polyamide represents a novel therapeutic drug for AML with high EVI1 expression. Sci Rep 8: 13741 10.1038/s41598-018-32205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone A, Najima Y, et al. 2010. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med 2: 17ra9 10.1126/scitranslmed.3000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakahata S, Yamakawa N, Kaneda K, Ichihara E, Suekane A, Morishita K. 2011. CD52 as a molecular target for immunotherapy to treat acute myeloid leukemia with high EVI1 expression. Leukemia 25: 921–931. 10.1038/leu.2011.36 [DOI] [PubMed] [Google Scholar]
- Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Recher C, et al. 2011. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J Clin Invest 121: 384–395. 10.1172/JCI41495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Caux C, Kitamura T, Watanabe Y, Arai K, Banchereau J, Miyajima A. 1993. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood 82: 752–761. [PubMed] [Google Scholar]
- Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, Small D, Burnett A, Levis M. 2011. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood 117: 3286–3293. 10.1182/blood-2010-01-266742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schessl C, Rawat VP, Cusan M, Deshpande A, Kohl TM, Rosten PM, Spiekermann K, Humphries RK, Schnittger S, Kern W, et al. 2005. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest 115: 2159–2168. 10.1172/JCI24225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri A, Will B, Steidl U, Verma A. 2017. Stem and progenitor cell alterations in myelodysplastic syndromes. Blood 129: 1586–1594. 10.1182/blood-2016-10-696062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI. 2018. Age-related clonal hematopoiesis. Blood 131: 496–504. 10.1182/blood-2017-07-746453 [DOI] [PubMed] [Google Scholar]
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, et al. 2014. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506: 328–333. 10.1038/nature13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A, Jaciw-Zurakowsky I, Marke R, et al. 2017. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 547: 104–108. 10.1038/nature22993 [DOI] [PubMed] [Google Scholar]
- Shouval R, Shlush LI, Yehudai-Resheff S, Ali S, Pery N, Shapiro E, Tzukerman M, Rowe JM, Zuckerman T. 2014. Single cell analysis exposes intratumor heterogeneity and suggests that FLT3-ITD is a late event in leukemogenesis. Exp Hematol 42: 457–463. 10.1016/j.exphem.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. 1999. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: A phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93: 3678–3684. [PubMed] [Google Scholar]
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, Dombret H, Karanes C, Theobald M, Bennett JM, Sherman ML, et al. 2001. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 19: 3244–3254. 10.1200/JCO.2001.19.13.3244 [DOI] [PubMed] [Google Scholar]
- Smith CC, Paguirigan A, Jeschke GR, Lin KC, Massi E, Tarver T, Chin CS, Asthana S, Olshen A, Travers KJ, et al. 2017. Heterogeneous resistance to quizartinib in acute myeloid leukemia revealed by single-cell analysis. Blood 130: 48–58. 10.1182/blood-2016-04-711820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. 2006. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 10: 257–268. 10.1016/j.ccr.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC. 2004. HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res 28: 1089–1096. 10.1016/j.leukres.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. 2015. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126: 9–16. 10.1182/blood-2015-03-631747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl U, Rosenbauer F, Verhaak RG, Gu X, Ebralidze A, Otu HH, Klippel S, Steidl C, Bruns I, Costa DB, et al. 2006. Essential role of Jun family transcription factors in PU.1 knockdown-induced leukemic stem cells. Nat Genet 38: 1269–1277. 10.1038/ng1898 [DOI] [PubMed] [Google Scholar]
- Steidl U, Steidl C, Ebralidze A, Chapuy B, Han HJ, Will B, Rosenbauer F, Becker A, Wagner K, Koschmieder S, et al. 2007. A distal single nucleotide polymorphism alters long-range regulation of the PU.1 gene in acute myeloid leukemia. J Clin Invest 117: 2611–2620. 10.1172/JCI30525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, et al. 2017. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377: 454–464. 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striz I. 2017. Cytokines of the IL-1 family: Recognized targets in chronic inflammation underrated in organ transplantations. Clin Sci 131: 2241–2256. 10.1042/CS20170098 [DOI] [PubMed] [Google Scholar]
- Sun GX, Wormsley S, Sparkes RS, Naeim F, Gale RP. 1991. Where does transformation occur in acute leukemia? Leuk Res 15: 1183–1189. 10.1016/0145-2126(91)90188-Y [DOI] [PubMed] [Google Scholar]
- Sutherland HJ, Blair A, Zapf RW. 1996. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood 87: 4754–4761. [PubMed] [Google Scholar]
- Takizawa H, Boettcher S, Manz MG. 2012. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood 119: 2991–3002. 10.1182/blood-2011-12-380113 [DOI] [PubMed] [Google Scholar]
- Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt WD, et al. 2017. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell 21: 225–240.e5. 10.1016/j.stem.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Tamura S, Kanamaru A, Takemoto Y, Kakishita E, Nagai K. 1993. Clonal evolutions during long-term cultures of bone marrow from de novo acute myeloid leukaemia with trilineage myelodysplasia and with myelodysplastic remission marrow. Br J Haematol 84: 219–226. 10.1111/j.1365-2141.1993.tb03055.x [DOI] [PubMed] [Google Scholar]
- Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, Rouce RH, Lulla P, Rooney CM, Gottschalk S, et al. 2017. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type lectin-like molecule 1. Mol Ther 25: 2202–2213. 10.1016/j.ymthe.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, Luongo JL, Danet-Desnoyers GA, Bonnet D. 2005. Hematopoietic stem cells express multiple myeloid markers: Implications for the origin and targeted therapy of acute myeloid leukemia. Blood 106: 4086–4092. 10.1182/blood-2005-03-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, Lillington D, Oakervee H, Cavenagh J, Agrawal SG, et al. 2010. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34− fraction. Blood 115: 1976–1984. 10.1182/blood-2009-02-206565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Duyvestyn JM, Dagger SA, Dishington EJ, Rinaldi CA, Dovey OM, Vassiliou GS, Grove CS, Langdon WY. 2017. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci Transl Med 9: eaam8060 10.1126/scitranslmed.aam8060 [DOI] [PubMed] [Google Scholar]
- Terpstra W, Ploemacher RE, Prins A, van Lom K, Pouwels K, Wognum AW, Wagemaker G, Lowenberg B, Wielenga JJ. 1996. Fluorouracil selectively spares acute myeloid leukemia cells with long-term growth abilities in immunodeficient mice and in culture. Blood 88: 1944–1950. [PubMed] [Google Scholar]
- Terwijn M, Feller N, van Rhenen A, Kelder A, Westra G, Zweegman S, Ossenkoppele G, Schuurhuis GJ. 2009. Interleukin-2 receptor α-chain (CD25) expression on leukaemic blasts is predictive for outcome and level of residual disease in AML. Eur J Cancer 45: 1692–1699. 10.1016/j.ejca.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Thomas D, Majeti R. 2017. Biology and relevance of human acute myeloid leukemia stem cells. Blood 129: 1577–1585. 10.1182/blood-2016-10-696054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traer E, Martinez J, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Kovacsovics T, Tyner JW, Wong M, Druker BJ. 2016. FGF2 from marrow microenvironment promotes resistance to FLT3 inhibitors in acute myeloid leukemia. Cancer Res 76: 6471–6482. 10.1158/0008-5472.CAN-15-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. 2015. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S, Ossenkoppele GJ, Schuurhuis GJ. 2007a. Aberrant marker expression patterns on the CD34+CD38− stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia 21: 1700–1707. 10.1038/sj.leu.2404754 [DOI] [PubMed] [Google Scholar]
- van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G. 2007b. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 110: 2659–2666. 10.1182/blood-2007-03-083048 [DOI] [PubMed] [Google Scholar]
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, et al. 1998. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab triple therapy study group. N Engl J Med 338: 161–165. 10.1056/NEJM199801153380304 [DOI] [PubMed] [Google Scholar]
- Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, Moehrle B, Brocks D, Bayindir I, Kaschutnig P, et al. 2015. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520: 549–552. 10.1038/nature14131 [DOI] [PubMed] [Google Scholar]
- Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, Wang W, Xu L, Liao S, Liu W, et al. 2018. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol 11: 7 10.1186/s13045-017-0553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei AH, Tiong IS. 2017. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood 130: 2469–2474. 10.1182/blood-2017-08-784066 [DOI] [PubMed] [Google Scholar]
- Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, et al. 2012. The origin and evolution of mutations in acute myeloid leukemia. Cell 150: 264–278. 10.1016/j.cell.2012.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Schlegl J, Hahne H, Gholami AM, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, et al. 2014. Mass-spectrometry-based draft of the human proteome. Nature 509: 582–587. 10.1038/nature13319 [DOI] [PubMed] [Google Scholar]
- Will B, Zhou L, Vogler TO, Ben-Neriah S, Schinke C, Tamari R, Yu Y, Bhagat TD, Bhattacharyya S, Barreyro L, et al. 2012. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood 120: 2076–2086. 10.1182/blood-2011-12-399683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will B, Vogler TO, Narayanagari S, Bartholdy B, Todorova TI, da Silva Ferreira M, Chen J, Yu Y, Mayer J, Barreyro L, et al. 2015. Minimal PU.1 reduction induces a preleukemic state and promotes development of acute myeloid leukemia. Nat Med 21: 1172–1181. 10.1038/nm.3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll PS, Kjallquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S, Erlandsson R, Ngara M, Anderson K, Deng Q, et al. 2014. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 25: 794–808. 10.1016/j.ccr.2014.03.036 [DOI] [PubMed] [Google Scholar]
- Wong TN, Miller CA, Klco JM, Petti A, Demeter R, Helton NM, Li T, Fulton RS, Heath SE, Mardis ER, et al. 2016. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood 127: 893–897. 10.1182/blood-2015-10-677021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Yang S, Zhu L, Wang Y, Zhang Y, Zhou J, Li D. 2016. Prognosis of patients with de novo acute myeloid leukemia resistant to initial induction chemotherapy. Am J Med Sci 351: 473–479. 10.1016/j.amjms.2016.02.034 [DOI] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, et al. 2014. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20: 1472–1478. 10.1038/nm.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcintepe L, Frankel AE, Hogge DE. 2006. Expression of interleukin-3 receptor subunits on defined subpopulations of acute myeloid leukemia blasts predicts the cytotoxicity of diphtheria toxin interleukin-3 fusion protein against malignant progenitors that engraft in immunodeficient mice. Blood 108: 3530–3537. 10.1182/blood-2006-04-013813 [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Wilkinson AC, Ooehara J, Lan X, Lai CY, Nakauchi Y, Pritchard JK, Nakauchi H. 2018. Large-scale clonal analysis resolves aging of the mouse hematopoietic stem cell compartment. Cell Stem Cell 22: 600–607.e4. 10.1016/j.stem.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sexauer A, Levis M. 2014. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br J Haematol 164: 61–72. 10.1111/bjh.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Ueno T, Fukumura K, Yamato A, Ando M, Yamaguchi H, Soda M, Kawazu M, Sai E, Yamashita Y, et al. 2014. Leukemic evolution of donor-derived cells harboring IDH2 and DNMT3A mutations after allogeneic stem cell transplantation. Leukemia 28: 426–428. 10.1038/leu.2013.278 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, et al. 2015. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525: 389–393. 10.1038/nature15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Singh S, Pardoux C, Zhao J, Hsi ED, Abo A, Korver W. 2010. Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia. Haematologica 95: 71–78. 10.3324/haematol.2009.009811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Ma C, O'Connell RM, Mehta A, DiLoreto R, Heath JR, Baltimore D. 2014. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell 14: 445–459. 10.1016/j.stem.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Yu SF, Del Rosario G, Leong SR, Lee GY, Vij R, Chiu CPC, Liang WC, Wu Y, Chalouni C, et al. 2019. An anti-CLL-1 antibody-drug conjugate for the treatment of acute myeloid leukemia. Clin Cancer Res 25: 1358–1368. 10.1158/1078-0432.CCR-18-0333 [DOI] [PubMed] [Google Scholar]