Abstract
Currently cancer is the second leading cause of death globally and worldwide incidence and mortality rates of all cancers of males and females are rising tremendously. In spite of advances in chemotherapy and radiation, metastasis and recurrence are considered as the major causes of cancer related deaths. Hence there is a mounting need to develop new therapeutic modalities to treat metastasis and recurrence in cancers. A significant amount of substantiation from epidemiological, clinical and laboratory research highlights the importance of diet and nutrition in cancer chemoprevention. Enterolactone (EL) is a bioactive phenolic metabolite known as a mammalian lignan derived from dietary lignans. Here in we review the reported anti-cancer properties of EL at preclinical as well as clinical level. Several in-vivo and in-vitro studies have provided strong evidence that EL exhibits potent anti-cancer and/or protective properties against different cancers including breast, prostate, colorectal, lung, ovarian, endometrial, cervical cancers and hepatocellular carcinoma. Reported laboratory studies indicate a clear role for EL in preventing cancer progression at various stages including cancer cell proliferation, survival, angiogenesis, inflammation and metastasis. In clinical settings, EL has been reported to reduce risk, decrease mortality rate and improve overall survival particularly in breast, prostate, colon, gastric and lung cancer. Further, the in-vitro human cell culture studies provide strong evidence of the anticancer and antimetastatic mechanisms of EL in several cancers. This comprehensive review supports an idea of projecting EL as a promising candidate for developing anticancer drug or adjunct dietary supplements and nutraceuticals.
Keywords: Enterolactone, Anticancer, Dietary Lignans, Mammalian Lignans, Anti-metastatic activity, Flax seeds
1. Introduction
Cancer is considered as the second leading cause of death globally according to WHO statistics and was responsible for 8.8 million deaths in 2015 (Global Burden of Disease Cancer Collaboration, 2017). As per GLOBOCAN 2012 data (Ferlay et al., 2015), there are 14 million new cancer cases per year which is expected to rise to 24 million new cases by 2035. The number of cancer-related deaths are also expected to rise from 8.2 million to 14.6 million annually (Stewart et al., 2016). The International Agency for Research on Cancer (IARC) estimated that the worldwide incidence and mortality rates of all cancers of males and females are very high in United States of America (USA), European Union (EU) and other developed regions (Ervik et al., 2016). As indicated in GLOBOCAN 2012 data based on incidence, mortality and prevalence worldwide in both sexes, the top 5 most frequent cancers occur in the lung, breast, colorectum, prostate and stomach tissues. Moreover, incidence and mortality rates of other cancers such as liver and bladder cancer in men and cervical, endometrial and ovarian cancer in females are also rising (Ferlay et al., 2015). Metastasis and recurrence are considered as the major causes of cancer related deaths in spite of advances in chemotherapy and radiation (Qian et al., 2017). Patients with metastatic tumors in all types of cancer are often unresponsive to existing therapies and strategies to achieve long-term remission than patients with localized cancer. In spite of more than 200 anticancer drugs presently approved for clinical use, none has been found to specifically and effectively inhibit cancer metastasis (Qian et al., 2017). Current diagnostic and treatment modalities to deal with this rising cancer burden worldwide are also inadequate and ineffective. Hence there is a mounting need to develop new therapeutic modalities to treat metastatic cancers. One approach is to prevent metastatic spread, which can be achieved by understanding the molecular and cellular mechanisms involved in malignancy and the aggressive spreading of the disease. In general, cancer cell metastasis comprises of an orderly sequence of pathological molecular events, collectively termed as metastatic cascade; starting with epithelial to mesenchymal transition (EMT) followed by extracellular matrix (ECM) remodeling; intravasation; survival of cancer cells in the blood stream; extravasation and colonization to prosper in a new compatible environment (Hegde et al., 2013). Future studies on different signaling pathways involved in these molecular events of metastatic cascade may provide novel therapeutic targets to develop new antimetastatic drugs against metastatic cancers.
The major risk factors associated with considerable deaths from cancer are: high body mass index, low fruit and vegetable intake, lack of physical activity, tobacco use, and alcohol use (Vineis and Wild, 2014). A remarkable amount of evidence from epidemiological, clinical and laboratory research indicates the importance of diet and nutrition in cancer chemoprevention (Iqbal et al., 2017; Kaur et al., 2018; Mayne et al., 2016; World Cancer Research Fund and American Institute for Cancer Research, 2007). The World Cancer Research Fund and American Institute for Cancer Research (WCRF/AIRC) have released evidence based preventive recommendations including physical activity, avoidance of energy-dense foods, consumption of variety of fruits, vegetables, whole grains and pulses, and avoiding consumption of alcohol (World Cancer Research Fund and American Institute for Cancer Research, 2007). In last few decades, a large number of scientific efforts have been made to discover effective dietary phytochemicals for instance; phenolic acids, monophenols, polyphenols, complex carbohydrates for their use as chemopreventive agents (Surh, 2003; Weng and Yen, 2012). Among these, the ones generated by human gut microbiota from dietary phenolic compounds like condensed tannins, lignans and lignans have been found to be most useful (Högger, 2013). One of these bioactive phenolic metabolites is an Enterolactone (EL) which is a mammalian lignan derived from the dietary lignans. Several studies have revealed that EL possesses potent anti-cancer and/or protective properties against different cancers viz. breast, prostate, colo-rectal, lung, ovarian, endometrial, cervical cancers and hepatocellular carcinoma. The anti-cancer effects of EL have been mainly attributed to its anti-proliferative, pro-apoptotic, anti-inflammatory, anti-angiogenic and anti-metastatic activities. In this review, we provide a summarized account of the reported anti-cancer effects of EL at preclinical as well as clinical level.
2. Enterolactone: a mammalian lignan derived from dietary lignans
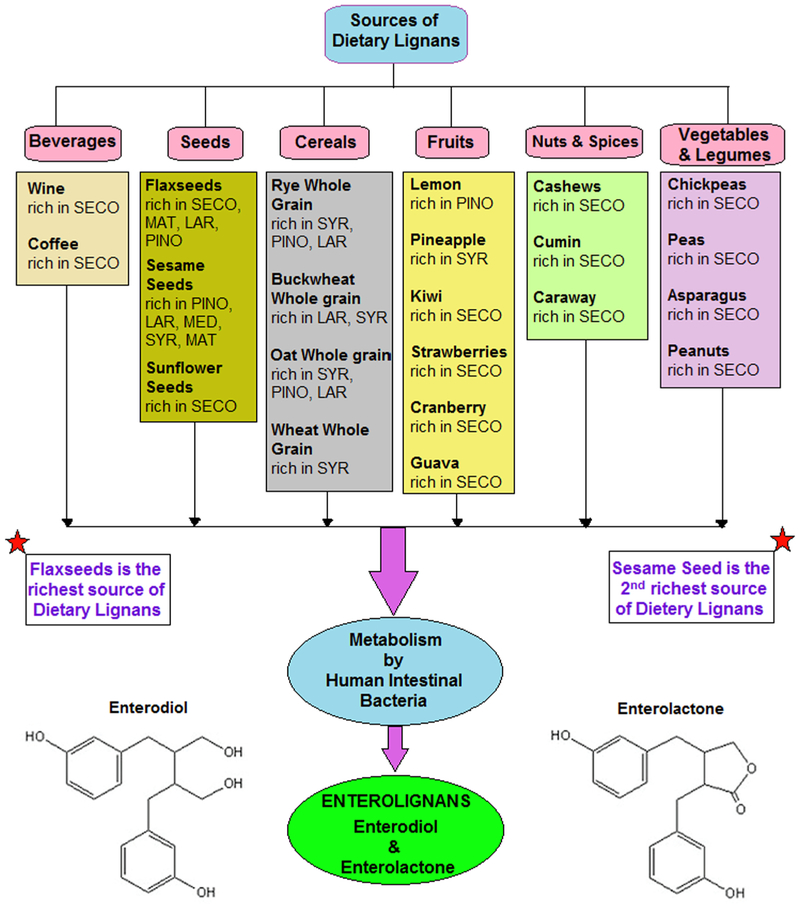
Plant lignans are large group of the PhE and regarded as an integral part of a healthy human diet (Adlercreutz, 2007). Dietary lignans are bioactive, non-caloric and phenolic compounds found in edible plants having structure similar to endogenous estrogen. Flax seeds and sesame seeds are the richest sources of lignans while other oil seeds, whole grain cereals, legumes, fruits, berries, vegetables, beverages and wine are also significant food sources (Fig. 1) of these dietary lignans (Peterson et al., 2010). Dietary lignans having significant health values include secoisolariciresinol (SECO), matairesinol (MAT), pinoresinol (PIN), medioresinol (MED), lariciresinol (LAR), syringaresinol (SYR), sesamin (SES, a lignan precursor), 7′- hydroxymatairesinol (HMR), arctigenin (ARC) and isolariciresinol (isoLAR) (Adlercreutz, 2007; Landete et al., 2016). These dietary lignans are metabolized to form nutritionally most significant enterolignans (also referred as mammalian lignans); enterodiol (ED) and enterolactone (EL) in various enzymatic steps including deglycosylation, demethylation, dehydroxylation, reduction, and dehydrogenation (Clavel et al., 2006b). Different strains of genera are involved in the metabolic conversion of dietary lignans into the enterolignans, which include Bacteroides, Clostridium, Eubacterium and Eggerthella lenta (Högger, 2013). Since EL appears to be the prime circulating enterolignan, its serum levels and urinary excretion levels are used as biomarkers for food lignan intakes (Peterson et al., 2010).
Fig. 1.
Metabolism of dietary lignans obtained from the major food sources to produce enterolignans in humans.
3. Pharmacokinetics of dietary and enterolignans in humans
To evaluate the absorption, distribution, metabolism and excretion (ADME) properties of dietary and enterolignans several pharmacokinetic (PK) studies have been carried out using lignan rich foods. However, very few studies have been carried out with isolated lignans in humans. Since flaxseeds and sesame seeds are the richest sources of dietary lignans, most of the PK studies have been carried out upon ingestion of secoisolariciresinol diglucoside (SDG) and sesamin (SES). Dietary lignans are present in edible plants both as aglycones (without sugars) and as glycosides (with sugars). Lignan glycosides after absorption in gastrointestinal tract (GIT), get metabolized in aglycones and subsequently to enterolignans. Flaxseed contains 0.05–0.2% SDG as a major glycoside lignan which is present in it as an oligomer of SDG molecules complexed with hydroxymethylglutaric acid (Setchell et al., 2014). Another rich source of lignans are the sesame seeds belonging to the oil seed crop, Sesamum indicum in which sesaminol triglucoside (STG) is the most abundant lignan glycoside and SES is present as a prime fat soluble lignan along with other lignans such as sesamolin, sesaminol, sesamolinol, PIN and HMR among which SES acts as one of the major precursors of mammalian lignans in sesame seeds (Liu et al., 2006; Majdalawieh et al., 2017; Pen et al., 2005). The SDG and STG glycoside lignans are considered to undergo hydrolysis in the large intestine after oral administration with subsequent deglycosylation of both in order to form SECO which is an aglycone lignan. Unabsorbed SECO undergoes microbial demethylation and dehydroxylation to produce the enterolignans; ED and EL respectively (Clavel et al., 2006a; Lampe et al., 2006; Roncaglia et al., 2011). Although available data suggests ambiguity about the metabolic pathway of SES in humans, EL is considered as the final product of the SES metabolism, which is independent of intermediates of the pathway (Pen et al., 2005; Tomimori et al., 2013). Besides SDG and SES, the aglycone dietary lignans like LAR, PIN, MAT and others are absorbed directly or converted finally to EL through intermediates. PIN is first metabolized to LAR, which is further converted to SECO, and then to ED and EL while MAT is directly converted to EL. Detection of both enterolignans and dietary lignans in human serum and urine indicates that both types of lignans are absorbed from the gut (Clavel et al., 2006a; Miles et al., 2017). A recent study in humans has shown that, following single-bolus oral administration of SDG, the SECO appeared rapidly in serum with peak concentrations reaching after 5–7 h independent of the dose ingested or the extent of purity of the extract while the peak serum ED and EL concentrations appeared at 19.2 ± 2.6 h and 26.7 ± 2.5 h, respectively (Setchell et al., 2014). These results are consistent with a previous study on 12 healthy young adults where enterolignans started to appear in plasma after 8–10 h after ingestion of the purified SDG (Kuijsten et al., 2005).
Once produced by intestinal bacteria, ED and EL are absorbed, conjugated in the gut epithelium or liver with sulfate or glucuronic acid, and excreted in the urine and bile. Since EL (~11.6 h) has a longer half-life than ED (~9 h), it constitutes the majority of enterolignans in circulation or in urine excretion (Kuijsten et al., 2005; Miles et al., 2017; Setchell et al., 2014). In order to get a better understanding of the oral absorption characteristics of SDG, SECO, ED & EL, a systematic evaluation of the intestinal permeation and conjugative metabolism was conducted using the polarized Caco-2 cell system in recent study. This study addressed the intestinal permeability of these lignans where authors suggested that SECO, ED and EL are able to undergo passive permeation and conjugative metabolism by the Caco-2 cells (Mukker et al., 2014). These findings are consistent with the previous in-vitro study involving three human colon epithelial cell lines where authors reported that the enterolignans (ED and EL) permeate human colon epithelial cells wherein they are conjugated with glucuronic acid or to a lesser extent with sulfate. The authors suggested that phase II metabolism of EL and ED may take place during uptake in the colon and colon epithelial cells may be responsible for this metabolism (Jansen et al., 2005). Although the conjugates of enterolignans are excreted in urine and bile usually, those that are re-excreted in bile undergo enterohepatic recycling (Kuijsten et al., 2005). In urine, EL and ED are excreted primarily as monoglucuronides (95% and 85%, respectively), with small percentages being excreted as monosulfates (2–10%) and free aglycones (0.3–1%). (Adlercreutz et al., 1995). It is important to note that there are obvious determinants of serum EL concentration which include dietary intake of EL precursors, health of gut microbiota along with other determinants like dietary habits, demographic factors, constipation, smoking, low or high body mass index, age, sex, education, fat intake and use of antibiotics (Adlercreutz, 2007).
4. Clinical evidence of cancer chemopreventive effects of EL
About 30 epidemiological studies supporting a protective role of dietary intake of EL and its plasma/serum/urine concentrations in several cancers are identified and summarized in Table 1. Among these 19 breast cancer, 5 prostate cancer, 3 colorectal cancer, 1 gastric cancer and 1 lung cancer study reported on the protective or inverse association between EL and risk of respective cancers. Most of these are case control studies with retrospective design and few are cohort studies with prospective design. Epidemiological studies of breast cancer reported that higher dietary intake of EL precursors and higher blood concentrations of EL are associated with the reduced risk of breast cancer, decreased mortality rate and better survival particularly in postmenopausal breast cancer patients but with little ambiguity among the data in relation to the estrogen receptor status particularly in estrogen receptor α + , estrogen receptor α− and estrogen receptor β+ breast cancers. However, a few studies have reported none or limited association between EL concentration derived from dietary lignans in plasma, serum or urine and risk of breast cancer (den Tonkelaar et al., 2001; Horn-Ross et al., 2001; Hultén et al., 2002; Kilkkinen et al., 2004; Kyrø et al., 2017; Touillaud et al., 2006; Verheus et al., 2007; Ward et al., 2008; Xie et al., 2013; Zeleniuch-Jacquotte et al., 2004). Prostate cancer studies also supported the hypothesis that higher dietary intake of PhE and blood concentrations of EL are associated with the reduced risk of prostate cancer in men. There are also studies that report no protective association between EL and risk of prostate cancer (Eriksen et al., 2017; Stattin et al., 2004, 2002; Travis et al., 2009; Wallström et al., 2017). Epidemiological studies suggest that high dietary intake of EL resulting in increased plasma concentrations of EL might protect against the risk of colon cancer particularly in women (Johnsen et al., 2010; Ward et al., 2010). While one nested case control study did not support the hypothesis that high plasma ED or EL concentrations are associated with reduced risk of colorectal cancer (Kuijsten et al., 2008), a Korean epidemiological study on gastric cancer concluded that interaction between CRK gene and PhE (genistein, daidzein, equol and EL) modify gastric cancer risk. It was also reported that high dietary intake of PhE producing EL and ED are associated with a decrease in risk in lung cancer. One case control study did not support a protective role of circulating lignans (EL) against endometrial cancer in both pre-and postmenopausal women (Zeleniuch-Jacquotte et al., 2006). However majority of the clinical evidence strongly support the protective role of EL in the risk of breast, prostate, colon, gastric and lung cancers and indicates EL as a potential anticancer nutrition molecule.
Table 1.
Epidemiologic studies based on plasma/serum/urine Enterolactone concentrations and intake of Enterolactone in association with several cancer risks.
| Types of cancer | Country/ Region |
Design | Cases/ Controls |
Methodology | Analysis of EL | Findings related to EL | Interpretation | Ref. |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | Boston, USA | Dietary Study | 10 omnivores, 10 vegetarians and 7 BC; PoM women | 72 h collection of urine, 3-day food record. | Nutrient composition, Urinary EL and ED by capillary GC. | Urinary excretion of EL in the BC group was significantly lower than in both the other groups. ED excretion was also lower in the BC group than in the vegetarian group. | PoM women with BC excreted significantly less EL in their urine than did healthy PoM women | (Adlercreutz et al., 1982) |
| Western Australia | CCS (RD) | 144 /144 women | SQ and FFQ, 72 h urine collection, Blood sample collection before the treatment. | Urinary PhE, ED, EL and MAT by isotope dilution GC-MS. | High excretion of both equol and EL was associated with a substantial reduction in BC risk, with significant trends through the quartiles: equol ORs were 1·00, 0·45 (95% CI 0·20, 1·02), 0·52 (0·23, 1·17), and 0·27 (0·10, 0·69)—trendp = 0·009—and EL ORs were 1·00, 0·91 (0·41, 1·98), 0·65 (0·29, 1·44),0·36 (0·15, 0·86)—trend p = 0·013. | High intake of equol and EL could be important in the prevention of BC. | (Ingram et al.,1997) | |
| Eastern Finland | CCS (RD) | 194 (68 PrM and 126 PoM)/208 women | FFQ, Serum samples collection before the examinations. | Serum EL by TR-FIA | The mean serum EL concentration in the lowest quintile was 3.0 nmol/l and 54.0 nmol/l in the highest (OR in the highest quintile of EL adjusted for all of the known risk factors for BC was 0.38 (95% CI, 0.18–0.77; P for trend, 0.03). The inverse association between serum EL and risk of BC was seen in both PrM and PoM women. | Serum EL level was significantly inversely associated with risk of BC | (Pietinen et al., 2001) | |
| Shanghai, China | CCS (RD) | 250 /250 women | SQ and FFQ Urine samples collection before cancer therapy. | Urinary isoflavonoids, ED, EL, and citrus flavonoids by LC-MS | The risk of BC was reduced with increasing excretion of total isoflavonoids and total mammalian lignans (ED and EL). The risk of BC was reduced with increasing excretion of total isoflavonoids (P for trend, 0.04) and total lignans (P for trend, <0.01), with adjusted ORs of 0.62 (95% CI, 0.39–0.99) and 0.40 (95% CI, 0.24–0.64) observed for the highest versus the lowest tertile of total isoflavonoid and lignan excretion, | Study suggested that high intake of certain PhE like flavonoids and lignans (dietary precursors of ED and EL) may reduce the risk of BC | (Dai et al., 2002) | |
| Western New York, USA | CCS (RD) | 96 / 86 PrM women 112 / 102l PoM women. |
SQ and FFQ Blood samples collection Lignan intake = EL + ED production from foods. |
Analysis of dietary lignans stratified by CYP17 genotype. | Women in the highest tertile of dietary lignans tended to have reduced breast cancer risk (OR 0.45, 95% CI 0.20–1.01 and OR 0.59, 95% CI 0.28–1.27, PrM and PoM women, respectively). Substantially reduced risks in the highest tertile of lignans were observed for PrM women with at least one A2 allele (OR 0.12, 95% CI 0.03–0.50). | Results suggested that CYP17 genotype may be important in modifying the effect on BC risk of ED and EL particularly for PrM women | (McCann et al., 2002) | |
| Western New York USA | CCS (RD) | 315 / 593 PrM women 807/ 1443 PoM women. |
Self administered 104-item FFQ Detailed in-person interviews. | ED and EL= SECO + MAT in diet. | PrM women in the highest quartile of dietary lignan intake had reduced breast cancer risk (OR= 0.66; 95% CI =0.44–0.98). No association was observed between lignan intakes and PoM BC. | Authors concluded that lignans may be important in the etiology of BC, particularly among PrM women. | (McCann et al., 2004) | |
| Southern Germany | CCS (RD) | 278 /666 PrM women | SARFQ, Validated FFQ. | By establishing the database of PhE containing foods usually consumed in Europe. | Both estimated mammalian lignans, ED and EL were inversely associated with BC risk, with ORs (95% CI) of 0.61 (0.39–0.98) and 0.57 (0.35–0.92), respectively | Authors suggested an important role of dietary intake of daidzein and genistein, MAT, Ed and EL to reduce PrM BC risk in this study population. | (Linseisen et al., 2004) | |
| Denmark | CCS (RD) | 381/381 PoM women | SQ and FFQ Blood samples collection | Plasma EL by TR-FIA | For estrogen receptor α-positive BC (n = 273) only a weak association was seen (IRR,0.97; 95% CI, 0.88-1.06), whereas for estrogen receptor α-negative BC (n = 80; IRR, 0.71; 95% CI, 0.53-0.94) a protective effect was seen per 20 nmol/L higher plasma EL. | Authors found a tendency toward a lower risk BC with higher concentrations of EL, which was restricted almost entirely to estrogen receptor α-negative BC | (Olsen et al., 2004) | |
| Genoa, Italy | Cohort Study (PD) | 383 women with palpable cysts | Serum samples collection at the time of first cyst aspiration. | Serum EL by TR-FIA | Median values of serum EL were significantly lower in women who subsequently developed BC: 8.5 nM/l versus 16.0 nM/l: P = 0.04. OR for BC were: 0.36 (P = 0.03), 0.57 (P = 0.3) and 0.38 (P = 0.25) for 25th (8 nM/l), 50th (16 nM/l) and 75th (24 nM/l) percentile values, respectively. | Authors concluded that the serum EL concentration was inversely correlated with the risk of BC in women with palpable cysts. | (Boccardo et al., 2004) | |
| Southern Germany | CCS (RD) | 220/ 237 PrM women | SARFQ and Validated FFQ, Blood samples collection. | Plasma EL by TR-FIA | PrM BC risk decreased with increasing plasma EL concentrations. Adjusted OR (95% CI) were 0.42 (0.20–0.90) and 0.38 (0.17–0.85) (P for trend 0.007) for women in the third and fourth quartile of plasma EL compared to those in the lowest quartile. | Authors concluded a strong inverse association between EL and PrM BC risk as found with dietary intake estimates. | (Piller et al., 2006) | |
| Breast cancer | France | Cohort Study (PD) | 1469 incident cases of primary invasive BC | SADHQ evaluating 208 food items. | Estimation of exposure to ED and EL from dietary lignan intake. | The inverse associations between PhE intakes and PoM BC risk were limited to estrogen receptor- and progesterone receptor-positive disease (e.g., RR for highest versus lowest quartiles of total plant lignan intake = 0.72, 95% CI = 0.58 to 0.88, Ptrend = .01, 174 versus 214 cases per 100 000 person-years, and RR for highest versus lowest quartiles of total enterolignan (ED and EL) level = 0.77, 95% CI = 0.62 to 0.95, Ptrend = .01, 164 versus 204 cases per 100 000 person-years) | High dietary intakes of plant lignans and high exposure to enterolignans (ED and EL) were associated with reduced risks of estrogen receptor and progesterone receptor positive PoM BC | (Touillaud et al., 2007) |
| Sweden | Nested CCS (PD) | 366 / 733 women | A modified diet history method, Direct anthropometric measurements, Blood samples collection. | Plasma EL by TR-FIA | EL concentrations above the median (16 nmol/L) were associated with reduced BC risk when compared with those below [OR, 0.75; 95% CI (95% CI), 0.58-0.98]. The reduced risk was only observed for estrogen receptor α ( + ); OR, 0.73; 95% CI, 0.55-0.97] and estrogen receptor β (−) tumors (OR, 0.60; 95% CI, 0.42-0.84), with significantly different risks for estrogen receptor β (−)and estrogen receptor β ( + ) tumors (P for heterogeneity = 0.04). | The protective association between EL and BC was significantly different between estrogen receptor β (−) and estrogen receptor β (+) tumors and most evident in tumors that express estrogen receptor α but not estrogen receptor β. | (Sonestedt et al., 2008) | |
| Germany | Meta Analysis | 11 prospective cohort studies and 10 CCS (total 21 studies) | A systematic MEDLINE search to identify epidemiologic studies published between 1997 and August 2009. | Pooled risk estimates (REs) for total lignan exposure, dietary lignan intake, enterolignan exposure, and EL in blood or urine were calculated. | BC risk was also inversely associated with enterolignan exposure (4 studies; RE: 0.84; 95% CI: 0.71, 0.97) but not with blood or urine EL concentrations. The associations were not significantly different between Estrogen receptor-status subgroups (6 studies). | Authors concluded that high lignan exposure may be associated with a reduced BC risk in PoM women | (Buck et al., 2010) | |
| Germany | Follow-up Study | 2653 PoM BC patients | A self-administered validated 176-items FFQ An intake in grams per day (g day–1) was calculated for each food item. | Bioavailable ED and EL were calculated per 100 g of ingested foods. | High estimated EL and ED levels were associated with significantly lower overall mortality (highest quintile, HR= 0.60, 95% CI= 0.40–0.89, PTrend= 0.02 and HR= 0.63, 95% CI= 0.42–0.95, PTrend= 0.02, respectively) | PoM BC patients with high estimated enterolignans (ED and EL) may have a better survival | (Buck et al., 2011a; 2011b) | |
| Denmark | Cohort Study (PD) | 24,697 PoM women | FFQ, A lifestyle questionnaire, Plasma samples collection. | Plasma EL by TR-FIA | When comparing women with EL levels above the median (20.5 nmol/l) to those with lower levels, decreased HR were seen for both ACM (HR: 0.47; 95% CI: 0.32–0.68) and BCRM (HR: 0.56; 95% CI: 0.36–0.87). | Higher prediagnostic plasma levels of ELwere found related to lower mortality among BC patients. | (Olsen et al, 2011) | |
| Germany | Prognosis Study | 1140 PoM BC patients | Clinical and pathologic records. Serum samples collection. | Serum EL by TR-FIA | Higher serum EL levels were associated with significantly reduced HRs for death (HR per 10 nmol/L increment, 0.94; P .04; HR for the highest quartile, 0.58; 95% CI, 0.34 to 0.99). The highest quartile of serum EL was associated with a significantly reduced risk of death only for estrogen receptor – tumors (HR, 0.27; 95% CI, 0.08 to 0.87). | PoM patients with BC who have high serum EL levels may have better survival. | (Buck et al., 2011a; 2011b) | |
| Italy | Cohort Study (RD) | 300 Patients operated on for BC | Operation and subsequent follow up, Blood samples collection. | Serum EL by TR-FIA. | An association between a decreased mortality risk and EL levels ≥ 10 nmol/l was found in respect to both all-cause and breast cancer-specific mortality. BCRM risk remained constantly lower in those patients with higher EL levels. | ED and EL might play an important role in reducing all-cause and cancer-specific mortality of the patients operated on for BC. | (Guglielmini et al., 2012) | |
| Germany | CCS (RD) | 1250/ 2164 PoM women | Self-administered FFQ, Serum samples collection. | Serum EL by TR-FIA. | Significant inverse association between serum EL and PoM BC risk, which was stronger for estrogen receptor− progesterone receptor− than for estrogen receptor + progesterone receptor + tumors but not differential by further expression of human epidermal growth factor receptor 2. | Study supported an inverse association between higher serum EL levels and PoM BC risk. | (Zaineddin et al.,2012) | |
| Germany | Prognosis Follow-up Study (PD) | 2182 PoM BC patients | Clinical and pathologic records, Serum samples collection. | Serum EL by TR-FIA. | High EL concentrations were significantly associated with lower ACM (per 10 nmol L−1: HR 0.94, 95% CI 0.90–0.98), BCSM(HR 0.94, 0.89–0.99), and DDFS (HR 0.94, 0.90–0.98). | Study showed that high lignan exposure (EL) is associated with reduced mortality in BC patients. | (Seibold et al.,2014) | |
| Prostate cancer | Sweden | CCS (RD) | 1499 / 1130 men Serum collection 209/214 men | FFQ Serum samples collection |
Serum EL by TR-FIA | Intermediate serum levels of EL were associated with a decreased risk of PC. The ORs comparing increasing quartiles of serum EL concentration to the lowest quartile were, respectively, 0.28 (95% CI: 0.15–0.55), 0.63 (95% CI: 0.35–1.14) and 0.74 (95% CI: 0.41–1.32) | Results supported the hypothesis that certain foods high in PhE are associated with a lower risk of PC | (Hedelin et al.,2006) |
| Scotland | CCS (RD) | 433/483 men | A validated FFQ Blood samples collection. | Serum EL by isotope dilution GC-MS | A significant inverse associations was found with increased serum concentrations of EL (adjusted OR 0·40, 95% CI 0·22, 0·71) and with the consumption of soy foods (adjusted OR 0·52, 95% CI 0·30, 0·91). | Study supported the hypotheses that soy foods and EL protect against PC in older Scottish men. | (Heald et al.,2007) | |
| Scotland | CCS (RD) | 247 PC/ 125 BPH/274 men | Serum samples collection, DNA extraction. | Serum EL by isotope dilution GC-MS | TT homozygotes who had low serum EL concentrations (below median) were more likely to have PC (OR= 2.90; 95% CI, 1.28–6.57) than individuals with CC/CT genotype and high serum EL concentrations (above median). | PC susceptibility was associated with TT genotype of SNP rs10993994 and increased risk of PC was modified by serum EL concentrations. | (Ho et al., 2012) | |
| USA | Multisite Phase II RCT | 147 PC patients | 30 g/day of whole-ground flaxseed supplementation for ~30 days before surgery. Pre and post surgery urine samples and tumor tissues collection. | Urinary EL by HPLC | Total urinary enterolignans and EL were significantly and inversely correlated with Ki67 in the tumor tissue (ρ= −0.217, P = .011, and ρ = −0.230, P = .007, respectively), and a near-significant inverse association was observed for ED (q =− 0.159, P = .064). An inverse association was observed between EL and VEGF (q= −0.143, P = .141), | Flaxseed-derived EL is inversely associated with tumor cell proliferation in men with localized PC | (Azrad et al.,2013) | |
| China | Meta-analysis | 2 Cohort and 9 CCS on PhE intake and 8 studies on serum concentrations of PhE | Relevant publications were identified in the MEDLINE database using PubMed, Web of Science, and the Cochrane Library up to June 2014 | The ORs were used as the common measure of association across studies by considering the RRs as ORs. | In stratified analysis, high genistein and daidzein intake and increased serum concentration of EL were associated with a significant reduced risk of PC. | Increased serum concentration of EL was associated with a significant reduced risk of PC | (He et al., 2015) | |
| Endometrial cancer | Denmark | Case Cohort Study | 173/149 women | SFQ, Blood samples collection. | Plasma EL by TR-FIA | A 20 nmol/l higher plasma concentration of EL was associated with a nonsignificant lower risk of EMC (IRR 0·93, 95% CI 0·84, 1·04) | Authors found some support for a possible inverse association between plasma EL concentration and EMC incidence. | (Aarestrup et al.,2013) |
| Colorectal cancer | Netherlands | CCS (RD) | 532/503 | SFQ, Blood samples collection. | Plasma EL by LC-MS | Plasma ED concentrations were associated with a reduction in CRA risk after adjustment for confounding variables, OR (95% CI) were 1.00, 0.69 (0.42-1.13), 0.60 (0.37-0.99), and 0.53 (0.320.88) with a significant trend (P = 0.01) through the quartiles. EL’s reduction in risk was not statistically significant (P for trend = 0.09). | Substantial reduction in CRA risk among subjects with high plasma concentrations of enterolignans | (Kuijsten et al.,2006) |
| United Kingdom | CCS (PD) | 221/886 | Prospective collection of lifestyle and 7-d records of diet | 509 food items by LC-MS | Among women, CRC risk was inversely associated with EL (OR: 0.33; 95% CI: 0.14, 0.74) and total enterolignans (OR: 0.32; 95% CI: 0.13, 0.79), with a positive trend detected for SECO (OR: 1.60; 95% CI: 0.96, 2.69). | EL, found at high concentration in eggs and dairy products, may influence the risk of CRC among women. | (Ward et al, 2010) | |
| Denmark | Case Cohort Study | 244 CoC/137 RC/370 | Lifestyle questionnaire, A 192-item FFQ, Blood samples collection. | Plasma EL by TR-FIA | For each doubling in EL concentration there was lower risk of colon cancer among women [IRR (95% CI) = 0.76 (0.60–0.96)] and a tendency toward lower risk of rectal cancer [IRR (95% CI) = 0.83 (0.60–1.14)]. | Study supported the hypothesis that EL may protect against colon cancer in women | (Johnsen et al.,2010) | |
| Gastric cancer | Korea | CCS | 462/670 | Meta-analysis. | Plasma EL by TR-FIA | Risk allele of CRK rs7208768 had a significantly increased risk for gastric cancer at low PhE levels (p interaction ≤0.05). | Interaction between CRK gene and PhE modify gastric cancer risk. | (Yang et al., 2012) |
| Lung cancer | USA | CCS (RD) | 1674/1735 | FFQs Quantification of dietary intake of 12 individual PhE | Intake of specific PhE was calculated using the DIETSYS + Plus version 5.9 dietary analysis program. | High intake of the EL and ED and use of hormone therapy were associated with a 50% (OR, 0.50; 95% CI, 0.31-0.68; P = .04 for interaction) reduction in risk of lung cancer. | Study supported growing epidemiologic evidence that PhE (EL, ED) are associated with a decrease in risk of lung cancer | (Schabath et al.,2005) |
CCS= Case Control Study, RD= Retrospective Design, PD= Prospective Design, PrM- Premenopausal, PoM= Postmenopausal, SQ = Structured Questionnaire, FFQ= Food Frequency Questionnaire, BC= Breast Cancer, PhE= Phytoestrogens, GC-MS = Gas Chromatography-Mass Spectroscopy, TR-FIA= Time Resolved-Fluoroimmunoassay, OR= Odds Ratio, CI= Confidence Interval, IRR= Incidence Rate Ratio, LC-MS = Liquid Chromatography-Mass Spectroscopy, SARFQ= self-administered risk factor questionnaire, SADHQ= Self-Administered Diet History Questionnaire, RR= Relative Risk, HR= Hazard Ratio, BCSS= Breast Cancer-Specific Survival, BCRM= Breast Cancer Related Mortality, BCSM= Breast Cancer Specific Mortality, BCUM= Breast Cancer Unrelated Mortality, ACM= All-Cause Mortality, PC= Prostate Cancer, BPH= Benign Prostatic Hyperplasia, RCT= Randomized Controlled Trial, SFQ= self-administrated questionnaires, HPLC= high-performance liquid chromatography, IHC= Immunohistochemistry, EMC= Endometrial Cancer, CRA= Colorectal adenoma, CRC= Colorectal Cancer, CoC= Colon Cancer, IRR= Incidence Rate Ratios, RC= Rectal Cancer.
5. Pre-clinical evidence of anticancer effects of EL on different types of cancer
5.1. Evidence from studies of EL in animal models of cancer
About 12 in-vivo animal studies evaluating the anticancer potential of EL was performed by its direct administration (Table 2). These studies include human cancer cell xenografts as well as chemically induced carcinogenesis by 7, 12-Dimethylbenz(a) anthracene (DMBA) and (N-ethyl-N′-nitro-N nitroso-guanidine) ENNG, and knockout models of ovariectomized mice and rats. In these studies EL was administered via oral or subcutaneous (SC) routes and intra tumor injections. EL was reported to exert its anticancer potential in breast cancer by both estrogen dependent and independent mechanisms (Jungeström et al., 2007). Consistent anticancer effects observed in breast cancer studies included reduction in tumor volume and size, increase in apoptosis, decrease in both stroma and cancer cell derived vascular endothelial growth factor (VEGF), inhibition of estradiol (E2) induced growth and angiogenesis, decrease in in-vivo release of interleukin-1 beta (IL-1β) and increase in interleukin-1 receptor antagonist (IL-1Ra) levels (Jungeström et al., 2007; Lindahl et al., 2011; Saarinen et al., 2010). One study confirmed that EL reduced the growth and metastasis of solid AH109A hepatomas in rats (Miura et al., 2007). Other studies on uterine cancer and colon cancer reported that EL exerts chemopreventive effects in the rat ENNG-uterine carcinogenesis model while it inhibits cell proliferation and induces cell death in COLO 201 human colon cancer xenografts, respectively (Danbara et al., 2005; Katsuda et al., 2004). All these preclinical animal studies provide strong evidence in favor of the chemopreventive or anticancer potential of EL in breast, liver, uterine and colon cancers.
Table 2.
In vivo studies investigating the exclusive effects of EL in cancer bearing animal models.
| Type of cancer | Experimental Cancer Model | Animals used | Dosing | ROA | Principle findings | Ref. |
|---|---|---|---|---|---|---|
| Breast cancer | DMBA induced mammary carcinoma | Female Sprague Dawley rats | EL 1 mg/kg and 10 mg/kg of BW starting 9 weeks after the DMBA-induction. | Oral |
|
(Saarinen et al.,2002) |
| MCF-7 human breast cancer xenografts | Ovariectomized athymic nude female mice | 10 mg/kg body weight of EL, ED, GEN and mixture daily for 22 weeks. | SC |
|
(Power et al.,2006a) | |
| MCF-7 human breast cancer xenografts | Ovariectomized athymic nude female mice | 10 mg/kg body weight of EL, ED, Genistein and mixture daily for 22 weeks. | SC |
|
(Power et al.,2006b) | |
| MCF-7 human breast cancer xenografts | Female athymic mice, BALB/c nu/nu | Either 10% flaxseed diet or BD with daily injections of ED, EL (15 mg/kg BW). | SC |
|
(Jungeström et al., 2007) | |
| MCF-7 human breast cancer xenografts | Ovariectomized athymic nude female mice | BD or BD supplemented with 100 mg/kg ENL, 100 mg/ kg GEN or their combination (EL+ GEN). | Oral |
|
(Saarinen et ah, 2010) | |
| MCF-7 human breast cancer xenografts | Athymic female mice, Balb/cA nu/nu | BD or BD supplemented with 100 mg/kg ENL, 100 mg/ kg GEN or 10% ground flax. TAM (1 mg for every 2 days) | OralSC |
|
(Lindahl et al., 2011) | |
| Gnotobiotic rat model (LCC rats) of DMBA induced breast cancer | Female germ-free Sprague Dawley rats | Flaxseed-rich diet, contained 0.34 g/kg SDG for 13 weeks | Oral |
|
(Mabrok et al.,2012) | |
| Knockout ABCG2−/− and wild-type | Lactating female mice | BD supplemented with 1% of lignan-rich extract (SDG = 2 mg/g) for 7 days | Oral |
|
(GarcÍa-mateos et al., 2017) | |
| ES-2 human ovarian cancer xenografts | BALB/c nude mice | ED or EL at 1 mg/kg or 0.1 mg/kg separately, once per 2 days up to 32 days. | ITI |
|
(Liu et al., 2017) | |
| Liver cancer | Subcutaneous AH109A Hepatomas in Rats | Male Donryu rats | Basal diet or basal diet supplemented with either 0.15% of HMR for 14 days or 0.001% and 0.01% of EL, for 21 days. | Oral |
|
(Miura et al., 2007) |
| Uterine carcinogenesis | N-ethyl-N′-nitro-N nitrosoguanidine (ENNG), induced uterine carcinogenesis | Female Crj: Donryu rats | From 11th weeks of age animals were fed with 200 (11 ± 0.3 mg/kg/day), or 600 (32.7 ± 1.1 mg/kg/day) ppm HMR until 15 months of age. | Oral |
|
(Katsuda et al., 2004) |
| Colon cancer | Colo 201 human colon cancer xenografts | Male BALB/c-nu/nu mice | EL at a dose of 0, 1, or 10 mg/kg of body weight 1 day before tumor cell inoculation and treatment was continued 3 times a week for 23 days | SC |
|
(Danbara et al., 2005) |
ROA = Route of Administration, BW = Body Weight, DMBA = 7, 12-Dimethylbenz(a) anthracene, HMR = Hydroxymatairesinol, SC = Subcutaneous, GEN = Genistein, BMD = Bone Mineral Density, PoM = Postmenopausal, E2 = Estradiol, BD = Basal Diet, VEGF = Vascular endothelial growth factor, TAM = Tamoxifen, IL-1Ra = Interleukin-1 Receptor Antagonist, BCRP/ABCG2 = Breast Cancer Resistant Protein, ITI = Intra Tumor Injection.
5.2. Evidence from mechanistic studies of EL on human cancer cell lines
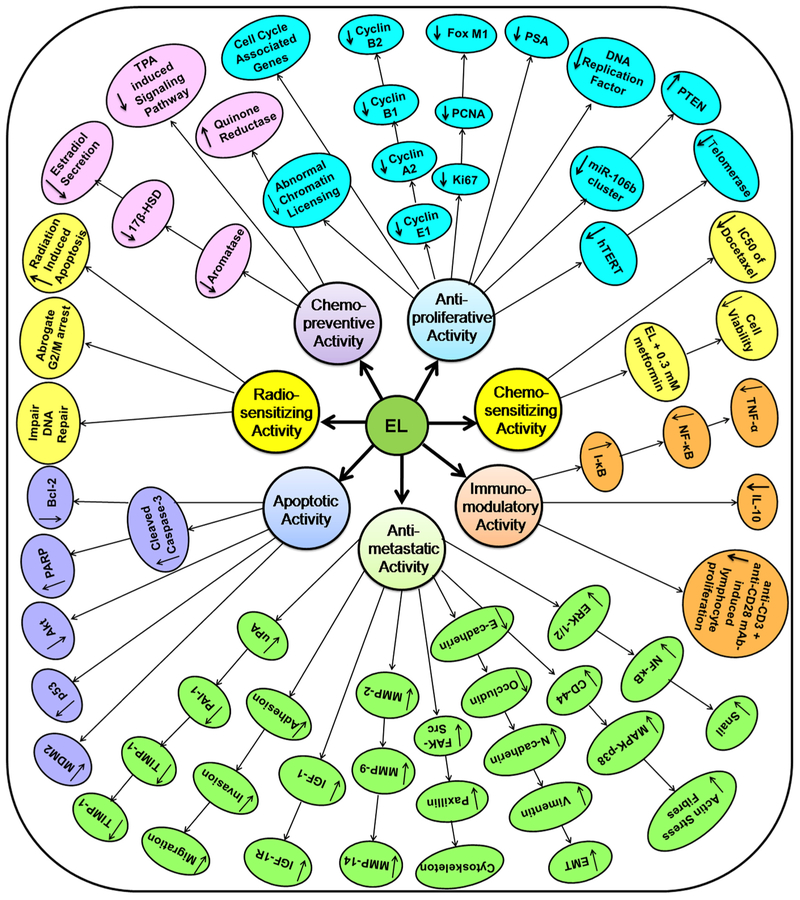
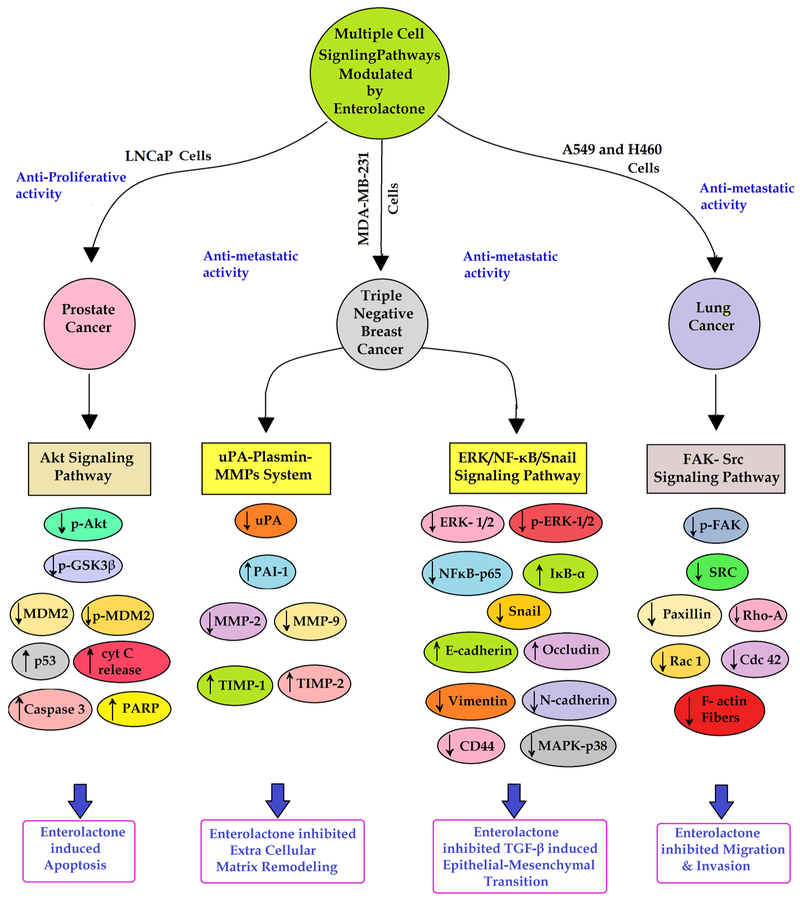
So far 30 in-vitro cell culture studies have been identified reporting on the anticancer activities of EL on several human cancer cell lines (Table 3). These studies reported various cellular and molecular mechanisms of EL against novel therapeutic targets in different types of cancers. EL was found to exhibit a host of activities including chemopreventive, antiproliferative, apoptotic, antimetastatic, immunomodulatory, chemosensitizing and radiosensitizing in different human cancer cell lines such as breast cancer, prostate cancer, colon cancer, lung cancer, ovarian cancer, choriocarcinoma, osteosarcoma and monocytic leukemia cell lines. Reported mechanistic end points on different therapeutic targets of EL in different cancer cells have been shown in Fig. 2 while major signaling pathways modulated by EL, in breast cancer and lung cancer have particularly been shown in Fig. 3. In prostate cancer, it was reported that EL induced apoptosis in human prostate carcinoma LNCaP cells by inhibiting Akt signaling pathway (Chen et al., 2007) while in the case of lung cancer, EL altered FAK-Src signaling pathway to suppress migration and invasion of A549 and H460 human lung cancer cell lines (Chikara et al., 2017a). In our recent work we have shown that EL exerts its antimetastatic breast cancer activity by suppressing invasion, migration, colonization in-vitro; via inhibition of uPA/Plasmin/MMPs mediated ECM remodeling and by reverting TGF-β induced EMT via modulation of ERK-NFκB-Snail signaling pathway in triple negative MDA-MB-231 human breast cancer cells (Mali et al., 2017, 2018). It is important to note that EL was reported to show estrogen receptor dependent as well as estrogen receptor independent mechanisms in different breast cancer cell lines (Bigdeli et al., 2016a; Brooks and Thompson, 2005; Chen and Thompson, 2003; Di et al., 2018; Mali et al., 2017, 2012, 2018; Pianjing et al., 2011). Collectively most of these in-vitro human cell culture studies provide strong evidence in support of strong anticancer and antimetastatic potential of EL in several cancers.
Table 3.
In vitro studies investigating the effect of EL in several human cancer cell lines.
| Activity | Cancer | Experimental Human Cell Culture Model |
Dose and Treatment Period of EL |
Mechanistic Endpoints of EL Investigated | Potential Mechanism(s) of EL |
Ref |
|---|---|---|---|---|---|---|
| Chemopreventive activity | CC | JEG-3 | 1, 10 and 100 μM |
|
EL acts as an aromatase inhibitor. | (Adlercreutz and Vickery, 1993) |
| BC | MDA-MB-468 | 100 μM EL for 30 min. |
|
EL interfere with TPA-induced signal transduction pathway. | (Dale et al., 1998) | |
| CoC | Colo 205 | 0.001 and 10 μM for 48 h |
|
EL is capable of QR induction in Colo205 cells by promoting QR mRNA expression. | (Wang et al., 1998) | |
| BC | MCF-7 | 1–50 μM for 6 h to 5 days |
|
EL modulates E2 synthesis via inhibiting aromatase and 17β-HSD in estrogen receptor + BC cells | (Brooks and Thompson, 2005) | |
| BC | T-47D T47D-KBluc |
10 μM for 24 h |
|
EL possesses antiestrogenic activity. | (Pianjing et al., 2011) | |
| ChoC BC |
JEG-3, BeWo MCF-7 | 10–100 μg/ml over 72 h |
|
EL may exhibit chemopreventive activity in estrogen receptor + BC. | (Schröder et al.,2016) | |
| Antiproliferative activity | CoC | LS174T, Caco-2, HCT-15, T-84 | 100 μM for 8–10 days |
|
EL inhibits the growth of colon cancer cells through a mechanism other than antiestrogenic activity. | (Sung et al., 1998) |
| OS | MG-63 | 0.01–10 mg/ml for 7 days |
|
EL exhibits antiproliferative effect on OS cells. | (Feng et al., 2008) | |
| PC | PC-3, DU-145, LNCaP. | 10–100 μM for 72 h |
|
EL suppresses the growth of PC cells via hormonally dependent and independent mechanisms. | (Lin et al., 2001) | |
| PC | LNCaP | 0–100 μM for 24 h to 6 days |
|
EL exhibits an antiproliferative effect as a consequence of altered expression of cell cycle associated genes. | (McCann et al.,2008) | |
| PC | RWPE-1, WPE1-NA22, WPE1-NB14, WPE1-NB11, WPE1-NB26, LNCaP, PC-3 | 0.01–10 μM for 24–72 h |
|
EL exhibits antiproliferative effect on PC cells. | (Mccann et al.,2013) | |
| PC | RWPE-1, WPE1-NA22, WPE1-NB14, WPE1-NB11, WPE1-NB26, LNCaP | 10 to 100 μM over 48 h |
|
Antiproliferative effects of EL in earlier stages of PC are mediated, in part, by microRNA-mediated regulation. | (McCann et al.,2014) | |
| BC | MCF-7 BT-20 |
1 × 10−3 to 1 × 10−7 mol/l for 24 h |
|
EL inhibits proliferation in BC cells. | (Abarzua et al.,2012) | |
| BC | MCF-7 | 1–100 μM for 48 h |
|
High concentration of EL inhibits the expression and activity of telomerase in BC cells to decrease viability. | (Ilbeigi et al.,2017) | |
| NSCLC | A549 H441 H520 Hs888Lu |
0–100 μM for 24 to 72 h |
|
EL inhibits cell proliferation by inducing G1-phase cell cycle arrest in NSCLC cells by down-regulating-cyclins and cyclin-dependent kinases | (Chikara et al., 2017b) | |
| Apoptotic activity | CoC | Colo 201 | 2–100 μM for 24–72 h |
|
EL Induces apoptosis and inhibits growth of Human CoC cells. | (Danbara et al.,2005) |
| CoC | SW480 | 0–200 μM for 24–72 h |
|
EL inhibits CoC cell growth via cytostatic and apoptotic mechanisms. | (Qu et al, 2005) | |
| CoC | CaCo-2 | 50–150 μM for 24–72 h |
|
EL inhibits proliferation and induces apoptosis in CoC cells. | (Bommareddy et al., 2010) | |
| PC | LNCaP | 0–100 μM for 24–72 h |
|
EL suppresses LNCaP cell growth by the induction of apoptosis via a mitochondrial mediated, caspase-dependent pathway which may be mediated by the inhibition of Akt-dependent phosphorylation and promotion of p53 expression. | (Chen et al., 2007) | |
| Antimetastatic activity | BC | MDA-MB-435, MDA-MB-231 | 1 to 10 μM for 24 h |
|
EL, ED and TAM, alone or in combination, can inhibit the steps involved in the metastasis cascade. | (Thompson, 2003) |
| PC | PC-3 | 0–80 μM for 20–24 h |
|
EL suppresses proliferation and migration of PC cells through inhibition of IGF-1/ insulin-like growth factor-1 receptor signaling. | (Chen et al., 2009) | |
| BC | MCF-7 MDA-MB-231 |
25–75 μM for 24–48 h |
|
EL exhibits in-vitro antimetastatic activity in BC cells. | (Mali et al, 2012) | |
| BC | MDA-MB-231 | 0–400 μM over 48 h |
|
EL shows antitumor effect by regulating the expression of genes associated with cell proliferation and the cell cycle. EL shows antimetastatic effect by blocking the FAK/paxillin signaling pathway. | (Xiong et al.,2015) | |
| BC | MDA-MB-231 | 25–75 μM for 24–72 h |
|
EL suppresses proliferation, migration and metastasis of MDA-MB-231 breast cancer cells by inhibiting uPA induced plasmin activation and MMPs mediated ECM remodeling | (Mali et al., 2017) | |
| BC | MDA-MB-231 | 25–75 μM for 48 h |
|
EL exhibits an antimetastatic activity in triple-negative BC via inhibiting the ERK/NF-κB/Snail signaling pathway to revert TGF-β-induced EMT. | (Mali et al, 2018) | |
| LC | A549H460 | 0–100 μM for 24–48 h |
|
EL shows antimetastatic potential by altering FAK-Src signaling and suppressing invasion, migration of LC cell lines. | (Chikara et al., 2017a) | |
| OC | ES-2 | 10−3 to 10−6 mol/L for 24–72 h |
|
EL possesses a more effective anti-cancer capability and less side effects than ED. | (Liu et al., 2017) | |
| Immunomodulatory Activity | ML CoC |
PBL THP-1 CaCo-2 |
1–1000 μM for 24–72 h |
|
EL modulates the immune response by acting on NF-κB signaling. | (Corsini et al.,2010) |
| Radiosensitizing activity | BC | MDA-MB-231 T47D |
0–500 μM over 48 h |
|
EL acts as a novel radiosensitizer for BC irrespective of estrogen receptor status. | (Bigdeli et al.,2016b) |
| Chemosensitizing activity | BC | MDA-MB-231 SKBR3 |
3.12–1,000 μM for 72 h |
|
EL acts as a novel chemosensitizer for BC irrespective of receptor status. | (Di et al., 2018) |
CC= Choriocarcinoma, BC= Breast Cancer, TPA= Phorbol Ester 12-O-tetradecanoylphorbol-13-acetate, IC50 = The half maximal inhibitory concentration, CoC= Colon Cancer, QR = NADPH:quinone reductase, conc. = concentration, PC= Prostate Cancer, TAM= Tamoxifen, 17β-HSD= 17β-hydroxysteroid dehydrogenase, ERE= Estrogen Responsive Element, PCNA= Proliferating Cell Nuclear Antigen, PARP= poly(ADP-ribose)-polymerase, OS= Osteosarcoma, PSA= Prostate Specific Antigen, PTEN= Phosphatase And Tensin Homolog, hTERT= Human Telomerase Reverse Transcriptase, NSCLC= Non Small Cell Lung Cancer, IGF-1R= Insulinlike Growth Factor-1 Receptor, ACC= adrenocorticocarcinoma, ML= Monocytic Leukemia, PBL= Peripheral Blood Leukocytes, I-κB= Inhibitory-κB, NF-κB= Nuclear Factor-κB, TNF-α= Tumor Necrosis Factor-α, ChoC= Chorion Carcinoma, PR= Progesterone Receptor, LC= Lung Cancer, OC= Ovarian Cancer, MDM2= Mouse Double Minute 2 homolog, IGF-1= Insulin-like Growth Factor-1, MMP= Matrix Metalloproteinases, PCNA= Proliferating Cell Nuclear Antigen, FoxM1 = Forkhead box protein M1, FAK= focal adhesion kinase, uPA= Urokinase Plasminogen Activator, PAI= Plasminogen Activator Inhibitor-1, TIMP= Tissue Inhibitor of Metalloproteinases, ECM= Extracellular Matrix, TGF-β= Transforming Growth Factor β, EMT= Epithelial-Mesenchymal Transition, CD44= Cluster of differentiation 44, MAPK-p38= mitogen-activated protein kinase-p38, ERK= Extracellular Signal-Regulated Kinase, NF-κB= nuclear factor kappa-light-chain-enhancer of activated B cells, LPS= Lipopolysaccharide, TNF-α= Tumor necrosis factor, I-κB= Inhibitor kappa b alpha.
Fig. 2.
Anticancer activities of EL with several molecular targets and mechanistic endpoints investigated in breast, prostate, colon, lung, osteosarcoma, monocytic leukemia human cancer cell lines.
Fig. 3.
Major signaling pathways modulated by Enterolactone to arrest carcinogenesis and metastasis, particularly in breast cancer and lung cancer.
6. Conclusions
Due to the high incidence and mortality rates and lack of new therapeutic agents targeting novel therapeutic strategies, cancer imposes a great burden of disease and challenges worldwide not only to the care providers but also to scientists. As mentioned earlier, diet, nutrition and physical activity are some of the most important determinants of cancer risk in humans through their in-part contribution to obesity, which is considered as a known risk factor for many malignancies (Mayne et al., 2016). By advance in nutrition-based research, it is now well accepted fact that diet not only plays a fundamental role in cancer prevention, but also contributes to the reduced treatment associated complications and the patient’s well-being (World Cancer Research Fund and American Institute for Cancer Research, 2007). One of the important categories of potential nutrients having chemopreventive action is dietary lignans and their metabolites (enterolignans). In this review, we focused on EL which is a prime, nutrition-derived, bioactive circulating metabolite of most of the dietary lignans and considered as having potential, nutritionally significant anticancer activity in several cancers of both men and women. Initial preclinical and clinical data based on several epidemiological studies supports the chemopreventive role of EL, particularly in hormonal cancers. Whereas, recent preclinical data based on mechanistic studies involving human cell cultures and xenografts revealed the therapeutic potential of EL not only in hormonal cancers but also in other types of cancer.
Several, but not all, epidemiologic studies report reduced risks of breast cancer, prostate cancer, colorectal cancer, lung cancer, endometrial cancer and gastric cancer associated with higher exposure to dietary lignans and their metabolites (enterolignans), expressed as either dietary intakes or as plasma, serum, or urinary EL concentrations. These clinical evidence strongly support the chemopreventive role of EL in respective cancers by considering its ability to play an important role in reducing the risk of developing the cancer, lowering cancer related mortality and better survival. However it is important to note that most of these studies were carried out on breast and prostate cancer and very few studies were conducted on other cancers, particularly in western and European populations. We did not find any intervention clinical trial of EL in cancer patients, however several flaxseed or SDG intervention clinical trials are reported particularly in breast cancer (Calado et al., 2018).
In the case of preclinical animal studies, we found that very few studies were carried out by direct administration of EL to the cancer animal models (summarized in this review) and many studies were carried out on flaxseed or sesame seed diet supplementation to evaluate their anticancer potential (not included in this review). Most of these in-vivo studies of EL are based on human xenografts, particularly of breast, liver, uterine and colon cancers and provide a strong evidence to support the anticancer and antimetastatic potential of EL by considering its ability to inhibit tumor growth and angiogenesis, to lower tumor burden, to promote tumor cell apoptosis, and to reduce metastasis. On the other hand, the in-vitro studies based on different human cancer cell lines provide deep insights into novel molecular and cellular mechanisms of EL which are capable of interfering the steps of carcinogenesis and metastasis by inhibiting adhesion, invasion, migration, proliferation, ECM remodeling, EMT, etc. Thus several in-vitro studies also provide a strong evidence to support anticancer and antimetastatic potential of EL. In this comprehensive review on EL and its role in cancer we propose this molecule as a potential candidate for anti cancer drug discovery or dietary supplements and nutraceuticals.
7. Future perspectives
In order to establish the protective and therapeutic association of EL with breast, prostate and other cancers, there is strong need to conduct more epidemiological studies and intervention trials in different ethnic populations. Future studies with a new wave of technology, based on novel approaches like genome-wide association studies (GWAS), metabolomics and nutritional epigenetics will provide more scientific clinical evidence to support the anticancer and antimetastatic potential of the EL. Moreover, very few clinical studies of EL in Asian and other populations project a great opportunity to both clinicians and scientists to carry out research in this diet-cancer research field.
Acknowledgments
Source(s) of support
This work was supported by an institutional funding.
Footnotes
Conflict of interest
This work was supported by an institutional funding. Authors declared no potential conflicts of interest.
References
- Aarestrup J, Kyro C, Knudsen KEB, Weiderpass E, Christensen J, Kristensen M, Würtz AML, Johnsen NF, Overvad K, Tjonneland A, Olsen A, 2013. Plasma enterolactone and incidence of endometrial cancer in a case-cohort study of Danish women. Br. J. Nutr 109, 2269–2275. 10.1017/S0007114512004424. [DOI] [PubMed] [Google Scholar]
- Abarzua S, Serikawa T, Szewczyk M, Richter DU, Pieehulla B, Briese V, 2012. Antiproliferative activity of lignans against the breast carcinoma cell lines MCF 7 and BT 20. Arch. Gynecol. Obstet 285, 1145–1151. 10.1007/s00404-011-2120-6. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, 2007. Lignans and human health. Crit. Rev. Clin. Lab. Sci 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Heikkinen R, Woods M, Fotsis T, Dwyer JT, Goldin BR, Gorbach SL, 1982. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 320, 1295–1299. 10.1016/S0140-6736(82)91507-0. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wähäla K, Mäkelä T, Hase T, Fotsis T, 1995. Lignan and isoflavonoid conjugates in human urine. J. Steroid Biochem. Mol. Biol 52, 97–103. 10.1016/0960-0760(94)00146-D. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Vickery LE, 1993. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol 44, 147–153. [DOI] [PubMed] [Google Scholar]
- Azrad M, Vollmer RT, Madden J, Dewhirst M, Polascik TJ, Snyder DC, Ruffin MT, Moul JW, Brenner DE, Demark-Wahnefried W, 2013. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J. Med. Food 16, 357–360. 10.1089/jmf.2012.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigdeli B, Goliaei B, Masoudi-Khoram N, Jooyan N, Nikoofar A, Rouhani M, Haghparast A, Mamashli F, 2016a. Enterolactone: a novel radiosensitizer for human breast cancer cell lines through impaired DNA repair and increased apoptosis. Toxicol. Appl. Pharmacol 313, 180–194. 10.1016/j.taap.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Bigdeli B, Goliaei B, Masoudi-Khoram N, Jooyan N, Nikoofar A, Rouhani M, Haghparast A, Mamashli F, 2016b. Enterolactone: a novel radiosensitizer for human breast cancer cell lines through impaired DNA repair and increased apoptosis. Toxicol. Appl. Pharmacol 313, 180–194. 10.1016/j.taap.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A, 2004. Serum enterolactone levels and the risk of breast cancer in women with palpable cysts. Eur. J. Cancer 40, 84–89. 10.1016/S0959-8049(03)00576-8. [DOI] [PubMed] [Google Scholar]
- Bommareddy A, Zhang XY, Kaushik RS, Dwivedi C, 2010. Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: possible mechanisms of flaxseed on colon cancer development in animals. Drug Discov. Ther. 4, 184–189. [PubMed] [Google Scholar]
- Brooks JD, Thompson LU, 2005. Mammalian lignans and genistein decrease the activities of aromatase and 17βhydroxysteroid dehydrogenase in MCF-7 cells. J. Steroid Biochem. Mol. Biol 94, 461–467. 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Buck K, Vrieling A, Zaineddin AK, Becker S, Huṡing A, Kaaks R, Linseisen J, Fleseh-Janys D, Chang-Claude J, 2011a. Serum enterolactone and prognosis of postmenopausal breast cancer. J. Clin. Oncol 29, 3730–3738. 10.1200/JCO.2011.34.6478. [DOI] [PubMed] [Google Scholar]
- Buck K, Zaineddin AK, Vrieling A, Heinz J, Linseisen J, Fleseh-Janys D, Chang-Claude J, 2011b. Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. Br. J. Cancer 105, 1151–1157. 10.1038/bjc.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J, 2010. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr 92, 141–153. 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- Calado A, Neves PM, Santos T, Ravasco P, 2018. The effect of flaxseed in breast cancer: a literature review. Front. Nutr 5, 4 10.3389/fnut.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Thompson LU, 2003. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res. Treat 80, 163–170. 10.1023/a:1024513815374. [DOI] [PubMed] [Google Scholar]
- Chen L-H, Fang J, Li H, Demark-Wahnefried W, Lin X, 2007. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol. Cancer Ther 6, 2581–2590. 10.1158/1535-7163.MCT-07-0220. [DOI] [PubMed] [Google Scholar]
- Chen L, Fang J, Sun Z, Li H, Wu Y, Demark-wahnefried W, Lin X, 2009. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J. Nutr 139, 653–659. 10.3945/jn.108.101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikara S, Lindsey K, Borowicz P, Christofidou-Solomidou M, Reindl KM, 2017a. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement. Altern. Med 17, 30 10.1186/s12906-016-1512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikara S, Lindsey K, Dhillon H, Mamidi S, Kittilson J, Christofidou-Solomidou M, Reindl KM, 2017b. Enterolactone Induces G1-phase cell cycle arrest in nonsmall cell lung cancer cells by downregulating cyclins and cyclin-dependent kinases. Nutr. Cancer 69, 652–662. 10.1080/01635581.2017.1296169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T, Doré J, Blaut M, 2006a. Bioavailability of lignans in human subjects. Nutr. Res. Rev 19, 187–196. 10.1017/S0954422407249704. [DOI] [PubMed] [Google Scholar]
- Clavel T, Henderson G, Engst W, Doré J, Blaut M, 2006b. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol 55, 471–478. 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- Corsini E, Dell’Agli M, Facchi A, De Fabiani E, Lucchi L, Boraso MS, Marinovich M, Galli CL, 2010. Enterodiol and enterolactone modulate the immune response by acting on nuclear factor-κB (NF-κB) signaling. J. Agric. Food Chem 58, 6678–6684. 10.1021/jf100471n. [DOI] [PubMed] [Google Scholar]
- Dai Q, Franke AA, Jin F, Shu X-O, Hebert JR, Custer LJ, Cheng J, Gao Y-T, Zheng W, 2002. Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol. Biomark. Prev 11, 815–821. [PubMed] [Google Scholar]
- Dale IL, Gant TW, Chipman JK, Kerr DJ, 1998. Regulation of c- fos transcription by chemopreventive isoflavonoids and lignans in MDA-MB-468 breast cancer cells. Eur. J. Cancer 34, 1425–1431. [DOI] [PubMed] [Google Scholar]
- Danbara N, Yuri T, Tsujita-Kyutoku M, Tsukamoto R, Uehara N, Tsubura A, 2005. Enterolactone induces apoptosis and inhibits growth of colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Res. 25, 2269–2276. [PubMed] [Google Scholar]
- den Tonkelaar I, Keinan-Boker L, Veer PV, Arts CJ, Adlercreutz H, Thijssen JH, Peeters PH, 2001. Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol. Biomark. Prev 10, 223–228. [PubMed] [Google Scholar]
- Di Y, De Silva F, Krol ES, Alcorn J, 2018. Flaxseed lignans enhance the cytotoxicity of chemotherapeutic agents against breast cancer cell lines MDA-MB-231 and SKBR3. Nutr. Cancer 5581, 1–10. 10.1080/01635581.2018.1421677. [DOI] [PubMed] [Google Scholar]
- Eriksen AK, Kyrø C, Nørskov N, Bolvig AK, Christensen J, Tjønneland A, Overvad K, Landberg R, Olsen A, 2017. Prediagnostic enterolactone concentrations and mortality among Danish men diagnosed with prostate cancer. Eur. J. Clin. Nutr 71, 1235–1240. 10.1038/ejcn.2017.42. [DOI] [PubMed] [Google Scholar]
- Ervik M, Lam F, Ferlay J, Mery L, Soerjomataram I, Bray F, 2016. Cancer Today. International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- Feng J, Shi Z, Ye Z, 2008. Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biol. Pharm. Bull 31, 1067–1070. 10.1248/bpb.31.1067. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- GarÍfa-mateos D, GarÍfa-villalba R, Angel J, Carlos J, Merino G, Álvarez AI, 2017. The Breast Cancer Resistance Protein (BCRP / ABCG2) influences the levels of enterolignans and their metabolites in plasma, milk and mammary gland. J. Funct. Foods J 35, 648–654. 10.1016/j.jff.2017.06.038. [DOI] [Google Scholar]
- Global Burden of Disease Cancer Collaboration, 2017. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol. 3, 524 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmini P, Rubagotti A, Boccardo F, 2012. Serum enterolactone levels and mortality outcome in women with early breast cancer: a retrospective cohort study. Breast Cancer Res. Treat 132, 661–668. 10.1007/s10549-011-1881-8. [DOI] [PubMed] [Google Scholar]
- He J, Wang S, Zhou M, Yu W, Zhang Y, He X, 2015. Phytoestrogens and risk of prostate cancer: a meta-analysis of observational studies. World J. Surg. Oncol 13 10.1186/s12957-015-0648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE, 2007. Phytooestrogens and risk of prostate cancer in Scottish men. Br. J. Nutr 98, 388–396. 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- Hedelin M, Klint Å, Chang ET, Bellocco R, Johansson JE, Andersson SO, Heinonen SM, Adlercreutz H, Adami HO, Grönberg H, Bälter KA, 2006. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the Cancer Prostate Sweden Study (Sweden). Cancer Causes Control 17, 169–180. 10.1007/s10552-005-0342-2. [DOI] [PubMed] [Google Scholar]
- Hegde MV, Mali AV, Chandorkar SS, 2013. What is a cancer cell? Why does it metastasize? Asian Pac. J. Cancer Prev 14, 3987–3989. 10.7314/APJCP.2013.14.6.3987. [DOI] [PubMed] [Google Scholar]
- Ho CKM, Halley L, Wei J, Habib FK, 2012. Analysis of prostate cancer association with four single-nucleotide polymorphisms from genome-wide studies and serum phytoestrogen concentrations. Prostate Cancer Prostatic Dis. 15, 365–368. 10.1038/pcan.2012.24. [DOI] [PubMed] [Google Scholar]
- Högger P, 2013. Nutrition-derived bioactive metabolites produced by gut microbiota and their potential impact on human health. Nutr. Med 1, 1–32. [Google Scholar]
- Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ, 2001. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am. J. Epidemiol 154, 434–441. 10.1093/AJE/154.5.434. [DOI] [PubMed] [Google Scholar]
- Hultén K, Winkvist A, Lenner P, Johansson R, Adlercreutz H, Hallmans G, 2002. An incident case-referent study on plasma enterolactone and breast cancer risk. Eur. J. Nutr 41, 168–176. 10.1007/s00394-002-0373-3. [DOI] [PubMed] [Google Scholar]
- Ilbeigi D, Nourbakhsh M, Khaghani S, Einollahi N, Kheiripour N, Gholinejad Z, Alaee M, Saberian M, 2017. Enterolactone reduces telomerase activity and the level of its catalytic subunit in breast cancer cells. Cell J. 19, 37–43. 10.22074/cellj.2017.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D, Sanders K, Kolybaba M, Lopez D, 1997. Case-control study of phytoestrogens and breast cancer. Lancet 350, 990–994. 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, Khalil AT, 2017. Plant-derived anticancer agents: a green anticancer approach. Asian Pac. J. Trop. Biomed 7, 1129–1150. 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- Jansen GHE, Arts ICW, Nielen MWF, Müller M, Hollman PCH, Keijer J, 2005. Uptake and metabolism of enterolactone and enterodiol by human colon epithelial cells. Arch. Biochem. Biophys 435, 74–82. 10.1016/j.abb.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Johnsen NF, Olsen A, Thomsen BLR, Christensen J, Egeberg R, Bach Knudsen KE, Loft S, Overvad K, Tjønneland A, 2010. Plasma enterolactone and risk of colon and rectal cancer in a case-cohort study of Danish men and women. Cancer Causes Control 21, 153–162. 10.1007/s10552-009-9445-5. [DOI] [PubMed] [Google Scholar]
- Jungeström MB, Thompson LU, Dabrosin C, 2007. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res 13, 1061–1067. 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- Katsuda S, Yoshida M, Saarinen N, Smeds A, Nakae D, Santti R, Maekawa A, 2004. Chemopreventive effects of hydroxymatairesinol on uterine carcinogenesis in Donryu rats. Exp. Biol. Med 229, 417–424. [DOI] [PubMed] [Google Scholar]
- Kaur V, Kumar M, Kumar A, Kaur K, Singh V, Kaur S, 2018. Pharmacotherapeutic potential of phytochemicals: implications in cancer chemoprevention and future perspectives. Biomed. Pharmacother 97, 564–586. [DOI] [PubMed] [Google Scholar]
- Kilkkinen A, Virtamo J, Vartiainen E, Sankila R, Virtanen MJ, Adlercreutz H, Pietinen P, 2004. Serum enterolactone concentration is not associated with breast cancer risk in a nested case-control study. Int. J. Cancer 108, 277–280. 10.1002/ijc.11519. [DOI] [PubMed] [Google Scholar]
- Kuijsten A, Arts ICW, Hollman PCH, Van’t Veer P, Kampman E, 2006. Plasma enterolignans are associated with lower colorectal adenoma risk. Cancer Epidemiol. Biomark. Prev 15, 1132–1136. 10.1158/1055-9965.EPI-05-0991. [DOI] [PubMed] [Google Scholar]
- Kuijsten A, Arts ICW, Vree TB, Hollman PCH, 2005. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr 135, 795–801. [DOI] [PubMed] [Google Scholar]
- Kuijsten A, Hollman PCH, Boshuizen HC, Buijsman MNCP, Van’t Veer P, Kok FJ, Arts ICW, Bueno-De-Mesquita HB, 2008. Plasma enterolignan concentrations and colorectal cancer risk in a nested case-control study. Am. J. Epidemiol 167, 734–742 . 10.1093/aje/kwm349. [DOI] [PubMed] [Google Scholar]
- Kyrø C, Hansen L, Frederiksen K, Nørskov NP, Bach Knudsen KE, Eriksen AK, Holm M, Tjønneland A, Olsen A, 2017. Pre-diagnostic plasma enterolactone concentrations and breast cancer prognosis among postmenopausal women - The Danish diet, cancer and health cohort. Clin. Nutr 1–9. 10.1016/j.clnu.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Atkinson C, Hullar MAJ, 2006. Assessing exposure to lignans and their metabolites in humans. J. AOAC Int 89, 1174–1181. [PubMed] [Google Scholar]
- Landete JM, Arqués J, Medina M, Gaya P, de Las Rivas BD, Muñoz R, 2016. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit. Rev. Food Sci. Nutr 56, 1826–1843. 10.1080/10408398.2013.789823. [DOI] [PubMed] [Google Scholar]
- Lin X, Switzer BR, Demark-Wahnefried W, 2001. Effect of mammalian lignans on the growth of prostate cancer cell lines. Anticancer Res. [PubMed] [Google Scholar]
- Lindahl G, Saarinen N, Abrahamsson A, Dabrosin C, 2011. Tamoxifen, flaxseed, and the lignan enterolactone increase stroma- and cancer cell-derived IL-1Ra and decrease tumor angiogenesis in estrogen-dependent breast cancer. Cancer Res. 71, 51–60. 10.1158/0008-5472.CAN-10-2289. [DOI] [PubMed] [Google Scholar]
- Linseisen J, Piller R, Hermann S, Chang-Claude J, 2004. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int. J. Cancer 110, 284–290. 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu J, Wang S, Zeng Z, Li T, Liu Y, Mastriani E, Li QH, Bao HX, Zhou YJ, Wang X, Hu S, Gao S, Qi Y, Shen Z, Wang H, Yu M, Gao T, Johnston RN, Liu SL, 2017. Enterolactone has stronger effects than enterodiol on ovarian cancer. J. Ovarian Res 10, 49 10.1186/s13048-017-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Saarinen NM, Thompson LU, 2006. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr 136, 906–912 (https://doi.org/136/4/906)(pii). [DOI] [PubMed] [Google Scholar]
- Mabrok HB, Klopfleisch R, Ghanem KZ, Clavel T, Blaut M, Loh G, 2012. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis 33, 203–208. 10.1093/carcin/bgr256. [DOI] [PubMed] [Google Scholar]
- Majdalawieh AF, Massri M, Nasrallah GK, 2017. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol 10.1016/j.ejphar.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Mali AV, Joshi AA, Hegde MV, Kadam SS, 2017. Enterolactone suppresses proliferation, migration and metastasis of MDA-MB-231 breast cancer cells through inhibition of uPA induced plasmin activation and MMPs-mediated ECM remodeling. Asian Pac. J. Cancer Prev 18, 905–915. 10.22034/APJCP.2017.18.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali AV, Wagh UV, Chandorkar SS, Patole MV, Hegde MV, Surve SV, 2012. In vitro anti-metastatic activity of enterolactone, a mammalian lignan derived from flax lignan, and down-regulation of matrix metalloproteinases in MCF-7 and MDA MB 231 cell lines. Indian J. Cancer 49, 181 10.4103/0019-509X.98948. [DOI] [PubMed] [Google Scholar]
- Mali AV, Joshi AA, Hegde MV, Kadam SS, 2018. Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB- 231 to revert the TGF-β-induced epithelial–mesenchymal transition. Cancer Biol. Med 15, 137–156. 10.20892/j.issn.2095-3941.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne ST, Playdon MC, Rock CL, 2016. Diet, nutrition, and cancer: past, present and future. Nat. Rev. Clin. Oncol 13, 504–515. 10.1038/nrclinonc.2016.24. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Gill CIR, Linton T, Berrar D, McGlynn H, Rowland IR, 2008. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell linein vitro. Mol. Nutr. Food Res 52, 567–580. 10.1002/mnfr.200700052. [DOI] [PubMed] [Google Scholar]
- Mccann MJ, Rowland IR, Roy NC, 2013. Anti-proliferative effects of physiological concentrations of enterolactone in models of prostate tumourigenesis. Mol. Nutr. Food Res 57, 212–224. 10.1002/mnfr.201200362. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rowland IR, Roy NC, 2014. The anti-proliferative effects of enterolactone in prostate cancer cells: evidence for the role of DNA licencing genes, Mi-R106b cluster expression, And PTEN dosage. Nutrients 6, 4839–4855. 10.3390/nu6114839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SE, Moysich KB, Freudenheim JL, Ambrosone CB, Shields PG, 2002. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J. Nutr 132, 3036–3041. [DOI] [PubMed] [Google Scholar]
- McCann SE, Muti P, Vito D, Edge SB, Trevisan M, Freudenheim JL, 2004. Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int. J. Cancer 111, 440–443. 10.1002/ijc.20262. [DOI] [PubMed] [Google Scholar]
- Miles FL, Navarro SL, Schwarz Y, Gu H, Djukovic D, Randolph TW, Shojaie A, Kratz M, Hullar MAJ, Lampe PD, Neuhouser ML, Raftery D, Lampe JW, 2017. Plasma metabolite abundances are associated with urinary enterolactone excretion in healthy participants on controlled diets. Food Funct. 8, 3209–3218. 10.1039/C7FO00684E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D, Saarinen NM, Miura Y, Santti R, Yagasaki K, 2007. Hydroxymatairesinol and its mammalian metabolite enterolactone reduce the growth and metastasis of subcutaneous AH109A hepatomas in rats. Nutr. Cancer 58, 49–59. 10.1080/01635580701308133. [DOI] [PubMed] [Google Scholar]
- Mukker JK, Michel D, Muir AD, Krol ES, Alcorn J, 2014. Permeability and conjugative metabolism of flaxseed lignans by caco-2 human intestinal cells. J. Nat. Prod 77, 29–34. 10.1021/np4004905. [DOI] [PubMed] [Google Scholar]
- Olsen A, Christensen J, Knudsen KEB, Johnsen NF, Overvad K, Tjønneland A, 2011. Prediagnostic plasma enterolactone levels and mortality among women with breast cancer. Breast Cancer Res. Treat 128, 883–889. 10.1007/s10549-011-1397-2. [DOI] [PubMed] [Google Scholar]
- Olsen A, Erik K, Knudsen B, Thomsen BL, Loft S, Stripp C, Overvad K, Møller S, Tjønneland A, 2004. plasma enterolactone and breast cancer incidence by estrogen receptor status plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol. Biomark. Prev 13, 2084–2089. [PubMed] [Google Scholar]
- Pen L, Heinonen S, Aura A, Adlercreutz H, 2005. Dietary Sesamin Is converted to enterolactone in humans. J. Nutr 135, 1056–1062 (https://doi.org/135/5/1056) (pii). [DOI] [PubMed] [Google Scholar]
- Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML, 2010. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr. Rev 68, 571–603. 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianjing P, Thiantanawat A, Rangkadilok N, Watcharasit P, Mahidol C, Satayavivad J, 2011. Estrogenic activities of sesame lignans and their metabolites on human breast cancer cells. J. Agric. Food Chem 59, 212–221. 10.1021/jf102006w. [DOI] [PubMed] [Google Scholar]
- Pietinen P, Stumpf K, Männistö S, Kataja V, Uusitupa M, Adlercreutz H, 2001. Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol. Biomark. Prev 10, 339–344. [PubMed] [Google Scholar]
- Piller R, Chang-Claude J, Linseisen J, 2006. Plasma enterolactone and genistein and the risk of premenopausal breast cancer. Eur. J. Cancer Prev 15, 225–232. 10.1097/01.cej.0000197449.56862.75. [DOI] [PubMed] [Google Scholar]
- Power KA, Saarinen NM, Chen JM, Thompson LU, 2006a. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int. J. Cancer 118, 1316–1320. 10.1002/ijc.21464. [DOI] [PubMed] [Google Scholar]
- Power KA, Ward WE, Chen JM, Saarinen NM, Thompson LU, 2006b. Genistein alone and in combination with the mammalian lignans enterolactone and enterodiol induce estrogenic effects on bone and uterus in a postmenopausal breast cancer mouse model. Bone 39, 117–124. 10.1016/j.bone.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Qian C-N, Mei Y, Zhang J, 2017. Cancer metastasis: issues and challenges. Chin. J. Cancer 36, 38 10.1186/s40880-017-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Madl RL, Takemoto DJ, Baybutt RC, Wang W, 2005. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J. Nutr 135, 598–602. [DOI] [PubMed] [Google Scholar]
- Roncaglia L, Amaretti A, Raimondi S, Leonardi A, Rossi M, 2011. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl. Microbiol. Biotechnol 92, 159–168. 10.1007/s00253-011-3338-8. [DOI] [PubMed] [Google Scholar]
- Saarinen NM, Abrahamsson A, Dabrosin C, 2010. Estrogen-induced angiogenic factors derived from stromal and cancer cells are differently regulated by enterolactone and genistein in human breast cancer in vivo. Int. J. Cancer 127, 737–745. 10.1002/ijc.25052. [DOI] [PubMed] [Google Scholar]
- Saarinen NM, Huovinen R, Warri A, Makela SI, Valentin-Blasini L, Sjoholm R, Ammala J, Lehtila R, Eckerman C, Collan YU, Santti RS, 2002. Enterolactone inhibits the growth of 7,12-Dimethylbenz(a) anthracene-induced mammary carcinomas in the rat. Mol. Cancer Ther 1, 869–876. [PubMed] [Google Scholar]
- Sehabath M, Hernandez L, Pillow P, Wu X, Spitz M, 2005. Dietary phytoestrogens and lung cancer risk. JAMA 294, 1493–1504. 10.1016/S0169-5002(05)80187-4. [DOI] [PubMed] [Google Scholar]
- Schröder L, Richter DU, Pieehulla B, Chrobak M, Kuhn C, Schulze S, Abarzua S, Jeschke U, Weissenbacher T, 2016. Effects of phytoestrogen extracts isolated from elder flower on hormone production and receptor expression of trophoblast tumor cells JEG-3 and BeWo, as well as MCF7 breast cancer cells. Nutrients 8, 616 10.3390/nu8100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold P, Vrieling A, Johnson TS, Buck K, Behrens S, Kaaks R, Linseisen J, Obi N, Heinz J, Fleseh-Janys D, Chang-Claude J, 2014. Enterolactone concentrations and prognosis after postmenopausal breast cancer: assessment of effect modification and meta-analysis. Int. J. Cancer 135, 923–933. 10.1002/ijc.28729. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE, 2014. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 5, 491–501. 10.1039/c3fo60402k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonestedt E, Borgquist S, Ericson U, Gullberg B, Olsson H, Adlercreutz H, Goran L, Wirfalt E, 2008. Enterolactone is differently associated with estrogen receptor B – negative and – positive breast cancer in a Swedish Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev 17, 3241–3252. 10.1158/1055-9965.EPI-08-0393. [DOI] [PubMed] [Google Scholar]
- Stattin P, Adlercreutz H, Tenkanen L, Jellum E, Lumme S, Hallmans G, Harvei S, Teppo L, Stumpf K, Luostarinen T, Lehtinen M, Dillner J, Hakama M, 2002. Circulating enterolactone and prostate cancer risk: a nordic nested case-control study. Int. J. Cancer 99, 124–129. 10.1002/ijc.10313. [DOI] [PubMed] [Google Scholar]
- Stattin P, Bylund A, Biessy C, Kaaks R, Hallmans G, Adlercreutz H, 2004. Prospective study of plasma enterolactone and prostate cancer risk (Sweden). Cancer Causes Control 15, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Stewart BW, Bray F, Forman D, Ohgaki H, Straif K, Ullrich A, Wild CP, 2016. Cancer prevention as part of precision medicine: “plenty to be done.”. Carcinogenesis 37, 2–9. 10.1093/carcin/bgv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MK, Lautens M, Thompson LU, 1998. Mammalian lignans inhibit the growth of estrogen-independent human colon tumor cells. Anticancer Res. 18, 1405–1408. [PubMed] [Google Scholar]
- Surh Y-J, 2003. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 3, 768–780. 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tomimori N, Tanaka Y, Kitagawa Y, Fujii W, Sakakibara Y, Shibata H, 2013. Pharmacokinetics and safety of the sesame lignans, sesamin and episesamin, in healthy subjects. Biopharm. Drug Dispos 34, 462–473. 10.1002/bdd.1862. [DOI] [PubMed] [Google Scholar]
- Touillaud MS, Thiebaut ACM, Fournier A, Niravong M, Boutron-Ruault M-C, Clavel-Chapelon F, 2007. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J. Natl. Cancer Inst 99, 475–486. 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touillaud MS, Thiebaut ACM, Niravong M, Boutron-Ruault M-C, Clavel-Chapelon F, 2006. No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French Cohort Study. Cancer Epidemiol. Biomark. Prev 15, 2574–2576. 10.1158/1055-9965.EPI-06-0543. [DOI] [PubMed] [Google Scholar]
- Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, Johnsen NF, Olsen A, Kaaks R, Linseisen J, Boeing H, Nöthlings U, Bueno-De-Mesquita HB, Ros MM, Sacerdote C, Palli D, Tumino R, Berrino F, Trichopoulou A, Dilis V, Trichopoulos D, Chirlaque MD, Ardanaz E, Larranaga N, Gonzalez C, Suárez LR, Sánchez MJ, Bingham S, Khaw KT, Hallmans G, Stattin P, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ, 2009. Plasma phytooestrogens and prostate cancer in the European prospective investigation into cancer and nutrition. Br. J. Cancer 100, 1817–1823. 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheus M, Van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PHM, 2007. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol 25, 648–655. 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- Vineis P, Wild CP, 2014. Global cancer patterns: causes and prevention. Lancet 383, 549–557. 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- Wallström P, Drake I, Sonestedt E, Gullberg B, Bjartell A, Olsson H, Adlercreutz H, Tikkanen MJ, Wirfält E, 2017. Plasma enterolactone and risk of prostate cancer in middle-aged Swedish men. Eur. J. Nutr 1–12. 10.1007/s00394-017-1530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Liu LQ, Higuchi CM, Chen H, 1998. Induction of NADPH: quinone reductase by dietary phytoestrogens in colonic Colo205 cells. Biochem. Pharmacol 56, 189–195. 10.1016/S0006-2952(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Ward H.a., Kuhnle GGC, Mulligan A.a., Lentjes M.a.H., Luben RN, Khaw K, 2010. Breast, colorectal, and prostate cancer risk in the European prospective investigation into cancer and nutrition – Norfolk in relation to phytoestrogen intake derived from an improved database 1 – 4. Am. J. Clin. Nutr 91, 440–448. 10.3945/ajcn.2009.28282.1. [DOI] [PubMed] [Google Scholar]
- Ward H, Chapelais G, Kuhnle GGC, Luben R, Khaw KT, Bingham S, 2008. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European prospective into cancer-Norfolk cohort study. Breast Cancer Res. 10, 1–9. 10.1186/bcr1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng CJ, Yen GC, 2012. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev 38, 76–87. 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund, American Institute for Cancer Research, 2007. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Cancer Res (https://doi.org/978-0-9722522-2-5). [Google Scholar]
- Xie J, Tworoger SS, Franke AA, Terry KL, Rice MS, Rosner BA, Willett WC, Hankinson SE, Eliassen AH, 2013. Plasma enterolactone and breast cancer risk in the Nurses' Health Study II. Breast Cancer Res. Treat 139, 801–809. 10.1007/s10549-013-2586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X-Y, Hu X-J, Li Y, Liu C-M, 2015. Inhibitory effects of enterolactone on growth and metastasis in human breast cancer. Nutr. Cancer 67,1326–1334. 10.1080/01635581.2015.1082113. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Cho LY, Ko KP, Shin A, Ma SH, Choi BY, Han DS, Song KS, Kim YS, Lee JY, Han BG, Chang SH, Shin HR, Kang D, Yoo KY, Park SK, 2012. Genetic susceptibility on cagA-interacting molecules and gene-environment interaction with phytoestrogens: a putative risk factor for gastric cancer. PLoS One 7, e31020 10.1371/journal.pone.0031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaineddin AK, Vrieling A, Buck K, Becker S, Linseisen J, Fleseh-Janys D, Kaaks R, Chang-Claude J, 2012. Serum enterolactone and postmenopausal breast cancer risk by estrogen, progesterone and herceptin 2 receptor status. Int. J. Cancer 130, 1401–1410. 10.1002/ijc.26157. [DOI] [PubMed] [Google Scholar]
- Zeleniueh-Jaequotte A, Adlercreutz H, Shore RE, Koenig KL, Kato I, Arslan AA, Toniolo P, 2004. Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br. J. Cancer 91, 99–105. 10.1038/sj.bjc.6601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleniueh-Jaequotte A, Lundin E, Micheli A, Koenig KL, Lenner P, Muti P, Shore RE, Johansson I, Krogh V, Lukanova A, Stattin P, Afanasyeva Y, Rinaldi S, Arslan AA, Kaaks R, Berrino F, Hallmans G, Toniolo P, Adlercreutz H, 2006. Circulating enterolactone and risk of endometrial cancer. Int. J. Cancer 119, 2376–2381. 10.1002/ijc.22140. [DOI] [PubMed] [Google Scholar]