Abstract
The pathology of traumatic brain injury (TBI) adversely affects many brain regions, often resulting in the development of comorbid psychiatric disorders including substance use disorders (SUD). Although traditionally thought to be an epidemic that predominantly affects males, recent clinical studies report females have higher rates of concussions and longer recovery times than males. Yet, how neurotrauma, particularly deep within the brain, between the sexes is differentially manifested remains largely unknown. The risk of TBI peaks during adolescence when neuronal networks that regulate reward behaviors are not fully developed. Previously, using the conditioned place preference (CPP) assay, we found that adolescent TBI increased susceptibility to the rewarding effects of cocaine in male mice. Further, we observed augmented inflammatory profiles, increased microglial phagocytosis of neuronal proteins, and decreased neuronal spine density in the NAc. Notably, the extent of sex differences in SUD susceptibility following TBI has not be investigated. Thus, here we ask the central question of whether the adolescent TBI-induced neuroinflammatory profile at reward centers is divergent in a sex-dependent manner. Using the CPP assay, we found that female mice with high levels of female sex hormones at the time of adolescent TBI demonstrated neuroprotection against increased sensitivity to the rewarding effects of cocaine. These studies also provide evidence of significantly reduced microglial activation and phagocytosis of neuronal proteins within the NAc of females. Overall, our results offer crucial insight into how adolescent TBI impacts the reward pathway in a sex depending manner that could explain a vulnerability to addiction-like behavior.
Keywords: Traumatic Brain Injury, Sex Differences, Addiction, Behavior, Neuroinflammation
1. Introduction
Among adolescents and young adults, the risk of traumatic brain injury (TBI) has conventionally been thought to be male dominated as the absolute number of TBIs occur more frequently in males. However, epidemiological reports in comparable sports indicate that rates of TBIs are higher and recovery time is longer following brain injury in females[1-3]. Data from the injury surveillance system known as the High School Reporting Information Online, determined that brain injuries comprised a greater percentage of total injuries for high school female athletes and that females also sustained higher rates of brain injuries compared to male athletes in sports played by both sexes[4]. In a separate study, pre- and post-injury cognitive performance was compared in high school and collegiate athletes who experienced a mild TBI from contact sports, found that females demonstrated significantly greater declines in reaction time tests along with cognitive impairments that were 1.7 times more frequent in females than males[5]. Furthermore, a report on the rates of brain injuries and recovery among high school athletes indicated increased symptom frequency in females but with recovery trajectories similar to those compared to males[6]. Although research into female TBI is increasing, much remains unknown regarding how the progression of neurotrauma is affect by fluctuating hormones.
Sex differences in preclinical investigations of TBI-induced neuroinflammation have also been observed. Males typically show a more aggressive neuroinflammatory profile following brain injury when compared to females[7]. Although females demonstrate reduced peripheral macrophage infiltration, cell death, and lesion volume at the acute phase, the female inflammatory response becomes indistinguishable from that of males when analyzed at chronic stages, suggesting neuroprotection following TBI in females may be a transient effect[7]. Epidemiological studies have established a link between TBI and the likelihood of developing Substance Use Disorders (SUD). A link that is also reproduced by combining experimental TBI and behavioral assays for the analysis of the reward properties of drugs of abuse [8-10]. To date, both clinical and preclinical research has suggested that females are more vulnerable to addiction than males. Females demonstrate a higher propensity of telescoping, a phenomenon where users go from initial drug use to maintenance of drug-taking at faster rates than males[11, 12]. Also, female rats self-administer more cocaine while male rats are better able to control cocaine intake over time[13]. Importantly, female sex hormones appears to play a role in the observed behavior of cocaine intake since the behavior could be eliminated (ovariectomized animals) and restored in ovariectomized mice with exogenous estrogen replacement [14]. Similarly, a study in ovariectomized female rats treated with exogenous estrogen found increased rates of cocaine-seeking and reinstatement, a correlate of drug relapse [15]. These findings point to a role of sex hormones in SUD vulnerability and are consistent with clinical data reporting higher rates of cocaine use disorder among adolescent females than males[16]. However, the sensitivity to the rewarding effects of drugs of abuse following brain injury in females has yet to be investigated.
TBI is still a major cause of morbidity and mortality among females and therefore, females should be better represented in clinical studies of long-term psychiatric effects of earl-life TBI and in preclinical investigations of potential underlying mechanisms. Therefore, the following studies were designed to investigate the magnitude of the influence of sex differences in SUD vulnerability following adolescent TBI. Here in study 1, freely-cycling adolescent female mice were exposed to our model of CCI-TBI, tested for motor deficits as exclusionary criteria, and tested for sensitivity to the rewarding efficacy of subthreshold doses of cocaine two weeks later using the CPP assay based on estrous phase. Next, study 2 and 3 sought to determine sex differences in acute and chronic neuropathology and characterize the inflammatory profile including microglial morphology, microglial phagocytosis status, neuronal loss, and permeability of the blood-brain barrier (BBB) following adolescent CCI-TBI in females compared to males by without estrous phase stratification.
2. Materials and Methods
2.1. Animals
For all experiments, six-week old male and female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Upon arrival, mice were group housed under a 12 h/12 h light/dark cycle with ad libitum access to food and water. Mice were given a 5-7-day period of acclimation to the University Laboratory Animal Research facility at the Lewis Katz School of Medicine at Temple University (Philadelphia, PA) prior to the administration of experimental traumatic brain injury. All experiments were approved by the IACUC at Temple University and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865- 23).
2.2. Methods for Study 1
2.2.1. Rotarod
Motor performance was assessed at 48 hrs and 7 days following induction of experimental TBI. A rotarod apparatus equipped with a 3 cm rotating rod and five lanes each with an automatic timer and falling sensor was used (40 cm W x 30 cm D x 38 cm H) (Ugo Basile, Mt. Laurel, NJ). Before the testing sessions, mice were habituated to stay on the rod at a constant speed of 4 rpm for two 3 min trials. During testing sessions, mice were placed on the rod with the speed set to accelerate from 4-40 rpm over a duration of 5 min. Each testing session consisted of three 5 min trials. Latency to fall from the rotating rod was recorded. Post-injury motor performance was compared to within-subject baseline performance recorded the day before injury to evaluate any motor deficits resulting from TBI. For each TBI severity, there were N=8 mice per group.
2.2.2. Traumatic Brain Injury by Controlled Cortical Impact
Clinically, severe TBI is the least common severity of injury that occurs, and typically results in significant damage, lengthy hospital stays, extreme rehabilitation, and extensive care. In fact, patients that sustain severe TBI can even remain unconscious or severely disabled until death[17]. The extent of damage and recovery times can impact patients’ likelihood to experiment with drugs of abuse and therefore, studies investigating increased risk of developing neuropsychiatric complications following severe TBI are contradictory. Several studies report increased risk of depression and anxiety disorders while others report decreased risk for alcohol and substance use disorders compared to less severe injuries[18, 19]. Therefore, severe TBI was not included in the behavioral analyses conducted in Study 1. Following the acclimation period, experimental TBI of three severities (Sham, Mild, and Moderate), was administered to different groups of mice using an Impact One™ Stereotaxic CCI Instrument (Leica Microsystems, Buffalo Grove, IL) outfitted with a piston (2-mm diameter) as previously described [8, 9, 20]. Mice were anesthetized using vaporized isoflurane (5% induction, 2% maintenance) and placed in a Just for Mouse™ Stereotaxic Instrument (Stoelting Co., Wood Dale, IL). An Ideal Micro-Drill™ (CellPoint Scientific Inc., Gaithersburg, MD) with a 0.5-mm, rounded burr was used to perform a craniectomy removing a 4-mm bone flap located to the right of the sagittal suture between lambda and bregma under a Zeiss Stemi 2000-C stereomicroscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) equipped with a SCHOTT EasyLED Ringlight (SCHOTT North America Inc., Elmsford, NY). The electro-magnetically-driven piston was placed at the surface of the exposed parenchyma and a single discharge (Mild: speed: 2m/s, dwell time: 0.5 sec, depth: 1mm; Mod: speed: 4.5 m/s, dwell time: 0.5 sec, depth: 2 mm) produced Mild- or Mod-CCI-TBI in 6-week-old, adolescent male and female mice. The craniectomy site was then covered by a sterile, 6-mm glass cover slip (ProSciTech Pty Ltd, Kirwan, Australia) adhered to the skull using VetbondTM tissue adhesive (3M, St. Paul, MN). Post-operatively, mice were individually-housed to ensure surgical recovery and weighed and monitored daily for 7 days. Sham controls (craniectomy only) underwent identical surgical procedures except impactor discharge. Naþve controls were individually housed at the same time as experimental mice.
2.2.3. Cocaine CPP
Two weeks following TBI, behavior was assessed using a biased CPP assay as previously described [8, 9, 20]. A biased CPP design takes into consideration the fact that animal strains may demonstrate an initial preference for one of the compartments prior the experimental conditioning [21, 22]. As such, during the preconditioning phase, animals can enter either compartments and the innate preference of each animal is determined. The drug then is paired with the preferred or nonpreferred environment depending on whether the drug is assumed to produce aversive or positive reinforcing effects (such as cocaine), respectively. Therefore, in a biased CPP assay, the compartmental shift to the drug-paired side can be interpreted as a positive rewarding effect. Compartment bias was determined during the pre-test, when mice had access to both compartments through a door opening in the divider wall for 30 min and time spent in each compartment was recorded. Baseline preference shifts (average and SD) during preconditioning for female mice were as follows: Naive (compartment A (white)=692±105, compartment B (black)= 1108±105); Sham (compartment A (white)=729±95, compartment B (black)=1071±95); mod-CCI-TBI (compartment A (white)=752±88, compartment (black)=1048±88). Males and females had no differences between baseline initial preferences. The compartment with a lower residence time was assigned as the drug-paired, least-preferred compartment. During the conditioning phase, mice were randomly assigned to receive one of 5 doses of cocaine, administered in the morning by intraperitoneal (IP) injection (vehicle, 1.25, 2.5, 10, or 30 mg/kg). Each mouse was immediately placed in its least-preferred compartment for 30 min following IP cocaine without access to the preferred compartment. After a minimum of 4 hours, mice were injected with 0.9% saline and placed in the preferred compartment for 30 min. This schedule persisted for 6 days. During the post-test, mice were placed in the chamber again for 30 min with free access to both compartments without any injections. The rewarding effects of cocaine were evaluated by a place preference shift, which was calculated by subtracting the time spent in the drug-paired compartment during the pre-test from the time spent in the drug-paired compartment during the post-test. When testing or conditioning occurred on the same day, mice assigned to various injury severity conditions were distributed equally to control for any influence of time of test. For each TBI severity, there were N=7-13 mice per group.
2.2.4. Vaginal Cytology
Stage of estrous cycle (proestrus, estrus, metestrus, and diestrus) was determined by epithelial cell morphology after vaginal lavage using a protocol previously described by others [23-26]. Female mice were placed on the lid of the cage or procedure bench and gently grasped by the base of the tail to gently lift the hind legs and expose the vaginal area. The tip of a sterile cotton swab dipped in ambient temperature sterile saline was briefly inserted and gently twisted to collect vaginal epithelial cells. Vaginal epithelial cells were then transferred to a glass microscope slide (12-550-15 Fisherbrand™ Superfrost™ Plus Microscope Slides) by rolling the swab across the slide allowing for later determination of estrous stage by microscopic analysis of cellular composition and morphology. This procedure was performed at the same time each day, for a minimum of five days prior to and on the day of CCI-TBI or sham surgical procedures. Naïve females were swabbed for the same number of days as experimental mice.
For microscopic analysis of cellular composition and morphology, slides were placed in a 0.1% crystal violet solution (C581-25, Certified Biological Stain, Fisher) for 1 min, removed and then washed for 1 min in ddH20 twice. Slides were allowed to dry thoroughly before being imaged under a light microscope. Stage of estrous cycle was determined by the following cellular composition: Proestrus: mostly nucleated cells, some cornified epithelial cells may be present and some leukocytes may be present in early proestrus; Estrus: mostly cornified epithelial cells are present; Metestrus: some cornified epithelial cells and polymorphonuclear leukocytes present; Diestrus: primarily polymorphonuclear leukocytes, some epithelial cells may be present during late diestrus.
2.3. Methods for Study 2
2.3.1. Flow Cytometry
Brain tissue was harvested from a separate cohort of mice at either 2 (acute) or 14 days (chronic) post- CCI-TBI and analyzed for both the impacted and non-impacted hemispheres. Tissue was placed into HBSS and held on ice until processing. The cerebellum was removed, and hemispheres were separated using a mouse brain matrix (Zivic Instruments, Pittsburgh, PA) and clean razor blades. Each hemisphere was homogenized in RPMI in a 5-mL ounce homogenizer (12-16 strokes) using a previous protocol [27]. In short, homogenized brains were combined to a final 30% Percoll solution (9 mL percoll, 1 mL 10x PBS without calcium/magnesium, 10 mL RPMI) and then centrifuged in a fixed angle rotor at 7800g for 30 min RT. Floating myelin debris was removed, and the remaining cell solution was passed through a 40 μm cell strainer. The volume was brought up to 50 mL with RPMI and centrifuged at 1500 rpm for 5 min at RT. The cell pellet was resuspended in 1 mL FACS Buffer and transferred to 5 mL polystyrene flow cytometry tubes. 1 mL of Ficoll-Paque PREMIUM was carefully layered under the cell solution and then spun at 2500 rpm for 25 min at RT without brake. The white layer at the interface (~1 mL) was transferred to a new flow cytometry tube, washed with FACS buffer and pelleted at 1500 rpm for 5 min. Fc receptors were blocked for 15 min and then cells labeled using the following antibodies/reagents purchased from eBioscience Inc./ Thermo Fisher Scientific unless indicated otherwise: CD11b (Pacific Blue), CD45 (AmCyan), Ly6G (APC), Fixability dye and lineage markers (FVD, CD3, CD9, Siglec F, NK1.1, APC-Cy7), Ly6C (PerCP-5.5), CX3CR-1 (Pe-Cy7, Biolegend). The intracellular protein PSD95 (Abcam ab76115, PE secondary) was labeled after fixation and permeabilization. Samples were read on a BD FACS Canto II and data analysis was performing using FlowJo® software (FlowJo, LLC). Our gating strategy involved gating on singlet populations on FSC-H vs FSC-A and then on live lineage negative populations. Then microglial and leukocytes were gated as CD11b+, CD45+. Neutrophils were then gated by positive expression of Ly6G. Microglia were then gated for CX3CR-1low and PSD95+ cells.
Punch-outs of the nucleus accumbens (NAc) were processed using a modified version of the above protocol with reduced volumes. Brains were sectioned using the mouse matrix and tissue containing the NAc was excised using a brain microdissection tool 1.25 mm in diameter (Stoelting Co., Wood Dale, IL). 1.25 mm punchouts of the NAc from the right (impacted) hemisphere were homogenized using a pestle. Punchouts were resuspended in a 30% Percoll solution as above and centrifuged at 7800g for 30 min RT. Myelin debris was removed, and cells were washed, centrifuged, and underlaid with Ficoll. The interface was collected after a spin at 2500 rpm for 25 min at RT without brake. Cells were then washed and stained as described above. For each TBI severity, there were N=4-6 mice per group.
2.4. Methods for Study 3
2.4.1. Traumatic Brain Injury by Controlled Cortical Impact
Although severe TBI was not included in the behavioral assays conducted in Study 1, the goal of Study 3 was to recapitulate to what degree biological sex regulated severity-dependent inflammatory responses Therefore, methods for experimental TBI following the acclimation period were conducted exactly as described above in section 2.2.2 except the three severities now included Sham, Mod and Severe. Severity parameters were as follows: Mod: speed: 4.5 m/s, dwell time: 0.5 sec, depth: 2 mm; Severe: speed 6 m/s, dwell time: 0.5 sec, depth: 2mm). As described above, Post-operatively, mice were individually-housed to ensure surgical recovery and weighed and monitored daily for 7 days. Sham controls (craniectomy only) underwent identical surgical procedures except impactor discharge. Naïve controls were individually housed at the same time as experimental mice.
2.4.1. Tissue Preparation, Histology and Image Analysis
Immunohistochemistry was performed on brain tissue segments to evaluate the degree of neuropathology in male and female mice with and without adolescent TBI. At two weeks post injury, anesthetized mice were transcardially perfused with PBS followed by Poly/LEM fixative (Polysciences, Inc.; Warrington, PA). Brains were removed and placed in Poly/LEM fixative for 24hrs at 4°C. 2mm segments were post-fixed in Poly/LEM fixative at 4°C for an additional 24hrs. Next, segments were washed with PBS, processed using a Tissue-Tek® VIP® 6 (Sakura Finetek USA, Inc.; Torrance, CA), paraffin-embedded using a TN-1500 Embedding Console System (Tanner Scientific, Inc.; Sarasota, FL), and sectioned using a rotary microtome (Leica Microsystems, Inc.; Buffalo Grove, IL). 5μm paraffin-embedded sections from each experimental group (Naïve/Sham, Mod-CCI-TBI, and Severe-CCI-TBI) were cleared, rehydrated and stained for Ionized calcium binding adapter molecule 1 (Iba1), neuronal nuclei (NeuN), and Fibrinogen to determine the activation of microglial, survival of neurons, and degree of blood-brain barrier permeability in the region of impact. Sections stained for Iba1 were HIER pre-treated with 10mM citric acid buffer (pH 6.0) prior to primary antibody incubation, while those stained for Fibrinogen were PIER pre-treated with Proteinase K (Dako). Sections stained for NeuN did not receive any pre-treatment. All sections were incubated in primary antibody prepared in Dako Antibody Diluent for 1hr at RT at the following dilutions: Iba1(1:400, Wako Chemicals, Cat # 019-19741), NeuN (1:500, BioLegend, Cat # 834501), Fibrinogen (1:400, Dako). An HRP- or AP-conjugated labeled polymer system (ImmPRESS Staining Kits, Vector Laboratories) was used to detect positive antibody staining and subsequently visualized using Sigma DAB. Sections were then dehydrated, and cover slipped in preparation for imaging. For analysis of chromogen immunostaining of Iba1, slides containing tissue sections were scanned using an Aperio AT2 slide scanner (Leica Biosystems) and converted to tiff files using Aperio-ImageScope software. ImageJ analysis was performed by batch processing tiff files with an automated cell-counting macro. The macro was configured to perform particle counting on high-resolution images (ipsilateral hemisphere, 4/group, taken at 20X objective magnification) on immunolabeled cells. Calibrated images were sequentially processed for background subtraction, color threshold segmentation, binary conversion (including watershed function application) and particle analysis (based on diameter, area, and circularity) [28]. Briefly, the analysis relies on the identification of cells immunopositive for IBA-1. In the CNS, IBA-1 expression is restricted to microglial cells. Images of coronal sections labeled with the 3,3′-diaminobenzidine or DAB chromogen are processed for threshold segmentation in which any color outside of the range of the brown DAB stain (RGB color threshold segmentation min=20,100,100 to max=30,255,255) is excluded from analysis. The thresholded images are then converted to binary images for particle counting. The classification of particles corresponding to all microglia were based on the following particle counting parameters: diameter of >12 microns, particle area of >50 microns2, and circularity within 0-0.02. For the classification of particles corresponding to amoeboid/activated microglia the parameters were as follows: diameter of >18 microns, particle area of >72 microns2, and circularity within 0.05-0.5. The NeuN analysis was based on identifying non-viable neurons, that met the criteria of >90% loss of DAB density. For determinations of vascular leakiness, coronal section sections through the impact site were immunostained for fibrinogen as described above. The images were analyzed with NIS Elements AR (Nikon Instruments). Utilizing color thresholding on the DAB+ areas, the detection of fibrinogen in the CNS parenchyma was identified and measured using area tools calibrated for magnification and scanner pixel size. Thus, the fibrinogen positive areas are reported as mm2 of the total tissue section area. For each TBI severity, there were N=4 mice per group.
2.5. Statistical Analysis
Data were analyzed for statistical significance using Prism software (version 6.0h; GraphPad Software Inc., La Jolla, CA). One-way ANOVA with Tukey’s post-hoc tests were used to analyze the rotarod and CPP data stratified by hormone level in females. CPP data irrespective of hormone level data in females, analysis of ipsilateral and NAc immune infiltration, microglial phagocytosis, and neuropathological outcomes between males and females for each time point were each analyzed by two-way ANOVA followed by Tukey’s post-hoc tests. For all tests, statistical significance was defined at p < 0.05.
3. Results
3.1. Results for Study 1
3.1.1. Female Mice do not Demonstrate Increased Susceptibility to the Rewarding Effects of Cocaine after Adolescent TBI
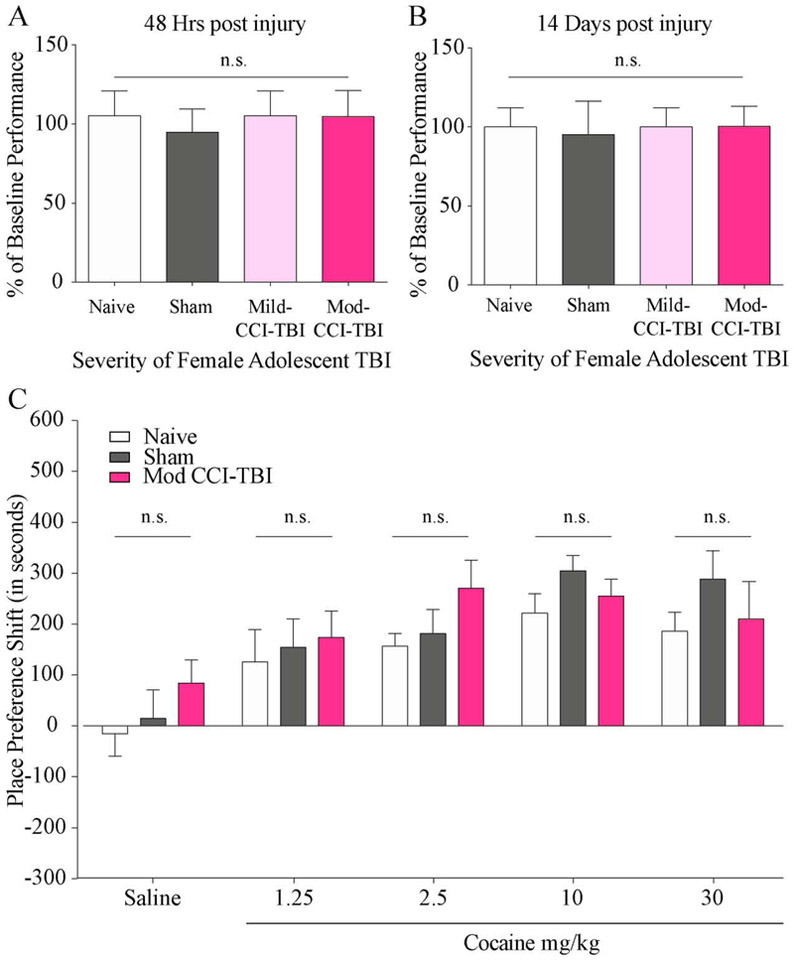
As was previously done with males[9], first we assessed freely-cycling adolescent female mice utilizing the Rotarod assay to exclude any mice with TBI-induced motor deficits from further behavioral testing. Pre-injury baseline performance was used as a percentage standard to show progression of ambulatory function. Mice were considered impaired and excluded from the CPP assay if they fell below 1.5 times the standard deviation from the mean latency to fall of no craniectomy control mice. Under these parameters, no female mice were considered impaired or excluded. As demonstrated in Figure 1 we report no differences in rotarod performance at either 48 hrs (Fig. 1A) (One-way ANOVA, injury severity effect: F (3, 43) = 1.299, p = 0.2869) or 7 days (Fig. 1B) (One-way ANOVA, injury severity effect: F (3, 43) = 0.3138, p = 0.8153) post Sham, Mild, or Mod injury in adolescent female mice. These results indicate no change in motor function or coordination following CCI-TBI in females.
Figure 1. Adolescent TBI in female mice does not induce motor deficits or increase sensitivity to the rewarding effects of cocaine.
Results from the rotarod assay indicate no motor deficits following adolescent TBI in female mice 48 hours (A) or 7 days (B) after injury, justifying exclusion of subjects exhibiting rotarod deficits. Data are normalized to pre-injury performance. (C) CPP shift for cocaine in adolescent female mice tested 2 weeks following Mod-CCI-TBI. No dose-related differences were detected in cocaine CPP shift. N = 8-14 per condition (two-way ANOVA, p>0.05).
Since we did not observe significant effects of mice exposed to a single CCI-TBI of mild severity, only Mod-CCI-TBI parameters were used in females from this point forward. Similar to our previous studies[8, 9], a biased CPP test was administered two weeks after CCI-TBI in order to observe behavioral responses to the rewarding effects of cocaine in injured adolescent females (see methods for a description of the biased CPP designed). Doses of cocaine we chosen to represent ascending and descending portions of the expected inverted-U dose response curve. However, in contrast to male mice and due to increased sensitivity to low doses of psychostimulants that has been previously established, cocaine was administered once-daily at doses of 30, (descending), 10, 2.5 (ascending), and 1.25 (subthreshold) mg/kg for 6 consecutive days [15, 29, 30]. Control mice received an equivalent volume of 0.9% saline. Without considering the phase of estrous cycle at the time of injury, the CPP shift of injured adolescent females given any dose of cocaine did not differ from naïve mice, unlike our observations in male mice[9] (Fig. 1C) (two-way ANOVA with Tukey’s post-hoc tests; cocaine dose effect: F (4, 138) = 12.60, p<0.0001, TBI severity effect: F (2, 138), p=0.0795). Notably, the dose considered subthreshold for males of 2.5 mg/kg cocaine trended towards a higher CPP shift in females that received Mod-CCI-TBI compared to Naïve controls, but this effect did not reach statistical significance[9]. These results suggest that the effect of adolescent TBI on the rewarding efficacy of cocaine may present in a sex-specific manner, which has implications for individual treatment strategies in patients with a history of early-life TBI.
3.1.2. The Role of Sex Hormones in Cocaine Reward Following Adolescent CCI-TBI
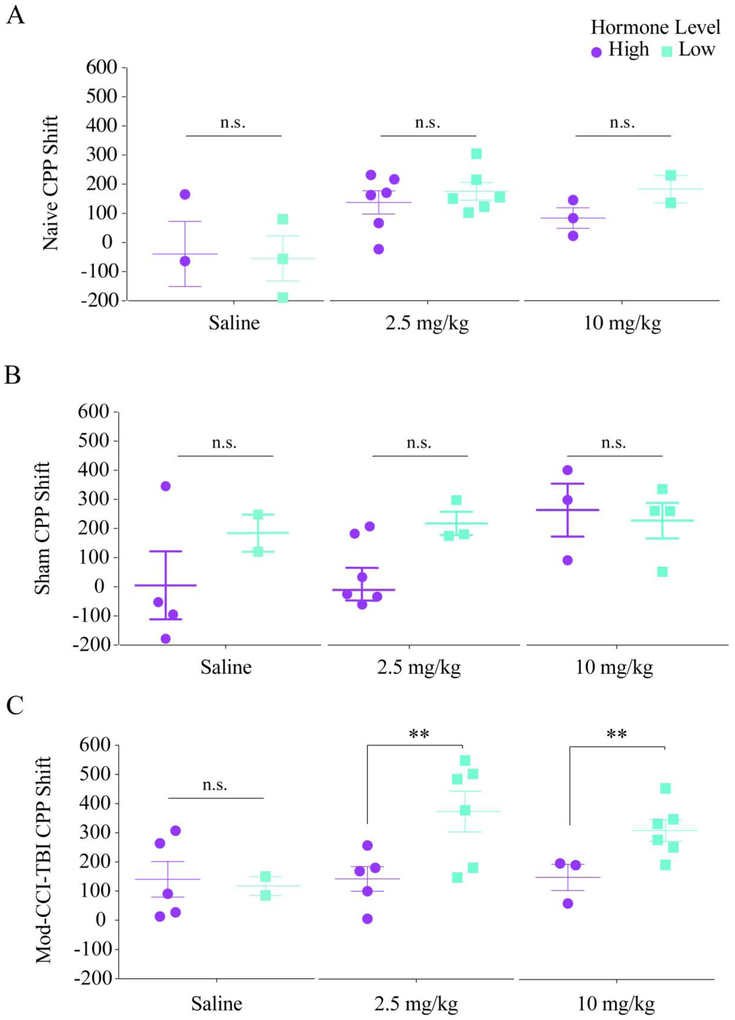
Since high levels of female hormones, particularly 17β-estradiol (estrogen in humans) and progesterone which peak during the estrus phase and proestrus phase of the estrus cycle, respectively, has been shown to be neuroprotective after TBI, we next wanted to determine the influence of sex hormones on the increased sensitivity to rewarding effects of cocaine seen in adolescent males [31-33]. Vaginal cytology samples were collected for a minimum of five days prior to and on the day of CCI-TBI surgery to determine phase of estrous cycle at the time of impact (Supplemental Fig. 1 A-D). As can be expected in freely-cycling mice, post-hoc analyses of vaginal cytology samples from Mod-CCI-TBI mice revealed unequal and low numbers of mice in each estrus phase per condition (e.g. for 10 mg/kg, total of 1 mouse in estrus, 2 mice in proestrus, 2 mice in metestrus, and 3 mice in diestrus) on the day of injury (Supplemental Table 1). Since these numbers are underpowered for appropriate statistical comparisons, the phases were collapsed into relatively high hormone levels: High (mice in either estrus or proestrus on day of TBI), and relatively low hormone levels: Low (mice in either metestrus or diestrus on day of TBI) (Supplemental Table 2). Due to the importance of maintaining the age of injury fixed, and goal of abiding by institutional guidelines to reduce the number of animals used in each experiment, vaginal cytology was demonstrated as an impractical assay and was not included in Study 2 or 3. When CPP scores were stratified by High or Low hormone levels, the effect of Mod-CCI-TBI on CPP shift appears equivalent to what was previously seen in adolescent injured males[8, 9]. Specifically, when stratified by High (purple) or low (teal) we found that behavioral CPP shifts of either Naïve (Fig 2A) or Sham (Fig 2B) adolescent females were not affected by levels of circulating hormones after being conditioned to saline, 2.5 mg/kg, or 10 mg/kg cocaine (Naïve: two-way ANOVA with Tukey’s post-hoc tests; cocaine dose effect: F (2, 17) = 7.47, p = 0.0047; effect of Hormone level: F (1, 17) = 0.6893, p = 0.4179; Sham: two-way ANOVA with Tukey’s post-hoc tests; cocaine dose effect: F (2, 18) = 3.086, p = 0.0704; effect of Hormone level: F (1, 18) = 3.566, p = 0.0752). However, females exposed to Mod-CCI-TBI with relatively low levels of circulating estrogen and progesterone demonstrated significantly higher CPP shifts in response to doses of 2.5mg/kg and 10 mg/kg cocaine, similar to what was observed in adolescent males[9] (Fig. 2C) (two-way ANOVA with Tukey’s post-hoc tests; effect of Hormone Level: F (1, 21) = 5.808, p = 0.0252; Cohen’s d coefficient: 2.5mg/kg d = 1.66, 10 mg/kg d = 1.90). In contrast, females with relatively high levels of circulating 17β-estradiol and progesterone, hormones not produced in male mice, had significantly lower CPP shifts in response to these doses of cocaine (Fig. 2C). These results suggest a neuroprotective effect of high levels of female hormones at the time of injury against increased sensitivity to the rewarding effects of cocaine after Mod-CCI-TBI.
Figure 2. High levels of female hormones at the time of adolescent TBI may protect against increased cocaine rewarding efficacy.
Individual female CPP responses stratified by high or low hormone levels determined by estrous phase indicates fluctuating levels of female hormones on the day of TBI may be protective against increased cocaine CPP shifts. Data presented is CPP shift shown for saline, 2.5, and 10 mg/kg cocaine. No significant differences were observed in CPP responses of Naïve (A) or Sham (B) control female mice when stratified by high or low levels of female hormones. CPP shifts for 2.5 and 10 mg/kg are significantly higher in female mice exposed to Mod-CCI-TBI with relatively low (teal) fluctuating female hormones on the day of injury. Lower CPP shifts in Mod-CCI-TBI female mice with high (purple) levels of hormones on day of TBI suggests protection against increased efficacy of cocaine after TBI (C). These results suggest TBI during adolescence may enhance the abuse liability of cocaine in adulthood and the threshold for the rewarding effects of cocaine could be lower in TBI patients in a sex specific manner. Two-way ANOVA, *= p<0.05, **= p<0.01.
3.2. Results for Study 2
3.2.1. Sex Differences in TBI-Induced Neuroinflammation
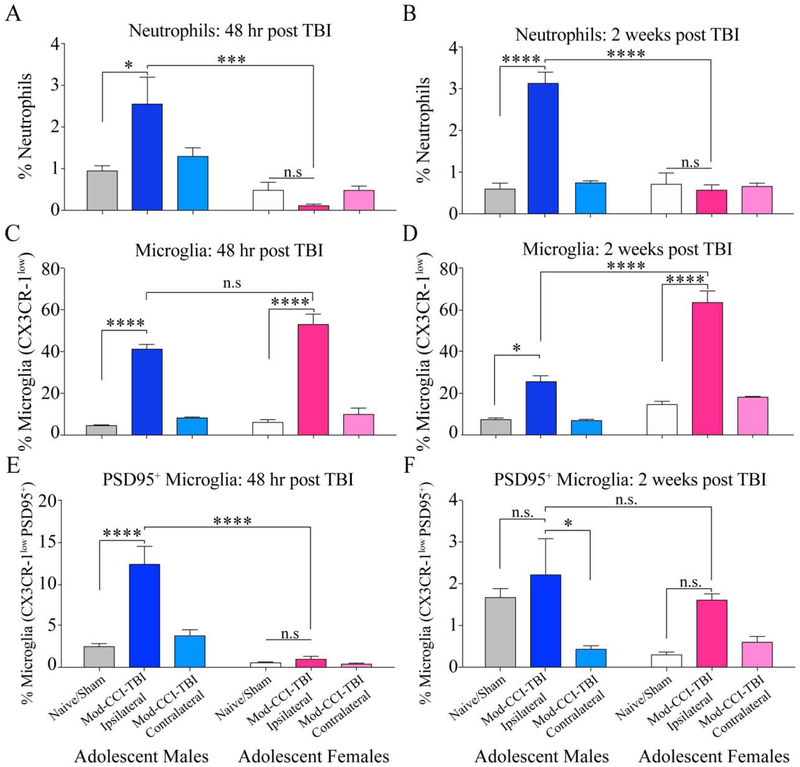
Knowing that TBI induces a persistent and inflammatory response within the reward pathway and that sex differences have been established in the neuroinflammatory response following TBI at acute time points, we utilized flow cytometry to characterize the infiltration of immune cells and phagocytosis status of microglia at two weeks following Mod-CCI-TBI[7, 20]. Using Two-way ANOVA, we report an acute Interaction effect p=0.0084 (F (2, 18) = 6.312), and Effect of Sex p<0.001 (F (1, 18) = 26.15) (Fig 3A.). At 48 hours post injury, Tukeys post-hoc analysis revealed a significant increase of infiltrating neutrophils in the ipsilateral cortex following Mod-CCI-TBI versus Naïve/Sham in males (p< 0.05) and compared to Mod-CCI-TBI in females (p<0.001) but no differences in Naïve/Sham vs Mod-CCI-TBI in females (p>0.05). In both sexes, we report no significant infiltration in the contralateral hemisphere. Figure 3 B demonstrates using Two-way ANOVA, a chronic Interaction effect p<0.001 (F (2, 18) = 34.51). Tukey’s post-hoc test also showed a persistently significant increase in infiltrating neutrophils in the ipsilateral cortex two weeks after Mod-CCI-TBI compared to Naïve/Sham in males (p<0.0001) and compared to Mod-CCI-TBI in females (p<0.0001) but no effect of Mod-CCI-TBI in females compared to Naïve/Sham females (p>0.05). Additionally, the percentage of Neutrophils at 48 hrs and 2-weeks were not statically different in males (supplemental figure 2).
Figure 3. Sex differences in TBI induced immune response in the ipsilateral hemisphere at acute and chronic time points post adolescent injury.
(A) The percentage of infiltrating neutrophils identified as CD11b+, CD45+ and Ly6G+ at 48 hours post adolescent CCI-TBI. Mod-CCI-TBI resulted in a higher percentage of infiltrated neutrophils only in the ipsilateral cortex in males compared to Naïve/Sham and Mod-CCI-TBI in females. (B) Increased neutrophil infiltration persisted in the ipsilateral cortex two weeks post TBI in males. (C) Microglia were identified as CD11b+ CD45+ Ly6G− Ly6C+ and CX3CR-1low. We report a significant increase in microglia in the impacted hemisphere at 48 hrs post injury with no difference between injured males or females. (D) This effect persisted and was more pronounced in females 2 weeks post-TBI. (E) Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia in males compared to Naïve/Sham or injured females (p<0.00001). (F) At two-weeks post Mod-CCI-TBI the sex differences in PSD95 positive microglia appears to resolve, however there is a persistently higher percentage of these cells in the ipsilateral cortex of TBI males compared to the contralateral cortex. (two-way ANOVA, * = p<0.05, ***= p<0.001, **** = p<0.0001) N=4-6 per condition.
With regard to the percentage of microglia gated as CX3CR-1low expression at 48 hrs post Mod-CCI-TBI, Figure 3C demonstrates significant effect of injury severity (F (2, 17) = 145.3 p<0.0001) and of sex (F (1, 17) = 5.021 p=0.0387) using two-way ANOVA. Multiple comparisons corrected by Tukey’s post-hoc test reveal a significant increase in CX3CR-1low expressing microglia in the ipsilateral cortex 48 hrs after Mod-CCI-TBI compared to Naïve/Sham in adolescent males (p<0.0001) and after Mod-CCI-TBI compared to Naïve/Sham in adolescent females (p<0.0001). However, we report no significant difference between injured males compared to females at this acute time point (p>0.05). Similarly, there is no significant difference in CX3CR-1low expressing microglia in the contralateral hemisphere compared to Naïve/Sham in either sex (p>0.05). Chronically, we report a significant interaction effect of TBI severity and sex on CX3CR-1low microglial analyzed by two-way ANOVA (F (2, 18) = 19.76, p<0.0001). Tukey’s multiple comparisons test further demonstrate a chronically significant increase in microglia in the ipsilateral cortex two weeks following Mod-CCI-TBI compared to Naïve/Sham in males (p<0.01). Unexpectedly, the ipsilateral cortex of females exposed to Mod-CCI-TBI showed persistent microglia to a higher degree than males (p<0.0001) and Naïve/Sham females (p<0.0001) (Fig. 3D). Comparisons as a function of time showed that the percentage of CX3CR1low microglia was statistically reduced between 48hrs and 2-weeks for males (p<0.001) but not females (supplemental figure 2). As with neutrophils, we found no difference in CX3CR-1low microglia in the contralateral hemisphere compared to Naïve/Sham in either sex at either time point (p>0.05).
Microglia play an important role in the remodeling of synapses and PSD-95 has been found to be localized inside microglia by confocal, superresolution and electron microscopy [34]. Consequently, the detection of PSD-95 positive microglia represent a snapshot of microglia phagocytosis and remodeling of dendritic spines. Thus, in order to determine the impact of sex on not only the activation status but also the phagocytosis status of microglia and following Mod-CCI-TBI in males and females, CX3CR-1low microglia from injured (ipsilateral) and non-injured (contralateral) hemispheres were also gated on expression of the non-endogenous neuronal synaptic protein, PSD95. As shown in Figure 3E, two-way ANOVA indicated a significant interaction effect of TBI severity and sex (F (2, 18) = 14.08, p=0.0002). Post-hoc analyses using Tukey’s multiple comparisons test revealed a significant increase in microglia positive for the expression of PSD95 in the ipsilateral cortex 48 hrs following Mod-CCI-TBI in males compared to Naïve/Sham (p<0.0001) and compared to Mod-CCI-TBI adolescent females (p<0.0001). We found no difference in co-expression of CX3CR-1low and PSD95 in the ipsilateral cortex of Mod-CCI-TBI females compared to Naïve/Sham females at 48 hrs after injury (p>0.05). Similarly, there was no difference in the contralateral hemisphere compared to Naïve/Sham in either sex (p>0.05). At two weeks-post Mod-CCI-TBI, we only report a significant effect of TBI severity on CX3CR-1low PSD98+ microglia using two-way ANOVA (F (2, 17) = 6.833, p=0.0066). Chronically, the effect of increased PSD95 positive microglia seems to resolve as we no longer report significantly different between Naïve/Sham and Mod-CCI-TBI for males or females (Fig. 3F). Additionally, PSD95 positive microglia was statistically different between 48hrs and 2 weeks for both males and females (supplemental figure 2). However, we report a significantly higher percentage of PSD95 positive microglia in the ipsilateral cortex compared to the contralateral cortex two weeks after Mod-CCI-TBI in males (p<0.05), an effect not observed in females.
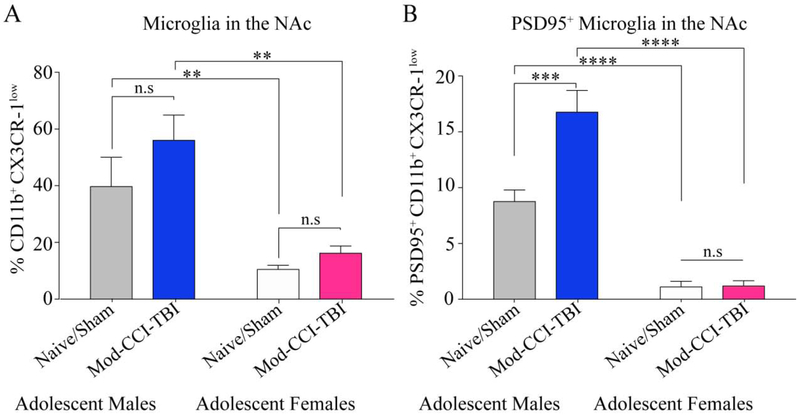
Considering the differences in behavioral responses to the rewarding effects of cocaine, we next wanted to determine the influence of sex on microglial interactions with neuronal complexity within reward regions, particularly the NAc. Figure 4A demonstrates a significant effect of sex on the percentage of CX3CR-1low microglia in the NAc two weeks post Mod-CCI-TBI as determined by two-way ANOVA (F (1, 14) = 3.624, p<0.0001). Tukey’s multiple comparisons test did not demonstrate and increase in microglia in the NAc following Mod-CCI-TBI but did reveal a significant effect of sex in that we report a higher percentage of CX3CR-1low microglia in male Naïve/Sham mice than female Naïve/Sham mice (p<0.01). Similarly, post-hoc analyses determined that Mod-CCI-TBI males displayed a significantly higher percentage of microglia in the NAc than Mod-CCI-TBI females (p<0.01). However, with regard to PSD95+ microglia in the NAc two weeks post Mod-CCI-TBI, two-way ANOVA revealed a significant interaction effect of TBI severity and sex (F (1, 15) = 18.80, p=0.0006) (Fig. 4B). Using Tukey’s multiple comparisons test, we found a significant increase in PSD95+ microglia in the NAc in Mod-CCI-TBI males compared to Naïve/Sham males (p<0.001). We again report an inherent sex difference demonstrated by an increase in PSD95 microglia in Naïive/Sham males compared to Naïive/Sham females (p<0.0001) as well as Mod-CCI-TBI males compared to Mod-CCI-TBI females (p<0.0001). We found no significant difference in PSD95 positive microglia in the NAc two weeks following Mod-CCI-TBI compared to Naïive/Sham females (p<0.05). It is important to note that a limitation of Study 2 is that although these findings were significant with low N, these data were underpowered to sort by estrous phase.
Figure 4. Adolescent Mod-CCI-TBI does not increase microglial phagocytosis of neuronal proteins in the nucleus accumbens in females.
Two-weeks following adolescent Mod-CCI-TBI in males and females, the NAc was excised and processed for CX3CR-1low PSD95+ microglia. (A) Mod-CCI-TBI did not increase CX3CR-1low microglia compared to Naïve/Sham in either males or females. However, we report an inherent sex difference with a higher percentage of CX3CR-1low microglia in males compared to females in both conditions. (B) Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia only in the NAc of males compared to Naïve/Shams. Inherent sex differences were also apparent in PSD95+ microglia for both conditions. (two-way ANOVA, **= p<0.01, ***=p<0.001, ****=p<0.0001). N=4-6 per condition.
3.3. Results for Study 3
3.3.1. Sex Differences in Severity of TBI Pathology
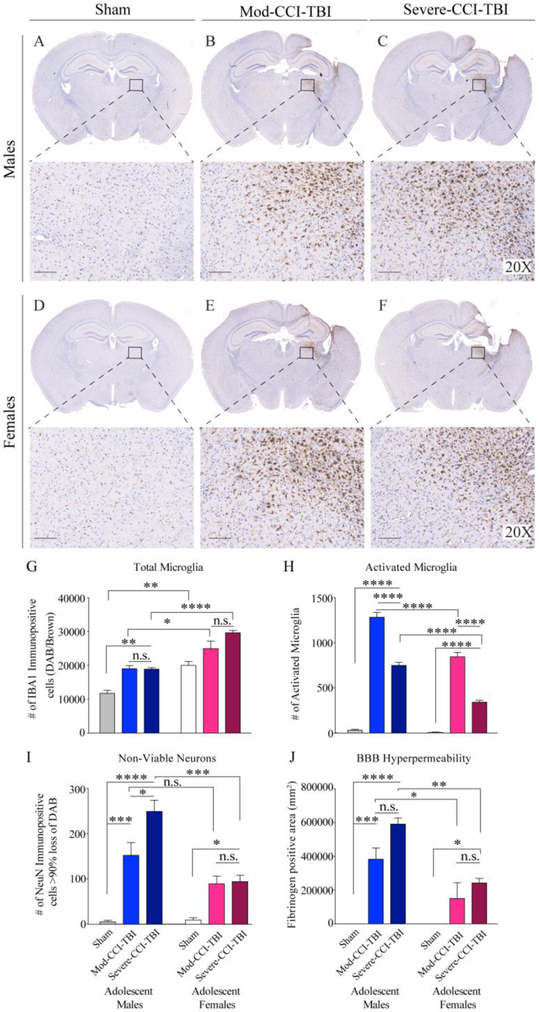
Although our behavioral data initially did not reveal TBI-mediated increases in rewarding efficacy of cocaine in adolescent females, the doses of 2.5 and 10 mg/kg Mod-CCI-TBI female mice were approaching higher CPP responses at trend levels. Notably, this is equivalent to what we observed in adolescent male mice exposed to Mild-CCI-TBI[8, 9]. Changes in microglial morphology, neuronal loss, and permeability of the BBB are all hallmark pathological consequences that reflect severity of injury to the brain[10, 35]. Therefore, we next wanted to investigate whether induction of CCI-TBI using identical speed, compression depth, and dwell time parameters, actually produced sexually-divergent neuropathology severity as a consequence of the injury. In other words, does Mod-CCI-TBI in females, known to demonstrate neuroprotection against TBI-induced inflammation, better represent the Mild-CCI-TBI pathology seen in males, and thus would Severe-CCI-TBI in females more closely correspond to the Mod-CCI-TBI pathology of males? Histological analysis of increased Iba1 expression as a consequence of severity of injury in the ipsilateral hemisphere at the chronic, two-week timepoint (Fig. 5 A-F) demonstrates a significant effect of both TBI severity (two-way ANOVA F: (2, 18) = 25.57 p<0.0001) and sex (two-way ANOVA F: (1, 18) = 70.67 p<0.0001) for total number of microglia (Fig. 5G). Tukey’s multiple comparisons test revealed no difference in Mod-CCI-TBI versus Severe-CCI-TBI in total number of Iba1 positive microglia in either males or females. Generally, our findings indicate an inherent sex difference as females display significantly higher number of microglia in Naïve/Sham (p<0.01), Mod-CCI-TBI (p<0.05) and Severe-CCI-TBI (p<0.0001). Analysis of activated microglia revealed the opposite effect. Figure 5H shows a significant interaction effect of TBI severity and sex (two-way ANOVA F: (2, 18) = 23.55, p<0.0001). Tukey’s multiple comparisons test revealed a significant increase in activated microglia in both severities of TBI for both males and females (p<0.0001). However, for both severities, females had significantly less activated microglia than males (p<0.0001). Notably, quantification of histological detection of NeuN, identifying non-viable neurons following increased severity of injury (data not shown) also revealed a significant interaction effect of TBI severity and sex (two-way ANOVA F (2, 18) = 9.915, p=0.0012) (Fig 5I). Using Tukey’s multiple comparisons test we report a more pronounced step-wise increase in non-viable neurons following Mod-CCI-TBI (p<0.001) and Severe-CCI-TBI (p<0.0001) versus naþve in males compared to female injured versus naþve (p<0.05). Our findings revealed only a significant sex difference in number of non-viable neurons following Severe-CCI-TBI with no differences in non-viable neurons between males and females exposed to Mod-CCI-TBI. Similarly, quantification of Fibrinogen immunopositive tissue area, identifying permeability of the blood-brain barrier (BBB) following increased severity of injury (Figure 5J and Supplemental Figure 3) also revealed a significant interaction effect of TBI severity and sex (two-way ANOVA F (2, 18) = 6.321, p=0.0083) (Fig 5J). Using Tukey’s multiple comparisons test we report greater BBB hyperpermeability following either TBI condition compared to Naïve in males (Mod-CCI-TBI: p<0.001, Severe CCI-TBI: p<0.0001) but only after Severe CCI-TBI in females (p<0.05). We again found no difference between Severe CCI-TBI compared to Mod-CCI-TBI in either sex suggesting our parameters did not induce significantly different pathological immune responses (p>0.05). Interestingly, we report a significant sex difference for each severity of injury in BBB permeability where males exposed to either Mod-CCI-TBI or Severe CCI-TBI demonstrated greater loss of BBB integrity than females (p<0.05 and p<0.01, respectively). It is important to note that a limitation of Study 3 is that although these findings were significant with low N, these data were underpowered to sort by estrous phase.
Figure 5. Chronic sex differences in adolescent CCI-TBI-induced severity of neuropathology in the ipsilateral cortex.
Representative images and 20x magnified areas of immunohistochemical detection of Iba1 in the ipsilateral cortex in males (A-C) and females (D-F) two-weeks following Naïve/Sham, Mod-CCI-TBI, or Severe-CCI-TBI. Microglia are finely ramified with small cell bodies in the Naïve/Sham condition for both sexes. (G) Associated bar graph quantifying that following CCI-TBI, the total number of microglia is significantly higher in all female conditions compared to males. (H) Significantly less activated microglia following either severity of CCI-TBI were detected in females compared to males. (I) Associated bar graph display imaging quantification of immunohistochemical detection of NeuN identifying non-viable neurons in the ipsilateral cortex two-weeks post injury (not shown). Sex-specific differences reveal increased loss viable neurons following Mod- and Severe-CCI-TBI. (H) Associated bar graph display imaging quantification of immunohistochemical detection of Fibrinogen staining identifying blood-brain barrier (BBB) permeability in the ipsilateral cortex two-weeks post injury (not shown). Sex-specific differences reveal increased BBB hyperpermeability following either severity of CCI-TBI, but no differences between Moderate versus Severe for either sex. Data are presented as mean ±SEM. (two-way ANOVA, *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001) N=4 per condition.
4. Discussion
Sex as a biological factor is an important consideration in the context of TBI-induced SUD vulnerability particularly in light of observations that suggests that females demonstrate neuroprotection in the context of brain injury, but increased propensity in the context of psychostimulant addiction. Indeed, the initial behavioral outcomes of our CPP assay following TBI in adolescent female mice indicated corroboration of protection against negative outcomes of TBI when compared to males. However when analyzed as a function of hormonal levels our studies are in agreement with observations reported by Weil and colleagues who found increased substance consumption (alcohol) following TBI in female mice [36]. To date the investigations reported here and those by Weil and colleagues are the only two investigations into sex difference in SUD following TBI, specific behavioral outcome differences are likely attributed to mouse age differences and the category of substance of abuse used in the studies.
In order to determine the influence of estrous cycle on increased sensitivity to the rewarding effects of cocaine after TBI, post-hoc analyses based on vaginal cytology recorded on the day of injury were performed to stratify behavioral CPP data by relative level of fluctuating female hormones. Interestingly, we found that females with relatively low levels of hormones such as progesterone or estrogen, which are not considerable in males, demonstrated significantly higher CPP shifts at the same doses we observed to be augmented in adolescent male TBI mice[8, 9]. The resulting decreased CPP shifts when female mice had high levels of sex hormones on the day of injury replicates the established concept of neuroprotection in females in preclinical TBI research [31-33]. However, our findings from this study are limited by the inability to distinguish between the effects of specific female hormones. Succeeding studies will employ ovariectomized mice treated with exogenous hormones to better understand the role of specific sex hormones in SUD following exposure to TBI during adolescence.
With regard to the TBI-induced neuroinflammatory response in adolescent females compared to males, our finding of a sex-specific increase in the infiltration of neutrophils acutely at 48 hours after injury that persisted for at least two weeks exclusively in males supports the established preclinical notion that females demonstrate protection against inflammatory responses after impact. These results are also consistent with those of Semple and colleagues who reported decreased neuroinflammation, cell death, and rescued behavioral phenotypes after juvenile CCI when neutrophils are inhibited [37]. Neutrophils, which are inflammatory cells known to be one of the first-responders in the context of inflammation and specifically in TBI, are typically short-lived and resolve within hours or a few days after injury. Interestingly, our results suggest a different rate of clearance of infiltrating neutrophils in male mice compared to females, showing that neutrophils remain significantly increased after two weeks post injury.
Soon after neutrophils are recruited to sites of injury, microglia become activated [38]. A known functional role of activated microglia is to target and phagocytose infiltrating neutrophils as part of the process of resolution of inflammation[39]. Our analysis of microglia activation by TBI in the impacted ipsilateral cortex did not reveal acute sex-specific effects. Recently, Doran and colleagues also used flow cytometry to characterize potential sex differences in morphological and functional features of microglia after TBI, although in a model of CCI-TBI in adult mice. The authors reported that in both males and females, TBI resulted in an increase in the percentage of microglia and decreased phagocytic activity in female microglia in the ipsilateral hemisphere at acute time points, which is consistent with our results [40]. However, at our chronic time point, two-weeks post injury, our findings demonstrated an increased percentage of microglia that have downregulated CX3CR-1 (a subpopulation of protracted hyperactive microglia) in females compared to males after injury. At this chronic time point there was no sex-specific difference in phagocytic microglia. A result that is in line with the results from the Villapol laboratory showing that neuroinflammation becomes indistinguishable between males and females at chronic time points after TBI [7]. Alternatively, the increase in percentage of microglia in the ipsilateral cortex of injured females at chronic time points could potentially be accounted for by inherent sex differences in microglia colonization patterns during development compounded by TBI. specifically, Schwarz and colleagues demonstrated that while no differences in number or morphology exist in microglia during development at embryonic day 17 or post-natal day 4 of rat male and female pups, female rats show increased total numbers, particularly of non-ameboid microglia with thick processes at adolescent and adult ages at post-natal days 30 and 60 [41].
As part of our indices of neuropathology, we investigated possible changes to BBB permeability in relationship to sex differences. As previously established, a persisting loss of BBB integrity can lead to chronic inflammation which could contribute to a greater risk for behavioral complications after the initial injury [10, 42]. As such, our analysis with fibrinogen demonstrated acute sex-specific neuroprotection to BBB compromise. The above is corroborated by Umeano and colleagues in which following a closed-head injury model, the effect of BBB protection in females was diminished with the absence of sex hormones as a result of ovariectomy [43]. Together with our behavioral data, this finding implies that it is not necessarily the injury parameters that are responsible for inducing sex-specific effects of TBI-induced neuroinflammation, but rather the sex-specific biological response to injury that can account for differences observed. In other words, a moderate injury in females does not produce an immune response that more closely represents what ensues in a mild injury in males, but rather that biological sex can perhaps discriminate the manner by which the insult is resolved. Notably, our neuropathological findings did not account for estrous phase at the time of injury, which is a limitation of the current study and must be considered when interpreting these results. However, previous preclinical models of TBI have also reported significant neuroprotection in females compared to males without examining the impact of sex hormones at the time of injury. For example, Villapol et al. performed a comprehensive immunohistochemical analysis over a time course up to 30 days post CCI-TBI in adult male and randomly cycling female mice. They demonstrated males displayed more severe pathology at acute time points post injury but were comparable to females at chronic time points, irrespective of estrous phase in females [7]. Similar to our findings, investigating sex differences in motor and cognitive performance following increased severity of CCI-TBI in mice, Tucker and colleagues reported no difference in lesion volume following mild or severe injury, but sex-specific effects in a battery of behaviors irrespective of estrous phase [44]. It is important to point out that differences in lesion volumes (as a result of TBI) between the sexes could be resolved, depending on how these measures are performed[45]. Taken together, here we provide (for the first time) evidence that sex differences during the chronic phase of injury displays severity-dependent neuropathological outcomes in the context of adolescent injury where females had less activated microglia and less pronounced neuronal loss.
Following isolation and analysis of microglia in the NAc, an essential nucleus of reward-motivated behaviors, we again found intrinsic sex differences in the number of phagocytic microglia at chronic time-points after brain injury, which are both substantially reduced in females compared to males. Additional studies are needed to assess the morphology and functional proxy of neurons in the NAc of females as a possible consequence of microglial phagocytosis. However, as our behavioral data suggests neuroprotection against increased sensitivity to the rewarding effects of cocaine when mice had relatively high levels of female hormones at the time of injury, it would be expected that the presence of female hormones would attenuate the decreased spine density in the reward circuitry of females as well. Admittedly, future studies that incorporate ovariectomy procedures and include the administration of exogenous estrogen replace will help to discern whether the effect on spine desnity is due to female hormones acting directly on neurons to confer neuroprotection [46] or via microglial phagocytosis or both. Lastly, the analysis show in this report open the door to future experiments aimed at understanding the mechanisms through which the immune system interacts with the endocrine system and CNS to shape normal and aberrant inflammatory responses during the developmental period of adolescence.
Supplementary Material
Supplemental Figure 1. Representative images of vaginal cytology samples. Morphology of (A) nucleated epithelial cells present in proestrus, (B) cornified epithelial cells present in estrus, (C) cornified epithelial cells and polymorphonuclear leukocytes present in metestrus, and (D) primarily polymorphonuclear leukocytes and a few epithelial cells present in diestrus when female mice are in each respective phase of the estrous cycle. When stages are collapsed, proestrus and estrus are referred to as High (purple) and metestrus and diestrus are considered as Low (teal).
Supplemental Figure 2. TBI induced immune response comparison of acute and chronic time points post adolescent injury. (A) The percentage of infiltrating neutrophils identified as CD11b+, CD45+ and Ly6G+ at 48 hours post adolescent CCI-TBI. Mod-CCI-TBI resulted in a higher percentage of infiltrated neutrophils only in the ipsilateral cortex in males compared to Naïve/Sham at both acute and chronic timepoints. (B) Females did not have a significant neutrophil infiltration at either the acute or chronic timepoint (C) Microglia were identified as CD11b+ CD45+ Ly6G− Ly6C+ and CX3CR-1low. We report a significant increase in microglia in the impacted hemisphere at 48 hrs post injury that was statistically reduced but still elevated at 2 weeks post-injury in males. (D) This effect was also seen in females but persisted 2 weeks post-TBI. (E) Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia in males compared to Naïve/Sham at 48 hrs but had resolved by 2 weeks post injury (F). In contrast, females did not demonstrate elevated CX3CR-1low PSD95+ microglia at 48 hrs but had a small but statistically significant increase at two-weeks post injury (two-way ANOVA, * = p<0.05, ***= p<0.001, **** = p<0.0001) N=4-6 per condition.
Supplemental Figure 3. Immunohistochemistry of fibrinogen in brain parenchyma. Representative images of immunohistochemical detection of fibrinogen in the ipsilateral cortex in males (A,C,E) and females (B,D and F) two-weeks following Naïve/Sham, Mod-CCI-TBI, or Severe-CCI-TBI. Fibrinogen is a bloodborne clotting protein that is restricted to the vasculature and does not cross the BBB. Loss of BBB integrity is shown by positive immunolabeling for fibrinogen using 3,3′-diaminobenzidine or DAB (brown). Fibrinogen detection can observed in the perivascular space and throughout the brain parenchyma of TBI animals. Females with TBI appear to show less fibrinogen “leakiness” into the CNS than males. Scalebar = 1mm.
HIGHLIGHTS.
Cocaine sensitivity increases after traumatic brain injury (TBI) in male mice
High levels of female hormones protect against cocaine sensitivity after TBI
Females display decreased immune cell infiltration and microglial activation after TBI
Neuropathological outcomes after TBI are sex- and severity-dependent
Acknowledgements
This work was supported by National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) P30 DA013429-16 (SHR, SMR), R01DA046833 01 (SHR), T32 DA007237 (LAC), NIH/National Institute of Neurological Disorders and Stroke (NINDS), R01 NS086570-01 (SHR), and the PA-CURE program (Pennsylvania Department of Health).
Footnotes
Author conflict of interest statement: The authors of this manuscript have no competing financial interests or any other conflict of interest to declare.
Author disclosure statement: No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Covassin T, Swanik CB, Sachs ML, Sex Differences and the Incidence of Concussions Among Collegiate Athletes, J Athl Train 38(3) (2003) 238–244. [PMC free article] [PubMed] [Google Scholar]
- [2].Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS, Predictors of clinical recovery from concussion: a systematic review, Br J Sports Med 51(12) (2017) 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Semrud-Clikeman M, Klipfel KM, TBI and Concussions in Student Athletes: How do Severity of Injury, Age, and Gender Influence Recovery, Journal of Pediatric Neuropsychology 2(1-2) (2016) 58–69. [Google Scholar]
- [4].Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD, Concussions among United States high school and collegiate athletes, J Athl Train 42(4) (2007) 495–503. [PMC free article] [PubMed] [Google Scholar]
- [5].Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT, Sex differences in outcome following sports-related concussion, J Neurosurg 102(5) (2005) 856–63. [DOI] [PubMed] [Google Scholar]
- [6].Ono KE, Burns TG, Bearden DJ, McManus SM, King H, Reisner A, Sex-Based Differences as a Predictor of Recovery Trajectories in Young Athletes After a Sports-Related Concussion, Am J Sports Med 44(3) (2016) 748–52. [DOI] [PubMed] [Google Scholar]
- [7].Villapol S, Loane DJ, Burns MP, Sexual dimorphism in the inflammatory response to traumatic brain injury, Glia 65(9) (2017) 1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Merkel SF, Razmpour R, Lutton EM, Tallarida CS, Heldt NA, Cannella LA, Persidsky Y, Rawls SM, Ramirez SH, Adolescent Traumatic Brain Injury Induces Chronic Mesolimbic Neuroinflammation with Concurrent Enhancement in the Rewarding Effects of Cocaine in Mice during Adulthood, J Neurotrauma (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cannella LA, Andrews AM, Tran F, Razmpour R, McGary H, Collie C, Tsegaye T, Maynard M, Kaufman MJ, Rawls SM, Ramirez SH, Experimental Traumatic Brain Injury during Adolescence Enhances Cocaine Rewarding Efficacy and Dysregulates Dopamine and Neuroimmune Systems in Brain Reward Substrates, Journal of Neurotrauma In press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cannella LA, McGary H, Ramirez SH, Brain interrupted: Early life traumatic brain injury and addiction vulnerability, Exp Neurol 317 (2019) 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE, Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus, Psychopharmacology (Berl) 182(2) (2005) 245–52. [DOI] [PubMed] [Google Scholar]
- [12].Anker JJ, Gliddon LA, Carroll ME, Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake, Behav Pharmacol 19(5-6) (2008) 615–29. [DOI] [PubMed] [Google Scholar]
- [13].Lynch WJ, Taylor JR, Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure, Neuropsychopharmacology 29(5) (2004) 943–51. [DOI] [PubMed] [Google Scholar]
- [14].Lynch WJ, Taylor JR, Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones, Neuropsychopharmacology 30(5) (2005) 927–35. [DOI] [PubMed] [Google Scholar]
- [15].Doncheck EM, Urbanik LA, DeBaker MC, Barron LM, Liddiard GT, Tuscher JJ, Frick KM, Hillard CJ, Mantsch JR, 17beta-Estradiol Potentiates the Reinstatement of Cocaine Seeking in Female Rats: Role of the Prelimbic Prefrontal Cortex and Cannabinoid Type-1 Receptors, Neuropsychopharmacology 43(4) (2018) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen K, Kandel D, Relationship between extent of cocaine use and dependence among adolescents and adults in the United States, Drug Alcohol Depend 68(1) (2002) 65–85. [DOI] [PubMed] [Google Scholar]
- [17].Ahmed S, Venigalla H, Mekala HM, Dar S, Hassan M, Ayub S, Traumatic Brain Injury and Neuropsychiatric Complications, Indian J Psychol Med 39(2) (2017) 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Diaz AP, Schwarzbold ML, Thais ME, Hohl A, Bertotti MM, Schmoeller R, Nunes JC, Prediger R, Linhares MN, Guarnieri R, Walz R, Psychiatric disorders and health-related quality of life after severe traumatic brain injury: a prospective study, J Neurotrauma 29(6) (2012) 1029–37. [DOI] [PubMed] [Google Scholar]
- [19].Beaulieu-Bonneau S, St-Onge F, Blackburn MC, Banville A, Paradis-Giroux AA, Ouellet MC, Alcohol and Drug Use Before and During the First Year After Traumatic Brain Injury, J Head Trauma Rehabil 33(3) (2018) E51–E60. [DOI] [PubMed] [Google Scholar]
- [20].Merkel SF, Andrews AM, Lutton EM, Razmpour R, Cannella LA, Ramirez SH, Dexamethasone Attenuates the Enhanced Rewarding Effects of Cocaine Following Experimental Traumatic Brain Injury, Cell Transplantation epub ahead of print (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].dela Cruz AM, Herin DV, Grady JJ, Cunningham KA, Novel approach to data analysis in cocaine-conditioned place preference, Behav Pharmacol 20(8) (2009) 720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spyraki C, Fibiger HC, Phillips AG, Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions, Brain Res 253(1–2) (1982) 195–203. [DOI] [PubMed] [Google Scholar]
- [23].Byers SL, Wiles MV, Dunn SL, Taft RA, Mouse estrous cycle identification tool and images, PLoS One 7(4) (2012) e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goldman JM, Murr AS, Cooper RL, The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies, Birth Defects Res B Dev Reprod Toxicol 80(2) (2007) 84–97. [DOI] [PubMed] [Google Scholar]
- [25].Cora MC, Kooistra L, Travlos G, Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears, Toxicol Pathol 43(6) (2015) 776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McLean AC, Valenzuela N, Fai S, Bennett SA, Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification, J Vis Exp (67) (2012) e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].LaFrance-Corey RG, Howe CL, Isolation of brain-infiltrating leukocytes, J Vis Exp (52) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De Groef L, Characterizing microglia activation: a spatial statistics approach to maximize information extraction, Sci Rep 7(1) (2017) 1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schenk S, Horger BA, Peltier R, Shelton K, Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats, Brain Res 543(2) (1991) 227–35. [DOI] [PubMed] [Google Scholar]
- [30].Zakharova E, Wade D, Izenwasser S, Sensitivity to cocaine conditioned reward depends on sex and age, Pharmacol Biochem Behav 92(1) (2009) 131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM, Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury, Brain Res 628(1-2) (1993) 271–8. [DOI] [PubMed] [Google Scholar]
- [32].O'Connor CA, Cernak I, Vink R, Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats, Brain Res 1062(1-2) (2005) 171–4. [DOI] [PubMed] [Google Scholar]
- [33].Roof RL, Duvdevani R, Stein DG, Gender influences outcome of brain injury: progesterone plays a protective role, Brain Res 607(1-2) (1993) 333–6. [DOI] [PubMed] [Google Scholar]
- [34].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, Synaptic pruning by microglia is necessary for normal brain development, Science 333(6048) (2011) 1456–8. [DOI] [PubMed] [Google Scholar]
- [35].Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV, Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities, Neuropsychiatr Dis Treat 11 (2015) 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weil ZM, Karelina K, Gaier KR, Corrigan TE, Corrigan JD, Juvenile Traumatic Brain Injury Increases Alcohol Consumption and Reward in Female Mice, J Neurotrauma 33(9) (2016) 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Semple BD, Trivedi A, Gimlin K, Noble-Haeusslein LJ, Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain, Neurobiol Dis 74 (2015) 263–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loane DJ, Byrnes KR, Role of microglia in neurotrauma, Neurotherapeutics 7(4) (2010) 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG, Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege, J Neurosci 28(23) (2008) 5965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ, Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells, J Neurotrauma 36(7) (2019) 1040–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schwarz JM, Sholar PW, Bilbo SD, Sex differences in microglial colonization of the developing rat brain, J Neurochem 120(6) (2012) 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ichkova A, Rodriguez-Grande B, Bar C, Villega F, Konsman JP, Badaut J, Vascular impairment as a pathological mechanism underlying long-lasting cognitive dysfunction after pediatric traumatic brain injury, Neurochem Int 111 (2017) 93–102. [DOI] [PubMed] [Google Scholar]
- [43].Umeano O, Wang H, Dawson H, Lei B, Umeano A, Kernagis D, James ML, Female gonadal hormone effects on microglial activation and functional outcomes in a mouse model of moderate traumatic brain injury, World J Crit Care Med 6(2) (2017) 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tucker LB, Fu AH, McCabe JT, Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research, J Neurotrauma 33(9) (2016) 880–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wright DK, O'Brien TJ, Shultz SR, Mychasiuk R, Sex matters: repetitive mild traumatic brain injury in adolescent rats, Ann Clin Transl Neurol 4(9) (2017) 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lenz KM, Nugent BM, Haliyur R, McCarthy MM, Microglia are essential to masculinization of brain and behavior, J Neurosci 33(7) (2013) 2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative images of vaginal cytology samples. Morphology of (A) nucleated epithelial cells present in proestrus, (B) cornified epithelial cells present in estrus, (C) cornified epithelial cells and polymorphonuclear leukocytes present in metestrus, and (D) primarily polymorphonuclear leukocytes and a few epithelial cells present in diestrus when female mice are in each respective phase of the estrous cycle. When stages are collapsed, proestrus and estrus are referred to as High (purple) and metestrus and diestrus are considered as Low (teal).
Supplemental Figure 2. TBI induced immune response comparison of acute and chronic time points post adolescent injury. (A) The percentage of infiltrating neutrophils identified as CD11b+, CD45+ and Ly6G+ at 48 hours post adolescent CCI-TBI. Mod-CCI-TBI resulted in a higher percentage of infiltrated neutrophils only in the ipsilateral cortex in males compared to Naïve/Sham at both acute and chronic timepoints. (B) Females did not have a significant neutrophil infiltration at either the acute or chronic timepoint (C) Microglia were identified as CD11b+ CD45+ Ly6G− Ly6C+ and CX3CR-1low. We report a significant increase in microglia in the impacted hemisphere at 48 hrs post injury that was statistically reduced but still elevated at 2 weeks post-injury in males. (D) This effect was also seen in females but persisted 2 weeks post-TBI. (E) Mod-CCI-TBI induced more CX3CR-1low PSD95+ microglia in males compared to Naïve/Sham at 48 hrs but had resolved by 2 weeks post injury (F). In contrast, females did not demonstrate elevated CX3CR-1low PSD95+ microglia at 48 hrs but had a small but statistically significant increase at two-weeks post injury (two-way ANOVA, * = p<0.05, ***= p<0.001, **** = p<0.0001) N=4-6 per condition.
Supplemental Figure 3. Immunohistochemistry of fibrinogen in brain parenchyma. Representative images of immunohistochemical detection of fibrinogen in the ipsilateral cortex in males (A,C,E) and females (B,D and F) two-weeks following Naïve/Sham, Mod-CCI-TBI, or Severe-CCI-TBI. Fibrinogen is a bloodborne clotting protein that is restricted to the vasculature and does not cross the BBB. Loss of BBB integrity is shown by positive immunolabeling for fibrinogen using 3,3′-diaminobenzidine or DAB (brown). Fibrinogen detection can observed in the perivascular space and throughout the brain parenchyma of TBI animals. Females with TBI appear to show less fibrinogen “leakiness” into the CNS than males. Scalebar = 1mm.