Abstract
Induced pluripotent stem cells are a patient-specific, proliferative cell source that can differentiate into any somatic cell type. Bipotent endothelial progenitors, which can differentiate into the cell types necessary to assemble mature, functional vasculature, have been derived from both embryonic and induced pluripotent stem cells. However, these cells have not been rigorously evaluated in three-dimensional environments, and a quantitative measure of their vasculogenic potential remains elusive. Here, the generation and isolation of iPSC-EPs via fluorescent-activated cell sorting are first outlined, followed by a description of the encapsulation and culture of iPSC-EPs in collagen hydrogels. This extracellular matrix (ECM)-mimicking microenvironment encourages a robust vasculogenic response; vascular networks form after a week of culture. The creation of a computational pipeline that utilizes open-source software to quantify this vasculogenic response is delineated. This pipeline is specifically designed to preserve the 3D architecture of the capillary plexus to robustly identify the number of branches, branching points, and the total network length with minimal user input.
Keywords: Stem Cell, Endothelial Progenitor, Vasculogenesis, Extracellular Matrix, Collagen, Computational Analysis, Vessel Networks
SUMMARY:
Endothelial progenitors derived from induced pluripotent stem cells (iPSC-EPs) have the potential to revolutionize cardiovascular disease treatments and to enable the creation of more faithful cardiovascular disease models. Herein, the encapsulation of iPSC-EPs in three-dimensional (3D) collagen microenvironments and a quantitative analysis of these cells’ vasculogenic potential are described.
INTRODUCTION:
Human umbilical vein endothelial cells (HUVECs) and other primary endothelial cell types have been utilized for two decades to model blood vessel sprouting and development in vitro1. Such vascular platforms promise to illuminate molecular and tissue-level mechanisms of cardiovascular disease and may present physiological insight into the development of primitive vascular networks2,3. Though the field of vascular modeling has witnessed significant advances, a “gold standard” assay that can quantitatively model and assess physiological vascular development remains elusive. Most published protocols (1) do not adequately recapitulate the vascular niche to encourage the formation of mature, functional blood vessels or (2) do not have a method to quantitatively compare the vasculogenic potential of the assessed cell types in three dimensions (3D).
Many current vascular models are limited in their ability to mimic the physiological vascular niche. One of the most commonly employed in vitro platforms is the gelatinous protein mixture-based tube formation assay. Briefly, HUVECs are seeded as single cells on a thin layer of gel that consists of proteins harvested from murine sarcoma extracellular matrix (ECM); within one to two days, the HUVECs self-assemble into primitive tubes4. However, this process occurs in two-dimensions (2D) and the endothelial cells (ECs) utilized in this assay do not form enclosed, hollow lumens, thereby limiting the physiological significance of these studies. More recently, ECs and supporting cells (e.g., mesenchymal stem cells (MSCs) and pericytes) have been co-cultured in 3D microenvironments that simulate the fibrous architecture of the native ECM, such as collagen or fibrin hydrogels5. To model vascular development in these microenvironment, polymeric beads coated with ECs are typically employed6. The addition of exogenous growth factors and/or growth factors secreted by other cells interstitially embedded in the hydrogel can induce the ECs, coating the polymeric beads, to sprout and form single lumen; the number and diameter of sprouts and vessels can then be computed. However, these sprouts are singular and do not form an enclosed, connected network as is seen in physiological conditions and thus is more reminiscent of a tumor vasculature model. Microfluidic devices have also been utilized to mimic the vascular niche and to promote the formation of vasculature in EC-laden hydrogels7,8. Typically, an angiogenic growth factor-gradient is applied to the circulating cell culture medium to induce EC migration and sprouting. ECs that constitute the lumen of developed vessels are sensitive to the shear stress induced by the application of fluid flow through the microfluid device; thus, these microfluidic devices capture key physiological parameters that are not accessible in the static models. However, these devices require costly microfabrication abilities.
Most importantly, all three vascular models (2D, 3D, microfluidic) overwhelmingly utilize primary ECs as well as primary supporting cell types. Primary cells cannot be developed into an effective cardiovascular therapy because the cells would engender an immune response upon implantation; furthermore, HUVECs and similar primary cell types are not patient-specific and do not capture vascular abnormalities that occur in patients with a genetic disposition or preexisting health conditions, e.g., diabetes mellitus. Induced pluripotent stem cells (iPSCs) have emerged in the past decade as a patient-specific, proliferative cell source that can be differentiated into all somatic cells in the human body9. In particular, protocols have been published that outline the generation and isolation of iPSC-derived endothelial progenitors (iPSC-EPs)10, 11; iPSC-EPs are bipotent and can, therefore, be further differentiated into endothelial cells and smooth muscle cells/pericytes, the building blocks of mature, functional vasculature. Only one study has convincingly detailed the development of a primary capillary plexus from iPSC-EPs in a 3D microenvironment12; though this study is critical to an understanding of iPSC-EP assembly and differentiation in natural and synthetic hydrogels, it did not quantitatively compare the network topologies of the resulting vasculature. Another recent study has used the polymeric bead model to compare the sprouting of HUVECs and iPSC-derived ECs5. Therefore, there is a clear need to further elucidate the physical and chemical signaling mechanisms that regulate iPSC-EP vasculogenesis in 3D microenvironments and to determine if these cells are suitable for ischemic therapy and cardiovascular disease modeling.
In the past decade, different open-source computational pipelines and skeletonization algorithms have been developed to quantify and compare vascular network length and connectivity. For example, Charwat et al. developed a Photoshop-based pipeline to extract a filtered, binarized image of vascular networks derived from a co-culture of adipose-derived stem cells and outgrowth ECs in a fibrin matrix13, 14. Perhaps the most widely used topology comparison tool is AngioTool, a program published online by the National Cancer Institute15; despite the program’s widespread adoption and well-documented fidelity, the program is limited to analyzing vessel-like structures in two dimensions; other programs, including AngioSys and Wimasis, share the same dimensionality limitation16. Powerful software suites such as Imaris, Lucis, and Metamorph have been developed to analyze the network topology of engineered microvasculature; however, these suites are cost-prohibitive for most academic labs and limit access to the source code, which may hinder the ability of the end-user to customize the algorithm to their specific application. 3D Slicer, an open-source magnetic resonance imaging/computed tomography package, contains a Vascular Modeling Toolkit that can effectively analyze the topology of 3D vascular networks17; however, the analysis is dependent on the user manually placing the end-points of the network, which may become tedious when analyzing a large dataset and can be influenced by the user’s subconscious biases. In this manuscript, a computational pipeline that can quantify 3D vascular networks is described in detail. To overcome the above-outlined limitations, this open source computational pipeline utilizes ImageJ to pre-process acquired confocal images to load the 3D volume into a skeleton analyzer. The skeleton analyzer uses a parallel medial axis thinning algorithm, and was originally developed by Kerschnitzki et al. to analyze the length and connectivity of osteocyte networks18; this algorithm can be effectively applied to characterize the length and connectivity of engineered microvasculature.
Altogether, this protocol outlines the creation of microvascular networks in 3D microenvironments and provides an open-source and user-bias free computational pipeline to readily compare the vasculogenic potential of iPSC-EPs.
PROTOCOL:
1. Preparation of Culture Media and Coating Solutions
1.1
To prepare vitronectin coating solution, dilute vitronectin 1:100 in Dulbecco’s phosphate-buffered saline (DPBS).
CAUTION: Once diluted, it is not recommended to store this solution for future use.
1.2
Upon receiving phenol red-free, growth factor-reduced gelatinous protein mixture (see Table of Materials) from the manufacturer, thaw on ice at 4°C until the mixture is transparent and fluid. Keeping the mixture on ice in a laminar flow hood, pipette 75 μL of the mixture into a1.8 mL microcentrifuge tube and freeze at −20 °C immediately. These aliquots can be stored for up to one year.
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| μ-Slide Angiogenesis | Ibidi | N/A | A flat, glass bottom tissue-culture plate with side walls enables facile confocal imaging |
| 96 well, round bottom, ultra low attachment microplate, sterile | Corning | 7007 | Prevents the binding of cell-laden collagen hydrogels to the cell culture dish |
| Accutase | STEMCELL Technologies | 7920 | Gentle cell detachment solution; does not degrade extracellular epitopes vital for FACS |
| Advanced DMEM/F12 | Thermo Scientific | 12634010 | The base media for iPSC-EP differentiation. |
| Barnstead GenPure xCAD Plus | Thermo Fisher Scientific | 50136165 | Water purification system; others can be readily substituted |
| Bovine Serum Albumin solution, 7.5% in DPBS, sterile-filtered, BioXtra, suitable for cell culture | Fisher Scientific | A8412 | To preserve cell viability when FACs sorting |
| CD34-PE, human (clone: AC136) | Miltenyi Biotec | 130-098-140 | Antibody used for FACs isolation of iPSC-EPs |
| CHIR99021 | LC Laboratories | C-6556 | Induces the formation of mesoderm from pluripotent stem cells |
| Collagen I Rat Tail High Protein 100 mg | VWR | 354249 | Main component of the 3D microenvironment |
| Conical centrifuge tubes (15/50 mL) | Fisher Scientific | 14-959-49D/A | Used to store and mix relatively large volumes of reagents and cell culture media |
| DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo Fisher Scientific | D1306 | To counterstain and visualize cell nuclei |
| DMEM/F12 | Thermo Fisher Scientific | 11320–082 | For dilution of Matrigel and thawing of pluripotent stem cells |
| Dulbecco’s phosphate-buffered saline (DPBS) | ThermoFisher | 14190–250 | To wash monolayer cultures |
| EDTA | Sigma-Aldrich | E8008 | For passaging of pluripotent stem cell colonies and to prevent cell aggregation when FACs sorting |
| Endothelial Cell Growth Medium 2 | PromoCell | C-22011 | Promotes endothelial cell viability and proliferation |
| Essential 8 Medium | Thermo Fisher Scientific | A1517001 | For maintenance of pluripotent stem cells |
| Glycine,BioUltra, for molecular biology, >=99.0% (NT) | Sigma-Aldrich | 50046 | Neutralizes remaining detergent |
| L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate,>=95% | Sigma-Aldrich | A8960 | Component of iPSC-EP differentiation medium |
| MATLAB | MathWorks | 1.8.0_152 | Multi-paradigm numerical computing environment (free available at most academic institutions) |
| Matrigel Matrix GFR PhenolRF Mouse 10 mL (gelatinous protein mixture) | Fisher Scientific | 356231 | Diluted in DMEM/F12 to coat plates for iPSC-EP differentiation |
| Medium-199 10X | Thermo Fisher Scientific | 1825015 | Used to balance final hydrogel osmolarity and pH |
| Microcentrifuge tubes (1.7 mL) | VWR | 87003–294 | Stores small volumes of reagents |
| Phosphate-buffered saline (PBS) | Sigma-Aldrich | P3813 | The main ingredient of the immunostaining solutions |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | 15140122 | Antibiotic used after sorting to remove possible contamination from FACS instrument |
| Recombinant Human VEGF 165 Protein | R&D Systems | 293-VE | Mitogen that stimulates endothelial cell proliferation and tubulogenesis |
| Rhodamine phalloidin | Themo Fisher Scientific | R415 | To identify F-actin deposition and therfore outline the borders of the vascular networks |
| Triton X-100 (nonionic surfactant) | Sigma-Aldrich | X-100 | Detergent used to gently permeabilize cells |
| Tween-20 (emulsifying reagent) | Fisher Scientific | BP337 | Increases the binding specificity of the added antibodies |
| VE-Cadherin (F-8) | Santa Cruz Biotechnology | sc-9989 | To identify 3D endothelial lumen in collagen hydrogels |
| Vitronectin | ThermoFisher | A14700 | For maintenance of pluripotent stem cells |
| Y-27632 | Selleck Chemicals | S1049 | Preserves pluripotent stem cell and iPSC-EP viability when dissociated and re-seeded |
CAUTION: This gelatinous protein mixture becomes very viscous when kept at room temperature and will solidify at higher temperatures. Keep this solution ice-cold.
1.3
To prepare this mixture for coating, thaw a 75 μL aliquot on ice and dilute with 6 mL of ice-cold Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12). This prepared volume is enough to coat a 12 well plate.
1.4
Prepare a 100 mg/mL stock solution of ascorbic acid magnesium-2-phosphate (a white lyophilized powder) in ultrapure water by adding 500 mg of powder to 5 mL of water in a 10 mL glass scintillation vial. Agitate with a stir bar until the solution is completely transparent (this may take up to an hour). This solution can be stored at −20 °C for up to one year.
1.5
Create a 10 mM stock of CHIR99021 (CHIR) by dissolving 10 mg of lyophilized powder in 1.9928 mL of dimethyl sulfoxide (DMSO) in a 15 mL conical centrifuge tube and warm in a 37 °C bead (or water) bath until the solution is transparent. Aliquot into 1.8 mL microcentrifuge tubes and store at −20 °C for up to one year.
1.6
Create a 10 mM stock of Y-27632, a ROCK inhibitor (ROCKi), by dissolving 10 mg of lyophilized powder in 3.1225 mL of dimethyl sulfoxide (DMSO) in a 15 mL conical centrifuge tube and warm in a 37 °C bead (or water) bath until the solution is transparent. Aliquot into 1.8 mL microcentrifuge tubes and store at −20 °C for up to one year.
1.7
To prepare Essential 8 (E8) medium, thaw frozen supplements, add to the 500 mL bottle of basal medium, and filter sterilize. This solution can be stored at 4 °C for up to two weeks.
CAUTION: It is very important not to warm this medium at 37 °C, as the proteins in the medium formulation may degrade with prolonged use. Instead, keep this medium at room temperature for 15 to 30 minutes before use.
1.8
To prepare sorting buffer, add 1.33 mL of 7.5% bovine serum albumin (BSA) in DPBS and 200 μL of 0.5 mM ethylenediaminetetraacetic acid (EDTA) to 48.5 mL of DPBS to create a final solution of 0.5% BSA and 2 mM EDTA.
1.9
To prepare LaSR Basal, add 300 μL of 100 mg/mL ascorbic acid magnesium-2-phosphate and 5 mL of GlutaMAX to 500 mL of Advanced DMEM/F12. This solution can be stored at 4 °C for up to one month.
1.10
To prepare Endothelial Cell Growth Medium-2 (EGM-2), thaw supplements and add to 500 mL bottle of basal medium. This solution can be stored at 4 °C for up to one month.
1.11
To prepare blocking buffer, add 500 mg of lyophilized bovine serum albumin (BSA) and 50 μL of an emulsifying reagent (see Table of Materials) to 48 mL of DPBS. Incubate at 37 °C for at least 15 minutes to ensure that the BSA does not clump and subsequently separate from the solution.
2. Thawing, Maintenance, and Passaging of iPSCs
2.1
Before thawing a cryopreserved iPSC vial, coat a single well of a 6-well plate with 1 mL of vitronectin solution (prepared as described in 1.1). Incubate for one hour at room temperature. Do not let this coating air dry before adding E8 medium to the plate.
2.2
To thaw iPSCs, retrieve a cryopreserved vial of iPSCs from its liquid nitrogen storage container and thaw at 37 °C until a small crystal of ice remains. Carefully (dropwise) transfer the content of the thawed vial to a 15 mL conical centrifuge tube containing 8 mL of ice-cold DMEM/F12. Wash the thawed vial with an addition 1 mL of ice-cold DMEM/F12 to minimize cell loss. Centrifuge for 5 minutes at 300G.
2.3
While waiting for the centrifuge, aspirate the vitronectin solution and add 1 mL of E8 medium (prepared as described in 1.6) to the well. Then, remove the DMEM/F12 supernatant from the centrifuged conical tube and re-suspend the pellet in 1 mL of E8 medium. Transfer the cells to the newly coated plate, being sure to agitate the suspension to prevent the colonies from clumping upon seeding.
2.4
There is usually substantial cell death after thawing. The next morning, remove the medium and replace with fresh E8 medium.
NOTE: Even under optimal conditions, iPSCs tend to recover poorly from cryopreservation. It is recommended to cryopreserve a near-confluent 6-well for future seeding into a single 6-well. Presence of cell debris is normal for the first 2–3 days during cell recovery.
2.5
Replace E8 medium daily until the cells are ready for passaging (70–80% confluency).
2.6
To passage, first prepare vitronectin-coated wells (as described in 2.1).
2.6.1
Once vitronectin-coated wells are ready (about a one hour), aspirate E8 medium from well to be passaged and wash twice with 1 mL of DPBS. Incubate with 1 mL of 0.5 mM EDTA for 5–7 minutes at 37 °C. While incubating, remove the vitronectin solution from the newly coated well and replace with 1–2 mL of E8 medium.
2.6.2
Remove the EDTA solution from the wells to be passaged and detach the colonies by gently washing once or twice with 1 mL of E8. Transfer 75–200 μL of cell suspension to the freshly coated E8-containing wells, agitate the plate to ensure even coverage, and place in the incubator immediately.
CAUTION: The incubation time for iPSC detachment is cell-line dependent; it is recommended that the user of this protocol tests a range of times upon sufficiently expanding a received/generated iPSC line. 75 μL of cell suspension generally results in 4 days between passages; 150 μL or more leads to 2–3 days between passages.
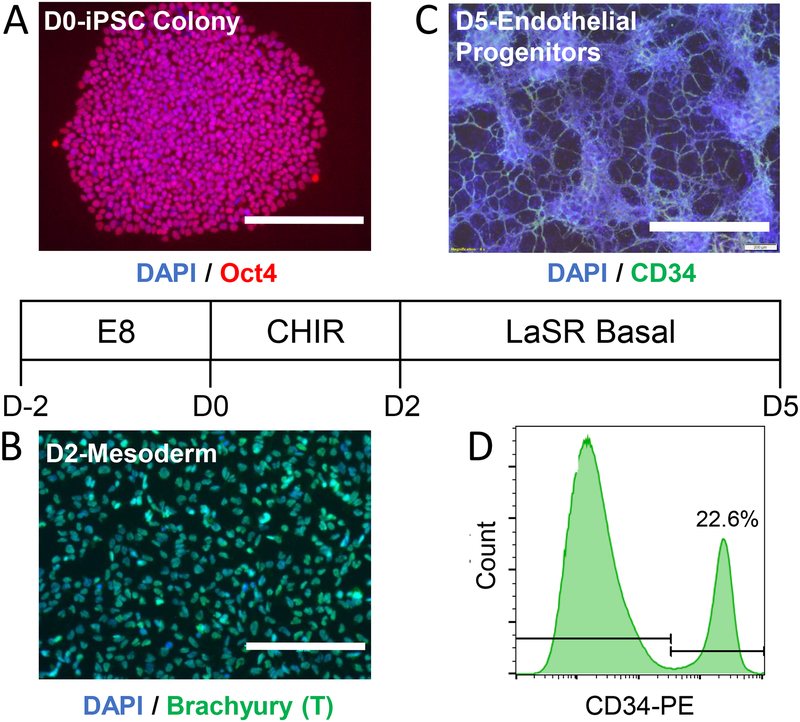
3. Generation of iPSC-derived Endothelial Progenitors (Figure 1).
Figure 1: Generation of iPSC-EPs from Pluripotent Stem Cells.
A. WiCell 19-9-11 iPSCs, which stained positive for Oct4, were cultured in E8 medium supplemented with 10 μM Y-27632 ROCK inhibitor for 48 hours. B. The iPSCs were then induced with 6 μM of CHIR99021 in LaSR Basal medium for 48 hours, at which point the cells were positive for Brachyury, a mesoderm marker. C. The cells were further cultured in LaSR Basal media until they expressed CD34, a marker for endothelial progenitors. D. Roughly 15–25% of the differentiated cells expressed CD34. All scale bars represent lengths of 200 μm.
3.1
When maintaining iPSCs, make sure the colonies are well compacted and that there are a low number of differentiated cells in culture. If the colonies begin to contact one another, the cells are most likely too confluent and need to be passaged immediately.
3.2
When iPSCs are 70–80% confluent, coat a 12-well plate with the gelatinous protein mixture (prepared as described in 1.2/1.3). Pipette 500 μL of this mixture into each well and keep for one hour at room temperature.
3.3
During the above one-hour incubation, remove the E8 medium and Y-27632 from the refrigerator and freezer, respectively. Once E8 medium and Y-27632 reach room temperature, prepare E8 + ROCKi by adding 13 μL of 10 μM Y-27632 to 13 mL of E8 in a 15 mL conical centrifuge tube.
3.4
Remove the E8 medium from the iPSC wells, wash twice with 1 mL of DPBS, and then incubate with 0.5 mM EDTA for 5–7 minutes at 37 °C. After the incubation period, remove the EDTA and rapidly shear the cells into a single-cell suspension with a P1000 pipette tip with 1 mL of E8 + ROCKi medium.
3.5
Count the cells with a hemocytometer. For a suspension that may contain millions of iPSCs, add 20 μL of cell suspension to 180 μL of trypan blue. Determine the number of total cells in the single-cell suspension.
3.6
Transfer 0.432*106 to 1.296*106 cells (105 to 3*105 cells/cm2) to the remaining E8 + ROCKi medium. Remove the gelatinous mixture coating from the newly-coated 12-well plate and add 1 mL of cell suspension to each well, ensuring that the cells are well-distributed and do not form small clumps.
NOTE: This range of densities is optimal for differentiation of CD34 positive cells; however, these seeding densities are cell line-dependent and may need to be optimized by the user of this protocol.
3.7
After 24 hours of culture, replace medium with 1 mL of fresh E8 medium. Incubate for an additional 24 hours under the same conditions.
3.8
Remove the E8 medium and replace with LaSR Basal supplemented with 6–12 μM of CHIR99021. This timepoint is defined as D0 of differentiation. After 24 hours of culture, replace with the same cell culture medium.
NOTE: As described previously, the amount of CHIR99021 added to this medium will depend on the iPSC line utilized. Please test a range of CHIR99021 concentrations to ensure optimal results.
3.9
After 48 hours of incubation with CHIR99021, replace with 2 mL of LaSR Basal.
3.10
Replace the medium daily with 2 mL of LaSR Basal for 2–3 additional days. At this time point (D5 of differentiation), a significant proportion of these cells will express CD34 and can be characterized as endothelial progenitors. A schematic of this protocol is outlined with representative results in Figure 1 and described in full by the investigators who first published this method11,19.
NOTE: These endothelial progenitors can be further differentiated along an endothelial lineage (35–99%) or a smooth muscle lineage (1–65%). Differentiation efficiencies vary depending on the type of ECM coating and the cell culture medium used during differentiation20.
4. FACS of Endothelial Progenitors
4.1
Before dissociating the D5/D6 differentiated cells, prepare sorting buffer as described in 1.5) and keep on ice.
4.2
Incubate the differentiated cells with 500 μL of cell detachment solution per well (see Table of Materials) for 10 minutes at 37 °C.
4.3
Dissociate the cells with a P1000 pipette tip into a single cell suspension in cell detachment solution. Consolidate the cell-cell detachment solution mixture in a 15 mL conical centrifuge tube and centrifuge for 5 minutes at 300G.
4.4
Remove the supernatant and resuspend in 200 μL of ice-cold sorting buffer (prepared as described in 1.7). Add 5 μL of the concentrated CD34-PE antibody to the cell-sorting buffer suspension and incubate for 10 minutes at 4°C.
4.5
Re-suspend in 5 mL of ice-cold sorting buffer. Filter this suspension through a cell strainer with a 40 μm cap before placing on the sorter.
4.6
On the appropriate fluorescent channel, sort 10,000 cells that have not been labeled with a fluorescent antibody. Gate a region at a high fluorescent intensity that does not contain any of these 10,000 cells (Figure 1D). This will serve as a negative control.
4.7
Run the sample containing iPSC-EPs labeled with a fluorescent solution and begin the sort. CD34 is highly expressed in these endothelial progenitors and is easily separated from the main population. After sorting, transfer the solution to a 15 mL conical centrifuge tube and centrifuge for 5 minutes at 300G. Remove the supernatant.
NOTE: If the solution is in a tube larger than a microcentrifuge tube (e.g., a 5 mL polystyrene tube commonly employed in many FACs protocols), re-suspend the sorted cell pellet in 1 mL of sorting buffer and transfer this solution to a microcentrifuge tube. Centrifuge at 300G for 5 minutes and remove the supernatant.
4.7.1
Optional: if further iPSC-EP characterization is desired, add other primary antibodies (e.g., CD31-APC) simultaneously with CD34-PE. Ensure that crosstalk between different fluorescent channels is minimized.
5. Encapsulation and Long-term Culture of iPSC-EP-laden Collagen Hydrogels
5.1
Prepare seeding medium by adding 40 μL of medium 199 10X to 400 μL of endothelial growth medium (EGM-2) supplemented with 10 μM Y-27632 in a 1.8 mL microcentrifuge and place tube on ice.
NOTE: while the use of antibiotics is discouraged for stem cell culture and the maintenance of differentiated cells, dosing with Pen/Strep is recommended after sorting because it is difficult to guarantee sterility in the sorting environment (this is more imperative if the sorter is shared among many laboratories).
5.2
Remove all the supernatant from the cell-suspension and add 200 μL of EGM-2 supplemented with 10 μM Y-27632. Transfer the cell suspension to seeding medium and mix vigorously.
5.3
Add 350 μL of collagen to the solution prepared in 5.2) and mix well. The solution will become pale yellow.
NOTE: the final concentration of collagen can have a significant impact on the formation of the capillary plexus. This protocol assumes that the collagen has been supplied at 10 mg/mL and that the hydrogels have a final concentration of 3.5 mg/mL. Adjust these volumes to achieve their final collagen concentration; it is recommended to restrict the final collagen concentration from 2 mg/mL to 4 mg/mL.
5.4
Add 5 μL of 1M NaOH to the solution prepared in 5.3 and mix well, avoiding the introduction of air bubbles. The solution will become bright pink.
5.5
Pipette 56 μL of neutralized collagen-cell solution prepared in 5.4 into individual wells of a 96-well ultra-low attachment U-bottom cell culture plate. Incubate at 37 °C for 30 minutes. Before adding media, check that the cells have been evenly distributed by examining the samples with a brightfield microscope.
5.6
Prepare 1 mL of culture medium comprised of EGM-2 supplemented with 10 μM Y-27632 and 50 ng/mL vascular endothelial growth factor (VEGF) by adding 1 μL of each stock solution to a microcentrifuge tube. After mixing well, pipette 100 μL of this cell culture medium onto the cell-laden hydrogels. Transfer the plate to 37°C for long-term culture.
5.7
Replace the culture medium daily, adding additional angiogenic growth factors or small molecule inhibitors as dictated by the goal of the study. To optimally remove the media, tilt the plate and use a P100 tip to gently aspirate medium without disturbing the hydrogel.
6. Fixing, Immunostaining, and Visualization of EP-Based Vascular Networks
6.1
After one week of culture, add 250 μL of 4% paraformaldehyde (PFA) solution to a 48-well plate. Fill as many wells as there are hydrogels. Remove the medium from the hydrogels (PFA) and use fine-tipped tweezers to transfer the hydrogels to PFA containing wells. Incubate for 10 minutes at room temperature and remove the PFA by washing rapidly with PBS.
6.2
Permeabilize by adding 250 μL of 0.5% of a nonionic surfactant (see Table of Materials) for 5 minutes at room temperature. Wash twice with 250 μL of PBS supplemented with 500 mM glycine. Incubate at room temperature for 5 minutes for each washing step to ensure the removal of excess detergent. Block by immersing the hydrogels in 250 μl of blocking buffer for 30 minutes.
6.3
Incubate with the desired primary antibodies diluted in blocking buffer overnight at 4°C. For example, if immunostaining with phalloidin and VE-cadherin, dilute 1:40 and 1:200, respectively, in blocking buffer.
6.4
The next day, remove the primary antibody solution and wash twice with 0.5% emulsifying reagent in DPBS. Cover with aluminum foil and incubate with a species-specific secondary antibody (e.g., 1:200 Alexa Fluor 488 in PBS) for 2 hours at room temperature.
6.5
Remove the secondary antibody solution and wash twice with 0.5% emulsifying reagent in DPBS. Incubate at room temperature for 5 minutes for each washing step to ensure the removal of free fluorophores.
6.6
To visualize cell nuclei, dilute 4’, 6-diamidino-2-phenylindole (DAPI) 1:10000 in PBS and add to the sample(s).
6.6.1
Incubate for 2 minutes at room temperature and wash twice with PBS. Incubate at room temperature for 5 minutes for each washing step to ensure the removal of free fluorophores.
6.7
Transfer the samples to an Angiogenesis μ-Slide or a glass bottom petri dish using fine-tipped tweezers. Ensure that no air bubbles are trapped underneath the hydrogel.
6.8
Image on a confocal microscope by acquiring z-stacks that extend from the bottom to the top of the sample. Ensure that the detector is not saturated and that the lowest magnification available is in use (a large field-of-view is desirable).
NOTE: For future processing, ensure that the z-stacks are saved with minimal compression (.czi is recommended on Zen instruments).
7. Using the Computational Pipeline to Analyze and Compare Vascular Network Topologies
7.1
Inspect each z-stack to ensure that slices only contain vessels. Open the z-stack in ImageJ and enhance the contrast by clicking “Image → Adjust → Brightness/Contrast.” The vessel borders are now clearly demarcated, and the background level is minimized.
CAUTION: ECs will migrate towards the edges of the gel and will form small cobblestone colonies. While these are useful to estimate the boundaries of the hydrogel, they will interfere with the final image analysis and should be deleted.
NOTE: ImageJ is a Java-based open-source image analysis software developed in concert by the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation. It is recommended to download Fiji, which is simply ImageJ bundled with useful plugins (https://fiji.sc/)
7.2
In ImageJ, blur the image in 3-dimensions clicking Process →Filters → Gaussian Blur 3D and then setting the sigma values in all 3 dimensions to 2.0 (this value might need to be adjusted by the end user).
7.3
In ImageJ, click Process → Binary → Make Binary and then select an appropriate thresholding algorithm. The cross-entropy thresholding algorithm developed by Li et al. is effective in separating vessels from the background21. Calculate the threshold for each image and set the background to “Default”.
7.4
In ImageJ, remove spurious noise and fill in “holes” in lumens by clicking Process → Noise → Remove Outliers.
7.4.1
Removing “bright” outliers will fill in small holes in connected vessels; removing “dark” outliers will remove dead cells. The size of the removal radius will vary based on the magnification and size of the vessels. For images acquired with a wide-field objective that are 512 × 512 pixels, the radii will typically range from 4–6 pixels.
7.5
Process all raw (e.g., czi extension) files and convert into .tif files before proceeding to Step 7.6). To aid in this processing, an ImageJ script, “Binarize and Filter.ijm” has been attached to this manuscript.
7.6
Save the processed .tif files in the same folder as the file “BatchProcessSkeleton.m”, available for download in the manuscript. This script, developed by the authors, calls the functions published by Phillip Kollmannsberger22 and conducts some additional file manipulation.
7.7
Download and unzip all files associated with the two main functions “Skel2Graph3D” (https://www.mathworks.com/matlabcentral/fileexchange/43527-skel2graph-3d) and “Skeleton3D” (https://www.mathworks.com/matlabcentral/fileexchange/43400-skeleton3d). Save all the functions into the same folder as the opened processed z-stack.
7.8
Open a multi-paradigm numerical computing environment (see Table of Materials) and navigate to the folder where all the files described above have been saved.
7.9
Run “BatchProcessSkeleton.m” either by typing “BatchProcessSkeleton” in the Command Window or by opening the script and hitting the Run button (right-facing green arrow) in the Editor.
NOTE: To analyze a large dataset with a single command, ensure that the string inside the dir function in line 6 contains an asterisk (e.g., ‘*.tif’). Otherwise, replace the asterisk with the file name if the analysis of a single file is desired.
7.10
The length of time that this script will take to execute will vary substantially based on the computing power available. It is recommended to conduct this analysis on a computer with at least 8 GB of RAM so that the user can access other programs while the script runs.
CAUTION: While not strictly necessary, graphing the original confocal acquisition (in gray) and overlaying with this image matrix with the skeleton matrix (in red) can aid in evaluating the performance of the skeletonization algorithm. In addition, the reader can create a nodal graph, as implemented by Kollmannsberger et al., to evaluate the accuracy of the network analysis. Creating both graphs, while useful, will dramatically increase the runtime of the script and require additional memory; if the user simply requires a final topology analysis and does not want to visualize data set, simply comment out lines XX to YY (skeleton graph) and lines YY to ZZ (topology graph).
7.11
Upon completion, the “Data” matrix, visible in the Workspace, now contains the processed data. Double click “Data” and open this cell in the Variable editor.
7.11.1
From left to right, this matrix will contain: 1) the file name, 2) the number of nodes (branch points + end points), 3) branch points, 4) links (i.e., vessels), 5) network length (including isolate vessels), and 6) the number of slices contained in the z-stack. This data can be saved as a .csv file and exported for further analysis to any software of choice.
REPRESENTATIVE RESULTS:
After differentiation (Figure 1), FACS, and encapsulating of iPSC-EPs in collagen hydrogels, the cells will typically remain rounded for 24 hours before beginning to migrate and form initial lumens. After about 6 days of culture, a primitive capillary plexus will be visible in the hydrogel when viewed with brightfield microscopy (Figure 2). After imaging the fixed, stained cell-laden hydrogels on a confocal microscope (Movie 1, Movie S.1), the pre-processed images are converted to a skeleton which enables an analysis of the overall length and connectivity of the network. These quantitative measures can then be used to determine which set of conditions are optimal for producing robust vascular networks.
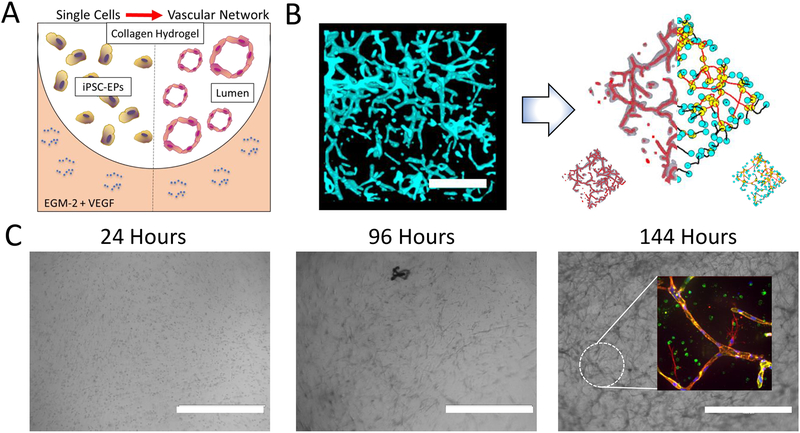
Figure 2: Generation and Analysis of iPSC-EP Vascular Networks in Collagen Hydrogels.
A. A cross-section of the 3D microenvironment used in this assay to promote vascular network formation from iPSC-EPs. A floating collagen hydrogel is seeded with iPSC-EPs and exposed to EGM-2 supplemented with 50 ng/mL VEGF and a temporal dose of Y-27632. B. The resulting capillary plexus is highly branched and interconnected, as visualized via staining with F-actin (cyan). The binarized image, shown on the left, is generated by pre-processing with ImageJ. This z-stack is then analyzed via a previously developed algorithm, which skeletonizes the network (shown in a collection of thin red lines) and then analyzes the network topology for branches (yellow), end points (blue), isolated vessels (black), and connected vessels (red). The scale bar represents a length of 100 μm C. Morphological changes of iPSC-EPs-laden collagen hydrogels: 24 hours after seeding, the iPSC-EPs remain spherical and within 96 hours gradually take on a more elongated phenotype. Further culture results in assembly of lumen-containing VE-Cadherin network, as shown in the inset at the 144-hour time point. The scale bars represent lengths of 400 μm; green = VE-Cadherin, red = F-actin, and blue = DAPI.
This protocol allows for the development of a robust, three-dimensional capillary plexus that is responsive to local physical and chemical cues. In previous work, this network formation has been shown to be sensitive to matrix density, matrix stiffness, matrix metalloprotease inhibition, and the type and concentration of various angiogenic mitogens20, 23.
DISCUSSION:
This protocol involves the long-term culture of cells in three types of cell culture media: E8, LaSR Basal, and EGM-2. Therefore, great care should be taken to appropriately sterilize all materials. Additionally, lab coats and ethanol-cleaned gloves should always be worn when working in the laboratory’s laminar flow hood. It is recommended to frequently test for mycoplasma contamination; if a large amount of debris is observed during iPSC culture or a sudden drop in differentiation efficiency is noted, mycoplasma contamination is a likely cause. iPSC-EPs generated with this protocol tend to deposit a significant amount of ECM, which lengthens the dissociation time and causes the single cell suspension to separate into small clumps. Do not use Trypsin-EDTA (0.25%), as the solution may disrupt CD34 epitopes on iPSC-EPs. However, treatment with collagenase/dispase solution may remove deposited ECM and ease dissociation with a standard cell detachment solution. After extensive dissociation, some small clumps of cells and ECM may remain; remove these clumps with a P100 pipette tip, as they are likely to clog the cell strainers or interfere with the FACS.
Pluripotent stem cells are sensitive to dissociation and will undergo apoptosis in a single-cell suspension unless a ROCK inhibitor (commonly Y-27632) is added to the medium24. iPSC-EPs are also sensitive to dissociation; including Y-27632 at 10 μM for the first 24 hours of culture is imperative to increase cell survival and proliferation. Therefore, it is critical that a ROCK inhibitor is included in both the hydrogel and surrounding medium immediately following FACS. The seeding density of iPSC-EPs also significantly impacts vessel development and total network size. Generally, a concentration of 500,000 cells/mL to 3 million cells/mL is an appropriate range of seeding densities. A further increase in seeding density often leads to hydrogel compaction and cell death.
The density of ECM structural proteins has been found to have a significant impact on the development of engineered microvasculature25, 26. Generally, increasing the density of an ECM-based hydrogel (often collagen or fibrin) limits vascular network length and connectivity. Therefore, it is critical that careful attention is paid to the collagen matrix density; concentrations below 2 mg/mL promotes the premature proteolytic degradation of collagen hydrogels, which results in an irreparable loss of the hydrogel’s structural integrity. In contrast, concentrations above 4 mg/mL induce the formation of short, wide lumen that exhibit poor connectivity; hydrogel deformability and a change in pore size are primarily responsible for this abrogation20.
Acellular and cell-laden collagen hydrogels do not bind to the ultra-low attachment plates; the hydrogels tend to remain suspended in the media and will occasionally float to the top of the well. If tissue culture-treated plates are employed in this protocol, the embedded progenitors will migrate to the bottom of the well and will form a near confluent monolayer at the bottom of the plate. The resulting decrease in cell seeding density inhibits the assembly of these progenitors into 3D networks. Additionally, if hydrogels are cast into the wells of a tissue culture treated plate, they will weakly bind the inner surface of the well; this binding applies strain to the hydrogel. Since this platform was developed to isolate the importance of physical and chemical cues, it is critical that no extraneous forces are imparted to the cells27. The floating hydrogels may be difficult to feed because most of the cell culture media lies below the hydrogel; to overcome this, tilt the plate and use a P100 pipette to remove media from the side of the wall.
When imaging the vascular networks on a confocal microscope, it is critical to follow all washing steps to ensure that excess fluorophore does result in a low signal to noise ratio. Excess fluorescence may confuse default thresholding algorithms and make the z-stack difficult to binarize. To overcome this issue, use phalloidin, a fungal toxin that selectively labels F-actin and displays limited off-target binding. In general, use primary antibodies that have been pre-conjugated to a secondary antibody to limit the concentration of free fluorophore that diffuses through the gel. When generating the z-stacks a slice depth of 10–20 microns is recommended to balance the time needed for acquiring a z-stack against the need for a high-resolution image.
Here a quantitative assay to assess the vasculogenic potential of iPSC-EPs in 3D microenvironments is described. This assay utilizes open source software and is unaffected by use biases. Still, it represents an oversimplified model of the physiological vascular niche. While iPSC-EPs can differentiate into the cells needed for mature, functional vasculature, this assay neglects the contributions of other cell types (e.g., fibroblasts and macrophages) that participate in vasculogenic processes. Furthermore, this system is static and does not expose the developing vessels to flow. Finally, while collagen I is one of the dominant proteins in the ECM 28 and maintains its fibrillar architecture in vitro, it is lot-dependent and is limited to weak physical crosslinking when neutralized at high temperatures. Despite these limitations, this assay represents a significant step forward in the quest to engineer functional vasculature for cardiovascular disease therapy and modeling.
Supplementary Material
Movie 1: Z-stack of Vasculature Generated from iPSC-EPs: Vascular networks were fixed, stained with F-actin, and visualized by acquiring z-stacks on a confocal microscope. Slices were acquired at 17 μm intervals.
Movie S.1: 3D Rendering of Vessels: Vascular networks were fixed, stained with F-actin (red) and VE-cadherin (green), and visualized by acquiring z-stacks on a confocal microscope.
ACKNOWLEDGMENTS:
We would like to acknowledge Prof. Jeanne Stachowiak (The University of Texas at Austin) for her technical advice on confocal microscopy. We are also grateful for discussions with Samuel Mihelic (The University of Texas at Austin), Dr. Alicia Allen (The University of Texas at Austin), Dr. Julie Rytlewski (Adaptive Biotech), and Dr. Leon Bellan (Vanderbilt University) for their insight on the computational analysis of 3D networks. Finally, we thank Dr. Xiaoping Bao (University of California, Berkeley) for his advice on differentiating iPSCs into iPSC-EPs.
This work was supported by the American Heart Association (grant number 15SDG25740035, awarded to J.Z.) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (grant number EB007507, awarded to C.C.).
Footnotes
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES:
- 1.Wang K, Lin R-Z, Melero-Martin JM Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. Cellular and Molecular Life Sciences. 1–19, doi: 10.1007/s00018-018-2939-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kant RJ, Coulombe KLK Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomaterialia. 69, 42–62, doi: 10.1016/j.actbio.2018.01.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briquez PS, Clegg LE, Martino MM, Gabhann F. Mac, Hubbell JA. Design principles for therapeutic angiogenic materials. Nature Reviews Materials. 1 (1), 1–15, doi: 10.1038/natrevmats.2015.6 (2016). [DOI] [Google Scholar]
- 4.Arnaoutova I, George J, Kleinman HK, Benton G The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis. 12 (3), 267–274, doi: 10.1007/s10456-009-9146-4 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Bezenah JR, Kong YP, Putnam AJ Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Scientific Reports. 8 (1), 2671, doi: 10.1038/s41598-018-20966-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakatsu MN et al. VEGF121 and VEGF165 regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Laboratory Investigation. 83 (12), 1873–1885, doi: 10.1097/01.LAB.0000107160.81875.33 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Shamloo A, Heilshorn SC Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab on a Chip. 10 (22), 3061, doi: 10.1039/c005069e (2010). [DOI] [PubMed] [Google Scholar]
- 8.Jeon JS et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integrative Biology. 6 (5), 555–563, doi: 10.1039/C3IB40267C (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Yamanaka S Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 126 (4), 663–676, doi: 10.1016/j.cell.2006.07.024 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Kusuma S, Macklin B, Gerecht S Derivation and network formation of vascular cells from human pluripotent stem cells. Methods in Molecular Biology. 1202, 1–9, doi: 10.1007/7651 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Lian X et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 3 (5), 804–816, doi: 10.1016/j.stemcr.2014.09.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusuma S, Shen Y-I, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proceedings of the National Academy of Sciences of the United States of America. 110 (31), 12601–6, doi: 10.1073/pnas.1306562110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charwat V et al. Potential and limitations of microscopy and Raman spectroscopy for live-cell analysis of 3D cell cultures. Journal of Biotechnology. 205, 70–81, doi: 10.1016/j.jbiotec.2015.02.007 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Holnthoner W et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. Journal of Tissue Engineering and Regenerative Medicine. 9 (2), 127–136, doi: 10.1002/term (2012). [DOI] [PubMed] [Google Scholar]
- 15.Zudaire E, Gambardella L, Kurcz C, Vermeren S A computational tool for quantitative analysis of vascular networks. PLoS ONE. 6 (11), 1–12, doi: 10.1371/journal.pone.0027385(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoo CP, Micklem K, Watt SM A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Engineering Part C: Methods. 17 (9), 895–906, doi: 10.1089/ten.tec.2011.0150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rytlewski JA, Geuss LR, Anyaeji CI, Lewis EW, Suggs LJ Three-dimensional image quantification as a new morphometry method for tissue engineering. Tissue Engineering Part C: Methods. 18 (7), 507–516, doi: 10.1089/ten.tec.2011.0417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerschnitzki M et al. Architecture of the osteocyte network correlates with bone material quality. Journal of Bone and Mineral Research. 28 (8), 1837–1845, doi: 10.1002/jbmr.1927 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Bao X, Lian X, Palecek SP Directed endothelial progenitor differentiation from human pluripotent stem cells via Wnt activation under defined conditions. Wnt Signaling: Methods and Protocols. 1481 (1), 183–196, doi: 10.1007/978-1-59745-249-6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby CO et al. Quantifying the vasculogenic potential of iPSC-derived endothelial progenitors in collagen hydrogels. Tissue Engineering Part A. (in press) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CH, Tam PKS An iterative algorithm for minimum cross entropy thresholding. Pattern Recognition Letters. 19 (8), 771–776, doi: 10.1016/S0167-8655(98)00057-9 (1998). [DOI] [Google Scholar]
- 22.Kollmannsberger P, Kerschnitzki M, Repp F, Wagermaier W, Weinkamer R, Fratzl P The small world of osteocytes: Connectomics of the lacuno-canalicular network in bone. New Journal of Physics. 19 (7), doi: 10.1088/1367-2630/aa764b (2017). [DOI] [Google Scholar]
- 23.Crosby CO, Zoldan J Mimicking the physical cues of the ECM in angiogenic biomaterials. Regenerative Biomaterials. (in press) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature biotechnology. 25 (6), 681–686, doi: 10.1038/nbt1310 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Kniazeva E, Kachgal S, Putnam AJ, Ph D Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Engineering Part A. 17 (7–8), 905–914, doi: 10.1089/ten.tea.2010.0275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghajar CM et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophysical Journal. 94 (5), 1930–1941, doi: 10.1529/biophysj.107.120774 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin KT, Smith AO, Davis GE, Tranquillo RT Aligned human microvessels formed in 3D fibrin gel by constraint of gel contraction. Microvascular Research. 90, 12–22, doi: 10.1016/j.mvr.2013.07.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frantz C, Stewart KM, Weaver VM The extracellular matrix at a glance. Journal of Cell Science. 123 (24), 4195–4200, doi: 10.1242/jcs.023820 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1: Z-stack of Vasculature Generated from iPSC-EPs: Vascular networks were fixed, stained with F-actin, and visualized by acquiring z-stacks on a confocal microscope. Slices were acquired at 17 μm intervals.
Movie S.1: 3D Rendering of Vessels: Vascular networks were fixed, stained with F-actin (red) and VE-cadherin (green), and visualized by acquiring z-stacks on a confocal microscope.