Our heat transfer model suggests that dinosaurs shrank in size as they evolved from cold-blooded reptiles to warm-blooded birds.
Abstract
The evolution of endothermy represents a major transition in vertebrate history, yet how and why endothermy evolved in birds and mammals remains controversial. Here, we combine a heat transfer model with theropod body size data to reconstruct the evolution of metabolic rates along the bird stem lineage. Results suggest that a reduction in size constitutes the path of least resistance for endothermy to evolve, maximizing thermal niche expansion while obviating the costs of elevated energy requirements. In this scenario, metabolism would have increased with the miniaturization observed in the Early-Middle Jurassic (~180 to 170 million years ago), resulting in a gradient of metabolic levels in the theropod phylogeny. Whereas basal theropods would exhibit lower metabolic rates, more recent nonavian lineages were likely decent thermoregulators with elevated metabolism. These analyses provide a tentative temporal sequence of the key evolutionary transitions that resulted in the emergence of small, endothermic, feathered flying dinosaurs.
INTRODUCTION
The evolution of endothermy in birds and mammals is regarded as one of the most important transitions in vertebrate evolution, providing an extraordinary case of evolutionary convergence between these groups that was pivotal to their widespread geographic distribution and ecological success (1). While several groups of invertebrates and vertebrates can raise their temperatures above ambient, the maintenance of high and constant body temperature (Tb) through endogenous heat production at rest is exclusive to birds and mammals and explains their greater mobility, stamina, and tolerance to a wider range of conditions. Nonetheless, because this strategy is energetically costly and leaves virtually no trace in the fossil record, the tempo and mode of the evolution of endothermy remains one of the most contentious subjects in vertebrate evolution (2–6). To understand how, when, and why endothermy arose during the evolution of birds and mammals, two fundamental questions must be considered: What are the costs and benefits of this strategy when compared against ectothermy, and, more importantly, under which conditions would the transition toward endothermy be favored?
Here, we address these questions using the Scholander-Irving model of heat transfer (Fig. 1) (7, 8). For any organism in thermal steady state, the model states that
| (1) |
where MR corresponds to metabolic rate (milliliters of O2 per hour), C corresponds to thermal conductance (milliliters of O2 per hour and degrees Celsius), and Tb − Ta constitutes the thermal gradient between body and ambient temperature (degrees Celsius), respectively. While this relationship has been used to study thermoregulation in endotherms for more than 60 years (7–9), it has been rarely used for ectotherms because of their low MR and high C that results, as the ratio MR/C tends to zero, in the rearranged approximation Tb = Ta [but see (10, 11)]. However, because all living organisms produce endogenous heat, the model remains applicable under thermal steady state, which is a crucial assumption to circumvent the use of complex dynamic models often applied to ectotherms that would render analyses below intractable.
Fig. 1. The evolution of endothermy and miniaturization in the theropod lineage leading to birds.
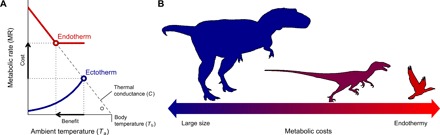
(A) The cost-benefit to switch from ectothermy to endothermy for different ranges of body size was quantified with the Scholander-Irving model, which describes how a rise in metabolism at rest (cost) increases the thermal niche Tb − Ta (benefit). Because there is no thermal gradient between the organism and the environment in the absence of heat production, this curve intersects the abscissa at Tb = Ta when MR = 0 (8). The solid blue and red lines depict the metabolic curves of a typical ectotherm and endotherm, respectively, and the open symbols depict the maximal thermal gradient Tb − Ta possible with resting metabolic rates, used in our model (Eq. 2). (B) A reduction in body size, consistent with the one described from ancestral theropods to basal birds (22), constitutes the evolutionary path of least resistance as the energy costs of being large are traded for those of being endothermic.
The costs of endothermy can be quantified as mass-independent energy expenditure, whereas the benefits include greater mobility and foraging efficiency, predator avoidance, tolerance to, and colonization of a wider range of environmental conditions, increased growth rates, and homeostasis (1). Many of these benefits derive from an elevated Tb, which can only be maintained above a certain minimum Ta. Therefore, Tb − Ta quantifies the thermal niche that organisms can occupy, and its expansion can be used to estimate the net benefit of endothermy (Fig. 1). The cost-benefit of adopting an endothermic lifestyle may now be calculated as the fold increase in MR required to expand the thermal niche by 1°C (hereafter cost per degree), which can be worked out for a constant Tb as
| (2) |
with the subscripts referring to the ectothermic ancestor and the endothermic descendant (Fig. 1). This index of energy cost per degree Celsius is expected to change with body size because both MR and C vary allometrically (Fig. 2) (12, 13). For example, since a generic endotherm exhibits a 5-fold higher MR adjusted to Tb = 38°C (14) and a 2.5-fold lower C than an ectotherm (11) (allometric equations in Fig. 2), then the size reduction from the estimated ~370 kg for the basal Tetanurae to ~0.9 kg for the basal bird would result in reduction in total energy expenditure from 10,194.0 to 574.4 ml O2/hour and a thermal niche expansion of 12.1°C (from a thermal gradient of 6.7° to 18.8°C) (Fig. 2). This corresponds to a cost per degree of 4.65 × 10−3 per °C, or 7.1 and 1.6% of the predicted costs should endothermy have evolved in lineages with a constant size of 370 and 0.9 kg (cost per degree of 6.48 × 10−2 and 2.89 × 10−1 per °C, respectively) (Fig. 3). These calculations, which can be replicated with the exact body size estimates provided in the Supplementary Materials (see Methods), show that the evolution of smaller sizes reduces the energy costs to evolve endothermy, as originally proposed by McNab for mammals (3). In the next sections, we explore how this heat transfer model, combined with well-resolved phylogenies and body size reconstructions, can shed light on how endothermy evolved in birds from their theropod ancestors.
Fig. 2. Reconstruction of metabolic levels and thermal niche of theropods.
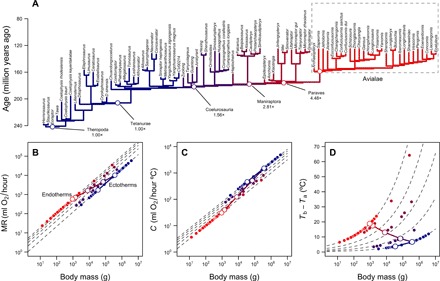
(A) Theropod phylogeny (15) with branches color-coded according to reconstructed metabolic levels. (B) Scaling of metabolic rate versus body mass (12) for ectotherms (MR = 0.68mass0.75) and endotherms (MR = 3.4mass0.75) and the predicted trajectory of the bird stem lineage during the transition from ectothermy to endothermy. Dashed lines show fold differences between ectotherms and endotherms (1× to 5×); open and closed symbols depict reconstructed values for the bird stem lineage and the tips of the phylogeny, respectively (see Methods). (C) Scaling of thermal conductance C and body mass (13) for ectotherms (C = 2.5mass0.5) and endotherms (C = 1.0mass0.5), fold differences from 2.5× to 1×. (D) Thermal gradient and fold differences calculated with Eq. 1 and values in (B) and (C). The log-log linear trajectories connecting MR and C of the ectothermic ancestor and the endothermic descendant, as well as the resulting trajectory in thermal gradient, are shown with the continuous lines.
Fig. 3. Body size evolution and the cost-benefit of endothermy.
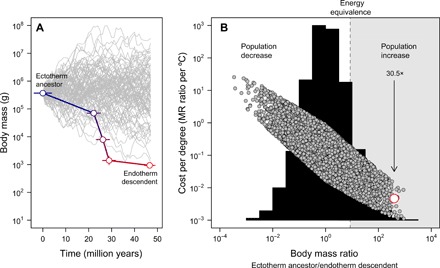
(A) The miniaturization from Tetanurae to basal birds inferred from the fossil record (15), contrasted against 100 simulated size trajectories starting from the same ancestral body size for illustrative purposes (note that for the subsequent full null model, the ancestral body size is allowed to vary). The error represents the SD in reconstructed values across 20 candidate trees (see Methods). (B) The frequency distribution of body mass ratios obtained across 10,000 simulated body size trajectories (histogram) and the energy costs to evolve endothermy expressed per degree Celsius (Eq. 2) under this null model (gray symbols). In this case, the ancestral body size was obtained from a uniform distribution ranging between 10 g and 100,000 kg. The empirical estimate in the bird stem lineage is shown in red. The region in which a reduction in body size would compensate for the energy costs of evolving endothermy, enabling the population to increase in a scenario of constant resources, is highlighted in gray. The arrow depicts the expected population fold increase, given the observed body size reduction in the bird stem lineage as endothermy evolved. These analyses indicate that the energy costs to evolve endothermy are reduced with miniaturization and, as a result, population size may have increased despite the metabolic costs of an endothermic lifestyle.
RESULTS
We estimated the costs of evolving endothermy along the bird stem lineage from reconstructed ancestral body sizes inferred from the fossil record (15). To quantify the energy costs in alternative scenarios, we simulated the evolution of body size along this lineage and obtained the distribution of cost per degree under this null model (Fig. 3). We assumed an undirected Ornstein-Uhlenbeck (OU) model of size evolution bounded between 10 g and 100,000 kg and a mean evolutionary rate equivalent to values reported for these theropods (see Methods). Estimates of cost per degree across different simulated size trajectories were log-normally distributed with a fold-change median of 0.124 per °C (≈10−0.9 in Fig. 3B), which implies that, relative to the metabolism of the ectothermic ancestor MRecto (Eq. 2), the energy cost to increase the thermal niche by 1°C as endothermy evolves amounts to roughly 12.4% at a constant body size. According to our null model, 95% of simulated costs per degree Celsius would fall between 275 and 0.576% depending on whether body size increases or decreases, respectively. In this context, simulations indicate that the energy costs per degree Celsius decrease markedly with miniaturization (Fig. 3). Two phenomena explain these reduced costs. First, the expansion in thermal niche following an increase in MR is disproportionally higher in larger ectotherms because they can maintain a high Tb with a relatively low mass-independent MR due to inertial homeothermy (also known as gigantothermy) (3, 16–18). Accordingly, the residuals of cost per degree controlling for the fold difference in size between ancestor and descendant are negatively related with the simulated ancestral size according to a linear regression (F1,9998 = 1.94 × 105, r2 = 0.95, P < 0.001). That is, the larger the starting size of the ectothermic ancestor, the cheaper the transition to endothermy should be. Second, during miniaturization, the energy costs of being large are traded for being endothermic, which helps to explain how high energy turnover rates evolved despite their impact on food and water requirements. Birds require between 15 and 20 times more food than a similar-sized reptile (6), which could be problematic because the proportional fold reduction in population size expected if resources were constant might jeopardize the population’s long-term persistence in evolutionary time (19, 20) (certainly, the benefits of being endothermic and capable of obtaining more resources might partly offset this limitation).
In contrast, in our model, energy equivalence between an ectotherm and an endotherm is attained with an 8.55-fold decrease in body size (Fig. 3); thus, a 43.3-kg bird should have the same requirements as its 370-kg Tetanurae ancestor, everything else being equal [life is certainly more complicated, and differences in activity patterns or home range size between ectotherms and endotherms should affect this rough estimate (21)]. Assuming that energy can be assigned to either body size or abundance, our analysis shows that, irrespective of the potential increment in food resources resulting from an expanded thermal niche or higher access to small prey as size decreased, populations could still exhibit a 30.5-fold increase as endothermy evolved (Fig. 3). This might explain how, despite the inherent variation in resource availability expected in evolutionary time, smaller sizes and higher energy turnover rates may have been systematically favored in this lineage. Accordingly, the estimated costs of 0.466% per °C estimated for the bird stem lineage are significantly lower than our null distribution (one-tailed P = 0.0172; Fig. 3) and, therefore, energetically cheaper than most simulated scenarios using realistic background rates of body size evolution for theropods. Results remained qualitatively identical for other null models with relaxed assumptions such as a smaller Tetanurae ancestor or assuming Brownian motion model of evolution (see Methods).
The size reduction in the bird stem lineage (15, 22, 23) closely matches the theoretical path of least resistance for endothermy to evolve. We now reconstruct how this phenomenon might have unfolded in the theropod phylogeny. Combining node dates and body size estimates (15) with the allometric shift that would describe the transition to endothermy, we interpolated MR and C of intermediate ancestors in this lineage (Fig. 2). This procedure indicates that the rise in MR spanned most of the Early-Middle Jurassic [~180 to 170 million years (Ma) ago] (Fig. 4) and involved theropod groups in which the occurrence of protofeathers and feathers was already ubiquitous (24). It also suggests that metabolic levels were highly diverse across contemporaneous lineages of Coelurosauria, Maniraptora, and Paraves, which might partly account for the emergence and diversification of these groups during the Late Jurassic (22, 25), and the abnormally high diversity of Coelurosauria at intermediate body sizes (between 30 and 300 kg) when compared against other dinosaur groups (15). A niche-filling model of adaptive radiation in Mesozoic dinosaurs also detected exceptional rates of body size reduction in the bird stem lineage, particularly in the basal nodes of Coelurosauria and Paraves, although no suitable evolutionary hypothesis was proposed to account for this result [table 3 in (23)]. Our analyses show that the evolution of an endothermic machinery, concomitantly with the resulting thermal niche expansion, provides a plausible explanation for both the radiation and the reduction in size detected in these lineages.
Fig. 4. Tempo and mode in the evolution of endothermy.
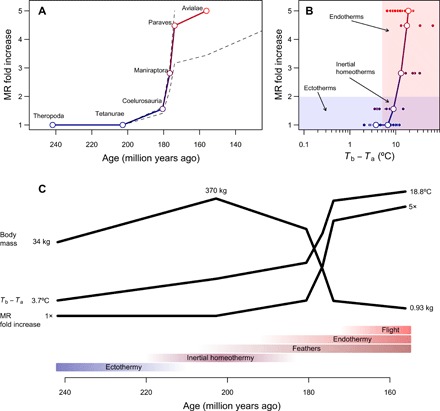
(A) Reconstructed temporal course of metabolic evolution in the bird stem lineage, with dashed lines showing how reconstructions change assuming that either Paraves or Neornithes were fully endothermic instead of the basal bird [for calculations with Neornithes, we assumed a body size of 150 g based on estimates for Vegavis (22) and a time estimate of 100 Ma ago (39)]. The fold increase in MR was calculated by dividing the reconstructed MR during the transition to endothermy by the MR expected for a similar-sized ectotherm and is therefore dimensionless and independent of body size. (B) The evolutionary path of least resistance from ectothermy to endothermy includes inertial homeothermy as a transitional stage, followed by an increase in metabolism concomitantly with a reduction in size. (C) Hypothetical sequence of evolutionary transitions in the bird stem lineage, which combines results from this study with phylogenetic reconstructions of epidermal structures (24, 42) and capacity for active flight (38) (see the main text).
DISCUSSION
Two exceptional phenomena are observed during the evolution of birds: a sustained (but not necessarily gradual) miniaturization lasting millions of years and the emergence of endothermy. We argue that these phenomena are mechanistically linked. Our reconstruction suggests that endothermy evolved concomitantly with the decrease in size along the bird stem lineage, as originally proposed for mammals by McNab (3), and that related theropod clades should exhibit a whole spectrum of MR. Although it may be debatable to what extent energy costs are minimized in evolution, such a principle has been widely invoked or implicitly assumed to explain the diversity of thermoregulatory strategies across extant lineages (7–9) and may be equally useful to study how such a diversity evolved. The proposed scenario explains the conundrum of an expensive lifestyle being systematically favored despite its energy costs and explains the sustained miniaturization that preceded the origin of birds (15, 22, 23, 26), the so-called “mesothermy” (12), and intermediate to high growth rates of many dinosaurs (12, 27–32). Previously labeled “mesotherms” (12) are either inertial homeotherms such as tunas, leatherback sea turtles, and large dinosaurs or small endotherms such as echidnas and, according to our reconstruction, the maniraptor Troodon and Archaeopteryx (Fig. 2). In this context, mesothermy constitutes an ambiguous concept from a mechanistic perspective because elevated MR due to thermodynamic effects (i.e., a high Tb due to thermal inertia and a large body size) is confounded with high MR due to the evolution of increased mass-independent energy turnover rates and true endothermy.
Inertial homeothermy might, in fact, constitute a necessary transitional state (3, 16–18) in which homeothermy and a high thermal gradient Tb − Ta can be maintained at low metabolic costs. That is, we posit that the MR of the large ancestral theropods (>300 kg) would fall in the allometric curve for ectotherms (21) and, yet, these organisms would be able to maintain a thermal gradient more in line with that of extant endotherms (Fig. 4B). Subsequently, selection toward smaller sizes would favor elevated MR if these large ancestral theropods were physiologically committed to homeothermy, as discussed by McNab (3), which would explain the departure from the ectothermic metabolic allometry with miniaturization (Fig. 2A). While this proposition has been generally dismissed (6, 33, 34) on the basis that a “large size obviates thermoregulatory needs for high metabolic rates” (16), this counterargument is true only within a limited range of Ta and body sizes and neglects other ecological advantages of a high mass-independent aerobic capacity and the advantage to a high reproductive rate, growth rate, etc. In large terrestrial animals, selection on increased MR for sustained activity, parental care, or high growth rates, presumed drivers of the evolution of endothermy in birds and mammals according to different hypotheses (4, 5), should inevitably increase the thermal gradient Tb − Ta and give rise to larger thermal niches as a useful by-product (Fig. 2). In evolutionary time, it is only reasonable to expect lineages to exploit newly opened niches and eventually diversify (35). Under this scenario, thermoregulatory performance is expected to evolve regardless of the selective pressures favoring a high aerobic capacity, which reconciles alternative theories on the evolution of endothermy in birds and mammals (2–6).
While quantitative estimates are expected to vary with the allometric relations used, these patterns should be robust to variation in scaling and violations of the model’s assumptions. On the basis of Eq. 1, as long as the MR scaling exponent remains greater than that of C, the thermal gradient Tb − Ta should increase with size and energy costs should decrease. In addition, the greater empirical MR scaling exponent described for ectotherms (12, 14) would result in lower energy costs per degree Celsius at larger sizes, whereas the lower Tb of smaller ectotherms should disproportionally increase the energy requirements to attain endothermy within this size range. The thermodynamic constraint imposed by a lower Tb would also buffer any potential advantage of a higher aerobic capacity on performance (i.e., highly aerobic lizards remain inactive when they are cold); hence, some degree of homeothermy would be desirable for high MR to evolve. In this context, it is important to recall that our model assumes a constant Tb for practical reasons, whereas, in reality, Tb likely varied across groups and was possibly higher and more stable in larger lineages, everything else being equal, due to a reduced surface relative to volume (36). Inherently higher and stable Tb in larger dinosaurs might partly explain their intermediate metabolic levels between reptiles and extant mammals and birds and may have contributed to the evolution of endothermy by facilitating parental care and favoring higher growth rates in these lineages (32).
Admittedly, the size reduction immediately preceding the radiation of birds could also be related with the evolution of flight, and it is quite possible that paravians or earlier groups were fully endothermic. The intermediate growth rates of these groups suggest otherwise (12, 27–32), and given the accelerated rates of body size reduction in this period, this possibility does not alter the general trend of MR evolution reported here (Fig. 4). Moreover, relative forelimb elongation and increased flapping assisted locomotion are detected primarily within paravians (37, 38). Consequently, the stepwise evolution in body size reported by Benson et al. (15), with a first sustained reduction between the ancestors of Tetanurae and Paraves and a second shift within Avialae roughly 20 Ma later, might be associated with, respectively, the evolution of endothermy and flight. Alternatively, if basal birds were not fully endothermic and this derived condition emerged later in groups such as crown group birds (Neornithes) (39), as suggested by the slow growth rates of Archaeopteryx (27), then these traits may have coevolved in tandem, to some extent, within the avian lineage. Flightless birds exhibit lower MR (40), and there is substantial variation in metabolic levels among extant birds (41); hence, these alternatives are not mutually exclusive. On the contrary, one should perhaps envision a scenario in which aerobic capacity shifted multiple times along the theropod and avian phylogeny (Fig. 2), resulting in a gradual rise in MR above the allometry of extant reptiles and a whole gradient of metabolic levels in these groups.
In any case, comparative analyses suggest that feathers or protofeathers evolved before the emergence of Coelurosauria (24, 42) and the rise in MR reconstructed in our study (Fig. 4). Together, the available evidence indicates that the evolution of flight cannot explain the reduction in size around the Early-Middle Jurassic boundary interval (~180 to 170 Ma ago). More crucially, our results, combined with previous analyses on the evolution of body size, feathers, and flight in the bird stem lineage (15, 22–24, 26, 37, 38, 42), give rise to a relatively well-defined temporal sequence of key evolutionary transitions and a detailed working hypothesis for future studies (Fig. 4). This interpretation, that endothermy preceded the evolution of flight, is also consistent with descriptions of skeletal pneumaticity in derived, but not basal theropods (43), and mirrors the evolutionary sequence that can be inferred for bats since endothermy is a plesiomorphy present in virtually all mammals. Whether a similar scenario could explain the evolution of flight in pterosaurs, which have been recently described to exhibit feather-like integumentary structures (44), remains an open question. The elevated MR among bats and the enormous variation in metabolism across mammalian groups constitute a reminder that endothermy (and ectothermy, for that matter) constitutes a matter of degree rather than kind, which might explain why the earliest dinosaurs may have exhibited higher metabolic rates than those of extant reptiles (45) and early bird-like taxa growth rates that were not quite comparable to modern birds according to bone histology (27, 32). Thus, these findings are not necessarily at odds with our proposition that the marked reduction in body size during the evolution of the bird stem lineage was accompanied by a major shift in metabolic levels; they simply highlight that there is likely more to the evolution of endothermy in extinct archosaurs, dinosaurs, and birds than our analyses can convey.
METHODS
To quantify how the sustained reduction in body size along the avian lineage would affect energy expenditure during the evolution of endothermy, we used the theropod phylogeny and body mass estimates reconstructed from the fossil record reported by Benson et al. (23) to build a realistic null model. We cross-validated this dataset against an independent study (22), which includes other taxa and is based on different methods to estimate body mass, phylogenetic relations, and evolutionary trends. For the species shared between these studies (n = 94), body mass estimates were very similar according to a regular regression (slope, 1.10 ± 0.02 SE; r2 = 0.98, P < 0.001), and so was the structure of the phylogenies in both studies based on Baker’s γ, which estimates the similarity between two trees of hierarchical clustering and varies between −1 and 1 as a regular correlation (γ = 0.962, P < 0.001) (figs. S1 and S2). While both studies reach very similar conclusions and detect exceptional body size reduction in nodes along the bird stem lineage (22, 23), there are two remarkable differences: Lee et al. (22) reported a sustained, gradual decrease in body size along the bird stem lineage from a large ancestral theropod (175 kg), whereas Benson et al. (23) suggested that this miniaturization occurred in a stepwise fashion and later in time, following a period of size increase in early theropod evolution from a smaller ancestor (10 to 30 kg). Because the larger ancestral size seems to be an artifact of incomplete and biased sampling of early taxa (15) and the stepwise decrease in size was observed by Novas et al. (46), estimates used here correspond to unweighted averages of reconstructed ancestral body mass and divergence times obtained across 20 candidate phylogenies by Benson et al. [appendix S5 in (15)]. The dataset used does not change the general conclusions of this study, and analyses using body mass data and phylogeny of Lee et al. (22) are shown in the Supplementary Materials (figs. S3 to S5).
Allometric equations for endotherms were obtained from the literature for MR (12) and C (13). For mathematical tractability and to ensure that results were comparable across different ranges of body size, we obtained parallel curves for ectotherms by dividing the intercept of these equations by 5 (14) and 2.5 (11). On the basis of these allometric curves, we then assigned typical ectothermic and endothermic values for the Tetanurae node (~370 kg, range 290 to 420 kg) that corresponds to the largest theropod ancestor in most reconstructions (15, 22) and for the basal bird (~0.93 kg, range 0.78 to 1.10 kg). Results are expressed as fold change in MR or C with respect to the ectothermic allometric curve throughout the study, calculated as the ratio of the observed estimate/expectation for a similar-sized ectotherm. Subsequently, from linear log-log MR and C curves connecting these two taxa (Fig. 2, B and C), we interpolated how these variables evolved in the bird stem lineage using the body size estimates reconstructed for intermediate nodes. With the MR and C calculated for these nodes combined with divergence time estimates, we then reconstructed how metabolic levels evolved along the bird stem lineage. This approach assumes, for simplicity, a constant rate of fold change in MR and C as body size decreases, which is unlikely. Nonetheless, the exceedingly high rates of body size reduction observed primarily between Neotetanurae and Paraves (15, 22, 23) constrain the period in which metabolic levels increased in a narrow temporal window, which should remain largely unaffected by the general shape of the evolutionary path connecting the end points (i.e., the ectothermic ancestor and its and endothermic descendant). For the remaining species in the theropod phylogeny, we assumed that they inherited the fold change in MR and C from their most recent ancestor in the bird stem lineage, shifted the intercept of the allometric curves accordingly to obtain appropriate estimates of MR and C accounting for size effects, and then calculated Tb − Ta with Eq. 1.
Null model
After quantifying the energy costs of evolving endothermy for the basal stem lineage leading to birds (Eq. 2), we built a null distribution of energy costs by simulating 10,000 different body size trajectories along this lineage under a null model. The body size distribution for the ancestral ectotherms was sampled from a uniform distribution ranging between 10 g and 100,000 kg, which encapsulate the range of body sizes observed within the theropod phylogeny, and the distribution of their descendent endotherms was constrained within this same range by removing those replicates falling outside this range. These boundaries are included to ensure that the body size null distributions fall within the range observed across higher vertebrates, which presumably reflect biomechanical or physiological constraints (e.g., simulating a 1-g endotherm is not realistic as sustaining a thermal gradient at this size is not really possible).
Body size evolution was simulated with an OU process, using a code written ad hoc. We used the σ2 and α parameters fitted to 20 candidate theropod phylogenies by Benson et al. (15), which measure, respectively, the intensity of random fluctuations in the evolutionary process and the strength of selection toward a presumed optimal trait value θ. To be as conservative as possible, from the seven single-optimum OU models with the best fit reported by Benson et al. (15), we used the parameters that resulted in the highest phenotypic variance following 10,000 diagnostic simulations: σ2 = 0.025 and α = 0.005 (obtained from the Theropoda tree 1 in their appendix S5; see figs. S6 and S7 for additional details on the null model and selected parameters). In addition, to remove the contribution of directional trends in these models and obtain random variation comparable to the empirical data, we used the same α and σ2 fitted for each tree and setting θ to the ancestral body mass [appendix S5 in (15)]. The amount of time between the ectothermic ancestor and endothermic descendant was set to 47 Ma, or the average difference (46.6 ± 2.9 Ma) between the Tetanurae and basal bird nodes across the candidate phylogenies (15).
With this approach, we obtained a null distribution of energy costs to evolve endothermy from theropods to paravians under different body size trajectories, controlling for the amount of time available for this transition and using body size evolutionary rates inferred from fossil data. Subsequently, we tested whether the cost per degree estimated for the bird stem lineage was lower than that of 95% of the simulated datasets, which would indicate that the empirical trajectory is energetically cheaper than the null hypothesis holding type I error rates at 0.05, and suggest that the observed miniaturization constitutes an “evolutionary path of least resistance” from an energetic point of view. Results using a Brownian null model setting α = 0.0, which is more conservative as the body size variance under this null model is expected to increase, were qualitatively identical (fig. S8). Similarly, results from our null model were also statistically robust to uncertainty in the ancestral body size of the Tetanurae node, whose estimated range between 290 and 420 kg always resulted in reduced energy costs associated with miniaturization with a P < 0.05.
Supplementary Material
Acknowledgments
We are grateful to S. Finnegan, D. Rubilar-Rogers, A. Vargas, B. K. McNab, and three anonymous reviewers for comments on different drafts of this study. Silhouettes in Fig. 1 were made by Freepik from www.flaticon.com. Funding: This work was partly funded by a CONICITY PIA/BASAL FB 0002-2014 grant to F.B. and a FONDECYT grant 1170017 to E.L.R. Author contributions: All authors were involved in the design of the study. E.L.R. compiled the data, performed analyses, and wrote the first draft, which was then edited by L.D.B., R.F.N., and F.B. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. All the data and R scripts used in this study are available at DRYAD (https://doi.org/10.5061/dryad.76hdr7ss0).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/1/eaaw4486/DC1
Fig. S1. Relationship between body size reconstructions performed by Benson et al. (15, 23) and Lee et al. (22).
Fig. S2. Comparison between the topologies of the theropod phylogeny reconstructed by Lee et al. (22) and Benson et al. (15, 23).
Fig. S3. Replicate of Fig. 2, except that, in this case, analyses were replicated using the dataset and phylogeny by Lee et al. (22).
Fig. S4. Replicate of Fig. 4, except that, in this case, analyses were replicated using the dataset and phylogeny by Lee et al. (22).
Fig. S5. Comparison between reconstructed metabolic levels along the bird stem lineage using the dataset by Benson et al. (15) and Lee et al. (22), plotted against the 1:1 line.
Fig. S6. Phenotypic variance simulated with the difference parameters fitted by Benson et al. (15) for the theropod phylogeny (parameters available in their appendix S5).
Fig. S7. Simulated OU model overlapped against the empirical data from Benson et al. (15) (their appendix S5), which shows that this model can replicate the distribution of phenotypic data observed along the theropod phylogeny and provide a valid “null model” in the absence of directionality (see below).
Fig. S8. Results from the null model in the main text compared against expectations for a more conservative model assuming Brownian motion.
REFERENCES AND NOTES
- 1.Nespolo R. F., Bacigalupe L. D., Figueroa C. C., Koteja P., Opazo J. C., Using new tools to solve an old problem: The evolution of endothermy in vertebrates. Trends Ecol. Evol. 26, 414–423 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Crompton A. W., Taylor C. R., Jagger J. A., Evolution of homeothermy in mammals. Nature 272, 333–336 (1978). [DOI] [PubMed] [Google Scholar]
- 3.McNab B. K., Evolution of endothermy in the phylogeny of mammals. Am. Nat. 112, 1–21 (1978). [Google Scholar]
- 4.Bennett A. F., Ruben J. A., Endothermy and activity in vertebrates. Science 206, 649–654 (1979). [DOI] [PubMed] [Google Scholar]
- 5.Farmer C. G., Parental care: The key to understanding endothermy and other convergent features in birds and mammals. Am. Nat. 155, 326–334 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Clarke A., Pörtner H.-O., Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703–727 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Scholander P. F., Hock R., Walters V., Irving L., Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol. Bull. 99, 259–271 (1950). [DOI] [PubMed] [Google Scholar]
- 8.Rezende E. L., Bacigalupe L. D., Thermoregulation in endotherms: Physiological principles and ecological consequences. J. Comp. Physiol. B 185, 709–727 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Fristoe T. S., Burger J. R., Balk M. A., Khaliq I., Hof C., Brown J. H., Metabolic heat production and thermal conductance are mass-independent adaptations to thermal environment in birds and mammals. Proc. Natl. Acad. Sci. 112, 15934–15939 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartholomew G. A., Tucker V. A., Size, body temperature, thermal conductance, oxygen consumption, and heart rate in Australian varanid lizards. Physiol. Zool. 37, 341–354 (1964). [Google Scholar]
- 11.McNab B. K., Auffenberg W., Effect of large body size on temperature regulation of Komodo dragon Varanus komodoensis. Comp. Biochem. Physiol. A 55, 345–350 (1976). [DOI] [PubMed] [Google Scholar]
- 12.Grady J. M., Enquist B. J., Dettweiler-Robinson E., Wright N. A., Smith F. A., Evidence for mesothermy in dinosaurs. Science 344, 1268–1272 (2014). [DOI] [PubMed] [Google Scholar]
- 13.McNab B. K., Morrison P., Body temperature and metabolism in subspecies of Peromyscus from arid and mesic environments. Ecol. Monogr. 33, 63–82 (1963). [Google Scholar]
- 14.White C. R., Phillips N. F., Seymour R. S., The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson R. B. J., Hunt G., Carrano M. T., Campione N., Cope’s rule and the adaptive landscape of dinosaur body size evolution. Palaeontology 61, 13–48 (2018). [Google Scholar]
- 16.Paladino F. V., O'Connor M. P., Spotila J. R., Metabolism of leatherback turtles, gigantothermy, and thermoregulation of dinosaurs. Nature 344, 858–860 (1990). [Google Scholar]
- 17.Gillooly J. F., Allen A. P., Charnov E. L., Dinosaur fossils predict body temperatures. PLoS Biol. 4, e248 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour R. S., Maximal aerobic and anaerobic power generation in large crocodiles versus mammals: Implications for dinosaur gigantothermy. PLOS ONE 8, e69361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas C. D., What do real population dynamics tell us about minimum viable population sizes? Conserv. Biol. 4, 324–327 (1990). [Google Scholar]
- 20.Burness G. P., Diamond J., Flannery T., Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proc. Natl. Acad. Sci. U.S.A. 98, 14518–14523 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNab B. K., Resources and energetics determined dinosaur maximal size. Proc. Natl. Acad. Sci. 106, 12184–12188 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M. S. Y., Cau A., Naish D., Dyke G. J., Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345, 562–566 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Benson R. B. J., Campione N. E., Carrano M. T., Mannion P. D., Sullivan C., Upchurch P., Evans D. C., Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett P. M., Evans D. C., Campione N. E., Evolution of dinosaur epidermal structures. Biol. Lett. 11, 20150229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Zhou Z., Dudley R., Mackem S., Chuong C.-M., Erickson G. M., Varricchio D. J., An integrative approach to understanding bird origins. Science 346, 1253293 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Puttick M. N., Thomas G. H., Benton M. J., High rates of evolution preceded the origin of birds. Evolution 68, 1497–1510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson G. M., Rauhut O. W. M., Zhou Z., Turner A. H., Inouye B. D., Hu D., Norell M. A., Was dinosaurian physiology inherited by birds? Reconciling slow growth in Archaeopteryx. PLOS ONE 4, e7390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padian K., Horner J. R., De Ricqlès A., Growth in small dinosaurs and pterosaurs: The evolution of archosaurian growth strategies. J. Vertebr. Paleontol. 24, 555–571 (2004). [Google Scholar]
- 29.Sander P. M., Christian A., Clauss M., Fechner R., Gee C. T., Griebeler E. M., Gunga H. C., Hummel J., Mallison H., Perry S. F., Biology of the sauropod dinosaurs: The evolution of gigantism. Biol. Rev. 86, 117–155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padian K., de Ricqlès A. J., Horner J. R., Dinosaurian growth rates and bird origins. Nature 412, 405–408 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Werner J., Griebeler E. M., Allometries of maximum growth rate versus body mass at maximum growth indicate that non-avian dinosaurs had growth rates typical of fast growing ectothermic sauropsids. PLOS ONE 9, e88834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson G. M., On dinosaur growth. Annu. Rev. Earth Planet. Sci. 42, 675–697 (2014). [Google Scholar]
- 33.Seebacher F., Dinosaur body temperatures: The occurrence of endothermy and ectothermy. Paleobiology 29, 105–122 (2003). [Google Scholar]
- 34.Kemp T. S., The origin of mammalian endothermy: A paradigm for the evolution of complex biological structure. Zool. J. Linn. Soc. 147, 473–488 (2006). [Google Scholar]
- 35.Price T. D., Hooper D. M., Buchanan C. D., Johansson U. S., Tietze D. T., Alström P., Olsson U., Ghosh-Harihar M., Ishtiaq F., Gupta S. K., Niche filling slows the diversification of Himalayan songbirds. Nature 509, 222–225 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Seebacher F., Grigg G. C., Beard L. A., Crocodiles as dinosaurs: Behavioural thermoregulation in very large ectotherms leads to high and stable body temperatures. J. Exp. Biol. 202, 77–86 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Dececchi T. A., Larsson H. C. E., Body and limb size dissociation at the origin of birds: Uncoupling allometric constraints across a macroevolutionary transition. Evolution 67, 2741–2752 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Dececchi T. A., Larsson H. C. E., Habib M. B., The wings before the bird: An evaluation of flapping-based locomotory hypotheses in bird antecedents. PeerJ 4, e2159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayr G., The origins of crown group birds: Molecules and fossils. Palaeontology 57, 231–242 (2014). [Google Scholar]
- 40.Mcnab B. K., Energy-conservation and the evolution of flightlessness in birds. Am. Nat. 144, 628–642 (1994). [Google Scholar]
- 41.Swanson D. L., Garland T., The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution 63, 184–194 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Zelenitsky D. K., Therrien F., Erickson G. M., DeBuhr C. L., Kobayashi Y., Eberth D. A., Hadfield F., Feathered non-avian dinosaurs from North America provide insight into wing origins. Science 338, 510–514 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Benson R. B. J., Butler R. J., Carrano M. T., O'Connor P. M., Air-filled postcranial bones in theropod dinosaurs: Physiological implications and the ‘reptile’-bird transition. Biol. Rev. 87, 168–193 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Yang Z., Jiang B., McNamara M. E., Kearns S. L., Pittman M., Kaye T. G., Orr P. J., Xu X., Benton M. J., Pterosaur integumentary structures with complex feather-like branching. Nature Ecol. Evol. 3, 24 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Benson R. B. J., Dinosaur macroevolution and macroecology. Ann. Rev. Ecol. Evol. System. 49, 379–408 (2018). [Google Scholar]
- 46.Novas F. E., Ezcurra M. D., Agnolin F. L., Pol D., Ortíz R., New Patagonian Cretaceous theropod sheds light about the early radiation of Coelurosauria. Rev. Mus. Argent. Cienc. Nat. 14, 57–81 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/1/eaaw4486/DC1
Fig. S1. Relationship between body size reconstructions performed by Benson et al. (15, 23) and Lee et al. (22).
Fig. S2. Comparison between the topologies of the theropod phylogeny reconstructed by Lee et al. (22) and Benson et al. (15, 23).
Fig. S3. Replicate of Fig. 2, except that, in this case, analyses were replicated using the dataset and phylogeny by Lee et al. (22).
Fig. S4. Replicate of Fig. 4, except that, in this case, analyses were replicated using the dataset and phylogeny by Lee et al. (22).
Fig. S5. Comparison between reconstructed metabolic levels along the bird stem lineage using the dataset by Benson et al. (15) and Lee et al. (22), plotted against the 1:1 line.
Fig. S6. Phenotypic variance simulated with the difference parameters fitted by Benson et al. (15) for the theropod phylogeny (parameters available in their appendix S5).
Fig. S7. Simulated OU model overlapped against the empirical data from Benson et al. (15) (their appendix S5), which shows that this model can replicate the distribution of phenotypic data observed along the theropod phylogeny and provide a valid “null model” in the absence of directionality (see below).
Fig. S8. Results from the null model in the main text compared against expectations for a more conservative model assuming Brownian motion.


