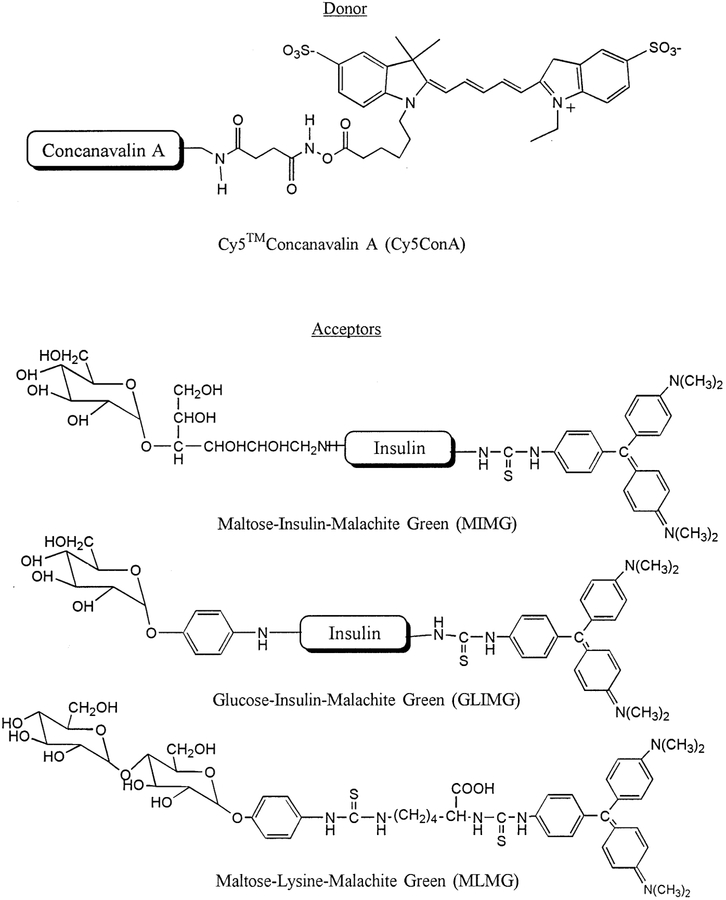
Abstract
An optical assay for glucose is described based on the luminescence decay time of a long wavelength dye (Cy5) which can be excited with currently available red laser diodes. Concanavalin A was covalently labeled with Cy5 which served as the donor in an assay based on fluorescence resonance energy transfer (FRET). The acceptor was Malachite Green which was covalently linked to insulin which served as a carrier protein. To provide binding affinity for ConA Malachite Green insulin was also covalently labeled with maltose (MIMG). Binding of Cy5ConA to MIMG resulted in a decreased intensity and decay time of Cy5 as observed by time-correlated single photon counting. Glucose was detected by competitive displacement of MIMG from Cy5ConA, resulting in increased intensity and decay time. This glucose assay has several features which can result in practical real world assays for glucose. The long absorption wavelength of Cy5 allows excitation with red laser diodes, which can be readily pulsed or amplitude-modulated for time-domain or frequency-domain decay time measurements. Additionally, decay times can be measured through skin using long wavelength excitation and emission, suggesting the possibility of an implanted glucose sensor. And finally, the assay affinity and reversibility can in principle be adjusted by controlling the extent and type of sugar labeling of the carrier protein.
Keywords: Lifetime-based sensing, Glucose assay, Cy5™, Energy transfer
1. Introduction
Blood glucose measurements are performed frequently in clinical labs, doctor’s offices and by diabetics using home test kits. At present, most glucose test strips rely on oxidation of glucose by glucose oxidase to yield a colored product [1,2]. Such methods require freshly drawn blood, which is unpleasant and precludes the use of this method as a feedback loop for an insulin pump. Hence, the development of non-invasive optical sensing of glucose has been pursued in numerous laboratories. Many methods have been suggested for optical measurements of glucose. These methods include measurement of oxygen consumption by glucose oxidase using an oxygen optrode [3,4], measurements of the changes in pH which accompany glucose oxidation [5,6], use of the intrinsic flavin fluorescence of glucose oxidase [7], and the use of optical activity [8–10]. Another approach to glucose sensing has been the use of near infrared (NIR) spectroscopy [11,12], which in principle could provide non-invasive optical sensing of tissue glucose. While promising [13,14], NIR spectroscopy has not successfully become available for use by diabetics.
We are presently attempting to develop glucose sensing methods which will allow quasi non-invasive glucose sensing using implantable sensors placed under the skin. This approach was chosen over a completely non-invasive method because of the difficulties encountered with such methods. Our goal is to develop a glucose patch in which the decay time changes in response to tissue glucose concentrations. Knowledge of the tissue glucose concentration, as compared to blood glucose, is likely to be adequate if not preferable given the close correlation between these two glucose levels [15]. The use of decay times, as opposed to intensities, is preferred because the decay times are mostly independent of probe concentrations or the intensity of the fluorescence signal. Importantly, lifetimes can be measured in highly scattering media [16,17], and have been successfully measured through several layers of chicken skin [18]. In previous reports we demonstrated that glucose could be measured using lifetimes based on the phenomenon of fluorescence resonance energy transfer [19,20]. Such assays are based on the reversible binding of ConA with sugars, as first used by Schultz and co-workers using dextrans as the sugars [21–23], except that our glucose assays are based on decay times rather than intensities. In our assay we used both short [19] and long lifetime metal–ligand complex [20] donors. However, to date, all available glucose assays require ultraviolet (UV) or visible excitation, and such wavelengths do not allow trans-dermal sensing. In the present report we describe a glucose assay based on the donor Cy5 (Scheme 1), which can be excited at wavelengths up to 700 nm, and thus using wavelengths which readily penetrate skin. Additionally, we used an acceptor other than dextran, a Malachite Green-labeled protein, which provided less aggregation and improved reversibility of the assay.
Scheme 1.
Donor-labeled ConA and sugar-acceptor labeled insulin and lysine.
2. Materials and methods
Concanavalin A (ConA), bovine insulin and maltose were purchased from Sigma, Malachite Green isothiocyanate from Molecular Probes, and Cy5™ Monofunctional dye from Amersham.
Cy5-labeled ConA (Cy5ConA) was prepared by dissolving 1 mg of ConA and 1.5 mg α-methyl mannoside in 1 ml of 0.1 M bicarbonate buffer, pH 9.0. This solution was added to the vial containing the pre-measured dye and mixed thoroughly. The mixture was incubated for two hours at room temperature with occasional stirring and the resulting conjugate was separated from the free dye by passing the reaction mixture thru a Sephadex G-50 column equilibrated with buffer containing 100 mM α-methyl mannoside and 10% dioxane. The product, Cy5ConA was freed of the sugar by repeated dialysis.
Preparation of the maltose–insulin conjugate was accomplished as reported by Jeong et al. [24]. The degree of glycosylation was determined by the phenol– sulfuric acid method [25], and was found to be 1 maltose per insulin molecule. The maltose–insulin– Malachite Green conjugate (MIMG) preparation was described in a previous paper [20]. Malachite Green isothiocyanate (5.9 mg) in DMF was added to insulin– maltose dissolved in 0.2 M bicarbonate buffer, pH 8.5. The reaction was stirred for an hour at room temperature and then quenched by the addition of hydroxylamine with incubation for an additional hour at room temperature. The conjugate was purified by gel filtration on a Biogel P6 column. The purified protein contained an average of 1.5 Malachite Green dyes per insulin molecule. All measurements were done in pH 6.8 buffer containing 0.25 M NaCl, 0.005 M NaH2PO4, 0.2 mM CaCl2, 0.1 mM MnCl2 and 0.02% sodium azide.
Emission spectra were recorded on an Aminco SLM AB2 spectrofluorometer using an excitation wavelength of 580 nm. Polarizers were used to eliminate the effect of Brownian rotation. Time-resolved measurements were performed by time-correlated single photon counting [26]. The light source was a cavity-dumped rhodamine 6G dye laser at 580 nm with a repetition rate of 1 MHz. The detection was a Hamamatsu R2809 microchannel plate PMT. The emission was isolated using a 620 nm cut off filter (Andover). The excitation was ventrically polarized and the emission detected through a polarizer at 54.7° from the vertical to eliminate the effects of Brownian rotation on the observed decay times.
The time-domain intensity data were fit to a multi-exponential decay law
| (1) |
where αi and τi are the preexponential factors and decay times, respectively. The parameters values (αi, τi) were determined by least square analysis using software from IBH. The values of αi and τi were used to calculate the mean lifetime, τ
| (2) |
The intensity decays of Cy5ConA were found to be multi-exponential both in the absence and presence of the MIMG acceptor. The mean decay time represent a weighted average of the decay times (τi) displayed by the sample.
3. Results
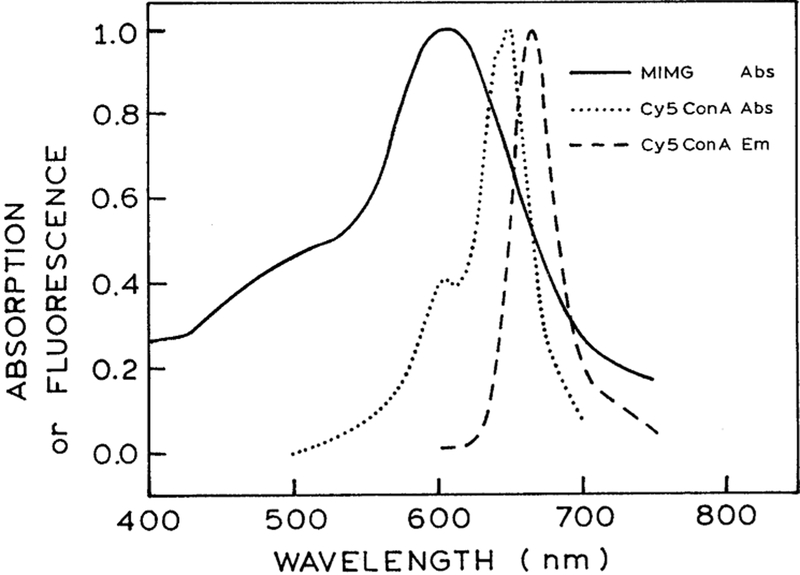
The absorption spectrum of Cy5ConA is shown in Fig. 1. This spectrum shows the favorable properties of the Cy5 as a luminescent probe for trans-dermal sensing. Cy5 displays an absorption maximum near 650 nm, with an absorption tail to 700 nm. Hence, it can be readily excited with red laser diodes. It is well known that the output of such diodes can be rapidly modulated or pulsed on a sub-nanosecond timescale. Additionally, wavelengths above 620 nm are weakly absorbed by skin and readily penetrate tissues. Hence, the use of Cy5 or similar long wavelength dyes [27,28] allow trans-dermal measurements, assuming a scheme for coupling the glucose concentrations to the decay time can be obtained.
Fig. 1.
Absorption spectrum of the acceptor MIMG, and the absorption and emission spectrum of the donor Cy5ConA.
To provide a lifetime change in the presence of glucose we used the phenomenon of fluorescence resonance energy transfer (FRET). For reasons described previously [20] we use insulin which was doubly labeled with maltose and the acceptor malachite green. The maltose acts as the recognition site for ConA binding. Upon binding to ConA the acceptor dye is in close proximity with the donor thereby allowing energy transfer to occur. In addition, it acts as the competitor for glucose, thus providing the basis for measuring glucose concentrations. We chose maltose for this purpose because when conjugated to insulin, it was reported to have comparable binding affinity for ConA as glucose [29]. The absorption spectrum of the acceptor, maltose–insulin–Malachite Green (MIMG), is shown in Fig. 1. It has an extinction coefficient of 30 000 M−1 cm−1 at 670 nm where it overlaps with the emission spectrum of Cy5ConA. Based on the spectral properties of Cy5ConA and Malachite Green, the estimated Forster distance is about 35Å.
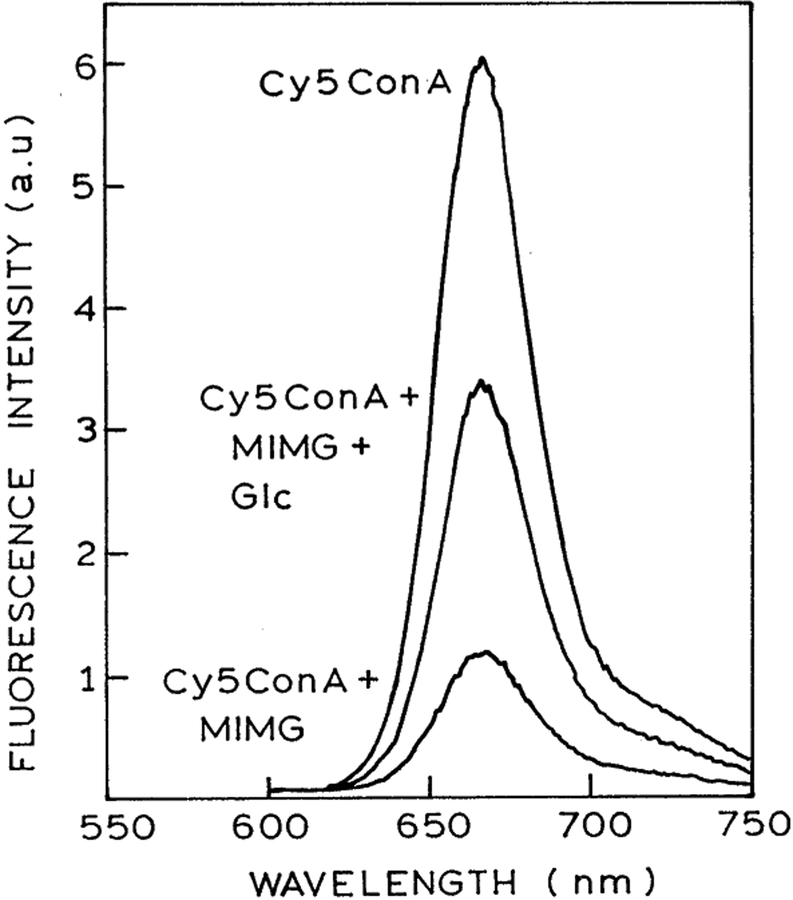
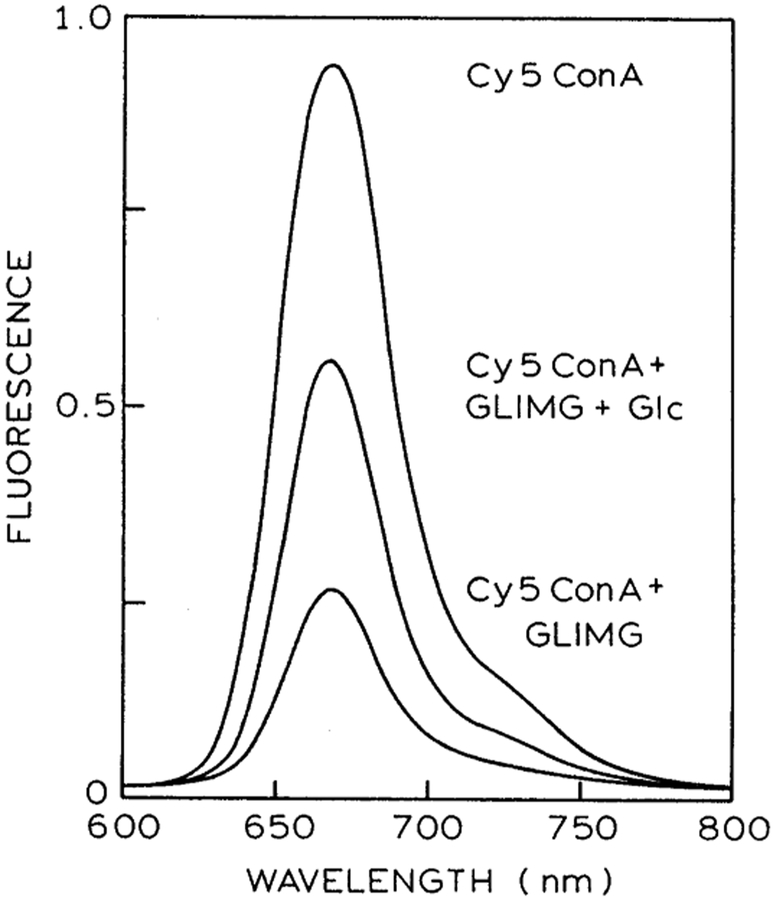
The emission spectrum of Cy5ConA in the presence of MIMG is shown in Fig. 2. Decreased intensity of Cy5ConA with increased concentration of MIMG is due to both energy transfer and inner filter effects. In order to confirm that a significant fraction of the quenching is due to energy transfer, intensity decay measurements were performed. Time-resolved data are unaffected by inner filter effects and can be used to estimate energy transfer parameters for a D–A system using appropriate models.
Fig. 2.
Emission spectrum of 2 μM Cy5ConA alone; in the presence of 4 μM MIMG; and in the presence of 4 μM MIMG plus 150 mM glucose.
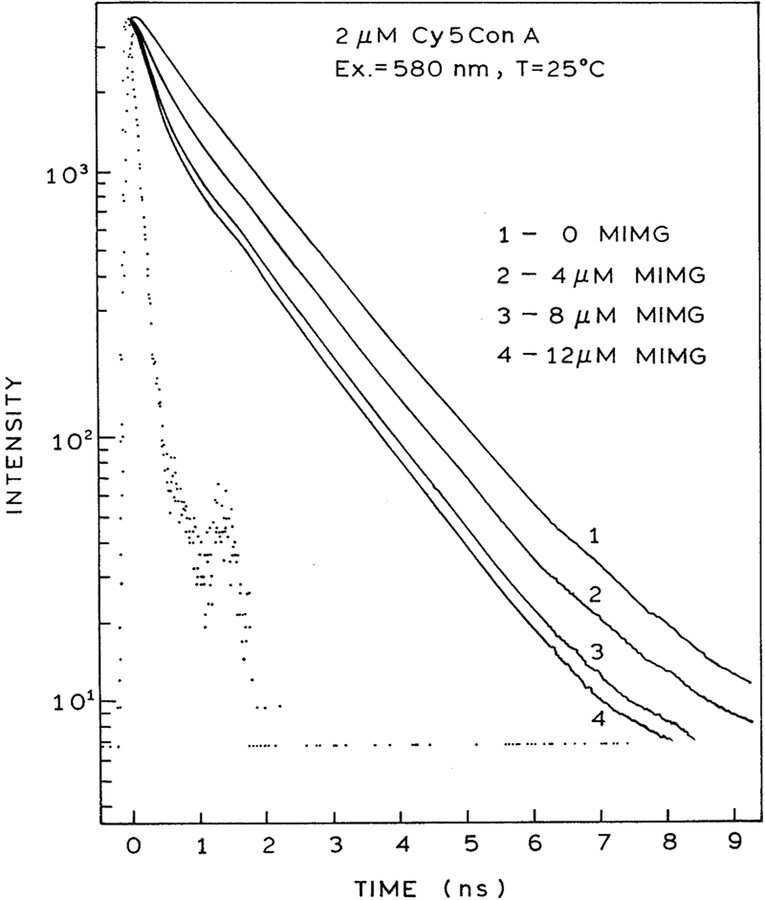
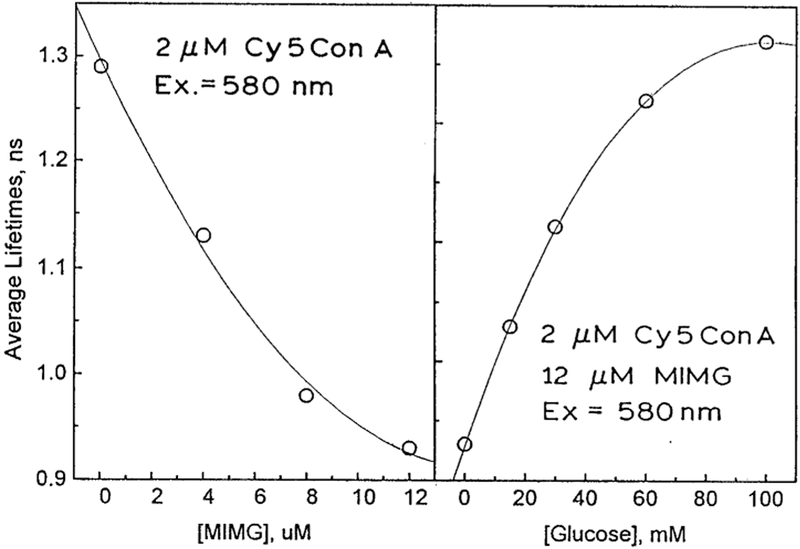
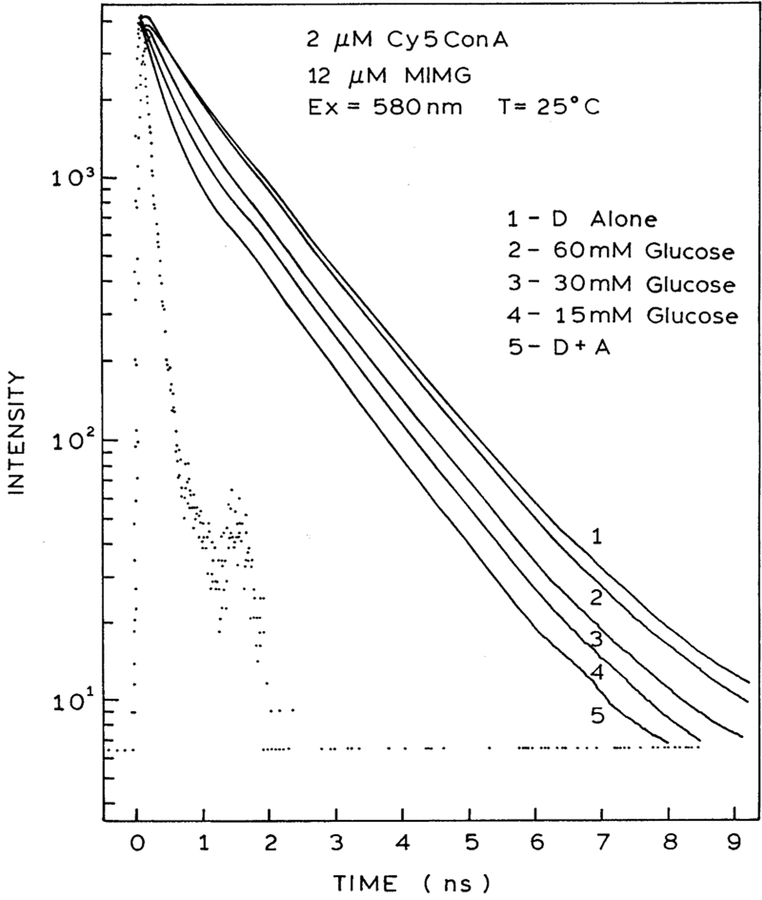
Time-domain intensity decays of Cy5ConA in the presence of increasing concentrations of MIMG are shown in Fig. 3. Close examination of the data reveals a short lifetime component whose amplitude increases with increasing concentrations of MIGM. Least squares analysis of the time-resolved data confirm this visual impression (Table 1). The intensity decay of Cy5ConA is at least double exponential in the absence or presence of MIMG. As the concentration of MIMG is increased, the fractional amplitude of the shorter decay time decreases. These effects are consistent with FRET from Cy5ConA to MIMG. The extent of energy transfer and/or MIMG binding can also be seen from the decrease in the mean lifetime of Cy5ConA (Fig. 5, left). In previous studies [20] we showed that there was no interaction of labeled ConA with non-glycosylated insulin-MG, showing that the maltose moiety was responsible for binding.
Fig. 3.
Excited state decay data of Cy5ConA (2 μM) with increasing concentrations of MIMG.
Table 1.
Excited state lifetimes of 2 μM Cy5ConA at various concentrations of MIMG
| τi (ns) | fi | ||
|---|---|---|---|
| 2 μM Cy5ConA | 0.66 | 0.221 | 1.12 |
| 1.48 | 0.779 | ||
| 2 μM Cy5ConA+ | 0.39 | 0.223 | 1.05 |
| 4 μM MIMG | 1.371 | 0.777 | |
| 2 μM Cy5ConA+ | 0.288 | 0.295 | 1.12 |
| 8 μM MIMG | 1.313 | 0.705 | |
| 2 μM Cy5ConA+ | 0.281 | 0.326 | 1.28 |
| 12 μm MIMG | 1.300 | 0.674 |
Fig. 5.
Excited state decay of Cy5ConA alone; Cy5ConA+MIMG and increasing concentrations of glucose.
The steady-state fluorescence spectra of 2 μM Cy5ConA in the presence of 4 μM MIMG and 150 mM glucose is shown in Fig. 2. This figure shows that the emission intensity of Cy5ConA is increased by the addition of glucose, suggesting competitive displacement of MIMG by glucose. We confirmed the decreased binding of Cy5ConA to MIMG in the presence of glucose by time-resolved measurements. Addition of glucose results in elimination of the shorter component due to MIMG (Fig. 4). In fact, visual inspection of the Cy5ConA intensity decays suggests that FRET is completely eliminated in the presence of 60 mM glucose. This impression is confirmed by the average lifetimes, which return to the unquenched value at 60 or 100 mM glucose (Fig. 5, right). Hence, in the present case the sensor appears to be completely reversible, which is an improvement over our previous glucose sensor [19,28]. The complete reversibility of this sensor is also seen by the multi-exponential analysis. The amplitude of the short component decreases with increasing concentrations of glucose (Table 2). Importantly, for 60 and 100 mM glucose the multi-exponential decay parameters are essentially identical to that seen in the absence of MIMG (Table 1).
Fig. 4.
Average lifetime of Cy5ConA (2 μM) at various concentrations of MIMG (left), and in the presence of 12 μM MIMG with increasing concentrations of glucose (right).
Table 2.
Excited state lifetimes of 2 μM cy5ConA in the presence of 12 μM MIMG and various concentrations of glucose
| τi (ns) | fi | ||
|---|---|---|---|
| 0 mM Glc | 0.281 | 0.326 | 1.28 |
| 1.300 | 0.674 | ||
| 15 mM Glc | 0.337 | 0.267 | 1.05 |
| 1.321 | 0.733 | ||
| 30 mM Glc | 0.480 | 0.286 | 1.13 |
| 1.388 | 0.714 | ||
| 60 mM Glc | 0.507 | 0.207 | 1.08 |
| 1.402 | 0.793 | ||
| 100 mM Glc | 0.531 | 0.168 | 1.09 |
| 1.418 | 0.832 |
All the data presented here were collected immediately upon addition of small aliquots of acceptor to the donor. The same is true to the titration of the donor–acceptor mixture with glucose. Aggregation of the donor and acceptor was observed with the appearance of a blue precipitate after about 3 h, although no time-resolved measurements were done at this time. In the presence of glucose, the precipitation takes a longer time to occur, about 24 h. This is consistent with the competitive binding of glucose for the binding site in ConA.
4. Discussion
In the previous paper [20], we described a glucose assay using Ru(bpy)3-labeled Concanavalin A (RuConA) as the donor, and MIMG as the acceptor. The differences between RuConA and Cy5ConA are listed in Table 3. The use of either RuConA or Cy5ConA in FRET-based glucose sensing has its advantages and disadvantages. RuConA has a long excited state lifetime and a large Stoke’s shift which simplifies instrumentation for phase-modulation lifetime measurements. From a practical point of view, RuConA may be useful for semi-noninvasive glucose sensing, such as an intradermal glucose sensing patch located on the skin. A low-cost phase-modulation lifetime measuring device can then be utilized to detect changes in interstitial glucose concentrations. On the down side, it has a low quantum yield which requires multiple labeling of the ConA molecule to obtain a better signal. This may increase the net charge of ConA which did not appear to affect the glucose-binding capacity of the protein but may increase the ability of ConA to agglutinate [30]. The latter is not desirable in devising a glucose sensor because it reduces the reversibility of the system. Indeed, full reversibility is not observed in the presence of glucose (20). However, control experiments suggests that this is partly due to diffusion dependent energy transfer, a consequence of the long lifetime of RuConA.
Table 3.
Properties of RuConA [20] and Cy5ConA
| Ru(bpy)3-ConA | Cy5-ConA | |
|---|---|---|
| Absorption maximum | 430 nm | 650 nm |
| Emission maximum | 650 nm | 675 nm |
| Average lifetime | ~460 ns | ~1.3 ns |
| Quantum yield | ~0.04 | ~0.15 |
The present report describes the use of Cy5-labeled ConA as the donor. Compared to RuConA, Cy5ConA has a smaller Stokes’ shift and a much shorter lifetime. However, it absorbs in the red region of the spectrum, thus the effects of scattered light can be minimized if the sensor is to be utilized in highly scattering media. Thus, Cy5ConA may find usefulness in whole blood assays. Additionally, it has a relatively high quantum yield. Thus, multiple labeling of the protein is not necessary. In this way, perturbation of the protein structure, particularly to its net charge, is minimized. As the lifetime data shows, we have greatly improved the reversibility of the glucose assay using Cy5 as the donor.
In the previous paper [19], the acceptor used was dye-labeled dextran with a molecular weight of about 10 000. This is similar to the strategy used in the Schultz glucose sensor [21–23]. The reversibility of the assay using this acceptor and RuConA is very poor, resulting in the precipitation of the ConA-polysaccharide aggregate. Initially, we tried using malachite green labeled ovalbumin, a natural glycoprotein, as the acceptor (unpublished data). Unfortunately, this acceptor agglutinated readily in the presence of ConA. In an effort to reduce agglutination, as well as increase the energy transfer efficiency, we designed a smaller acceptor by labeling insulin (MW 6000) with the disaccharide maltose and malachite green (MIMG). This acceptor exhibited better reversibility with RuConA and more so with Cy5ConA. In both cases, however, agglutination is not totally prevented, although the presence of glucose or mannose retards it.
With the success of this acceptor, attempts were made to optimize the structure. The maltose moiety was substituted with glucose in order to increase the binding affinity for the ConA. As expected, this acceptor (GLIMG) bound more tightly to ConA [29]. Reversibility in the presence of glucose was good when Cy5ConA (Fig. 6) was used as the donor, but with RuConA the assay was totally irreversible (data not shown). Decreasing the molecular weight by changing the insulin to lysine (MLMG) resulted in an acceptor that is minimally soluble in water. In addition, it was not eluted from a Sepharose-ConA column by 0.1 M a-methyl mannoside. It is likely that it binds to hydrophobic pockets in the ConA structure rather than the sugar binding site.
Fig. 6.
Emission spectrum of 2 μM Cy5ConA alone; in the presence of 4 μM GLIMG; and in the presence of 4 μM GLIMG plus 160 μM glucose.
The success of a glucose sensor using ConA as the glucose-binding protein depends on being able to eliminate agglutination. From the experiments that we did, it appears that the window of opportunity for a non-aggregating ConA–acceptor pair is very narrow indeed. This might explain why a working model of such a sensor has yet to be developed despite the many years that has been devoted to its design. Nevertheless, we have shown that a lifetime based sensor for glucose can be made practical with the right choice of donors and acceptors. In the future, other glucose-binding proteins other than ConA may also be utilized. Additionally, low-cost instrumentation has now been described for phase-modulation lifetime measurements [31,32]. It is well known that the output of laser diodes can be modulated at high frequencies and used in phase-modulation fluorometers [33,34]. Furthermore, it may even be possible to use still simpler amplitude-modulated LEDs as the light source. Amplitude modulation of LEDs has been described to frequencies near 60 MHz [35] or to above 100 MHz [36]. In summary, trans-dermal sensing of glucose appears to be possible with currently available probe chemistry and electro-optical technology.
Acknowledgements
This work was supported by grants from the National Institutes of Health RR-10955 to Dr. Govind Rao and from the National Center for Resource Resources (RR-08119) to Joseph R. Lakowicz. The authors thank Dr. Badri Maliwal for assistance with preparation of the Cy5 ConA.
References
- [1].Schier GM, Moses RG, Gan IET, Blair SC, An evaluation and comparison of reflolux II and glucometer II, two new portable reflectance meters for capillary blood glucose determination, Diabetes Res. Clin. Practice 4 (1988) 177–181. [DOI] [PubMed] [Google Scholar]
- [2].Clarke W, Becker DJ, Cox D, Santiago JV, White NH, Betschart J, Eckenrode K, Levandoski LA, Prusinski EA, Simineiro LM, Snyder AL, Tideman AM, Yaeger T, Evaluation of a new system for self blood glucose monitoring, Diabetes Res. Clin. Practice 4 (1988) 209–214. [DOI] [PubMed] [Google Scholar]
- [3].Trettnak W, Leiner MJP, Wolfbeis OS, Fibre-optic glucose biosensor with an oxygen optrode as the transducer, Analyst 113 (1988) 1519. [DOI] [PubMed] [Google Scholar]
- [4].Moreno-Bondi MC, Wolfbeis OS, Leiner MJP, Schaffar BPH, Oxygen optrode for use in a fiber-optic glucose biosensor, Anal. Chem 62 (1990) 2377. [DOI] [PubMed] [Google Scholar]
- [5].Trettnak W, Leiner MJP, Wolfbeis OS, Fibre-optic glucose sensor with an pH optrode as the transducer, Biosensors 4 (1988) 15–26. [DOI] [PubMed] [Google Scholar]
- [6].Shichiri M, Kawamori R, Yamasaki Y, Needle-type glucose sensor, Methods Enzymol. 137 (1988) 326–334. [DOI] [PubMed] [Google Scholar]
- [7].Tretnak W, Wolfbeis OS, Fully reversible fibre-optic glucose biosensor based on the intrinsic fluorescence of glucose oxidase, Anal. Chim. Acta 221 (1989) 195–203. [Google Scholar]
- [8].March WF, Rabinovitch B, Adams R, Wise JR, Melton M, Ocular glucose sensor, Trans. Am. Soc. Artif. Intern. Organ 28 (1982) 232–235. [PubMed] [Google Scholar]
- [9].Rabinovitch B, March WF, Adams RL, Noninvasive glucose monitoring of the aqueous humor of the eye: Part I. Measurement of very small optical rotations, Diabetes Care 5 (3) (1982) 254–258. [DOI] [PubMed] [Google Scholar]
- [10].Gunby P, Laser-implant contact lens could be glucose monitor, J. Am. Med. Assoc 243 (4) (1980) 317. [PubMed] [Google Scholar]
- [11].Bauer B, Floyd TA, Monitoring of glucose in biological fluids by Fourier–transform infrared spectrometry with a cylindrical internal reflectance cell, Anal. Chim. Acta 197 (1987) 295–301. [Google Scholar]
- [12].Kaiser N, Laser absorption spectroscopy with an AIR prismnoninvasive in vivo determination of glucose, J. Horm. Metabol. Res Suppl. 8 (1977) 30–33. [PubMed] [Google Scholar]
- [13].Heise HM, Marbach R, Koschinsky Th., Gries FA, Noninvasive blood glucose sensors based on near-infrared spectroscopy, Artif. Organs 18 (6) (1994) 439–447. [DOI] [PubMed] [Google Scholar]
- [14].Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, Robinson PL, Noninvasive glucose monitoring in diabetic patients: a preliminary evaluation, Clin. Chem 38 (9) (1992) 1618–1622. [PubMed] [Google Scholar]
- [15].Velho G, Froguel Ph., Thevenot DR, Reach G, In vivo calibration of a subcutaneous glucose sensor for determination of subcutaneous glucose kinetics, Diab. Nutr. Metab 3 (1988) 227–233. [Google Scholar]
- [16].Szmacinski H, Lakowicz JR, Fluorescence lifetime-based sensing and imaging, Sensors and Actuators B 29 (1995) 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Burch CL, Lakowicz JR, Sevick EM, Fluorescence lifetime-based sensing in tissues: a computational study, Biophys. J 68 (1995) 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bambot SB, Rao G, Romauld M, Carter GM, Sipior J, Terpetschnig E, Lakowicz JR, Sensing oxygen through skin using a red diode laser and fluorescence lifetimes, Biosens. Bioelectron 10 (6/7) (1995) 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lakowicz JR, Maliwal B, Optical sensing of glucose using phase-modulation fluorimetry, Anal. Chim. Acta 271 (1993) 155–164. [Google Scholar]
- [20].Tolosa L, Szmacinski H, Rao G, Lakowicz JR, Lifetime-based sensing of glucose using energy transfer with a long lifetime donor, Anal. Biochem 250 (1997) 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meadows D, Schultz JS, Fiber-optic biosensors based on fluorescence energy transfer, Talanta 35 (2) (1988) 145–150. [DOI] [PubMed] [Google Scholar]
- [22].Schultz JS, Mansouri S, Goldstein IJ, Affinity sensor: a new technique for developing implantable sensors for glucose and other metabolites, Diabetes Care 5 (3) (1982) 245–253. [DOI] [PubMed] [Google Scholar]
- [23].Schultz JS, Sims G, Affinity sensors for individual metabolites, Biotechnol. Bioeng. Symp 9 (1979) 65–71. [PubMed] [Google Scholar]
- [24].Jeong SY, Kim SW, Eenink MJD, Feijen J, Self-regulating insulin delivery systems, I. Synthesis and characterization of glycosylated insulin, J. Control. Release 1 (1984) 57–66. [Google Scholar]
- [25].Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F, Colorimetric method for determination of sugars and related substances, Anal. Chem 28 (1956) 350–356. [Google Scholar]
- [26].Birch DJS, Imhof RE, Time-domain fluorescence spectroscopy using time-correlated single-photon counting, Top. Fluoresc. Spectrosc. Techniques 1 (1991) 1–95. [Google Scholar]
- [27].Middendorf L, Bruce R, Brumbaugh J, Grone D, Jang G, Richterich P, Holtke HJ, Williams RJ, Peralta JM, A two-dimensional infrared fluorescence scanner used for DNA analysis, SPIE Proc. 2388 (1995) 44–55. [Google Scholar]
- [28].Papkovsky DB, Ponomarev GV, Wolfbeis OS, Longwave luminescent porphyrin probes, Spectrochim. Acta A 52 (1996) 1629–1638. [Google Scholar]
- [29].Sato S, Jeong SY, McRea JC, Kim SW, Glucose stimulated insulin delivery systems, Pure Appl. Chem 56 (10) (1984) 1323–1328. [Google Scholar]
- [30].Izhu S, Ake Y, Tanak I, Saito M, Alteration of quaternary structure and biological activity of ConA, J. Protein Chem 3 (1) (1984) 63–71. [Google Scholar]
- [31].Gruber WR, O’Leary P, Wolfbeis OS, Detection of fluorescence lifetime based on solid state technology and its application to optical oxygen sensing, SPIE Proc. 2388 (1995) 148–158. [Google Scholar]
- [32].Levy R, Guignon EF, Cobane S, Louis E. St., Fernandez SM, Compact, rugged and inexpensive frequency-domain fluorometer, SPIE Proc. (1997) in press. [Google Scholar]
- [33].Berndt KW, Gryczynski I, Lakowicz JR, Phase-modulation fluorometry using a frequency-doubled pulsed laser diode light source, Rev. Sci. Instrum 61 (7) (1990) 1816–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thompson RB, Frisoli JK, Lakowicz JR, Phase fluorometry using a continuously modulated laser diode, Anal. Chem (1998) submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fantini S, Franceschini MA, Fishkin JB, Barbieri B, Gratton E, Quantitative determination of the absorption spectra of chromophores in strongly scattering media: a light-emitting diode based technique, Appl. Optics 33 (22) (1994) 5204–5213. [DOI] [PubMed] [Google Scholar]
- [36].Sipior J, Carter GM, Lakowicz JR, Rao G, Single quantum well light emitting diode demonstrated as excitation sources for nanosecond phase-modulation fluorescence lifetime measurements, Rev. Sci. Instrum 67 (11) (1996) 3795–3798. [Google Scholar]