Abstract
Background/purpose:
Although inverse-planned intensity-modulated radiotherapy (IMRT) and deep inspiration breath hold (DIBH) may allow for more conformal dose distributions, it is unknown whether using these technologies reduces cardiac or pulmonary toxicity of breast radiotherapy.
Methods:
A randomized controlled trial compared IMRT-DIBH versus standard, free-breathing, forward-planned, three-dimensional conformal radiotherapy in patients with left-sided, node-positive breast cancer in whom the internal mammary nodal region was targeted. Endpoints included dosimetric parameters and changes in pulmonary and cardiac perfusion and function, measured by single photon emission computed tomography (SPECT) scans and pulmonary function testing performed at baseline and one-year post-treatment.
Results:
Of 62 patients randomized, 54 who completed all follow-up procedures were analyzed. Mean doses to the ipsilateral lung, left ventricle, whole heart, and left anterior descending coronary artery (LAD) were lower with IMRT-DIBH; the percent of left ventricle receiving ≥5 Gy (V5) averaged 15.8% with standard radiotherapy and 5.6% with IMRT-DIBH (p<0.001). SPECT revealed no differences in perfusion defects in the left anterior descending coronary artery territory, the study’s primary endpoint, but did reveal statistically significant differences (p=0.02) in left ventricular ejection fraction (LVEF), a secondary endpoint. No differences were found for lung perfusion or function.
Conclusion:
The small but statistically significant benefit in preservation of cardiac LVEF observed here should motivate future studies that include LVEF as a potentially meaningful endpoint. Future studies should disaggregate the impact of IMRT from that of DIBH. Clinical practice should recognize the importance of minimizing cardiac dose, even when already low in comparison to historical levels.
INTRODUCTION
Both tumor control and normal tissue complication probabilities depend on the dose of radiotherapy. Radiation oncologists are therefore driven to explore technologies that may allow an improvement in the therapeutic index. IMRT is a promising technology that allows the development of complex, conformal dose distributions that may be dosimetrically superior to conventional plans. This may yield improved tumor control due to increased target doses and/or reduced toxicity due to lower doses to adjacent normal tissues and improved dose homogeneity.
In the case of breast cancer treatment, standard radiation doses have been adequate for controlling microscopic disease in the routine adjuvant setting, and the goal of utilizing IMRT has largely been directed at minimizing treatment-related toxicities. Although radiotherapy is now firmly established as an integral part of the management of early stage breast cancer, both for patients undergoing breast-conserving therapy and for select patients after mastectomy, concerns remain regarding its potential long-term toxicities.1
Randomized controlled trials (RCTs) have compared simple forward-planned intensity modulation to 2-dimensional treatment planning and delivery, revealing benefits in skin and soft tissue toxicity within the breast due to improved dose homogeneity.2,3 However, modern technology now allows for considerably more complex IMRT plans than of the sort evaluated on those early RCTs.
Concerns about cardiac and pulmonary toxicity from breast radiotherapy, particularly when the targets of treatment include the regional lymph nodes, have motivated interest in evaluating the potential benefits of the more highly conformal plans that can be generated with inverse-planned, beamlet IMRT. Beamlet techniques have been developed that maintain target volume coverage with reduction in high doses to the lung, heart, and important substructures such as the left anterior descending coronary artery,4 without increasing contralateral breast dose.5 Given observational analyses that have suggested a linear dose-response relationship between mean heart dose and cardiac mortality risk,6 dosimetric improvements to minimize heart dose with IMRT have been hypothesized to result in improved clinical outcomes.
The additional geometrical advantages afforded by deep inspiration breath hold (DIBH) and the desire to limit motion in the context of the greater dose conformality with beamlet IMRT have encouraged investigation to determine whether the dosimetric improvements that can be obtained with IMRT in conjunction with DIBH yield the hypothesized improvements in clinical outcomes. Therefore, we conducted an RCT comparing inverse-planned beamlet IMRT with DIBH versus free-breathing forward-planned three-dimensional conformal radiotherapy (3DCRT) in patients with left-sided node-positive breast cancer in whom the internal mammary nodal region was targeted.
METHODS AND MATERIALS
Sample
After IRB-approval, we recruited patients who presented to the University of Michigan with node-positive breast cancer in whom radiotherapy was indicated to treat the left breast or chest-wall, as well as the internal mammary, infraclavicular, and supraclavicular nodal regions. The sample size for the randomized design was selected after considering the feasibility of patient accrual, the desire to yield unbiased estimates of the treatment effect for use in future research, and the hypothesis that IMRT-DIBH would be superior to 3DCRT.
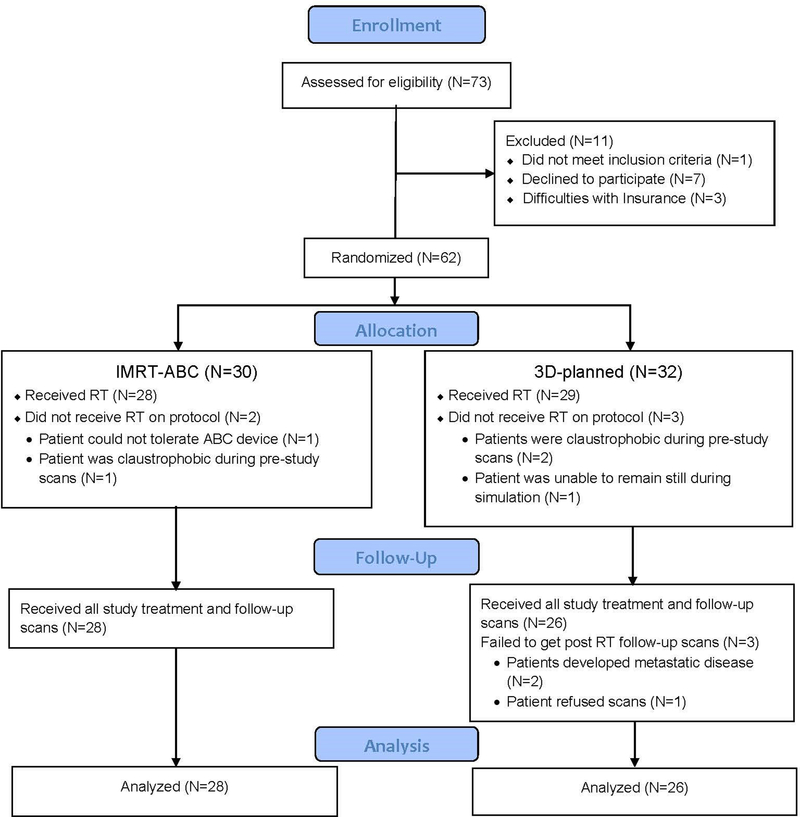
Between May 2006 and June 2012, 62 patients were randomized 1:1 (see Figure 1) between IMRT with DIBH and 3D-CRT using blocks of size 4 and 6. Treatment assignment lists were held confidentially by the study statistician and reported to clinical personnel only after a prospective patient was determined eligible and signed informed consent. Of these, 54 completed all follow-up procedures and were eligible for analysis.
Figure 1:
CONSORT diagram
*ABC=active breathing control, a device used for the deep inspiration breath hold required on the investigational arm of the trial.
Treatment Planning
The treating physician approved contours of the targets (breast/chestwall, regional lymph nodes, including supraclavicular, infraclavicular, and internal mammary nodes in interspaces 1–3) and critical normal tissues and created with two separate dosimetrists two plans on two different datasets for each patient enrolled: one using forward-planned 3D-CRT on a free-breathing scan, and one using inverse-planned beamlet IMRT on a DIBH scan.
Dose calculations used an inhomogeneity-corrected convolution/superposition algorithm. All patients treated on the standard arm received either 50 Gy in 2 Gy/fraction or 50.4 Gy in 1.8 Gy/fraction to the entire target volume once daily, five fractions per week (excluding holidays). A boost (10 Gy in 2 Gy fractions to the tumor bed or mastectomy scar) was then sequentially delivered, using electron beams of energies up to 12MeV or oblique photon beams when the tumor bed was too deep to cover with electrons. All patients treated with IMRT-DIBH were treated to the entire target volume to 52.2 Gy in 1.74 Gy/fraction (biologically equivalent to 50 Gy in 2 Gy/fraction). This allowed the 10 Gy boost to be incorporated and delivered simultaneously with the treatment to the volume such that the total dose to the tumor bed or mastectomy scar was biologically equivalent to 60 Gy in 2 Gy fractions. “
More specifically, for the 3D-CRT arm, treatment planning followed standard clinical practice at our institution at the time. PTVs were not generated for any structures; rather, dosimetrist and physician review of isodose distributions, field shapes, and flash were used to judge target coverage qualitatively. Approximately half (14/26) of the 3D-plans used electrons to simultaneously treat the internal mammary nodes and a portion of the medial breast tissue when a tangential only plan would have led to what the treating physician and dosimetrist viewed as excessive dose to the heart and lungs; the remainder of the plans encompassed the breast tissue and internal mammary region within the tangential beams.
The IMRT arm utilized restricted beam angles as described in our prior research, as nine-field equidistant beams had yielded unacceptable doses to contralateral structures.4 As shown in that prior research, coverage of the internal mammary nodes would be expected to be lower with the 3D-CRT technique than with the IMRT technique we used.4 For IMRT plans, an initial optimization was done with photon only beams. Electrons were added if deemed beneficial for balancing tradeoffs between target doses and doses to the heart and lungs, and not exclusively for the integrated boost portion of the IMRT plans.
For the IMRT arm, the left anterior descending coronary artery and brachial plexus were contoured as dose-limiting structures for the optimization; these structures were deliberately not contoured on the free-breathing dataset because it was not standard practice to contour those structures for standard 3D-CRT during the era of the study. There was no formal evaluation of LAD dose during plan review by treating physicians for patients treated on the 3D-CRT arm.
Endpoints/Measures
Pulmonary dosimetric parameters included mean dose to the ipsilateral lung and volume of the ipsilateral lung (in percent) receiving at least a certain dose (with the dose levels specified starting at 5Gy and increasing by 5Gy up to 60Gy). Cardiac dosimetric parameters included mean dose to the left ventricle and volume of the left ventricle (in percent) receiving at least a certain dose (again with the dose levels specified starting at 5Gy and increasing by 5Gy up to 60Gy).7 We also evaluated mean doses to the heart and the left anterior descending coronary artery.
The primary endpoint was the percentage decrease in attenuation corrected single photon emission computed tomography (SPECT/CT)-based perfusion of the heart, as evaluated one year post-treatment and compared to baseline pre-treatment SPECT. As described previously,8 SPECT image quantification was performed using Corridor4DM (INVIA Medical Imaging Solutions, Ann Arbor, MI) software. To evaluate changes in cardiac perfusion and function, one specialist blinded to treatment arm performed quantitative comparisons of one-year post-RT SPECT/CT scans with baseline scans. Perfusion defects were quantitatively assessed by comparing the normalized perfusion distribution for each subject’s scan without user intervention against normal polarmap databases using thresholds of 2.5 and 1.5 SD below the normal mean. On the basis of inter-test variability, perfusion defect increases greater than 5% and 10% were considered significant for 2.5- and 1.5-SD thresholds, respectively. Measurements of summed stress scores were performed automatically with the Corridor4DM algorithm.
Other key measures of cardiac function included changes in left ventricular ejection fraction (LVEF), summed stress scores, and peak filling rate, as measured with EKG-gated SPECT. LVEF was automatically calculated using the standard Corridor4DM algorithm. In brief, LV endocardial surface estimates throughout the cardiac cycle were used, with LV volumes calculated as the sum of the voxels within the contours for each frame. The end-diastolic and end-systolic volumes were determined from LV volume curves to derive LVEFs. Quantification of LVEFs for this study was conducted blinded to treatment arm. Baseline and follow-up studies were quantified side-by-side with both studies aligned and quantified in exactly the same way as was the perfusion quantified (above) with Corridor4DM but without attenuation correction, i.e., although perfusion data was obtained from the same gated SPECT/CT data sets and attenuation corrected, attenuation correction was not available for processing gated SPECT image reconstructions.
Measures of pulmonary outcomes included changes in SPECT perfusion of the left lung and pulmonary function testing, between baseline and one year. Lung perfusion SPECT/CT techniques have been described elsewhere.9 All lung perfusion SPECT/CT scans were read by the same expert nuclear medicine physician, with scans compared for each patient and qualitative perfusion changes recorded. Pulmonary function testing conformed to standard approaches used in clinical care.
Statistical analysis
We compared dosimetric measures, along with cardiac and pulmonary outcomes measures, by treatment arm. Fisher’s exact test and the Wilcoxon rank-sum test were used to compare treatment arms for categorical and continuous data, respectively. P-values ≤5% were considered evidence for significant difference.
RESULTS
Table 1 depicts the characteristics of the patients analyzed. Mean age was 50 years and 89% of the sample was white. Most characteristics were balanced between the arms. Although not significantly different (p=0.075), hypertension appeared more common on the standard arm. Also, while not significantly different, a numerically higher proportion of standard arm patients received doxorubicin (92% vs 86%, p=0.67) and trastuzumab.(31% vs 18%, p=0.35).
Table 1:
Clinical Characteristics of the Study Sample By Arm in a Trial of Radiotherapy Techniques in Patients with Node-Positive, Left-Sided Breast Cancer
| Characteristic: | Total (N=54) | Free- breathing 3DCRT (N=26) |
IMRT with DIBH (N=28) |
P-value |
|---|---|---|---|---|
| Age: Mean (SD) [Min.-Max] | 50.43 (10.41) [25.0, 74.0] | 51.69 (11.86)[25.0, 74.0] | 49.25 (8.91) [30.0, 69.0] | 0.38 |
| Race: N (%) | 0.67 | |||
| White | 48 (88.89) | 24 (92.31) | 24 (85.71) | |
| Other | 6 (11.11) | 2 (7.69) | 4 (14.29) | |
| BMI: Mean (SD) [Min.-Max] | 27.7 (5.0) [19.3, 38.9] | 28.5 (5.8) [20.7, 38.9] | 27.1 (4.2) [19.3, 35.3] | 0.49 |
| History of heart disease: N (%) | 2 (3.70) | 2 (7.69) | 0 | 0.49 |
| History of diabetes: N (%) | 4 (7.41) | 3 (11.54) | 1 (3.57) | 0.61 |
| History of hypertension: N (%) | 10 (18.52) | 8 (30.77) | 2 (7.14) | 0.08 |
| History of hypercholesterolemia: N (%) | 7 (12.96) | 3 (11.54) | 4 (14.29) | 0.99 |
| Family history of heart disease: N (%) | 30 (55.56) | 16 (61.54) | 14 (50.00) | 0.77 |
| Smoking history: N (%) | 0.96 | |||
| Never | 27 (50.00) | 13 (50.00) | 14 (50.00) | |
| Current | 2 (3.70) | 1 (3.85) | 1 (3.57) | |
| Past year | 5 (9.26) | 3 (11.54) | 2 (7.14) | |
| Ever | 20 (37.04) | 9 (34.62) | 11 (39.29) | |
| Lumpectomy: N (%) | 28 (51.85) | 11 (42.31) | 17 (60.71) | 0.28 |
| Mastectomy: N (%) | 26 (48.15) | 15 (57.69) | 11 (39.29) | |
| Chemotherapy before RT: N (%) | 52 (96.30) | 25 (96.15) | 27 (96.43) | 0.99 |
| doxorubicin-containing chemotherapy regimen: N (%) | 48 (88.89) | 24 (92.31) | 24 (85.71) | 0.67 |
| Trastuzumab receipt: N (%) | 13 (24.07) | 8 (30.77) | 5 (17.86) | 0.35 |
| Aromatase inhibitor receipt: N (%) | 17 (31.48) | 8 (30.77) | 9 (32.14) | 0.99 |
| Tamoxifen receipt: N (%) | 26 (48.15) | 12 (46.15) | 14 (50.00) | 0.80 |
Table 2 shows that the treatment plans for the two study arms differed significantly for numerous dosimetric parameters. Multiple metrics of pulmonary and cardiac doses were lower among patients on the IMRT-DIBH arm, including mean doses to the ipsilateral lung, left ventricle, whole heart, and left anterior descending coronary artery. The left ventricle V5 (volume receiving at least 5 Gy) averaged 15.8% among patients on the 3D arm and 5.6% among those on the IMRT-DIBH arm (p<0.001).
Table 2:
Dosimetric Parameters by Arm
| Mean (SD) | ||||
|---|---|---|---|---|
| Overall | Free-breathing 3DCRT (n=26) |
IMRT with DIBH (n=28) |
P-value | |
| Ipsilateral Lung: | ||||
| Mean dose | 14.02 (1.82) | 14.44 (1.96) | 13.62 (1.62) | 0.035 |
| V5 | 52.63 (7.06) | 53.08 (6.99) | 52.22 (7.23) | 0.47 |
| V10 | 40.53 (6.53) | 40.03 (5.98) | 41.00 (7.08) | 0.97 |
| V15 | 34.07 (5.15) | 33.81 (5.43) | 34.31 (4.96) | 0.99 |
| V20 | 29.40 (4.42) | 29.55 (4.88) | 29.26 (4.03) | 0.51 |
| V25 | 25.38 (4.06) | 26.07 (4.44) | 24.74 (3.63) | 0.08 |
| V30 | 21.30 (4.19) | 22.73 (4.16) | 19.96 (3.83) | 0.003 |
| V35 | 16.98 (4.56) | 19.07 (3.99) | 15.04 (4.23) | <0.001 |
| V40 | 11.82 (4.41) | 13.83 (3.83) | 9.95 (4.12) | <0.001 |
| V45 | 6.24 (3.46) | 7.18 (3.39) | 5.36 (3.35) | 0.06 |
| V50 | 2.06 (1.94) | 2.19 (1.99) | 1.95 (1.92) | 0.68 |
| V55 | 0.37 (0.70) | 0.43 (0.83) | 0.31 (0.56) | 0.97 |
| V60 | 0.06 (0.26) | 0.11 (0.36) | 0.02 (0.10) | 0.60 |
| Left Ventricle: | ||||
| Mean dose | 2.75 (0.99) | 3.22 (0.77) | 2.32 (0.98) | <0.001 |
| V5 | 10.53 (10.49) | 15.81 (8.65) | 5.62 (9.73) | <0.001 |
| V10 | 1.53 (2.47) | 2.09 (2.24) | 1.01 (2.60) | <0.001 |
| V15 | 0.39 (0.84) | 0.51 (0.74) | 0.28 (0.93) | 0.003 |
| V20 | 0.09 (0.24) | 0.16 (0.32) | 0.02 (0.06) | 0.002 |
| V25 | 0.03 (0.10) | 0.06 (0.13) | 0.00 (0.01) | 0.006 |
| V30 | 0.01 (0.03) | 0.01 (0.04) | 0.00 (0.00) | 0.009 |
| V35 | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.00) | 0.03 |
| V40 | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.15 |
| V45 (results were identical for V50, V55, and V60) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.32 |
| Whole Heart: | ||||
| Mean dose | 2.77 (1.18) | 3.22 (1.22) | 2.35 (0.98) | 0.006 |
| Left anterior descending coronary artery: | ||||
| Mean dose | 7.05 (3.31) | 8.95 (3.19) | 5.29 (2.32) | <0.001 |
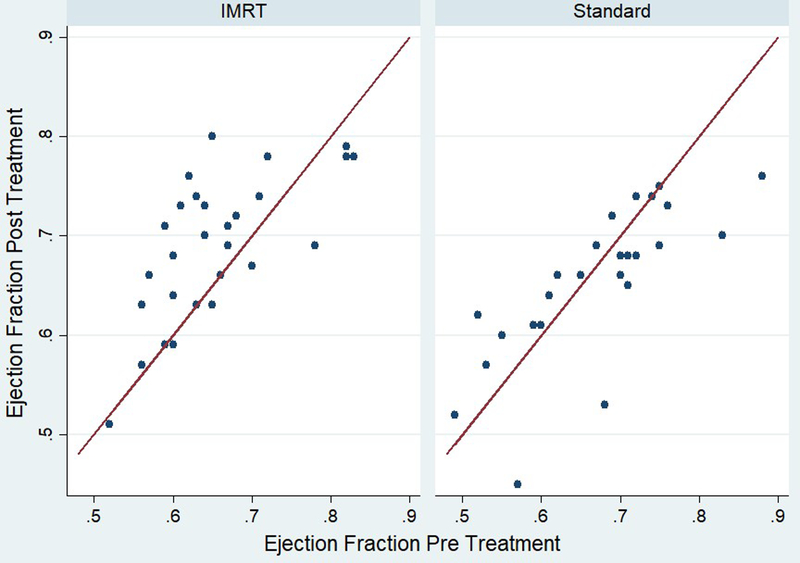
SPECT testing of the heart revealed no differences in perfusion defects in the left anterior descending coronary artery territory, regardless of threshold, and no difference in summed stress score or peak filling rates (Table 3). However, there were significant differences in LVEF. As detailed in Figure 2, six patients had changes >5% from baseline pre-treatment on the standard arm; those changes were 12% (88% to 76%), 15% (68% to 53%), 6% (75% to 69%), 6% (71% to 65%), 12% (57% to 45%), and 13% (83% to 70%). One patient in the IMRT arm had a change >5%; that change was 9% (78% to 69%). End-diastolic heart volumes were not different between the study arms (p=0.71), nor were baseline LVEFs (p=0.52). No differences were found for lung metrics on either SPECT or pulmonary function testing.
Table 3:
Cardiac and Pulmonary Test Results by Arm
| Mean (SD) | ||||
|---|---|---|---|---|
| Overall | Free- breathing 3DCRT (n=26) |
IMRT with DIBH (n=28) |
P-value | |
| Change in LAD perfusion defect (stricter threshold*) | −0.61 (5.85) | −1.19 (7.75) | −0.07 (3.31) | 0.70 |
| Change in LAD perfusion defect (less strict threshold*) | −2.57 (14.43) | −3.69 (16.59) | −1.54 (12.32) | 0.73 |
| Change in summed stress score | −0.61 (2.41) | −0.58 (2.39) | −0.64 (2.47) | 0.56 |
| Change in left ventricular ejection fraction percentage | 1.13 (6.62) | −1.46 (6.18) | 3.54 (6.19) | 0.016 |
| Change in peak filling rate | −0.03 (0.73) | −0.13 (0.53) | 0.05 (0.88) | 0.40 |
| N (%) | ||||
| New lung perfusion deficit in post-RT scan | ||||
| Missing | 1 (1.85) | 0 | 1 (3.57) | |
| No | 45 (83.33) | 21 (80.77) | 24 (85.71) | 0.41† |
| Yes | 8 (14.81) | 5 (19.23) | 3 (10.71) | |
| Mean (SD) | P-value | |||
| Change in percent predicted FVC | −0.01 (0.06) | −0.01 (0.06) | −0.01 (0.05) | 0.65 |
| Change in percent predicted FEV1 | −0.01 (0.06) | −0.01 (0.07) | −0.01 (0.05) | 0.96 |
| Change in percent predicted VC | −0.00 (0.05) | 0.00 (0.06) | −0.01 (0.04) | 0.53 |
| Change in percent predicted IC | 0.01 (0.12) | 0.01 (0.12) | 0.00 (0.12) | 0.81 |
| Change in percent predicted TLC | −0.01 (0.05) | −0.01 (0.06) | −0.02 (0.05) | 0.54 |
| Change in percent predicted DLCO | 0.07 (0.13) | 0.07 (0.13) | 0.08 (0.13) | 0.46 |
Perfusion defects were assessed by comparing normalized perfusion distributions against normal polarmap databases using thresholds of 2.5 (stricter threshold) and 1.5 SD (less strict threshold) below the normal mean. On the basis of inter-test variability, perfusion defect increases greater than 5% and 10% were considered significant for 2.5- and 1.5-SD thresholds, respectively.
Missing category excluded when calculating p-value.
Abbreviations: LAD=left anterior descending coronary artery; SD=standard deviation; FVC=forced vital capacity; FEV1=forced expiratory volume one second; VC=vital capacity; IC=inspiratory capacity; TLC=total lung capacity; DLCO=diffusing capacity of lungs for carbon monoxide
Figure 2:
This figure depicts the change in ejection fraction observed in patients treated on each arm.
DISCUSSION
In this RCT comparing 3D-CRT designed by experienced dosimetrists to inverse-planned, beamlet IMRT with DIBH, we found that doses to the lung and heart differed by technique; although we observed no differences between arms on pulmonary testing, we observed a statistically significant difference in LVEF change between baseline and one year. Although the observation of lower cardiac doses with IMRT-DIBH could be anticipated given the weighting of cardiac limits in the cost function for IMRT planning, this study is important in demonstrating that the reduction in cardiac dose with this technique might be associated with a potentially clinically meaningful outcome, i.e., better preservation of cardiac LV function. Comparative evaluation of outcomes and the impact of advances in radiation treatment planning and delivery is essential to inform clinical practice and policy.
The current study adds intriguing findings supporting the importance of restricting radiation dose to the heart. Several prior studies have suggested that the increased risk of heart disease associated with radiotherapy has decreased over time, coincident with the shift away from techniques that deliver substantial incidental cardiac doses.10,11 Yet recent studies have still found a significant increase in post-treatment development of ischemic cardiac disease in patients treated to the left side as compared with those treated to the right, even in series that included only patients treated with a contemporary tangential radiotherapy technique.12,13 Although contemporary radiotherapy exposes substantially less heart volume to radiation, CT-based studies have still shown delivery of high doses to small regions of the heart in some patients, including the left coronary vasculature. Although the results identified in the current study should be confirmed by others, they suggest a degree of short-term ventricular dysfunction following modest dose exposure. Since there are no known “safe” levels of radiation to the heart—especially in patients also exposed to potentially cardiotoxic systemic therapies—techniques that further minimize cardiac exposure to RT are important subjects for evaluation.
In a prior report, we noted that there were no significant perfusion deficits seen upon preliminary analysis of the cardiac SPECT scans performed in the first half of the patients enrolled on this trial. Those findings suggested that we would not ultimately observe a difference in perfusion by study arm. Indeed, we did not find any such difference in the present analysis. However, the prior study did not compare patients across arms (because only half of the subjects had been enrolled at the time and the comparative analysis was only appropriate after trial completion). Therefore, that prior study was unable to provide the detailed comparison that is provided in this primary outcomes analysis of the trial, including the analysis that revealed the difference in LVEF between the two arms.
Our observations of generally preserved myocardial perfusion after treatment contrasts with other studies, which have demonstrated significant defects within a year of radiotherapy.14 Although the clinical significance of perfusion defects remains unclear, and perfusion deficits can be artifacts when attenuation correction is not used,15 it is reassuring that in this study that did utilize attenuation correction, no new cardiac perfusion defects were observed. Nevertheless, the observation of differences in the change in LVEF, with slight improvement by one year in patients treated with IMRT and slight decline in those treated with standard therapy, even in the absence of perfusion defects, suggests that other mechanisms of radiation-induced cardiotoxicity, including microvascular compromise, merit further investigation.
The technique of SPECT scanning utilized in the current study is a well-established and valid approach to measurement of LVEF. The normal range for LVEF in women using the Corridor4DM software is 0.72±0.6.16 The vast majority of the ejection fractions measured in this study were technically within the normal range both before and after treatment, and the LV function assessments in this study are very similar to the normal range for LVEF using other imaging modalities including bi-plane and single plane contrast ventriculography17,18 and MRI.19–23 Still, it is noteworthy that within the IMRT-DIBH arm, most patients experienced stable or improved ejection fractions by one year (which may represent recovery of transient mild depression of ejection fraction at the time of the baseline scan, at which time patients had just completed chemotherapy24). By contrast, patients treated with standard therapy on the whole actually experienced a slight decline in ejection fraction by one year post-treatment. The difference observed was small and might represent variability in measurements or a chance spurious finding. Analyses have suggested that the 95% limits of agreement for EF by repeated Tc-99m-MIBI gated SPECT testing is 5.5%,25 but we minimized variability in measurement to the best of our abilities by reprocessing the studies from raw data projections, with motion correction and reconstructions side-by-side, followed by quantitative processing side-by-side by a single expert cardiologist to ensure precise reorientation and alignment of the two studies to ensure as precisely comparable quantification of the two studies as is possible.
Recent data have suggested that heart failure with “normal” ejection fraction is more common among those treated with radiotherapy.26 The observation in the current study that patients in the standard arm were more likely to experience a decline in ejection fraction supports further investigation of the mechanisms by which radiotherapy may contribute, particularly in combination with cardiotoxic systemic agents like anthracyclines and trastuzumab, to compromise heart function. After all, although a small decline in ejection fraction, especially if the ejection fraction remains within normal ranges, may not result in any signs, symptoms, or other experiences of clinical significance for a particular patient, the general observation of even small differences in ejection fraction on a population level may be meaningful to guide cardioprotective strategies.
The pulmonary toxicity of radiotherapy is another important concern. The overall risk of pneumonitis following breast radiotherapy increases with increasing lung volume irradiated.27 While most cases are self-limited or resolve with steroid therapy, some do develop chronic fibrosis.28 Of note, relatively few patients treated on modern trials of breast and regional radiotherapy have been found to experience radiation pneumonitis or pulmonary fibrosis.29,30 Our study results are similarly reassuring, demonstrating no patients with clinically significant pneumonitis and few patients with changes in perfusion or pulmonary function testing.
Most studies of IMRT and DIBH have been limited by primarily focusing on dosimetric differences, so this trial is important in providing the first data to our knowledge directly comparing outcomes on detailed cardiac and pulmonary function assessments of inverse-planned beamlet IMRT with DIBH versus best standard (forward-planned, 3D) therapy. Nevertheless, it has limitations that merit discussion. At the time of the trial, both IMRT and the use of breathing control for motion management were relatively uncommon, so our goal was to compare the most sophisticated form of treatment (adding both IMRT and DIBH) to standard therapy (3D). Since that time, many centers, including our own, have embraced breath-hold as a simple tool with which to reduce cardiac dose. Future studies will be necessary to evaluate whether the benefits observed in this trial can be replicated in other samples and whether they were primarily attributable to the treatment planning and delivery technique of IMRT itself, or whether similar outcomes can be achieved with the potentially less costly combination of 3D treatment with breath-hold techniques.31 Future research should also investigate whether simple mindfulness of LAD dose (thanks to contouring) or small differences in dose per treatment fraction might have meaningful impact on the cardiac toxicity of breast radiotherapy.
Other limitations include study size. Given the extent of effort associated with developing two complete plans for each enrolled patient and the costs of the extensive testing to evaluate pulmonary and cardiac status at baseline and in follow-up, it was not possible to conduct a larger study, which might have detected small differences in other outcomes between the two arms or allowed for us to stratify or control in the analyses for other risk factors that were not evenly balanced despite randomization. Therefore, further confirmatory research is essential in larger patient samples now that this initial study has suggested that there may be benefit. It is also important to note that the standard arm of this study included treatment planning by experienced dosimetrists in a specialized academic setting who were trained to minimize dose to the heart and lungs. These findings should not necessarily be generalized to settings in which care is not taken to optimize forward plans in this way; the benefits of advanced technology might be even greater than observed in the current study in such situations.
Finally, due to resource constraints, we conducted only one follow-up SPECT scan, at one year after treatment. It is possible that scans timed differently, for example at 6 months after treatment, might have detected larger differences. Also due to resource constraints, each scan was read by a single expert in nuclear medicine. However, we took great care to standardize the processing and used the highest standards of analysis. The cardiac SPECT scans were reconstructed side-by-side from the original raw data with motion correction and attenuation correction (for perfusion) by the same highly experienced research technologist using exactly the same reconstruction parameters and filters. Blinded study pairs were then processed by one highly experienced physician specialist with study pairs reoriented side-by-side within the same 4DM workflow from transverse space to cardiac long-axis and short-axis images to ensure precise alignment and then quantified automatically by the exact same 4DM algorithm. The quantitative results were automatically determined without user intervention. Only quantitative comparisons are reported. The only user intervention was to ensure that all studies were processed and aligned in exactly the same way. The expert physician who interpreted the results was blinded to study arm.
At the time the trial was conducted, IMRT-DIBH was considerably more costly than the standard alternative. Based on the Current Procedural Terminology codes and associated charges (derived from the 2009 Medicare Physician Fee Schedule from the Centers for Medicare and Medicaid Services, using the locality of the study, 0095399 Rest of Michigan, including global, professional, and technical fees, the total charges estimated for the typical IMRT-DIBH patient were $19,470 versus $9,397 in the typical 3D-planned radiotherapy patient. The difference was driven primarily by the difference in the daily rate of technical delivery ($478 versus $192). However, changes in fee schedules in more recent years have narrowed the difference in the Medicare schedule considerably, and the estimated difference using 2016 fee schedules is on the order of $2000 rather than $10,000, although some private insurers continue to provide more substantially different reimbursements by technique.
Taken together, this information is useful to guide the resource allocation decisions our society must make regarding the utilization of sophisticated forms of IMRT and the use of respiratory gating in treatment delivery. Although IMRT with DIBH is more costly than standard treatment, our study has suggested a potential benefit in terms of preservation of cardiac ejection fraction, among patients with left-sided disease in whom the internal mammary region was targeted. Still, the difference observed here was small, and the number of patients was insufficient to allow for us to stratify or control for potentially relevant other factors, so this finding should only be construed as hypothesis-generating. We hope that this work will motivate future studies to include rigorous measurements of LVEF as a potentially meaningful endpoint in an era of generally lower cardiac doses than historically delivered, in addition to the perfusion defects that have been the primary focus of studies to date. Gated perfusion studies with positron emission tomography (PET) or MRI might give more reproducible assessments of cardiac perfusion and function at significantly reduced radiation exposures for future studies. Future studies should also seek to disaggregate the impact of IMRT from that of breathing control. Finally, the findings support careful attention in clinical practice to the critical importance of minimizing cardiac dose, even when already low in comparison to historical levels.
Acknowledgments:
The authors gratefully acknowledge the contributions of Kathy Lash and Scott Wood, administrators within the Department of Radiation Oncology, for insights regarding the differences in billing reported in the discussion. We thank Jody Sharp for data management and study coordination. We further extend our gratitude to the patients who participated in this trial.
Funding: Funded, in part, by the National Cancer Institute through an R01 grant to LJP (5R01CA102435–3); in part, by the Breast Cancer Research Foundation to LJP; and, in part, by a Mentored Research Scholar Grant from the American Cancer Society to RJ. The funding bodies played no role in the conduct of the study or the decision to submit for publication.
Footnotes
Trial registration: ClinicalTrials.gov Identifier NCT00581256
https://clinicaltrials.gov/ct2/show/NCT00581256?term=NCT00581256&rank=1
The authors report no other relevant conflicts of interest.
REFERENCES
- 1.Taylor C, Correa C, Duane FK, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 2017;35:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukesh MB, Barnett GC, Wilkinson JS, et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J Clin Oncol 2013; 31:4488–4495. [DOI] [PubMed] [Google Scholar]
- 3.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008;26: 2085–2092. [DOI] [PubMed] [Google Scholar]
- 4.Jagsi R, Moran J, Marsh R, et al. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys 2010;78:1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams TM, Moran JM, Hsu SH, et al. Contralateral breast dose after whole-breast irradiation: an analysis by treatment technique. Int J Radiat Oncol Biol Phys 2012;82:2079–2085. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 7.van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 2017;35:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung E, Corbett JR, Moran JM, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys 2013;85:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liss AL, Marsh RB, Kapadia NS, et al. Decreased lung perfusion after breast/chest wall irradiation: quantitative results from a prospective clinical trial. Int J Radiat Oncol Biol Phys 2017;97:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366: 2087–2106. [DOI] [PubMed] [Google Scholar]
- 11.Giordano SH, Kuo Y-F, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005;97:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris EER, Correa C, Hwang W-T, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006;24:4100–4106. [DOI] [PubMed] [Google Scholar]
- 13.Jagsi R, Griffith KA, Koelling T, et al. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer 2007;109:650–657. [DOI] [PubMed] [Google Scholar]
- 14.Hardenbergh PH, Munley MT, Bentel GC, et al. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys 2001;49:1023–1028. [DOI] [PubMed] [Google Scholar]
- 15.Ficaro EP, Fessler JA, Shreve PD, Kritzman JN, Rose PA, Corbett JR. Simultaneous transmission/emission myocardial perfusion tomography. Diagnostic accuracy of attenuation-corrected 99mTc-sestamibi single-photon emission computed tomography. Circulation 1996;93:463–473. [DOI] [PubMed] [Google Scholar]
- 16.Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nuc Cardiol 2007;14:455–465. [DOI] [PubMed] [Google Scholar]
- 17.Wynne J, Green LH, Mann T, Levin D, Grossman W. Estimation of left ventricular volumes in man from biplane cineangiograms filmed in oblique projections. Am J Cardiol 1978;41:726–732. [DOI] [PubMed] [Google Scholar]
- 18.Rigaud M, Rocha P, Boschat J, Farcot JC, Bardet J, Bourdarias JP. Regional left ventricular function assessed by contrast angiography in acute myocardial infarction. Circulation 1979;60:130–139. [DOI] [PubMed] [Google Scholar]
- 19.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovac Magn Reson 2005;7:775–782. [DOI] [PubMed] [Google Scholar]
- 20.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 21.Salton C, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring Cohort. J Am Coll Cardiol 2002;39:1055–1060. [DOI] [PubMed] [Google Scholar]
- 22.Chuang ML, Gona P, Hautvast GL, et al. Correlation of trabeculae and papillary muscles with clinical and cardiac characteristics and impact on CMR measures of LV anatomy and function. JACC Cardiovasc Imaging 2012;5:1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–426. [DOI] [PubMed] [Google Scholar]
- 24.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing Stage II or III breast cancer: a pilot study. J Clin Oncol 2003;21(1):46–53. [DOI] [PubMed] [Google Scholar]
- 25.Hyun IY, Kwan J, Park KS, Lee WH. Reproducibility of Tl-201 and Tc-99m sestamibi gated myocardial perfusion SPECT measurement of myocardial function. J Nucl Cardiol 2001;8:182–187. [DOI] [PubMed] [Google Scholar]
- 26.Saiki H, Petersen IA, Scott CG. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 2017;135:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothwell RI, Kelly SA, Joslin CA. Radiation pneumonitis in patients treated for breast cancer. Radiother Oncol 1985;4:9–14. [DOI] [PubMed] [Google Scholar]
- 28.Movsas B, Raffin TA, Epstein AH, Link CJ Jr., Pulmonary radiation injury. Chest 1997;111:1061–1076. [DOI] [PubMed] [Google Scholar]
- 29.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015;373:317–327. [DOI] [PubMed] [Google Scholar]
- 31.Moran JM, Ben-David MA, Marsh RB, et al. Accelerated partial breast irradiation: what is the dosimetric effect of advanced technology approaches? Int J Radiat Oncol Biol Phys 2009;75:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]