Abstract
Background
Clinical impact of the Geriatric Nutritional Risk Index (GNRI) in patients with extensive‐stage disease small cell lung cancer (ED‐SCLC) have not previously been reported.
Methods
This study analyzed 352 patients enrolled in a previous randomized phase III trial comparing the efficacy of irinotecan plus cisplatin with that of etoposide plus cisplatin as the first‐line therapy for ED‐SCLC. GNRI values were calculated using serum albumin levels and actual and ideal bodyweights. Patients with a GNRI > 98, 92–98, and <92 were grouped into no, low, and moderate/major risk groups, respectively.
Results
The objective response rates were 63.2%, 52.6%, and 49.2% in the no, low, and moderate/major risk groups, respectively (P = 0.024). The median progression‐free survival (PFS) was shorter in patients with a lower GNRI than in those with a higher GNRI (no vs. low vs. moderate/major risk group; 6.5 vs. 5.8 vs. 5.9 months, respectively; P = 0.028). There were significant differences in median overall survival (OS) according to GNRI (no vs. low vs. moderate/major risk group; 13.2 vs. 10.3 vs. 8.4 months, respectively; P < 0.001). Multivariate analysis revealed that being in the moderate/major risk group was an independent poor prognostic factor for PFS (hazard ratio [HR]: 1.300, 95% confidence interval [CI]: 1.012–1.670; P = 0.040) and OS (HR: 1.539; 95% CI: 1.069–2.216; P = 0.020).
Conclusions
This prospective study shows that a low GNRI value was associated with a poor prognosis, and it supports the relationship between systemic inflammation, nutritional status, and clinical outcomes in patients with ED‐SCLC.Key points
Significant findings of the study
The lower GNRI group had a low response rate to chemotherapy for ED‐SCLC. The HRs for PFS and OS were 1.300 and 1.539 in the patients with GNRI < 92.
What this study adds
Low GNRI is associated with poor prognosis in ED‐SCLC.
Keywords: Cachexia, inflammation, nutrition assessment, small cell lung carcinoma
Introduction
Small cell lung cancer (SCLC) is a highly aggressive malignancy characterized by rapid tumor growth, early locoregional and distant metastases, and frequent presentation of paraneoplastic syndromes.1 Despite a high response rate to chemotherapy, the one‐year survival percentage in patients with extensive‐stage disease (ED) SCLC is only 25%–41%.2, 3, 4 Old age, poor performance status (PS), serum creatinine levels above the upper normal limit, and elevated serum lactate dehydrogenase levels are known to be poor prognostic factors in patients with ED‐SCLC.5, 6
Nutritional status is increasingly recognized as an important prognostic factor in cancer patients. Malnutrition and cachexia are associated with intolerance to anticancer therapy, reduced physical activity, and decreased survival.7, 8 Various markers for nutritional status and cachexia have previously been evaluated in SCLC. Sarcopenia and adipopenia measured by computed tomography have been reported to be related to early discontinuation of treatment and to reduced survival.9, 10 A low modified Glasgow prognostic score (GPS) consisting of serum albumin and C‐reactive protein levels was also reported to be associated with shorter overall survival (OS).11 The prognostic nutritional index (PNI) has been evaluated in several retrospective studies, which consistently reported an association between low PNI values and poor prognoses in patients with SCLC.11, 12, 13, 14 The Geriatric Nutritional Risk Index (GNRI) is another simplified parameter, which was developed to determine the risk of nutrition‐related morbidity and mortality in elderly noncancer patients.15 The low level of this index has been reported to be associated with frequent surgical complications16, 17, 18 and with reduced survival19, 20, 21 in various solid tumors. However, the clinical impact of the GNRI on SCLC have not previously been reported.
Etoposide plus platinum (EP) combination chemotherapy have been considered the standard first‐line treatment for ED‐SCLC.2, 22, 23 Additionally, the irinotecan plus platinum (IP) regimen has been suggested as a potent alternative therapy.24, 25, 26 A meta‐analysis showed a significant benefit of OS for IP over EP with a different toxicity profile.27 However, it is debatable whether irinotecan can substitute for etoposide because this meta‐analysis did not use individual patient data and showed only a relatively small absolute survival benefit without a benefit in terms of progression‐free survival (PFS). Furthermore, the results, which favored irinotecan in Asian patients, were not reproduced in non‐Asian patients.4, 28 Recently, our previous phase III study which was conducted in Korean patients reported that there was no significant difference in OS between IP and EP (median OS: 10.9 vs. 10.3 months, respectively, P = 0.120).29 Preplanned subgroup analysis showed that the IP regimen might be favorable in males and younger ages, with a good PS and a modest survival benefit of 5–6 weeks. This secondary analysis was conducted to evaluate the prognostic values of the GNRI and other nutritional markers in patients with ED‐SCLC, and to further select patients who would benefit from either the IP or EP regimens through stratification using the GNRI.
Methods
Patients and study design
This analysis used data from our previous randomized, multi‐center, phase III trial, which compared the efficacy of the IP regimen with that of the EP regimen in ED‐SCLC patients. The study design, eligibility, and treatment schedule have been previously described.29 Briefly, the study involved 362 eligible patients ≥18 years of age having histologically or cytologically confirmed ED‐SCLC, no previous chemotherapy, Eastern Cooperative Oncology Group (ECOG) PS ≤ 2, and measurable lesions as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0.30 Patients also had adequate organ function. Enrolled patients were randomly (1:1) treated with either the IP or EP regimen. The patients assigned to the IP arm received 65 mg/m2 of irinotecan on days 1 and 8 and 70 mg/m2 of cisplatin on day 1 every three weeks. Those assigned to the EP arm received 100 mg/m2 of etoposide on days 1–3 and 70 mg/m2 of cisplatin on day 1 every three weeks. Up to six cycles of chemotherapy were allowed in each arm. In the present study, 10 of 362 patients in whom the GNRI could not be calculated were excluded. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and approved by the Institutional Review Board of each participating institution. All patients provided written informed consent.
Evaluation and definition
Before treatment, all patients were evaluated using demographic information, physical measurements, ECOG PS, radiological studies, and laboratory tests, including a complete blood cell count and serum chemistry. Tumor response to treatment was assessed according to the RECIST version 1.0 every 2–3 cycles of treatment.30 Treatment‐related toxicity was evaluated using National Cancer Institute Common Toxicity Criteria, version 3.0, every treatment cycle. Body mass index (BMI) was calculated as bodyweight (kg) divided by the square of the height (m2). Underweight was defined as a BMI < 18.5 kg/m2 according to Asian criteria.31 PNI was calculated as 10 × serum albumin level (g/dL) + 0.005 × absolute lymphocyte count (/mm3). PNI values >45, 40–45, and <40 were categorized as low, intermediate, and high risk.32 GNRI values were calculated as 1.489 × serum albumin level (g/L) + 41.7 × (actual bodyweight [ABW]/ideal bodyweight [IBW] [kg]). The ABW/IBW ratio was set to one if the ABW exceeded the IBW. GNRI values >98, 92–98, and <92 were categorized as no, low, and moderate/major risk as described previously.15
Statistical analysis
All analyses were performed on an intention‐to‐treat population. The correlations between ordinal and continuous variables and those between ordinal and dichotomous variables were tested using Spearman's rank correlation and the chi‐square for trend tests, respectively. OS was calculated as the time from the date of beginning treatment to the date of death or the last follow‐up. PFS was calculated as the time from the date of beginning treatment to the date of progression, death, or last follow‐up. Survival was analyzed using the Kaplan‐Meier method and compared by the log‐rank test for trend. Cox regression analysis was performed to determine the influence of different variables on survival. All variables with a P‐value <0.05 on univariate analyses and treatment regimen were included in the multivariate Cox regression model. A two‐sided P‐value <0.05 was considered statistically significant. All analyses were performed with STATA software, version 14.2 (College Station, TX, USA).
Results
Baseline characteristics
The mean (± standard deviation) GNRI value was 94.7 (± 8.8) with a range of 68.5–113.2. Of 352 patients, 133 were assigned to the no risk group, 95 to the low risk group, and 124 to the moderate/major risk group. Their baseline characteristics according to GNRI are presented in Table 1. In total, the median age was 65 years (range: 36–81 years) and most patients were male (90.3%). A poor ECOG PS of two was observed in 51 patients (14.5%). Brain metastasis was detected at diagnosis in 94 patients (26.7%). In a comparison of the three GNRI groups, there was no statistically significant difference in age, sex, brain metastasis, chemotherapy regimen, or thrombocytopenia incidence. However, poor PS (P < 0.001), anemia (P < 0.001), and hyponatremia (P = 0.013) were more common in the moderate/major risk group. The values of other nutritional markers such as BMI (P < 0.001) and PNI (P < 0.001) were higher as the GNRI value increased.
Table 1.
Baseline characteristics
| Characteristics | GNRI > 98 (n = 133) | GNRI 92–98 (n = 95) | GNRI < 92 (n = 124) | P‐value |
|---|---|---|---|---|
| Age | ||||
| <65 years | 65 (48.9) | 46 (48.4) | 49 (39.5) | 0.136 |
| ≥65 years | 68 (51.1) | 49 (51.6) | 75 (60.5) | |
| Median, years (range) | 65 (36–80) | 65 (47–81) | 66 (48–81) | 0.066 |
| Sex | ||||
| Male | 123 (92.5) | 84 (88.4) | 111 (89.5) | 0.414 |
| Female | 10 (7.5) | 11 (11.6) | 13 (10.5) | |
| ECOG PS | ||||
| 0–1 | 122 (91.7) | 86 (90.5) | 93 (75.0) | < 0.001 |
| 2 | 11 (8.3) | 9 (9.5) | 31 (25.0) | |
| Brain metastasis | ||||
| Absent | 58 (43.6) | 39 (41.1) | 46 (37.1) | 0.143 |
| Present | 34 (25.6) | 32 (33.7) | 28 (22.6) | |
| Not evaluated | 41 (30.8) | 24 (25.3) | 50 (40.3) | |
| Regimen | ||||
| IP | 63 (47.4) | 47 (49.5) | 60 (48.4) | 0.867 |
| EP | 70 (52.6) | 48 (50.5) | 64 (51.6) | |
| Anemia | ||||
| Absent | 93 (69.9) | 54 (56.8) | 35 (28.2) | < 0.001 |
| Present | 40 (30.1) | 41 (43.2) | 89 (71.8) | |
| Thrombocytopenia | ||||
| Absent | 128 (96.2) | 87 (91.6) | 118 (95.2) | 0.682 |
| Present | 5 (3.8) | 8 (8.4) | 6 (4.8) | |
| Hyponatremia | ||||
| Absent | 105 (79.0) | 77 (81.1) | 81 (65.3) | 0.013 |
| Present | 28 (21.1) | 18 (19.0) | 43 (34.7) | |
| Median BMI (range), kg/m2 | 23.9 (17.9–34.8) | 22.8 (15.0–28.3) | 20.9 (14.8–27.8) | < 0.001 |
| Median PNI (range) | 54.7 (41.8–71.3) | 47.9 (37.7–60) | 41.4 (26.8–56.5) | < 0.001 |
BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; EP, etoposide/cisplatin; GNRI, Geriatric Nutritional Risk Index; IP, irinotecan/cisplatin; PNI, Prognostic Nutritional Index.
Treatment response
Treatment response assessments were available for 287/352 patients (Table 2). There were three complete responses (CRs) in the no risk group, compared with only one CR in the other two groups. The objective response rates (ORRs) were 63.2%, 52.6%, and 49.2% in the no, low, and moderate/major risk groups, respectively (P = 0.024). Similar to our previous study,29 the ORR was significantly higher in the IP arm compared with the EP arm (62.4% vs. 48.9%, respectively; P = 0.011). Regardless of the chemotherapy regimen, the ORR tended to be lower in the moderate/major risk group, although there was no statistical significance (no vs. low vs. moderate/major risk group, 71.4% vs. 57.5% vs. 56.7% in the IP arm [P = 0.090]; 55.7% vs. 47.9% vs. 42.2% in the EP arm [P = 0.118], respectively).
Table 2.
Best overall response
| Confirmed best response | GNRI > 98 (n = 133) | GNRI 92–98 (n = 95) | GNRI < 92 (n = 124) |
|---|---|---|---|
| Complete response | 4 (3.0) | 0 (0.0) | 1 (0.8) |
| Partial response | 80 (60.2) | 50 (52.6) | 60 (48.4) |
| Stable disease | 25 (18.8) | 24 (25.3) | 23 (18.6) |
| Progressive disease | 6 (4.5) | 4 (4.2) | 10 (8.1) |
| Not evaluable | 18 (13.5) | 17 (17.9) | 30 (24.2) |
| Objective response rate (CR + PR) | 84 (63.2) | 50 (52.6) | 61 (49.2) |
CR, complete response; GNRI, Geriatric Nutritional Risk Index; PR, partial response.
Toxicity
Grade 3 or more adverse events occurred in >2% of patients were reviewed (Table 3). The mean (± standard deviation) treatment cycles were 4.7 (± 1.9), 4.4 (± 2.0), and 4.1 (± 2.2) in the no, low, and moderate/major risk groups (P = 0.014). The no risk group had significantly fewer incidences of anemia (14.3% vs. 25.3% vs. 26.6%; P = 0.016) and thrombocytopenia (4.5% vs. 17.9% vs. 17.7%; P = 0.001) compared with the low and moderate/major risk groups, respectively. Nausea was less common in the moderate/major risk group than the other two groups, but only small numbers of patients experienced this toxicity. Otherwise, there were no differences in neutropenia, neutropenic fever, infection, vomiting, diarrhea, and liver function test abnormalities among the three groups. Treatment‐related deaths and treatment discontinuations caused by toxicity occurred in three, six, and nine patients (2.3%, 6.3%, and 7.3%; P = 0.068) and in 13, 10, and 13 patients (9.8%, 10.5%, and 10.5%; P = 0.850) in the no, low, and moderate/major risk groups, respectively.
Table 3.
Grade ≥ 3 adverse events in more than 2% of subjects
| Adverse event | GNRI > 98 (n = 133) | GNRI 92–98 (n = 95) | GNRI < 92 (n = 124) | P‐value |
|---|---|---|---|---|
| Anemia | 19 (14.3) | 24 (25.3) | 33 (26.6) | 0.016 |
| Neutropenia | 92 (69.2) | 55 (57.9) | 82 (66.1) | 0.588 |
| Thrombocytopenia | 6 (4.5) | 17 (17.9) | 22 (17.7) | 0.001 |
| Neutropenic fever | 23 (17.3) | 15 (15.8) | 21 (16.9) | 0.935 |
| Infection | 20 (15.0) | 16 (16.8) | 28 (22.6) | 0.119 |
| Nausea | 5 (3.8) | 3 (3.2) | 0 (0.0) | 0.045 |
| Vomiting | 4 (3.0) | 3 (3.2) | 1 (0.8) | 0.242 |
| Diarrhea | 8 (6.0) | 8 (8.4) | 6 (4.8) | 0.711 |
| AST elevation | 3 (2.3) | 5 (5.3) | 2 (1.6) | 0.780 |
| ALT elevation | 3 (2.3) | 4 (4.2) | 1 (0.8) | 0.453 |
AST, aspartate transaminase; ALT, alanine transaminase; GNRI, Geriatric Nutritional Risk Index.
Survival
The median follow‐up duration was 50.1 months (range: 17.6–83.2 months) in all patients. The median PFS was shorter in the moderate/major risk group than in the lower risk groups (no vs. low vs. moderate/major risk group, 6.5 [95% CI: 6.0–7.2] vs. 5.8 [95% CI: 5.5–0.5] vs. 5.9 [95% CI: 4.8–6.4] months; respectively; P = 0.028; Fig 1a). The difference was more apparent in the median OS among the three groups (no vs. low vs. moderate/major risk group, 13.2 [95% CI: 11.7–4.7] vs. 10.3 [95% CI: 8.8–1.5] vs. 8.4 [95% CI: 7.4–10.0] months, respectively; P < 0.001; Fig 1b). In a comparison according to chemotherapy regimen, there was no significant difference in median PFS (6.5 vs. 5.9 months; P = 0.105) and OS (10.9 vs. 10.3 months; P = 0.241) between the IP and EP arms, respectively, as shown in our previous study.29 In the IP arm, there was no difference in median PFS among the three groups (no vs. low vs. moderate/major risk group, 6.9 [95% CI: 6.3–7.4] vs. 5.9 [95% CI: 4.7–7.7] vs. 6.2 [95% CI: 5.3–7.2] months, respectively; P = 0.307; Fig 2a), while the median OS was shorter in the moderate/major risk group compared with the lower risk groups (no vs. low vs. moderate/major risk group, 12.7 [95% CI: 9.4–15.3] vs. 10.3 [95% CI: 7.3–15.6] vs. 9.6 [95% CI: 7.1–12.7] months, respectively; P = 0.033; Fig 2b). In the EP arm, there were significant differences among the three groups both in median PFS (no vs. low vs. moderate/major risk group, 6.0 [95% CI: 5.2–7.1] vs. 5.7 [95% CI: 4.5–6.7] vs. 4.8 [95% CI: 3.5–6.2] months, respectively; P = 0.027; Fig 2c) and OS (no vs. low vs. moderate/major risk group, 13.3 [95% CI: 11.8–16.0] vs. 10.0 [95% CI: 7.8–11.2] vs. 7.7 [95% CI: 6.7–9.1] months, respectively; P < 0.001; Fig 2d).
Figure 1.
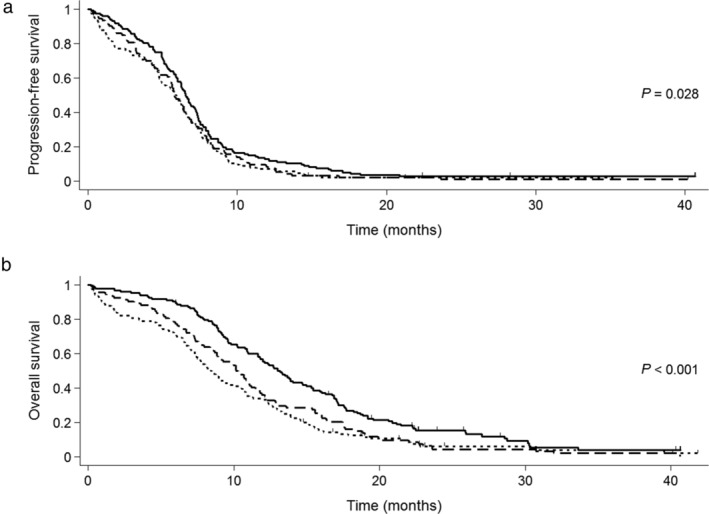
Kaplan‐Meier curves for (a) progression‐free survival and (b) overall survival according to GNRI. GNRI, Geriatric Nutritional Risk Index. ( ) GNRI > 98 (n = 133), (
) GNRI > 98 (n = 133), ( ) GNRI 92–98 (n = 95) and (
) GNRI 92–98 (n = 95) and ( ) GNRI < 92 (n = 124).
) GNRI < 92 (n = 124).
Figure 2.
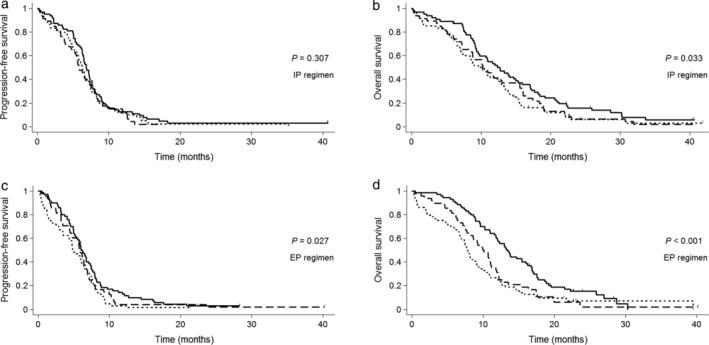
Kaplan‐Meier curves for (a) progression‐free survival, (b) overall survival in the IP arm, and (c) progression‐free survival and (d) overall survival in the EP arm according to GNRI. EP, etoposide/cisplatin; GNRI, Geriatric Nutritional Risk Index; IP, irinotecan/cisplatin. (a, b) ( ) GNRI > 98 (n = 63), (
) GNRI > 98 (n = 63), ( ) GNRI 92–98 (n = 47) and (
) GNRI 92–98 (n = 47) and ( ) GNRI < 92 (n = 60). (c, d) (
) GNRI < 92 (n = 60). (c, d) ( ) GNRI > 98 (n = 70), (
) GNRI > 98 (n = 70), ( ) GNRI 92–98 (n = 48) and (
) GNRI 92–98 (n = 48) and ( ) GNRI < 92 (n = 64).
) GNRI < 92 (n = 64).
In a multivariate analysis of PFS (Table 4), being part of the moderate/major risk group was an independent poor prognostic factor (hazard ratio [HR]: 1.300; 95% CI: 1.012–1.670; P = 0.040). The low risk group did not show a statistically significant difference in PFS compared with the no risk group (P = 0.085). In a multivariate analysis of OS, being a member of either the low risk group (HR: 1.446; 95% CI: 1.086–1.925; P = 0.012) or the moderate/major risk group (HR: 1.539; 95% CI: 1.069–2.216; P = 0.020) was an independent poor prognostic factor, compared with the no risk group. In contrast, the PNI lost statistical significance after adjusting for potential prognostic factors, including the GNRI.
Table 4.
Cox regression for PFS and OS
| Factor | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age | ||||||||||||
| <65 years | Ref. | Ref. | Ref. | |||||||||
| ≥65 years | 1.065 | 0.861–1.318 | 0.563 | 1.328 | 1.067–1.652 | 0.011 | 1.305 | 1.045–1.629 | 0.019 | |||
| Sex | ||||||||||||
| Male | Ref. | Ref. | ||||||||||
| Female | 1.257 | 0.882–1.793 | 0.206 | 1.153 | 0.799–1.662 | 0.447 | ||||||
| ECOG PS | ||||||||||||
| 0–1 | Ref. | Ref. | Ref. | |||||||||
| 2 | 1.208 | 0.892–1.635 | 0.221 | 1.767 | 1.294–2.413 | <0.001 | 1.686 | 1.217–2.336 | 0.002 | |||
| Brain metastasis | ||||||||||||
| Absent/not evaluated | Ref. | Ref. | ||||||||||
| Present | 1.056 | 0.831–1.341 | 0.658 | 1.149 | 0.900–1.467 | 0.265 | ||||||
| Regimen | ||||||||||||
| IP | Ref. | Ref. | Ref. | Ref. | ||||||||
| EP | 1.191 | 0.963–1.473 | 0.106 | 1.210 | 0.978–1.497 | 0.080 | 1.139 | 0.916–1.417 | 0.242 | 1.123 | 0.900–1.402 | 0.303 |
| Anemia | ||||||||||||
| Absent | Ref. | Ref. | Ref. | |||||||||
| Present | 1.157 | 0.937–1.430 | 0.176 | 1.288 | 1.037–1.602 | 0.022 | 1.106 | 0.863–1.418 | 0.426 | |||
| Thrombocytopenia | ||||||||||||
| Absent | Ref. | Ref. | ||||||||||
| Present | 1.072 | 0.675–1.704 | 0.768 | 1.104 | 0.683–1.782 | 0.687 | ||||||
| Hyponatremia | ||||||||||||
| Absent | Ref. | Ref. | Ref. | Ref. | ||||||||
| Present | 1.394 | 1.093–1.780 | 0.008 | 1.382 | 1.081–1.767 | 0.010 | 1.240 | 0.968–1.588 | 0.089 | 1.100 | 0.835–1.448 | 0.498 |
| BMI | ||||||||||||
| Normal to obese | Ref. | Ref. | ||||||||||
| Underweight | 1.175 | 0.798–1.731 | 0.414 | 1.370 | 0.908–2.067 | 0.134 | ||||||
| PNI | ||||||||||||
| >45 | Ref. | Ref. | Ref. | |||||||||
| 40–45 | 1.221 | 0.920–1.622 | 0.167 | 1.416 | 1.061–1.892 | 0.018 | 1.050 | 0.750–1.470 | 0.777 | |||
| <40 | 1.264 | 0.943–1.694 | 0.117 | 1.457 | 1.078–1.968 | 0.014 | 0.953 | 0.625–1.454 | 0.823 | |||
| GNRI | ||||||||||||
| >98 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 92–98 | 1.242 | 0.951–1.623 | 0.112 | 1.266 | 0.968–1.654 | 0.085 | 1.463 | 1.114–1.920 | 0.006 | 1.446 | 1.086–1.925 | 0.012 |
| <92 | 1.315 | 1.026–1.687 | 0.031 | 1.300 | 1.012–1.670 | 0.040 | 1.696 | 1.311–2.194 | <0.001 | 1.539 | 1.069–2.216 | 0.020 |
BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EP, etoposide/cisplatin; GNRI, Geriatric Nutritional Risk Index; HR, hazard ratio; IP, irinotecan/cisplatin; OS, overall survival; PFS, progression‐free survival; PNI, Prognostic Nutritional Index.
Discussion
This report provides the first evidence of an association between the GNRI and the prognoses of patients with ED‐SCLC. The moderate/major risk group had a 14% reduction in the treatment response percentage and decreased survival by five months (median OS: 13.2 vs. 8.4 months) compared with the no risk group. Although poor PS, anemia, and hyponatremia in baseline characteristics were more commonly observed in the higher risk group, the GNRI remained an independent prognostic factor for survival after adjusting for these variables.
The underlying mechanism resulting in SCLC patients with a low GNRI having a poor prognosis is unclear. The GNRI consists of the serum albumin level and bodyweight (ABW/IBW). Because the ABW/IBW ratio is set to one if the ABW is greater than the IBW in the GNRI formula, the value of albumin outweighs that of bodyweight in the GNRI.15 Hypoalbuminemia is known to reflect a systemic inflammatory condition. Inflammatory cytokines such as interleukin‐1 and ‐6 reduce the hepatic synthesis of albumin and its mRNA content.33, 34 Tumor necrosis factor‐alpha increases the albumin permeability of glomeruli through the generation of superoxides.35 The transcapillary escape of albumin from the intravascular space to the tissue space is increased under inflammatory conditions, and is promoted by interleukin‐2.36, 37 Oxidative stress results in an increase in denatured albumin, which is likely to be degraded by endocytosis in hepatic endothelial cells.38 Systemic inflammation is the main contributor to malnutrition and cachexia in cancer patients.39, 40, 41 Furthermore, there is a close relationship between systemic inflammation and tumor progression and metastasis.42, 43, 44 In SCLC, many studies have reported the association of inflammatory markers with the prognoses of patients.10, 45, 46, 47, 48, 49 Because the GNRI reflects both systemic inflammation and cachexia, which result in adverse clinical outcomes, the GNRI may be a prognostic factor in SCLC patients.
We identified nutritional markers that were prognostic in ED‐SCLC. The GNRI was closely and positively correlated with the PNI and BMI. In addition to the GNRI, a low PNI was also associated with worse OS in a univariate analysis, but not associated with PFS. In a multivariate analysis, the PNI was not an independent prognostic factor for OS after adjusting for the GNRI. BMI did not show any prognostic value for PFS or OS. Several studies have compared the clinical impacts of nutritional markers, including the GNRI, in various malignancies. Patients with a low GNRI had 3.4 times more postoperative respiratory complications than those with a high GNRI after esophagectomy for esophageal cancer, whereas there was no difference between the low and high PNI groups.50 Another study of esophageal cancer patients who underwent curative esophagectomy reported that the GNRI and PNI had similar prognostic values.20 In nonmetastatic renal cell carcinoma, the low GNRI group had 3.2 times longer cancer‐specific survival compared with the high GNRI group, but BMI did not have any prognostic value.51 A study of surgically‐treated elderly patients with non‐SCLC reported that the GNRI was the only independent prognostic factor for OS when compared with the PNI, BMI, and controlling nutritional status.52 Because of a scarcity of data and discordant cutoff values of the markers, it was difficult to conclude which one was the most appropriate prognostic factor among the nutritional markers. However, when considering the results of present and previous studies, the GNRI may be as good as other nutritional markers as a prognostic indicator in SCLC.
Another purpose of this study was to determine whether the GNRI could identify patients having a favorable outcome from either the IP or EP regimens. In the EP arm, there was a significant difference in PFS among the GNRI groups, and this trend was clearer in OS (no risk vs. moderate/major risk group, 13.3 vs. 7.7 months). In contrast, no difference in PFS was observed regardless of the GNRI, and the discrimination of OS was also less clear in the IP arm. Given the higher response rate of the IP regimen, the IP regimen may be more effective than the EP regimen in patients with a low GNRI value who have a poor prognosis. However, this finding was obtained from a subgroup analysis, and there was no statistical difference in median OS between the IP and EP arms when the analysis was performed only in the moderate/major risk group (9.6 vs. 7.7 months; P = 0.174). In the original cohort, although the favorable OS of the IP arm was observed in males <65 years of age and the ECOG PS 0–1 patient groups, the survival benefit was only 5–6 weeks in these populations.29 We suggest that the IP regimen does not clearly improve the prognoses of ED‐SCLC patients compared with the EP regimen, so additional studies are needed to identify patients who are likely to benefit from each regimen.
This study has some limitations. First, this was an unplanned subset analysis which was not powered to determine the prognostic role of the GNRI. In cancer patients, few studies have investigated the clinical impact of the GNRI using a prospective nonrandomized cohort.21, 53 To overcome the potential bias in an observational study, a randomized controlled trial comparing each GNRI risk group is needed. Second, nutritional and inflammatory assessments by other factors than the GNRI, PNI, and BMI were not performed due to limited information in this cohort. For example, mini‐nutritional assessment data, sarcopenia, GPS, and prognostic inflammatory and nutritional indices could not be assessed because of a lack of data regarding recent weight loss, muscle mass and quality, inflammatory cytokines, C‐reactive protein, and prealbumin.7, 54, 55
In conclusion, this prospective study suggests that a low GNRI value was associated with poor prognoses for ED‐SCLC patients. The results support the importance of systemic inflammation and nutritional status in the clinical outcomes of SCLC patients. However, additional studies with comprehensive nutritional assessments are warranted to confirm our findings.
Disclosure
The authors do not have any competing interests.
Acknowledgments
We thank the participating patients and their families, all study coinvestigators, and research coordinators. This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A040151).
References
- 1. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small‐cell lung cancer. Lancet 2011; 378: 1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Tiseo M, Boni L, Ambrosio F et al Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first‐line treatment in extensive‐disease small‐cell lung cancer: The GOIRC‐AIFA FARM6PMFJM trial. J Clin Oncol 2017; 35: 1281–7. [DOI] [PubMed] [Google Scholar]
- 3. Rossi A, Di Maio M, Chiodini P et al Carboplatin‐ or cisplatin‐based chemotherapy in first‐line treatment of small‐cell lung cancer: The COCIS meta‐analysis of individual patient data. J Clin Oncol 2012; 30: 1692–8. [DOI] [PubMed] [Google Scholar]
- 4. Lara PN Jr, Natale R, Crowley J et al Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive‐stage small‐cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009; 27: 2530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster NR, Mandrekar SJ, Schild SE et al Prognostic factors differ by tumor stage for small cell lung cancer: A pooled analysis of North Central Cancer Treatment Group trials. Cancer 2009; 115: 2721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Determinants of improved outcome in small‐cell lung cancer: An analysis of the 2,580‐patient Southwest Oncology Group data base. J Clin Oncol 1990; 8: 1563–74. [DOI] [PubMed] [Google Scholar]
- 7. Fearon K, Strasser F, Anker SD et al Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011; 12: 489–95. [DOI] [PubMed] [Google Scholar]
- 8. Zhang X, Tang T, Pang L et al Malnutrition and overall survival in older adults with cancer: A systematic review and meta‐analysis. J Geriatr Oncol 2019; 10: 874–83. [DOI] [PubMed] [Google Scholar]
- 9. Kim EY, Lee HY, Kim YS et al Prognostic significance of cachexia score assessed by CT in male patients with small cell lung cancer. Eur J Cancer Care (Engl) 2018; 27: e12695. [DOI] [PubMed] [Google Scholar]
- 10. Go SI, Park MJ, Song HN et al Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016; 24: 2075–84. [DOI] [PubMed] [Google Scholar]
- 11. Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Pretreatment Glasgow prognostic score and prognostic nutritional index predict overall survival of patients with advanced small cell lung cancer. Lung Cancer (Auckl) 2017; 8: 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong S, Zhou T, Fang W et al The prognostic nutritional index (PNI) predicts overall survival of small‐cell lung cancer patients. Tumour Biol 2015; 36: 3389–97. [DOI] [PubMed] [Google Scholar]
- 13. Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum‐based chemotherapy. Clin Respir J 2018; 12: 2433–40. [DOI] [PubMed] [Google Scholar]
- 14. Go SI, Jeon H, Park SW, Kang MH, Kim HG, Lee GW. Low pre‐treatment nutritional index is significantly related to poor outcomes in small cell lung cancer. Thorac Cancer 2018; 9: 1483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouillanne O, Morineau G, Dupont C et al Geriatric Nutritional Risk Index: A new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–83. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki H, Nagano S, Taniguchi N, Setoguchi T. Risk factors for surgical site infection after soft‐tissue sarcoma resection, including the Preoperative Geriatric Nutritional Risk Index. Nutrients 2018; 10: 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kushiyama S, Sakurai K, Kubo N et al The Preoperative Geriatric Nutritional Risk Index predicts postoperative complications in elderly patients with gastric cancer undergoing gastrectomy. In Vivo 2018; 32: 1667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubo N, Sakurai K, Tamura T et al The impact of geriatric nutritional risk index on surgical outcomes after esophagectomy in patients with esophageal cancer. Esophagus 2019; 16: 147–54. [DOI] [PubMed] [Google Scholar]
- 19. Okamoto T, Hatakeyama S, Narita S et al. Impact of nutritional status on the prognosis of patients with metastatic hormone‐naive prostate cancer: A multicenter retrospective cohort study in Japan. World J Urol 2019; 37: 1827–35. doi: 10.1007/s00345-018-2590-2 [DOI] [PubMed] [Google Scholar]
- 20. Migita K, Matsumoto S, Wakatsuki K et al The prognostic significance of the Geriatric Nutritional Risk Index in patients with esophageal squamous cell carcinoma. Nutr Cancer 2018; 70: 1237–45. [DOI] [PubMed] [Google Scholar]
- 21. Gu W, Zhang G, Sun L et al Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle 2015; 6: 222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans WK, Shepherd FA, Feld R, Osoba D, Dang P, Deboer G. VP‐16 and cisplatin as first‐line therapy for small‐cell lung cancer. J Clin Oncol 1985; 3: 1471–7. [DOI] [PubMed] [Google Scholar]
- 23. Fink TH, Huber RM, Heigener DF et al Topotecan/cisplatin compared with cisplatin/etoposide as first‐line treatment for patients with extensive disease small‐cell lung cancer: Final results of a randomized phase III trial. J Thorac Oncol 2012; 7: 1432–9. [DOI] [PubMed] [Google Scholar]
- 24. Noda K, Nishiwaki Y, Kawahara M et al Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002; 346: 85–91. [DOI] [PubMed] [Google Scholar]
- 25. Hermes A, Bergman B, Bremnes R et al Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small‐cell lung cancer: A randomized phase III trial. J Clin Oncol 2008; 26: 4261–7. [DOI] [PubMed] [Google Scholar]
- 26. Zatloukal P, Cardenal F, Szczesna A et al A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small‐cell lung cancer patients with extensive disease. Ann Oncol 2010; 21: 1810–6. [DOI] [PubMed] [Google Scholar]
- 27. Shao N, Jin S, Zhu W. An updated meta‐analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive‐stage small cell lung cancer. J Thorac Oncol 2012; 7: 470–2. [DOI] [PubMed] [Google Scholar]
- 28. Hanna N, Bunn PA Jr, Langer C et al Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive‐stage disease small‐cell lung cancer. J Clin Oncol 2006; 24: 2038–43. [DOI] [PubMed] [Google Scholar]
- 29. Kim DW, Kim HG, Kim JH et al Randomized phase III trial of irinotecan plus cisplatin versus etoposide plus cisplatin in chemotherapy‐naive Korean patients with extensive‐disease small cell lung cancer. Cancer Res Treat 2019; 51: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 31. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–63. [DOI] [PubMed] [Google Scholar]
- 32. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984; 85: 1001–5. [PubMed] [Google Scholar]
- 33. Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest 1987; 79: 1635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Y, Shinzawa H, Togashi H et al Interleukin‐6 down‐regulates expressions of the aldolase B and albumin genes through a pathway involving the activation of tyrosine kinase. Arch Biochem Biophys 1995; 320: 203–9. [DOI] [PubMed] [Google Scholar]
- 35. McCarthy ET, Sharma R, Sharma M et al TNF‐alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol 1998; 9: 433–8. [DOI] [PubMed] [Google Scholar]
- 36. Ballmer‐Weber BK, Dummer R, Kung E, Burg G, Ballmer PE. Interleukin 2‐induced increase of vascular permeability without decrease of the intravascular albumin pool. Br J Cancer 1995; 71: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fleck A, Raines G, Hawker F et al Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet 1985; 1: 781–4. [DOI] [PubMed] [Google Scholar]
- 38. Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress‐induced denatured albumin in rat liver endothelial cells. Am J Physiol Cell Physiol 2005; 289: C531–42. [DOI] [PubMed] [Google Scholar]
- 39. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: Understanding the molecular basis. Nat Rev Cancer 2014; 14: 754–62. [DOI] [PubMed] [Google Scholar]
- 40. Han J, Meng Q, Shen L, Wu G. Interleukin‐6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis 2018; 17: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 2016; 54: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J Clin Oncol 2016; 34: 4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coffelt SB, de Visser KE. Cancer: Inflammation lights the way to metastasis. Nature 2014; 507: 48–9. [DOI] [PubMed] [Google Scholar]
- 45. Go SI, Kim RB, Song HN et al Prognostic significance of the lymphocyte‐to‐monocyte ratio in patients with small cell lung cancer. Med Oncol 2014; 31: 323. [DOI] [PubMed] [Google Scholar]
- 46. Kang MH, Go SI, Song HN et al The prognostic impact of the neutrophil‐to‐lymphocyte ratio in patients with small‐cell lung cancer. Br J Cancer 2014; 111: 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim EY, Kim YS, Seo JY et al The relationship between sarcopenia and systemic inflammatory response for cancer cachexia in small cell lung cancer. PLOS One 2016; 11: e0161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou T, Hong S, Hu Z et al A systemic inflammation‐based prognostic scores (mGPS) predicts overall survival of patients with small‐cell lung cancer. Tumour Biol 2015; 36: 337–43. [DOI] [PubMed] [Google Scholar]
- 49. Zhou T, Zhan J, Hong S et al Ratio of C‐reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small‐cell lung cancer. Sci Rep 2015; 5: 10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamana I, Takeno S, Shibata R et al Is the geriatric nutritional risk index a significant predictor of postoperative complications in patients with esophageal cancer undergoing esophagectomy? Eur Surg Res 2015; 55: 35–42. [DOI] [PubMed] [Google Scholar]
- 51. Miyake H, Tei H, Fujisawa M. Geriatric nutrition risk index is an important predictor of cancer‐specific survival, but not recurrence‐free survival, in patients undergoing surgical resection for non‐metastatic renal cell carcinoma. Curr Urol 2017; 10: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shoji F, Miura N, Matsubara T et al Prognostic significance of immune‐nutritional parameters for surgically resected elderly lung cancer patients: A multicentre retrospective study. Interact Cardiovasc Thorac Surg 2018; 26: 389–94. [DOI] [PubMed] [Google Scholar]
- 53. Balzano G, Dugnani E, Crippa S et al A preoperative score to predict early death after pancreatic cancer resection. Dig Liver Dis 2017; 49: 1050–6. [DOI] [PubMed] [Google Scholar]
- 54. Caillet P, Liuu E, Raynaud Simon A et al Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin Nutr 2017; 36: 1473–82. [DOI] [PubMed] [Google Scholar]
- 55. Fruchtenicht AV, Poziomyck AK, Kabke GB et al Nutritional risk assessment in critically ill cancer patients: Systematic review. Rev Bras Ter Intensiva 2015; 27: 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


