Abstract
Purpose
Elevated aryl hydrocarbon receptor (AhR) transactivating (AHRT) activity and uremia in chronic kidney disease (CKD) may interact with each other, further complicating the disease course. In this study, we prospectively estimated serum AHRT activity using a highly sensitive cell-based AhR-dependent luciferase activity assay in CKD patients and compared differences therein according to treatment modality.
Materials and Methods
Patients undergoing peritoneal dialysis (PD) (n=22) and hemodialysis (HD) (n=38) and patients with pre-dialysis CKD stage IV or V (n=28) were included. AHRT activity and intracellular adenosine triphosphate (ATP) levels were measured. We performed a correlation analysis for AHRT activity, ATP levels, and various clinical parameters.
Results
AHRT activity and intracellular ATP levels were inversely correlated and differed according to treatment modalities. AHRT activity was higher in non-dialysis CKD patients than in patients undergoing dialysis and was higher in patients undergoing HD, compared to PD. AHRT activity decreased after HD treatment in HD patients. ATP levels were higher in healthy controls than in patients with pre-dialysis CKD and PD and were further decreased in patients with HD. We noted significant correlations between multiple clinical parameters associated with cardiovascular risk factors and AHRT activity.
Conclusion
AHRT activity was elevated in CKD patients, while dialysis treatment reduced AHRT activity. Further studies are warranted to specify AHRT activity and to evaluate the precise roles thereof in patients with CKD.
Keywords: Aryl hydrocarbon receptor transactivating activity, chronic kidney disease, dialysis
INTRODUCTION
Recently, attention on endocrine disrupting chemicals has increased due to rising concerns over the health risks of environmental pollutants, such as fine dust and microplastics. However, it has been proven difficult to demonstrate a causal relationship between serum levels of these materials and disease development, one of the most important reasons being the inadequacy of methods with which to evaluate the direct toxic effects of chemical mixtures that people are exposed to.
Persistent organic pollutants (POPs) are some of the most important and highly divergent endocrine disrupting chemicals. Among POPs, dioxin and dioxin-like substances are the most infamous, as they are resistant to degradation in the environment and bioaccumulate in our body through the food chain.
Many POPs bind to the aryl hydrocarbon receptor (AhR), leading to transcriptional activation of multiple genes, including cytochrome p450 1A1 (CYP 1A1), by binding to dioxin-response elements (DRE) of their promoters, eliciting increases in inflammatory proteins.1,2,3 The representative ligands of AhR are dioxins, which have a very high affinity for this receptor.4 The standard method of dioxin measurement is high resolution chromatography coupled to high resolution mass spectrometry system, which requires a large amount of serum, expensive facilities, and experts.
We previously developed a highly sensitive cell-based AhR-dependent luciferase activity (CALA) assay with which to determine serum levels of dioxin and dioxin-like substances as reflected by AhR transactivating (AHRT) activity. The assay shows good correlation with dioxin toxic equivalency values derived from chemically measured concentrations.5 Moreover, AHRT was correlated with serum mitochondrial inhibitor (MTI) activity, as ascertained by measurement of intracellular adenosine triphosphate (ATP) content and reactive oxygen species generation in cells treated with serum samples from patients.5 Our CALA assay can also be reliably used to assess dioxin-like polychlorinated biphenyl levels in clinical studies, as some polychlorinated biphenyls showed good correlation with AHRT activities and ATP levels in 911 senior adult subjects in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) cohort.6
We previously reported that AHRT activities as measured by CALA assay were quantitatively associated with the prevalence of diabetes and metabolic syndrome in a cross-sectional study.5 Furthermore, AHRT activity was positively correlated with diabetic kidney disease progression and severity of renal dysfunction in patients with diabetic kidney disease.7 Among many AhR ligands, gut bacteria metabolites of tryptophan, indoxyl sulfates, have been reported as uremic toxins associated with complex inflammatory conditions and cardiovascular comorbidities in uremic patients.8,9 According to these considerations, we hypothesized that patients with pre-dialysis chronic kidney disease (CKD) and end-stage renal disease (ESRD) patients would show high AHRT activity as measured by CALA analysis. One reason is the nature of POPs that accumulate in the body; the second is because CKD patients are not allowed to eat a lot of fresh vegetables, which help to get rid of chemicals; and the third is because kidney function itself is decreased. We measured AHRT activity and MTI activity in CKD patients and dialysis patients, investigated the effect of dialysis modality and time on AHRT activity, and evaluated the relationship between AHRT activity and clinical parameters of comorbid diseases in CKD patients.
MATERIALS AND METHODS
Patient characteristics
A total of 118 patients were prospectively recruited from Eulji Medical Center: 22 peritoneal dialysis (PD) and 38 hemodialysis (HD) patients currently being treated by dialysis for at least 36 months, as well as 28 pre-dialysis CKD stage IV or V patients [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2]. Thirty patients with normal kidney function (eGFR >60 mL/min/1.73m2) who visited the hospital for regular health checkups were included as normal controls. eGFR was calculated with the Modification of Diet in Renal Disease equation using sex, ethnicity, age, and serum creatinine. Dialysis patients, treated with alternative methods for over 3 months (in the case of HD patients, treated with PD for the last 3 months) were excluded. Patients exhibiting acute and chronic inflammation and were taking immunosuppressants were also excluded. We reviewed comorbid conditions, body mass index (BMI), blood pressure, and echocardiography. To compare differences in blood pressure among groups, we used the mean of 20 random blood pressure values of HD patients just before dialysis and the mean of 20 blood pressure values in PD patients during their visit to the clinic. Dialysis adequacy (Kt/V) in peritoneal equilibrium tests were quantified, and differences according to Kt/V were compared in PD patients. Serum samples for measuring AHRT activity and intracellular ATP content were collected before initiation and after termination of dialysis on weekdays among HD patients and at monthly check ups for PD and CKD stage IV and V patients. Two-dimensional echocardiography was performed to evaluate left ventricular ejection fraction (LVEF) in the patients undergoing HD or PD (n=46). LVEF was calculated using modified Simpson's rule. Informed consent was obtained from all 118 participants. All data were compared according to dialysis modality. This protocol was approved by the Institutional Review Board of the Eulji Medical College Hospital in accordance with the Declaration of Helsinki (IRB No. 2017-05-008).
Laboratory assay and definitions
Complete blood cell counts, BUN, creatinine, HbA1c, and serum lipid levels were measured. Fasting plasma glucose was measured using a glucose oxidase method, and total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) levels were measured using enzymatic colorimetric procedures with an auto-analyzer (Hitachi-747; Hitachi, Tokyo, Japan). Glycated hemoglobin (HbA1c) was measured with high-performance liquid chromatography using HLC-723G7 (Tosoh, Tokyo, Japan). Blood samples from all subjects were collected after >8 h of fasting.
Serum AHRT activity assay
We measured AHRT activity as described previously with some modifications.5 Serum was prepared by allowing the blood to clot and then removing the clot. Each serum sample was heatinactivated by incubation at 65℃ for 30 min. Fetal bovine serum (Gibco BRL, Grand Island, NY, USA) or control human serum (charcoal stripped serum, CSS) was treated with activated charcoal (Sigma Co., St. Louis, MO, USA) overnight at 4℃ and filtered to remove all small molecules and AhR agonists that might have been present. 2,3,7,8-tetrachlorodibenzodioxin (TCDD), a positive control for AhR agonist, was purchased from Sigma Co. TCDD was considered extremely hazardous, so appropriate personal protective methods and materials were used. pGL4-DRE-luc(puromycin+)/pRL-mTK double-positive stable cells and heat-inactivated serum samples were prepared as described previously.6 The AHRT activity assay is similar to the CALUX assay, except it utilizes different recombinant cell lines and heat-inactivation method instead of organic solvent extraction for sample preparation.10 All cell-based assays were performed in duplicate on blinded samples. pGL4-DRE-luc/pRL-mTK-transfected mouse Hepa1c1c7 cells (1×105/well) in a 96-well plate were treated for 24 h with 10 µL of serum sample (10% culture media) or control CSS in phenol red-free Dulbecco's Modified Eagle's Medium. Luciferase activity was measured using a Dual-Glo Luciferase assay system (Promega, Madison, WI, USA) and a luminometer (Berthold, Bad Wildbad, Germany), and subsequently normalized against Renilla luciferase activity. Relative AhR-dependent luciferase reporter activity reflects the AHRT activity of serum sample-treated cells, which was calculated as a fold induction (FI), with the AHRT activity of 10% CSS-treated cells being set to 1. Then, AHRT values were converted to 2,3, 7,8-TCDD equivalents (TCDDeq, pM) using a standard curve (0–10 pM of TCDD), prepared using cells exposed to serially diluted TCDD (0–50 pM) for 24 hours in the presence of 10% CSS. According to the standard curve, a 0.1-FI change in AHRT activity was equivalent to 0.37 pM TCDDeq. The intra- and interassay coefficients of variation for these methods were <5.0%.
Mitochondria inhibiting activity assay
The effects of human serum on the mitochondrial function of cultured cells was evaluated by measuring intracellular ATP content as described previously with minor modifications.5 In short, we selected G418 resistant stable colonies from Hepa1c1c7 cells that had been transfected with pRL-TK using SuperFect (Qiagen, Germantown, MD, USA) for 3 weeks and stored. Cells showed stable Renilla luciferase activity. pRL-mTK-transfected mouse Hepa1c1c7 cells (5×104/well) in a 96-well plate were treated with 10 µL of heat-inactivated-serum samples for 48 h. The ATP content of the treated cells was determined using the luciferin-luciferase reaction with the CellTiter-Glo luciferase kit (Promega), with the output being normalized to Renilla luciferase activity. The intracellular ATP content of CSS-treated control cells was 65.1±2.7 nM. The ATP concentration of 10% sample serum-treated cells could be calculated from a standard curve of ATP concentrations (nM)=(% control+18.24)/1.817. ATP content was expressed as a percentage of CSS-treated control. The intra- and interassay coefficients of variation for these methods were <6.0%.
Statistical analysis
This study was designed as an exploratory study through which to generate new evidence for our thesis. Mean values and standard deviations were calculated for individual variables using SPSS statistics software version 18.0 (SPSS Inc., Chicago, IL, USA). Comparisons between frequencies were tested by the chi-squared test. Differences between groups were tested using the unpaired Student's t-test for normally distributed variables and Mann–Whitney U-test for variables with a skewed distribution. Differences among the three groups were investigated by one-way analysis of variance for normally distributed variables and the Kruskal–Wallis test for variables with skewed distribution. Paired-t test was used to compare AHRT activity and ATP level before and after HD. Pearson's correlation test was used to evaluate the relationship among AHRT, MTI activity, and various metabolic parameters. The significance level was set at p<0.05.
RESULTS
Clinical characteristics of the study patients
There were a total of 118 participants, consisting of 30 subjects with normal kidney function, 28 with pre-dialysis CKD, 22 undergoing PD, and 38 undergoing HD. The mean age of all participants was 60.6±13.0 years. Of the participants, 77 (65.3%) were male, and 41 (34.7%) were female. The mean durations of dialysis were 62.9±39.6 months in PD patients and 81.4±35.2 months in HD patients. Table 1 lists the clinical characteristics of the study group. There were significant differences in BMI, systolic blood pressure (SBP), blood urea nitrogen (BUN), creatinine, hemoglobin, low-density-lipoprotein (LDL) cholesterol, and triglyceride levels between the study groups (Table 1).
Table 1. Comparison of Baseline Characteristics of the Study Groups.
| Normal | CKD | PD | HD | p value | |
|---|---|---|---|---|---|
| Age (yr) | 59.70±11.80 | 65.80±13.46 | 57.48±13.05 | 61.17±12.65 | 0.057 |
| Sex (M/F), n | 21/9 | 20/8 | 11/11 | 25/13 | 0.387 |
| Diabetes, n | 1 | 14 | 12 | 31 | <0.001 |
| BMI (kg/m2) | 24.86±3.27 | 24.16±2.89 | 24.64±3.58 | 22.14±3.68 | 0.005 |
| SBP (mm Hg) | 120.32±11.75 | 126.90±16.84 | 138.44±12.68 | 142.44±10.63 | <0.001 |
| DBP (mm Hg) | 73.53±7.40 | 73.20±9.13 | 78.81±8.90 | 70.69±7.09 | <0.001 |
| BUN (mmol/L) | 4.65±1.86 | 19.32±8.58 | 18.64±5.04 | 21.33±3.56 | <0.001 |
| Creatinine (µmol/L) | 85.78±23.03 | 336.99±109.86 | 960.42±312.74 | 856.80±252.23 | <0.001 |
| Hemoglobin (mmol/L) | 8.74±0.92 | 6.86±0.81 | 6.52±0.35 | 6.55±0.33 | <0.001 |
| HbA1c (%) | 6.02±1.25 | 6.81±1.34 | 7.01±1.66 | 7.16±1.55 | 0.364 |
| Fasting glucose (mmol/L) | 5.20±0.97 | 6.64±2.52 | 7.80±3.35 | 7.63±2.92 | <0.001 |
| LDL cholesterol (mmol/L) | 3.37±1.06 | 2.12±0.83 | 2.19±0.72 | 2.05±0.49 | <0.001 |
| Triglyceride (mmol/L) | 1.30±0.73 | 1.70±1.15 | 1.64±0.85 | 1.15±0.57 | 0.022 |
| AHRT activity (FI) | 1.63±0.23 | 2.36±0.32 | 1.73±0.36 | 2.56±0.31 | <0.001 |
| ATP (% control) | 61.91±6.00 | 54.37±6.18 | 54.28±4.35 | 49.68±4.40 | <0.001 |
CKD, chronic kidney disease; PD, peritoneal dialysis; HD, hemodialysis; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; BUN, blood urea nitrogen; LDL, low-density-lipoprotein; AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; FI, fold induction. Values are expressed as means±SDs unless otherwise noticed. Significance at p<0.05 by ANOVA and Tukey's test or χ2-test.
Comparisons of AHRT activity and intracellular ATP levels according to kidney function and dialysis modalities
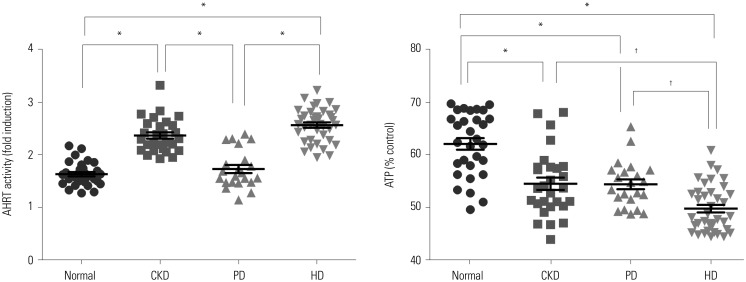
We noted statistical differences in AHRT activity among the study groups in the following order: normal kidney function<PD<CKD<HD. Intracellular ATP levels also differed significantly between groups: HD<PD=CKD<normal kidney function (Fig. 1). The same tendency was also confirmed when the mean values of AHRT activity before and after HD were analyzed.
Fig. 1. Comparison of AHRT activity and intracellular ATP levels among study groups. Significance at p<0.05 by ANOVA and Tukey's test . *p<0.001, †p<0.05. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; CKD, chronic kidney disease; PD, peritoneal dialysis; HD, hemodialysis.
Comparison of AHRT activities and intracellular ATP levels according to diabetes status in all study participants
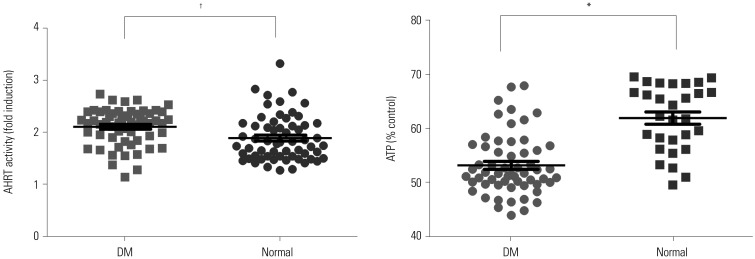
We analyzed serum AHRT activity and intracellular ATP levels in all study participants and compared these values according to diabetes status. The patients with diabetes had significantly higher serum levels of AHRT activity and lower intracellular ATP levels than the normal control group (Fig. 2).
Fig. 2. Comparison of AHRT activity and ATP levels according to DM status. *p<0.001, †p<0.05. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; DM, diabetes mellitus.
Comparison of AHRT activity and ATP levels according to dialysis treatment and dialysis adequacy
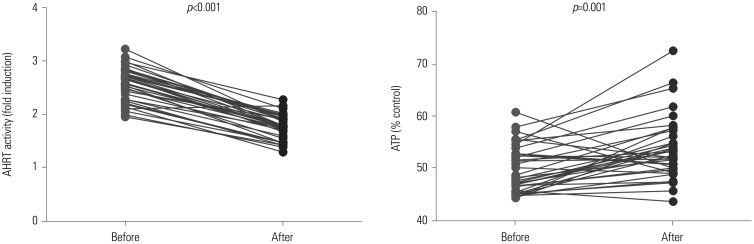
Serum levels of AHRT activity were reduced after dialysis in HD patients. On the contrary, intracellular ATP levels were elevated after dialysis, compared to a pre-dialysis state (Fig. 3). Among patients undergoing HD, over 97% achieve sufficient dialysis adequacy (>1.3 of Kt/V); however, among PD patients, only 60% reach an Kt/V ≥1.7. When we divided the PD patients into two groups according to their Kt/V values, as high Kt/V (≥1.7) and low Kt/V (<1.7) groups, there was no significant difference between two groups in regards to AHRT activity and ATP levels (low Kt/V vs. high Kt/V, AHRT activity, 1.81±0.42 FI vs. 1.63±0.16 FI, p=0.406; ATP level, 53.31±3.79% vs. 55.86±5.4%, p=0.327).
Fig. 3. Changes in AHRT activity and intracellular ATP levels after HD treatment in HD patients. Paired-t test was used. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; HD, hemodialysis.
Correlation analysis for AHRT activity, intracellular ATP levels, and various clinical parameters
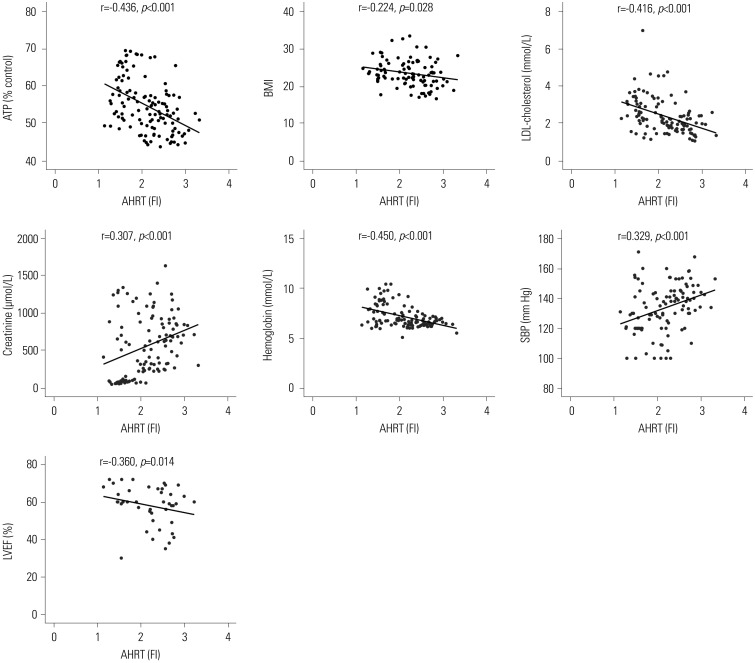
We performed linear regression analysis between AHRT activity and intracellular ATP, along with various clinical parameters. AHRT activities showed a negative correlation with intracellular ATP levels, LDL-cholesterol, hemoglobin, BMI, and LVEF (Table 2, Fig. 4). AHRT activity also showed a marginally significant positive correlation with SBP, BUN, creatinine, and duration of dialysis (Table 2, Fig. 4). ATP showed a significantly negative correlation with SBP, BUN, and creatinine and a positive correlation with multiple parameters, such as LDL cholesterol, hemoglobin, and LVEF (Table 2).
Table 2. Correlations among AHRT Activity, Intracellular ATP, and Various Clinical Parameters.
| Clinical parameters | AHRT activity | ATP | ||
|---|---|---|---|---|
| r | p value | r | p value | |
| AhR | - | - | -0.436 | <0.001 |
| ATP | -0.436 | <0.001 | - | - |
| Age | 0.088 | 0.342 | 0.008 | 0.928 |
| Duration of dialysis | 0.259 | 0.046 | 0.002 | 0.990 |
| BMI | -0.224 | 0.028 | 0.159 | 0.120 |
| SBP | 0.329 | 0.001 | -0.364 | <0.001 |
| DBP | -0.120 | 0.222 | 0.068 | 0.489 |
| Fasting glucose | 0.164 | 0.079 | -0.286 | 0.002 |
| HbA1c | 0.117 | 0.282 | -0.173 | 0.111 |
| LDL cholesterol | -0.416 | <0.001 | 0.381 | <0.001 |
| Triglyceride | -0.142 | 0.134 | -0.001 | 0.990 |
| Calcium | 0.103 | 0.441 | 0.085 | 0.527 |
| Phosphorus | -0.101 | 0.450 | 0.147 | 0.272 |
| PTH | 0.158 | 0.237 | 0.154 | 0.249 |
| BUN | 0.405 | <0.001 | -0.332 | <0.001 |
| Creatinine | 0.307 | 0.001 | -0.446 | <0.001 |
| hsCRP | -0.062 | 0.665 | 0.153 | 0.284 |
| Hemoglobin | -0.450 | <0.001 | 0.517 | <0.001 |
| Uric acid | 0.114 | 0.391 | -0.209 | 0.112 |
| LVEF | -0.360 | 0.014 | 0.408 | 0.005 |
AhR, aryl hydrocarbon receptor; AHRT, AhR transactivating; ATP, adenosine triphosphate; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LDL, low-density-lipoprotein; PTH, parathyroid hormone; BUN, blood urea nitrogen; hsCRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction.
Pearson's coefficient r and p values are presented.
Fig. 4. Significant correlations among AHRT activity and various clinical parameters. Pearson's coefficient r and p values are presented. AHRT, aryl hydrocarbon receptor transactivating; ATP, adenosine triphosphate; BMI, body mass index; LDL, low-density-lipoprotein; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; FI, fold induction.
DISCUSSION
As the number of patients with ESRD is increasing due partly to an increased prevalence of diabetes and partly with recent improvement in dialysis therapy and patient care, more patients are maintaining dialysis over the long term. However, the exact pathogenesis of cardiovascular complications, a main cause of death in ESRD patients, is still ill-defined, although chronic inflammation and some uremic toxins are suggested as primary culprits. In this study, we measured AHRT activity and ATP levels in patients with CKD and ESRD using a cell-based AhR-dependent luciferase activity assay. CKD patients showed higher levels of AHRT activity and lower intracellular ATP levels than control patients with normal renal function and ESRD patients under dialysis. We speculate that the elimination of AhR ligands is reduced in CKD patients and that increases in AhR ligands may be causes of kidney disease progression. AhR, a ligand-inducible transcription factor, mediates its toxic renal effect by inducing cytochrome p450 proteins, which contribute significantly to renal dysfunction through formation of reactive oxygen species.11,12 Furthermore, after classifying patients according to diabetes status, regardless of CKD stage, we found AHRT activity to be higher and intracellular ATP levels to be lower in patients with diabetes than in those without. These results coincide with findings from our previous epidemiologic study.5
In this study, patients with ESRD under HD and PD showed higher AHRT activity and lower levels of intracellular ATP than patients with normal renal function. Unexpectedly, ESRD patients treated by dialysis showed less elevation of AHRT activity than pre-dialysis CKD patients, suggesting that dialysis may reduce AHRT activity. To evaluate the effect of dialysis on serum AHRT activity, we obtained serial serum samples before and after HD sessions and found that serum AHRT activity decreased after HD. Indoxyl-sulfate and indole-3-acetic acid, well known uremic toxins known to be involved in renal progression, are also known as AhR ligands.9,13,14 They exist in the form of albumin-bound molecules in the body and are unlikely to be removed by dialysis.15 However, whether and how other individual AhR ligands are affected by dialysis treatment are still ill-defined. As high flux dialysis membranes may have different effects on AHRT activity, further research is mandated to identify the individual molecules among AhR ligands and to compare the effects of HD and hemodiafiltration on AHRT activity in order to determine the exact role of dialysis treatment. Regarding the relationship between Kt/V and AHRT activity in PD patients, our results showed no relationship between them. It is well-known that PD is more effective in removing larger molecules than HD, and AHRT activity was lower in PD patients than HD patients in this study. There is the possibility that larger protein-bound molecules, including indoxyl sulfate and indole-3 acetic acid, influenced this result.
Our data suggest that increased AHRT activity may have an important relationship with the cardiovascular complications of ESRD patients as evidenced by meaningful associations with multiple clinical parameters. AHRT activity was negatively correlated with LDL-cholesterol, hemoglobin, and LVEF. These results suggest that there may be some relationships between malnutrition, inflammation, and atherosclerosis (MIA) syndrome in CKD patients and AHRT activity.16 In MIA syndrome, malnutrition status adversely affects the development of cardiovascular events.17 Our study did not show an association between AHRT activity and the inflammatory marker CRP, although we suspect that chronic inflammation is the main link between AHRT activity and CKD. In addition, a complex variation in oxidative stress marker levels in CKD has been reported.18 Therefore, it is necessary to measure and analyze various oxidative stress markers and inflammatory cytokines further to clarify the association between inflammation and AHRT activity. As a reference, in our previous study, AHRT activity exhibited a negative correlation with serum adiponectin, which is known to have significant anti-inflammatory and anti-atherogenic effects.19 Recently, Dou, et al.20 showed that AhR is increased in patients and mice with CKD, and increased cardiovascular events were observed in CKD patients with increased AhR activities in their survival analyses.
In our study, AHRT activity showed a strong negative correlation with intracellular ATP levels. Given that ATP levels are potent biomarkers of mitochondrial function, our data suggest a possible mechanism linking mitochondrial dysfunction and increased AHRT activity, as well as their harmful effects on cardiovascular complications, in ESRD patients.
The present study has several limitations. First, this crosssectional observation study did not allow for concluding a causal relationship between AHRT activity and CKD. Second, this was a single-center study with a small sample size. Nationwide and multinational cooperation to clarify the observed association is needed. Third, there are several ‘non-classical’ AhR ligands other than dioxins and dioxin-like compounds. These include tryptophan and its metabolites and phytochemicals, and the list is growing.21
In conclusion, we found AHRT activity to be associated with CKD, ESRD, and cardiovascular complications, and dialysis treatment played a role in reducing AHRT activity. Further studies are needed to specify the precise role of AHRT activity and to demonstrate the direct cause and consequence relationships for complications in patients with CKD.
ACKNOWLEDGEMENTS
This research was supported by the Korean Health Technology R&D Project (HI14C2700) through the Korea Health Industry Development Institute and by the Basic Science Research Program (2018R1A6A1A03025124) through the National Research Foundation of Korea funded by the Korean government (to YKP). The funding source had no role in the collection of data or in the decision to submit the manuscript for publication.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jin Taek Kim, Hong Kyu Lee, So Young Lee, Youngmi Kim Pak.
- Data curation: Sang Hyuk Kim, Hyang Ki Min, Sang Jin Jeon, Su-Ah Sung.
- Formal analysis: Jin Taek Kim, So Young Lee, Youngmi Kim Pak, Hoon Sung Choi.
- Funding acquisition: Youngmi Kim Pak.
- Investigation: Jin Taek Kim, Wook Ha Park, So Young Lee, Youngmi Kim Pak.
- Methodology: So Young Lee, Youngmi Kim Pak.
- Project administration: So Young Lee, Youngmi Kim Pak.
- Resources: So Young Lee, Youngmi Kim Pak.
- Software: Wook Ha Park.
- Supervision: Hong Kyu Lee, Youngmi Kim Pak, So Young Lee.
- Validation: Wook Ha Park, Hoon Sung Choi.
- Visualization: Jin Taek Kim, Wook Ha Park, Hoon Sung Choi.
- Writing—original draft: Jin Taek Kim, Hong Kyu Lee, So Young Lee, Youngmi Kim Pak.
- Writing—review & editing: Jin Taek Kim, Hong Kyu Lee, So Young Lee, Youngmi Kim Pak, Hoon Sung Choi.
References
- 1.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 2.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:40–53. doi: 10.2183/pjab.86.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackowiak B, Wang H. Mechanisms of xenobiotic receptor activation: direct vs. indirect. Biochim Biophys Acta. 2016;1859:1130–1140. doi: 10.1016/j.bbagrm.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park WH, Jun DW, Kim JT, Jeong JH, Park H, Chang YS, et al. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors. 2013;39:494–504. doi: 10.1002/biof.1092. [DOI] [PubMed] [Google Scholar]
- 6.Park WH, Kang S, Lee HK, Salihovic S, Bavel BV, Lind PM, et al. Relationships between serum-induced AhR bioactivity or mitochondrial inhibition and circulating polychlorinated biphenyls (PCBs) Sci Rep. 2017;7:9383. doi: 10.1038/s41598-017-09774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JT, Kim SS, Jun DW, Hwang YH, Park WH, Pak YK, et al. Serum arylhydrocarbon receptor transactivating activity is elevated in type 2 diabetic patients with diabetic nephropathy. J Diabetes Investig. 2013;4:483–491. doi: 10.1111/jdi.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49:393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vondráček J, Pěnčííková K, Neča J, Ciganek M, Grycová A, Dvořák Z, et al. Assessment of the aryl hydrocarbon receptor-mediated activities of polycyclic aromatic hydrocarbons in a human cell-based reporter gene assay. Environ Pollut. 2017;220(Pt A):307–316. doi: 10.1016/j.envpol.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 11.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, et al. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25:2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- 13.Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brito JS, Borges NA, Esgalhado M, Magliano DC, Soulage CO, Mafra D. Aryl hydrocarbon receptor activation in chronic kidney disease: role of uremic toxins. Nephron. 2017;137:1–7. doi: 10.1159/000476074. [DOI] [PubMed] [Google Scholar]
- 15.Leong SC, Sirich TL. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins (Basel) 2016;8:E358. doi: 10.3390/toxins8120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 17.Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–960. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 18.Annuk M, Fellström B, Akerblom O, Zilmer K, Vihalemm T, Zilmer M. Oxidative stress markers in pre-uremic patients. Clin Nephrol. 2001;56:308–314. [PubMed] [Google Scholar]
- 19.Roh E, Kwak SH, Jung HS, Cho YM, Pak YK, Park KS, et al. Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes. Acta Diabetol. 2015;52:489–495. doi: 10.1007/s00592-014-0674-z. [DOI] [PubMed] [Google Scholar]
- 20.Dou L, Poitevin S, Sallée M, Addi T, Gondouin B, McKay N, et al. Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int. 2018;93:986–999. doi: 10.1016/j.kint.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]