Abstract
The regulation of glycemia is under a tight neuronal detection of glucose levels performed by the gut-brain axis and an efficient efferent neuronal message sent to the peripheral organs, as the pancreas to induce insulin and inhibit glucagon secretions. The neuronal detection of glucose levels is performed by the autonomic nervous system including the enteric nervous system and the vagus nerve innervating the gastro-intestinal tractus, from the mouth to the anus. A dysregulation of this detection leads to the one of the most important current health issue around the world i.e. diabetes mellitus. Furthemore, the consequences of diabetes mellitus on neuronal homeostasis and activities participate to the aggravation of the disease establishing a viscious circle. Prokaryotic cells as bacteria, reside in our gut. The strong relationship between prokaryotic cells and our eukaryotic cells has been established long ago, and prokaryotic and eukaryotic cells in our body have evolved synbiotically. For the last decades, studies demonstrated the critical role of the gut microbiota on the metabolic control and how its shift can induce diseases such as diabetes. Despite an important increase of knowledge, few is known about 1) how the gut microbiota influences the neuronal detection of glucose and 2) how the diabetes mellitus-induced gut microbiota shift observed participates to the alterations of autonomic nervous system and the gut-brain axis activity.
Keywords: Gut microbiota, Entero-endocrine hormones, Peripheral nervous system, Glucose, Diabetic neuropathy
Introduction
The blood glucose level or glycemia is one of the most regulated physiological parameters. It has to be maintained around 5.5 mM whitin the day, including the fasting periods as well as the prandial and post-prandial periods. Insulin and glucagon are both pancreatic hormones playing a critical role in the regulation of glycemia and their secretion depend on an efficient glucose detection. On the first hand, variation of blood glucose level is directly detected by the pancreatic beta cell i.e. when the increased arterial glycemia favors glucose entry into the beta cell through the glucose transporter GLUT2, the phosphorylation of glucose by the glucokinase leads to the triggering of insulin exocytosis. On the other hand, the regulation of glycemia is under nervous influences 1) from the central nervous system, particularly the hypothalamus which detects glycemia variations throughout the day in the systemic blood and 2) from the autonomic nervous system including the enteric nervous system (ENS) and the vagus nerve (VN) which detect the glycemia variations within the gastro-intestinal tract, from the mouth to the colon, and the portal vein during post-prandial period. When a variation of glycemia is detected, all the nervous systems send neuronal efferent messages to the organs involved in glucose metabolism (peripheral organs) as the liver, the muscles, the pancreas and the adipose tissue to regulate the gluconeogenesis, the storage of glycogen or lipids, the secretion of hormones as insulin and glucagon resulting then to a stabilization of the glycemia.
Type 2 diabetes (T2D) is characterized by a dysregulation of glucose metabolism leading to fasting (>7 mM) and postprandial (>11 mM, 2 h after a meal) hyperglycemia. This dysregulation results from a large panel of different organ alterations notably an impaired insulin and glucagon secretion and action. Such impairments could originates from a lack of glucose awareness where hyperglycemia is not correctly detected by the body and therefore, glucose regulatory signals are not sent properly to the organs. During T2D, the neuron activities and homeostasis are highly impacted, particularly by the diabetic neuropathy, i.e. neurodegeneration induced by a long-time period of diabetic state, leading to a dysregulation of glucose detection and an inadequate response from the peripheral organs. It participates then to the disease aggravation.
For the last decade the role of gut microbiota in the regulation of glucose metabolism and the corresponding metabolic diseases as diabetes has been well-described. However, the molecular mechanisms involving the host/microbiota interactions require to be elucidated. Up to now, only few articles described the molecular impact of microbiota on the glucose neuronal detection. Therefore, we will 1) review how the glucose is detected by the nervous system; 2) discuss how the gut microbiota can influence it; 3) how we can use these microbiota-host interactions to improve the glucose detection during T2D and the efficiency of some therapies requiring the nervous system to act and to treat the diabetic neuropathy.
Nervous glucose detection within the gut and its alteration during type 2 diabetes
Glucose-induced intestinal neuro-hormone secretions
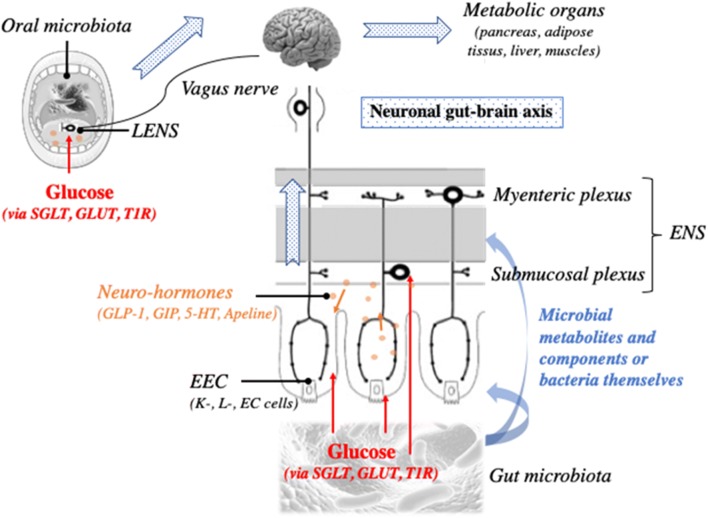
After a meal, entero-endocrine cells (EEC) within the intestinal mucosa secret a panel of intestinal hormones in response to nutritional and luminal glucose. It is detected in the luminal border of the EEC by specific receptors or transporters, namely the Taste Receptor 1 (T1R) and Sodium Glucose Cotransporter (SGLT) 1/3 [1–3] which induce secretion of intestinal neuro-hormones. Among them, there are Glucagon-Like Peptide 1 (GLP-1), Glucose dependent Insulinotropic Polypeptide (GIP), 5-hydroxytryptamine (5-HT) or serotonin respectively secreted by L and K cells and enterochromaffin cells [4, 5]. Apelin was also recently described as an intestinal hormone playing a critical role in metabolism regulation but the type of secreting cells is not identified yet [6, 7]. By immunohistochemistry, Wang et al. observed that the apelin immuno-signal colocalized with chromogranine A immuno-signal, which is a general marker of EEC, within the stomacal and ileal mucosa [7]. After their release, each neuro-hormone acts on a specific receptor express by the intestinal and portal innervations of the VN and the enteric neurons: GLP-1 on GLP-1 receptor, GIP on GIP receptor, 5-HT on 5-HT receptors 3 and 4 and apelin on APJ (Fig. 1).
Fig. 1.
The gut microbiota to the brain axis in the metabolic control. The gut microbiota influences the intestinal system of glucose detection involving the entero-endocrine cells (EEC) and the intestinal neuro-hormones, the enteric nervous system (ENS) and the vagus nerve, through its metabolites, its components and some bacteria themselves. A neuronal glucose detection begins inside the mouth, in the gustative papilleae of the tongue innervated by the vagus nerve and the lingual enteric nervous system (LENS) and where reside also a prokaryotic community, the oral microbiota. The neuronal glucose detection is critical for the metabolism control
During early stage of T2D, the intestinal genetic expressions of SGLT1 and GLUT2 are increased in rodents and humans as well as the secretions of intestinal neuro-hormones in response to glucose in prediabetic rat [8–10]. However, these compensatory mechanisms decrease with the diabetes duration in rodents suggesting a development of glucose resistance over time [11, 12]. During T2D, the neuro-hormone blood levels are particularly altered: the concentrations of GLP-1, GIP and 5-HT are maintained high revealing a resistance to the intestinal neuro-hormones as “incretin resistance” [12–15].
Vagus nerve, enteric nervous system and their alterations during type 2 diabetes
Within the intestine
The VN innervates the intestinal tractus and the portal vein. After a meal, it nervously detects the nutritional glucose reaching the intestinal tissue and the portal blood and informs the brain via a nervous axis called the gut-brain axis. Numerous studies showed that glucose infusions within the intestinal lumen and the portal vein controlled the food intake, the glucose consumption of liver and muscles and the endocrine secretion of pancreas through either sympathetic or parasympathetic (VN) innervations [16–19]. GLUT2 plays a critical role on the direct detection of glucose by the VN [16, 20]. However, the glucose detection can be indirect, i.e. through the intestinal neuro-hormones since the VN expresses also their specific receptors (Fig. 1). Thus, it detects directly the neuro-hormones after their release during the prandial and post-prandial periods ([21–24]. During a meal, the level of food intake plays a critical role on the neuro-hormone sensitivity since the nutrient levels influence the neuro-hormone receptor exposure in the vagal synapses [21, 24].
The ENS is an important network of neurons within the intestine. Its first described function was the regulation of the intestinal peristalsis involved in digestion processes [25]. Then, a lot of functions were attributed to the ENS as the control of the intestinal blood flow through the angiogenesis and the vessels motility; the intestinal immune system activities; the ion secretions within the lumen and the intestinal permeability [25]. The enteric neurons are organized in ganglions communicating with each other by innervations and divided in 2 plexi: the myenteric plexus localized between the inner and the outer muscular layers and involved in the control of intestinal transit and the submucosal plexus localized under the intestinal epithelium, within the lamina propria interacting with the blood vessels, the mucosa or the immune system [25]. Interesting studies demonstrated that glucose is detected through SGLT1 expressed by the enteric neurons, inducing then the phosphorylation of the protein Ca2+/calmoduline-dependant kinase II (CamKII) reflecting an activated neuronal state [26, 27]. Similarly, the intestinal neuro-hormones secreted in response to glucose, as GLP-1, 5-HT or apelin, activate enteric neurons and modulate the intestinal transit through their specific receptors [11, 14, 26–29]. Recent studies demonstrated that enteric neurons can play a critical role in the process of neuronal glucose detection (Fig. 1). When they are activated, they could transmit a neuronal message to the VN participating to the glycemia regulation through the gut-brain axis [14, 28, 30]. Furthermore, when the enteric neurons are pharmacologically destroyed, the GLP-1-induced gut-brain axis stimulation is decreased, impairing neuronal glucose detection [14]. Finally, during T2D, the vagal detection of the neuro-hormones is particularly altered [11, 14]. It was observed 1) fewer phosphorylation of CamKII, cell activation marker, in the EEC (enterochromaffin cells and L-cells), the enteric neurons and the nodose ganglia of the VN in response to glucose, in diabetic rats and 2) less neuronal expression of c-fos in response to glucose and GLP-1 in the brain stem of diabetic mice.
Within the tongue
The neuronal detection of glucose can be done before the small intestine, inside our mouth and particularly by the taste papillae, including the taste buds, within the tongue (Fig. 1). As it was observed in the EEC, the cells within the mucosa of taste papillae expresse the mRNA as well as the protein of T1R, GLUT1,3 and 4 and SGLT1 and expose them into the cell surface [31]. Endocrine cells secreting GLP-1 as L cells can be found inside the taste papillae and able to activate the VN [32]. The VN innervates the tongue but also some authors describe a sort of neuronal network comparable to the ENS within the gut called lingual ENS (LENS). The identified neurons are comparable to those involved in regulation of secretion and vasomotility within the gut. They can also regulate systemic reflexes on the digestive and respiratory apparatus through their innervations inside the pharynx or larynx. The LENS can modulate the activity of the annexed glands [33, 34]. Thus, the LENS and the VN innervating the tongue are the first neuronal network located at the beginning of the digestive apparatus which analyses the foods before their ingestion and diffuses this information distally (Fig. 1). It was recently demonstrated that the GLP-1 secretion by the taste buds is induced by the lipids trough CD36 and GPR120 and the aim is to regulate the palatability and the preference for the sweet water [35]. The stimulation of the taste buds with some long chain fatty acid induces a preference for the sweet water in a GLP-1r-dependant manner, since such behavior is not observed in the GLP-1r KO mice. Altogether few studies describes the role of the oral glucose detection in the regulation of glucose metabolism.
During T2D, the mechanisms involving the taste detection are modified. CD36 as well the gustudicine receptor are overexpressed in the taste buds of diabetic rats [36, 37] while their signaling, and particularly CD36 signaling, are altered [38] suggesting a mechanism of resistance for the nutrient detections within the tongue. An increase of apoptosis as well as a decrease of the innervation were observed by TUNNEL staining, western blot of BCL2, BAX and activated caspase 3 and 9 and immunostaining of PGP9.5, a neuronal marker in the taste buds of diabetic rats [39, 40]. Thus, these alterations of the tongue detection described by the decrease of the sensor cells or neuronal innervation inside the taste buds or an alteration of some intracellular signaling can induce an alteration of the food intake and food preferences leading to aggravate metabolic diseases.
Diabetic neuropathy
More than 50% of diabetic patients suffer from diabetic neuropathy. It is characterized by numerous alterations as axonal atrophies, demyelinisation, decrease of regeneration capacities, neuronal inflammation and an important decrease of distal, peripheral innervations called peripheral neuropathy. It could be considered as a nutritional neurodegenerative disease. These alterations participate to a worse neuronal conduction and a decrease of the amplitude of neuronal signals. A lot of mechanisms are involved as: 1) the intranerve angiopathy induced by a modification of vessel structure; 2) the production of glycated proteins, as glycated hemoglobin, increasing the blood viscosity and by chelation, limiting the nitric oxide biodisponibility affecting thus the vasomolitity; 3) a default of production and effects of neurotrophic factors induced by a intracellular stress of neurons and 4) the polyols flux modifying the function of glial cell as neuronal supporting cells, and affecting the electrical activity of neurons. Then, intranerve ischemia and oxidative stress induced by the polyol flux and glycated proteins are observed and participate the neurodegeneration process [41, 42]. A better knowledge about the involved mechanisms was permitted by studies on diabetic animal models as treated with streptozotocin, fed with high-fat diet, genetically-modified as db/db or ob/ob mice.
The diabetic neuropathy affects numerous organs giving rise to an intestinal transit and gastric emptying alterations (diarrhea and/or constipation; gastroparesis; nausea; …) [43], cardiovascular defaults (tachycardia, orthostatic hypotension, erectile troubles, etc), uncontrolled sweating, a hyper or hypo sensitivity during exercises or hormonal alterations. Despite of an important alteration of the quality of life, no efficient treatment is available in the pharmaceutical market. Associated to all these phenotypes, the diabetic neuropathy aggravates diabetes and alteration of glycemia regulation: gastroparesis and intestinal transit alteration affecting the nutrient entrance within the intestinal lumen and then into the blood, intestinal glucose malabsorption, a loss of nutrient detection by the neuronal gut-brain axis within the intestine as well as the portal vein, a default of energetic metabolism regulation in the central nervous system and in the periphery by a loss of neuronal conduction.
On the other hand, as diabetic neuropathy is a long-term complication of diabetes metillus, it occurs at the late stage of the disease and becomes even worst with aging [44]. By working on aging-associated neurodegenerative disorders, as Alzheimer or Parkinson, we can understand better as the neuronal homeostasis is critical for glycemia regulations. It is known that 30% of Parkinson patients have impaired glucose tolerance, among whom 5.6% were newly diagnosed T2D and 26% were insulin resistant [45, 46]. Inversely, the neurodegenerative disorders-suffering patients have more risk to develop obesity and T2D [47]. It was recently demonstrated that feeding a mouse models of Alzheimer disease (APP/PS1 transgenic mice) with an HFD induced a worsening of insulin and leptin resistance leading to an aggravation of the diabetic state [48]. A possible cause could be related to the mechanisms of the neurodegenerative process on the autonomic nervous system altering then the glucose detection. An atrophy of the VN was observed during Parkinson diseases [49] while a delay of vagus somatosensory evoked potentials or a dysfunctional nucleus tractus solitaries (the nucleus of the VN localized in the brain stem) are observed in Alzheimer’s disease [50, 51]. On the other hand, the ENS is also impacted. In APP/PS1 transgenic mice, the number of neuronal nitric oxide synthase (nNOS)-positive and acetylcholine transferase-positive neurons are decreased within the intestine [52]. During Parkinson disease, it is observed gastroparesis and intestinal transit alterations [53–55]. It could be due to an abnormal accumulation of α-synuclein inside the ENS and functional and neurochemical changes of the gastrointestinal tract [54–57]. Finally, taste sensations are highly impacted by all neurodegenerative disorders [58, 59].
Intestinal neuro-hormone and their potential role on neurogenesis/neuroprotection
Several studies observed that some intestinal neuro-hormones, whome are secreted in response to glucose, have neuroprotection and neurogenesis potentials as GLP-1 or 5-HT. GLP-1 induced axonal formation in primary culture of neurons from dorsal root ganglion (sympathetic branches of the autonomic nervous system) [60]. In a diabetic context, in streptozotocine-treated rats, a chronic treatment with GLP-1 or a GLP-1r agonist, exendin 4, improved the innervation within the periphery and the motor nerve activity (sciatic nerve) through a phosphoERK1/2 signaling [61]. A similar effect was observed in another animal model of sciatic nerve impairment [62]. By using serotonin transporter (SERT) inhibitor treatments, some authors observed that 5-HT had a similar effect on diabetic neuropathy [63, 64]. Within the intestine, liraglutide, a GLP-1 receptor agonist blocked lipopolysaccharide-induced visceral allodynia, which may be a -nitric oxide dependent response [65]. GLP-1, as well as GLP-2, significantly and concentration-dependently enhanced neuronal survival of myenteric neurons [66]. In other hand, 5-HT, through the 5-HT4r, is also able to promote the neuronal survival and maturation of myenteric neurons [67].
The neuroprotective effect can be indirect, through an effect on microglia and astrocytes. They expressed GLP-1r or GIPr and both hormones, GLP-1 and GIP, improved their survival, induced the neuronal growth factors expression, GDNF and NGF and reduced the oxidative stress [68].
In different animal model of neurodegenerative diseases, similar results were also observed. For example, in an animal model of neurodegenerative disease induced by ATP depleting-drug glucosamine, affecting the neuron metabolism and signaling, exendin 4 protected against the drug effect through an EPAC mechanism [69]. On the other hand, liraglutide, lixisenatide, as GLP-1r agonists able to cross the blood-brain barrier and a GIPr agonist reduced amyloid plaques, neurofibrillary tangles, the induced inflammation as well as stimulated neuronal progenitor proliferation and neurogenesis in a mouse model of Alzheimer [70–72]. Similar effect was observed in a mouse model of Parkinson disease [73]. 5-HT is also able to improve neurodegenerative state during Alzheimer disease through the 5-HT4r ([74].
Through their neuroprotective actions, the neuro-hormone involved in glucose detection seems to be important to send a neuronal message indicating that glucose is coming in prandial period but also to protect the nerve and neurons involved in this neuronal sending.
Microbiota-host interactions involved in the nervous control of glucose metabolism
Microbiota and metabolism
From the mouth to the large intestine, a large, rich, diverse and complex prokaryotic community live in straight contact with the host (Fig. 1). Between 2000 and 2010, numerous studies pointed out the importance of the microbiota in the energetic metabolism regulation. It was showed that germ-free (GF) mice are leaner and have a better glucose tolerance than their conventionally-raised counterpart (Conv-R). The colonization of GF mice with an intestinal microbiota coming from a Conv-R resulted in a worse insulin tolerance and a 60%-increase of body fat induced by a higher lipogenic activity within the liver [75–77].
The microbiota can interact with the host through different mechanism (Fig. 1). By digesting fibers and nutrients, the microbiota can produce different molecules as 1) short chain fatty acids (SCFA) with the most well-known byturate, propionate and lactate but also succinate 2) indole and its derivatives, 3) some neuro-hormones or mimetics as GABA [78], ClpB (αMSH mimetic) [79], and 4) others molecules affecting the host as imidazole propionate [80]. The microbiota also modifies and transforms the biliary acids secreted in the intestinal lumen to secondary biliary acids by deconjugation, dehydrogenation, dihydroxylation or epimerization [81]. One the other hand, the microbiota interacts through its own components as lipopolysaccharides (LPS) or RNA and DNA fragments through the transmembrane and cytosolic receptors TLR and as peptidoglycanes through the receptor NOD. During the last decades, numerous studies contributed to improve the knowledge about the microbiota-host interactions particularly in the metabolism context. It was shown 1) that LPS, through TLR4 and CD14, participates to the low-grade inflammation observed during the metabolic diseases and aggravating the diseases and it was called endotoxemia [82, 83] and peptidoglycans through NOD2 can modulate colonization and intestinal inflammation influencing the sensitivity to insulin [84]; 2) the importance of dietary fibers and the SCFA production in the metabolism regulation through a GLP-1-dependant mechanism [85–88] and 3) the role of secondary biliary acid through TGR5 and FXR [81, 89, 90]. More recently, it was observed that succinate is a critical molecule produced essentially by Prevotella in the regulation of glucose metabolism and the weight in mice [91]. Also, it was observed that E. coli produces ClpB, an αMSH mimetic, controlling then the food intake [79]. Indole and its interaction with the receptor AhR plays also a critical role in the regulation of intestinal immune system activity and glucose metabolism through a GLP-1-dependant mechanism [92]. Similarly, a study observed that Akkermansia, a mucin-degradating bacteria, and particularly a membrane protein, prevented obesity and associated complications in mice [93]. Finally, recently, a new bacterially-produced molecule in a context of type 2 diabetes, imidazole propionate, was identified to modulate the liver activity and impair glucose metabolism [80].
Relation between the microbiota and the entero-endrocrine and nervous systems
The EEC, VN and ENS express different receptors or channels able to recognize either bacterial components or molecules produced by the microbiota (Fig. 1). The receptors TLR binding to LPS and some bacterial DNA and RNA fragments [94, 95], the FFAR binding to SCFA [96] or TGR5 binding secondary biliary acids LCA and DCA [97] to cite a few are expressed by these tissues. Interestingly, inside the taste buds of the tongue, TLR and TGR5 are expressed also [98, 99].
It is known that microbiota influence the neuronal activities modifying then the transit, regulating the neuronal homeostasis particularly the critical ratio neuron/glial cell [100–102]. To go further, GF mice as well as antibiotic-treated mice have an immature ENS with a weak basal activity. Their ENS, as their VN, cannot be activate by some neuro-hormones like GLP-1 [14, 103–105]. The colonization of GF mice with a normal and healthy gut microbiota restores homeostasis and neuronal activities of ENS and VN ([14, 103–105]. It is not true if the gut microbiota comes from diabetic mice [14].
Some lactobacilli (L. reuteri and rhamnosus) improve ENS and VN activations [106, 107] while others (L farciminis, plantarum and fermentum) can produce a neurotransmitter, the nitric oxide, and thus control the intestinal transit [108, 109]. The bacterially-produced nitric oxide can also influence the neuronal response to GLP-1 and thus the glucose metabolism [14]. It is recently showed that Bacteroides can produce another intestinal inhibitory neurotransmitter, GABA [78]. The microbial influence on the ENS homeostasis and activity can be mediated by TLR4 [100]. NOD2 as TLR4 are critical for the ENS sensitivity to the intestinal neuro-hormone as GLP-1 [14]. In the tongue level, LPS, through TLR4, decrease the neuronal response of taste buds to saccharose [99].
Another kind of microbial influence should be more investigated. It is recently observed that SCFA can modulate epigenetics of the cells of the liver, adipose tissue and the colon [110]. On the other hand, epigenetics is critical for the neuronal homeostasis and activity. For example, MeCP2 (methyl CpG bonding protein) is a protein influence by epigenetic modifications and a mediator of synaptic development and plasticity. An alteration of MeCP2 expression induces Rett syndrome in humans characterizing by important neuronal deficiency but also because it is expressed by enteric neurons [111], an important dysregulation of the intestinal transit and nitric oxide production [112]. Since nitric oxide is an important mediator of GLP-1 action (14) for the control of glucose metabolism, MeCP2 could influence glucose metabolism. On the other DNA methylation is also critical for the expression of N-myc Downstream-Regulated Gene 4 (NDRG4). This protein is critical for brain morphogenesis through BDNF production and neurites outgrowth and myelinisation. Its expression level is reduced in Parkinson disease and this protein is expressed in the enteric neurons, particularly the nNOS-positive neurons [113]. The other epigenetic modification is histone deacetylations. Histone deacetylase (Hdac) can be critical for neuronal homeostasis and plasticity [114]. Hdac6 modulate alpha-tubulin, a critical protein in axon formation, expression [115]. Hdac dysregulation are also observed in neurodegenerative disorders [116–118]. Interestingly, it was observed that Hdac6 inhibition protects against vincristine-induced peripheral neuropathy [115]. Thus, by understanding better the microbiota-induced epigenetic modifications within the neurons of the VN or of the intestine could increase the knowledge about the molecular link between microbiota and neurons.
Finally, a recent work demonstrated that the influence of gut microbiota on the ENS and the VN homeostasis and activity can then influence the regulation of glucose metabolism [14].
The microbiota during type 2 diabetes and other neurodegenerative diseases
In humans as well as in animals, an important dysbiosis occurs during metabolic diseases, obesity and diabetes first described as an increase of the ratio Firmicutes/Bacteroidetes [119–122]. The gut microbiota impoverishes of Gram+ bacteria or bacteria producing SCFA as butyrate and gets rich of pathogenic bacteria [83, 123, 124]. Numerous authors demonstrated that the dysbiose can be a critical mechanism involved in metabolic disease development since the colonization of GF mice with a gut microbiota coming from diabetic/obese animals induce metabolic disease, despite of a normal chow diet [119, 125]. On the other hand, the bacteria involved in a better glucose metabolism, as Akkermensia, are reduced during obesity or diabetes [126]. Thus, target the microbiota with specific treatment as prebiotic or probiotic is an option widely studied around the world to treat metabolic diseases.
An important mouth dysbiosis is also observed. This dysbiosis induces dental pathologies observed during obesity and T2D but also influence the plasma cholesterol level, a blood parameter altered during metabolic diseases [127–130]. Some authors recently showed that Porphyromonas gingivalis-induced periodontitis is able to modulate immune system and promote glucose intolerance in mice fed with a high fat diet [131]. However, a lot is currently missing about the exact role of mouth dysbiosis in the metabolic alterations observed during type 2 diabetes.
The influence of the intestinal dysbiosis on the neuronal glucose detection was recently showed. The dysbiotic microbiota alters the neuronal homeostasis and the neuronal activities of the gut-brain axis in response to GLP-1 for the glucose metabolism regulation. These alterations can be transferred to GF mice when they are colonized with the flore coming from diabetic mice. Interestingly, an antibiotic treatment abolishes these alterations [14]. To go further, a probiotic treatment using different Lactobacilli is enough to restore the GLP-1 sensitivity in diabetic mice (data not published).
These set of data demonstrate clearly the importance of a good neuronal communication and it is highly influence by the gut microbiota and its metabolism. In neurodegenerative disorders, data showed that patients have an important dysbiosis [53, 132, 133] and it can influence the state of diseases. It was shown that 1) the presence of microbiota is determinant for the over-expression of alpha-synuclein; 2) colonization of GF mice with a gut microbiota coming from a Parkinson patient is able to induce motor dysfunction in mice and 3) SCFA plays a critical role in the pathology [134]. Along the same way, it was observed that a mouse model of Alzheimer disease, the APP/PS1 transgenic mice, without gut microbiota showed less beta-amyloid plaque formation and its colonization with gut microbiota coming from APP/PS1 transgenic mice is worst in term of beta amyloid plaque formation compared to its colonization with a gut microbiota coming from healthy mice [135].
Current treatment of type 2 diabetes: How can we improve their efficiency
Some anti-diabetic treatments require a high efficiency of the autonomic nervous system as for example, the GLP-1 based therapies as dipeptidyl peptidase 4 (DPP-4, “gliptine”) inhibitors or GLP-1 analogues (“tides”). GLP-1 acts in different organs involved in the regulation of glucose metabolism: the pancreas by stimulating the insulin secretion and by inhibiting the glucagon secretions; the stomach and the intestine by inhibiting the gastric emptying; intestinal absorption, and the intestinal transit; and the brain by decreasing the food intake. The most described and important pathway involved requires the autonomic nervous system and the neuronal gut-brain axis. It recruits the vagal innervations within the intestine and portal vein walls. Indeed, the nodose ganglions of the VN expressed the GLP-1r [22, 23]. When the GLP-1r gene is specifically deleted in the nodose ganglion, it induces a dysregulation of glucose metabolism, particularly the food intake, the gastric emptying and the insulin secretion [136]. Animal experiences testing the effect of mechanically-induced or chemically-induced vagotomy [14, 137, 138] as well as measuring electrophysiologically the VN activity induced by GLP-1 agonist or DPP-4 inhibitors [137, 139] demonstrated clearly the importance of the neuronal message sent to the brain by the VN. Additionally, a recent study suggests an important intermediate role of the ENS in this neuronal message since the chemically induced-ENS neuropathy alters the GLP-1 actions as observed in vagotomized animals [14]. However, the role of the ENS in the regulation of glucose metabolism should me more studied and explored. It could be interesting to study the impact of enteric neuropathy in the regulation of glycemia. Different animal models are available to answer this question as: 1) chemically-induced enteric neuropathy [14], 2) genetically-induced enteric neuropathy [140, 141], 3) gliopathy-induced enteric neuropathy [142]. In troncally-vagotomized-humans, the glucose metabolism is highly altered and characterized by dysregulations of gastric emptying and glucose-induced insulin secretion [143]. All the later data suggest the important role of the neuronal gut brain axis in the efficiency of anti-diabetic treatments. Furthermore, a recent study demonstrated that a co-treatment with neurturin, a glial cell-line derived neurotrophic factor, with a GLP-1 analogue improved the diabetic state of Zucker fatty rats [144].
The bariatric surgery, whose effectiveness on metabolic diseases is well-accepted and described, have a positive effect on the glucose metabolism through an important GLP-1 effect but also an important modification of the gut microbiota [145]. The VN could be involved in the beneficial effects observed after the surgery [146]. However, the real effect of bariatric surgery on the autonomic nervous system, including the VN or the ENS, and the diabetic neuropathy is not described yet and requires further experiments. According to the type of undergone surgery and the tissue analyzed post-surgery, the vagal innervations can be differently impacted: it was observed an important apoptosis of the vagal neurons for some surgeries while for the others, it was observed an important neurogenesis of these neurons [147]. No publications studied the long-term impact of the bariatric surgery on vagal or enteric neuron activities, homeostasis, neurogenesis or apoptosis processes or the innervation level of the gut and other tissues. It could be interesting to study further the impact of these surgery on the neuronal state and understand the role played by the gut microbiota.
The fecal microbiota transplantation (FMT) showed also an important and efficient effect to treat some diseases. From the beginning, this therapy was used to treat Clostridium difficile infection inducing hemorrhagic diarrhea and an important bowel inflammation. However, some research group try to extend this therapy in other diseases as metabolic diseases. It was also recently demonstrated that lean donor fecal microbiota transplantation in obese metabolic syndrome patients was efficient to treat insulin resistance through bacterial metabolites modification. However, the efficiency of the treatment is dependent of baseline fecal microbiota composition [148]. On the other hand, in a mouse model of Parkinson diseases, the FMT showed a neuroprotective effect through a suppression of the TLR4/TNFα pathway [149]. A beneficial effect of FMT through a better the gut-brain activity is not explored yet and we should go further to understand the involved mechanism.
The diabetic neuropathy is an important feature that needs to be diagnosed before applying a treatment to a diabetic patient since it can influence the efficacy of the treatment. Thus, research on alternative or co-treatment is required and necessary. As the microbiota dysbiosis can alters neuronal activities and homeostasis, by treating this dysbiosis could improve first the neuropathy state by also improve the efficiency of some current anti-diabetic treatment.
Conclusion
This review highlights the importance of autonomic nervous system, including the VN and the ENS, 1) on the physiological mechanism involved in glucose detection and glycemia regulation, from the mouth to the colon; 2) during the metabolic diseases either in the aggravation mechanisms or in the efficiency of some anti-diabetic treatment. Nevertheless, no efficient treatment is currently available to treat diabetic neuropathy. Diabetic neuropathy occurs after a middle to a long-time period of diabetes metillus (type 1 and type 2) and is usually associated to the aging. Along the same line, aging is also characterized by an increased risk of neurodegenerative diseases. Few is known about the problem of glucose metabolism during neurodegenerative diseases.
This review also proclaims how the gut microbiota is critical either in the neurogenesis, neuroplasticity and neuronal activities or neuronal alteration observed during T2D or neurodegenerative diseases. New studies are required to better understand the molecular mechanisms involved in the relation between microbiota and neuronal tissues. Thus, pro and pre-biotic treatment could be used to treat neuropathy or improve the current anti-diabetic treatments. Recent clinical trials demonstrated the role of dextrin [150], inulin-enriched oligofructose [151] or inuline alone [152] as well as L. acidophilus, Bifidobacterium animals [153, 154] and showed that these molecules improved glycemia control, insulin sensitivity and low-grade inflammation. However, none of these studies relate the effect on diabetic neuropathy. On the other hand, a lot of new animal works showed that current anti-diabetic treatment as metformin, GLP-1 agonist, DPP-4 inhibitors can positively modify the gut microbiota improving then the glucose metabolism [155–157]. However, although these anti-diabetic therapies induced an improvement of diverse neuropathies, no results permit to demonstrate how the gut microbiota shifts induced by the same therapies could influence and improve the neurons homeostasis and activities. Thus, this review suggests new mechanisms by how the gut microbiota influences glucose metabolism and opens new perspectives on how we can better treat T2D .
Funding information
Open access funding provided by University of Gothenburg. E.G. is funded by a postdoctoral fellowship from the Wenner-Gren foundations. RB is the recipient of a grant from the European Foundation for the Study of Diabetes Lilly-2.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 2.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 3.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dube PE, Rowland KJ, Brubaker PL. Glucagon-like peptide-2 activates beta-catenin signaling in the mouse intestinal crypt: role of insulin-like growth factor-I. Endocrinology. 2008;149:291–301. doi: 10.1210/en.2007-0561. [DOI] [PubMed] [Google Scholar]
- 5.Raybould HE. Sensing of glucose in the gastrointestinal tract. Auton Neurosci. 2007;133:86–90. doi: 10.1016/j.autneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Dray C, Sakar Y, Vinel C, Daviaud D, Masri B, Garrigues L, Wanecq E, Galvani S, Negre-Salvayre A, Barak LS, et al. The intestinal glucose-apelin cycle controls carbohydrate absorption in mice. Gastroenterology. 2013;144:771–780. doi: 10.1053/j.gastro.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Anini Y, Wei W, Qi X, O’Carroll A-M, Mochizuki T, Wang H-Q, Hellmich MR, Englander EW, Greeley JGH. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145:1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 8.Dyer J, Garner A, Wood IS, Sharma AK, Chandranath I, Shirazi-Beechey SP. Changes in the levels of intestinal Na+/glucose co-transporter (SGLT1) in experimental diabetes. Biochem Soc Trans. 1997;25:479S. doi: 10.1042/bst025479s. [DOI] [PubMed] [Google Scholar]
- 9.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 10.Fedorak RN, Cheeseman CI, Thomson AB, Porter VM. Altered glucose carrier expression: mechanism of intestinal adaptation during streptozocin-induced diabetes in rats. Am J Phys. 1991;261:G585–G591. doi: 10.1152/ajpgi.1991.261.4.G585. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657–R666. doi: 10.1152/ajpregu.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young, R.L., Lumsden, A.L., Martin, A.M., Schober, G., Pezos, N., Thazhath, S.S., Isaacs, N.J., Cvijanovic, N., Sun, E.W.L., Wu, T., et al. (2018). Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). [DOI] [PubMed]
- 13.Alssema M, Rijkelijkhuizen JM, Holst JJ, Teerlink T, Scheffer PG, Eekhoff EM, Gastaldelli A, Mari A, Hart LM, Nijpels G, et al. Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and ALT. Eur J Endocrinol. 2013;169:421–430. doi: 10.1530/EJE-13-0487. [DOI] [PubMed] [Google Scholar]
- 14.Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Terce F, Burcelin R. A specific gut microbiota Dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain Axis mechanism. Cell Metab. 2017;25(1075–1090):e1075. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Yeow TP, Pacini G, Tura A, Hor CP, Lim SL, Tan FH, Tong CV, Hong JY, Md Zain F, Holst JJ, et al. Preserved glucagon-like peptide-1 responses to oral glucose, but reduced incretin effect, insulin secretion and sensitivity in young Asians with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5:e000352. doi: 10.1136/bmjdrc-2016-000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–1648. doi: 10.2337/diabetes.49.10.1643. [DOI] [PubMed] [Google Scholar]
- 17.Gardemann A, Strulik H, Jungermann K. A portal-arterial glucose concentration gradient as a signal for an insulin-dependent net glucose uptake in perfused rat liver. FEBS Lett. 1986;202:255–259. doi: 10.1016/0014-5793(86)80697-4. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton-Wessler M, Bergman RN, Halter JB, Watanabe RM, Donovan CM. The role of liver glucosensors in the integrated sympathetic response induced by deep hypoglycemia in dogs. Diabetes. 1994;43:1052–1060. doi: 10.2337/diab.43.8.1052. [DOI] [PubMed] [Google Scholar]
- 19.Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80. doi: 10.1038/197079b0. [DOI] [PubMed] [Google Scholar]
- 20.Burcelin R, Thorens B. Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Diabetes. 2001;50:1282–1289. doi: 10.2337/diabetes.50.6.1282. [DOI] [PubMed] [Google Scholar]
- 21.Babic T, Troy AE, Fortna SR, Browning KN. Glucose-dependent trafficking of 5-HT3 receptors in rat gastrointestinal vagal afferent neurons. Neurogastroenterol Motil. 2012;24:e476–e488. doi: 10.1111/j.1365-2982.2012.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronveaux CC, de Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav. 2014;135:222–229. doi: 10.1016/j.physbeh.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 26.Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol Motil. 2011;23:e282–e293. doi: 10.1111/j.1365-2982.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournel A, Drougard A, Duparc T, Marlin A, Brierley SM, Castro J, et al. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 2015. [DOI] [PMC free article] [PubMed]
- 29.Michel K, Zeller F, Langer R, Nekarda H, Kruger D, Dover TJ, Brady CA, Barnes NM, Schemann M. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology. 2005;128:1317–1326. doi: 10.1053/j.gastro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Abot A, Lucas A, Bautzova T, Bessac A, Fournel A, Le-Gonidec S, Valet P, Moro C, Cani PD, Knauf C. Galanin enhances systemic glucose metabolism through enteric nitric oxide synthase-expressed neurons. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011;345:243–252. doi: 10.1007/s00441-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 32.Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, Shigemura N, Yanagawa Y, Drucker DJ, Margolskee RF, Ninomiya Y. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29:2268–2280. doi: 10.1096/fj.14-265355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, Elmquist JK. Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol. 2011;519:3085–3101. doi: 10.1002/cne.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sbarbati A, Osculati F. Extending the enteric nervous system. Biomed Pharmacother. 2007;61:377–382. doi: 10.1016/j.biopha.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53:2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Primeaux SD, Braymer HD, Bray GA. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig Dis Sci. 2013;58:363–370. doi: 10.1007/s10620-012-2364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou LH, Liu XM, Feng XH, Han LO, Liu GD. Expression of alpha-gustducin in the circumvallate papillae of taste buds of diabetic rats. Acta Histochem. 2009;111:145–149. doi: 10.1016/j.acthis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Chevrot M, Bernard A, Ancel D, Buttet M, Martin C, Abdoul-Azize S, Merlin JF, Poirier H, Niot I, Khan NA, et al. Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J Lipid Res. 2013;54:2485–2494. doi: 10.1194/jlr.M039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng B, Pan S, Liu X, Zhang S, Sun X. Cell apoptosis of taste buds in circumvallate papillae in diabetic rats. Exp Clin Endocrinol Diabetes. 2011;119:480–483. doi: 10.1055/s-0031-1279714. [DOI] [PubMed] [Google Scholar]
- 40.Pai MH, Ko TL, Chou HC. Effects of streptozotocin-induced diabetes on taste buds in rat vallate papillae. Acta Histochem. 2007;109:200–207. doi: 10.1016/j.acthis.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 42.Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;36:144–166. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]
- 43.Horowitz, M., and Samsom, M. (2004). Gastrointestinal function in diabetes mellitus. (Chichester, West Sussex, England ; Hoboken, NJ: J. Wiley).
- 44.Belmin J, Valensi P. Diabetic neuropathy in elderly patients. What can be done? Drugs Aging. 1996;8:416–429. doi: 10.2165/00002512-199608060-00003. [DOI] [PubMed] [Google Scholar]
- 45.Chen YL, Weng SF, Yang CY, Wang JJ, Tien KJ. Diabetic ketoacidosis further increases risk of Alzheimer's disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;147:55–61. doi: 10.1016/j.diabres.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Morsi M, Maher A, Aboelmagd O, Johar D, Bernstein L. A shared comparison of diabetes mellitus and neurodegenerative disorders. J Cell Biochem. 2018;119:1249–1256. doi: 10.1002/jcb.26261. [DOI] [PubMed] [Google Scholar]
- 47.Salas IH, De Strooper B. Diabetes and Alzheimer's disease: a link not as simple as it seems. Neurochem Res. 2018. [DOI] [PubMed]
- 48.Lee, Y.H., Hsu, H.C., Kao, P.C., Shiao, Y.J., Yeh, S.H., Shie, F.S., Hsu, S.M., Yeh, C.W., Liu, H.K., Yang, S.B., et al. (2018). Augmented insulin and Leptin resistance of high fat diet-fed APPswe/PS1dE9 transgenic mice exacerbate obesity and glycemic Dysregulation. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed]
- 49.Walter U, Tsiberidou P, Kersten M, Storch A, Lohle M. Atrophy of the Vagus nerve in Parkinson's disease revealed by high-resolution ultrasonography. Front Neurol. 2018;9:805. doi: 10.3389/fneur.2018.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daulatzai MA. Dysfunctional nucleus tractus solitarius: its crucial role in promoting neuropathogenetic cascade of Alzheimer's dementia--a novel hypothesis. Neurochem Res. 2012;37:846–868. doi: 10.1007/s11064-011-0680-2. [DOI] [PubMed] [Google Scholar]
- 51.Polak T, Dresler T, Zeller JB, Warrings B, Scheuerpflug P, Fallgatter AJ, Deckert J, Metzger FG. Vagus somatosensory evoked potentials are delayed in Alzheimer's disease, but not in major depression. Eur Arch Psychiatry Clin Neurosci. 2014;264:263–267. doi: 10.1007/s00406-013-0415-2. [DOI] [PubMed] [Google Scholar]
- 52.Han X, Tang S, Dong L, Song L, Dong Y, Wang Y, Du Y. Loss of nitrergic and cholinergic neurons in the enteric nervous system of APP/PS1 transgenic mouse model. Neurosci Lett. 2017;642:59–65. doi: 10.1016/j.neulet.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 53.Barboza JL, Okun MS, Moshiree B. The treatment of gastroparesis, constipation and small intestinal bacterial overgrowth syndrome in patients with Parkinson's disease. Expert Opin Pharmacother. 2015;16:2449–2464. doi: 10.1517/14656566.2015.1086747. [DOI] [PubMed] [Google Scholar]
- 54.Manfredsson FP, Luk KC, Benskey MJ, Gezer A, Garcia J, Kuhn NC, Sandoval IM, Patterson JR, O'Mara A, Yonkers R, et al. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol Dis. 2018;112:106–118. doi: 10.1016/j.nbd.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan F, Chen Y, Li M, Wang Y, Zhang W, Chen X, Ye Q. Gastrointestinal nervous system alpha-synuclein as a potential biomarker of Parkinson disease. Medicine (Baltimore) 2018;97:e11337. doi: 10.1097/MD.0000000000011337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blandini F, Balestra B, Levandis G, Cervio M, Greco R, Tassorelli C, Colucci M, Faniglione M, Bazzini E, Nappi G, et al. Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson's disease. Neurosci Lett. 2009;467:203–207. doi: 10.1016/j.neulet.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, Tache Y. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24:e425–e436. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardiner J, Barton D, Vanslambrouck JM, Braet F, Hall D, Marc J, Overall R. Defects in tongue papillae and taste sensation indicate a problem with neurotrophic support in various neurological diseases. Neuroscientist. 2008;14:240–250. doi: 10.1177/1073858407312382. [DOI] [PubMed] [Google Scholar]
- 59.Yamagishi M, Takami S, Getchell TV. Innervation in human taste buds and its decrease in Alzheimer's disease patients. Acta Otolaryngol. 1995;115:678–684. doi: 10.3109/00016489509139386. [DOI] [PubMed] [Google Scholar]
- 60.Anand U, Yiangou Y, Akbar A, Quick T, MacQuillan A, Fox M, Sinisi M, Korchev YE, Jones B, Bloom SR, et al. Glucagon-like peptide 1 receptor (GLP-1R) expression by nerve fibres in inflammatory bowel disease and functional effects in cultured neurons. PLoS One. 2018;13:e0198024. doi: 10.1371/journal.pone.0198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jolivalt CG, Fineman M, Deacon CF, Carr RD, Calcutt NA. GLP-1 signals via ERK in peripheral nerve and prevents nerve dysfunction in diabetic mice. Diabetes Obes Metab. 2011;13:990–1000. doi: 10.1111/j.1463-1326.2011.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuyucu E, Gumus B, Erbas O, Oltulu F, Bora A. Exenatide promotes regeneration of injured rat sciatic nerve. Neural Regen Res. 2017;12:637–643. doi: 10.4103/1673-5374.205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelrahman SA, Samak MA, Shalaby SM. Fluoxetine pretreatment enhances neurogenic, angiogenic and immunomodulatory effects of MSCs on experimentally induced diabetic neuropathy. Cell Tissue Res. 2018;374:83–97. doi: 10.1007/s00441-018-2838-6. [DOI] [PubMed] [Google Scholar]
- 64.Tawfik MK, Helmy SA, Badran DI, Zaitone SA. Neuroprotective effect of duloxetine in a mouse model of diabetic neuropathy: role of glia suppressing mechanisms. Life Sci. 2018;205:113–124. doi: 10.1016/j.lfs.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Nozu T, Miyagishi S, Kumei S, Nozu R, Takakusaki K, Okumura T. Glucagon-like peptide-1 analog, liraglutide, improves visceral sensation and gut permeability in rats. J Gastroenterol Hepatol. 2018;33:232–239. doi: 10.1111/jgh.13808. [DOI] [PubMed] [Google Scholar]
- 66.Voss U, Sand E, Hellstrom PM, Ekblad E. Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol. 2012;12:30. doi: 10.1186/1471-230X-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018a. [DOI] [PMC free article] [PubMed]
- 68.Spielman LJ, Gibson DL, Klegeris A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur J Cell Biol. 2017;96:240–253. doi: 10.1016/j.ejcb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Lim JG, Lee JJ, Park SH, Park JH, Kim SJ, Cho HC, Baek WK, Kim DK, Song DK. Glucagon-like peptide-1 protects NSC-34 motor neurons against glucosamine through Epac-mediated glucose uptake enhancement. Neurosci Lett. 2010;479:13–17. doi: 10.1016/j.neulet.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Cai HY, Yang JT, Wang ZJ, Zhang J, Yang W, Wu MN, et al. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2017. [DOI] [PubMed]
- 71.Holscher C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer's disease. Alzheimers Dement. 2014;10:S47–S54. doi: 10.1016/j.jalz.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng P, Zhang X, Li D, Ji C, Yuan Z, Wang R, Xue G, Li G, Holscher C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson's disease. Neuropharmacology. 2018;133:385–394. doi: 10.1016/j.neuropharm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Baranger K, Giannoni P, Girard SD, Girot S, Gaven F, Stephan D, Migliorati M, Khrestchatisky M, Bockaert J, Marchetti-Gauthier E, et al. Chronic treatments with a 5-HT4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer's disease. Neuropharmacology. 2017;126:128–141. doi: 10.1016/j.neuropharm.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 75.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 77.Wichmann A, Allahyar A, Greiner TU, Plovier H, Lunden GO, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Backhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, et al. GABA-modulating bacteria of the human gut microbiota. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breton J, Legrand R, Akkermann K, Jarv A, Harro J, Dechelotte P, Fetissov SO. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int J Eat Disord. 2016;49:805–808. doi: 10.1002/eat.22531. [DOI] [PubMed] [Google Scholar]
- 80.Koh A, Molinaro A, Stahlman M, Khan MT, Schmidt C, Manneras-Holm L, Wu H, Carreras A, Jeong H, Olofsson LE, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175(947–961):e917. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 81.Wahlstrom A, Kovatcheva-Datchary P, Stahlman M, Backhed F, Marschall HU. Crosstalk between bile acids and gut microbiota and its impact on Farnesoid X receptor Signalling. Dig Dis. 2017;35:246–250. doi: 10.1159/000450982. [DOI] [PubMed] [Google Scholar]
- 82.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 83.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 84.Denou E, Lolmede K, Garidou L, Pomie C, Chabo C, Lau TC, Fullerton MD, Nigro G, Zakaroff-Girard A, Luche E, et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7:259–274. doi: 10.15252/emmm.201404169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–465. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- 86.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–37. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- 87.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484–1490. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 88.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 89.Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(1679–1694):e1673. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 90.Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 92.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 94.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 96.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 97.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(814–825):e227–e818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giaretta PR, Suchodolski JS, Blick AK, Steiner JM, Lidbury JA, Rech RR. Distribution of bile acid receptor TGR5 in the gastrointestinal tract of dogs. Histol Histopathol. 2018;18025. [DOI] [PubMed]
- 99.Zhu X, He L, McCluskey LP. Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience. 2014;258:47–61. doi: 10.1016/j.neuroscience.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. 2012;143(1006–1016):e1004. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palu G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 102.Patel V, Patel AM, McArdle JJ. Synaptic abnormalities of mice lacking toll-like receptor (TLR)-9. Neuroscience. 2016;324:1–10. doi: 10.1016/j.neuroscience.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 103.De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci. 2018b. [DOI] [PMC free article] [PubMed]
- 104.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183–e188. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 105.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 2015;27:627–636. doi: 10.1111/nmo.12534. [DOI] [PubMed] [Google Scholar]
- 106.Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut-brain axis rewired: adding a functional vagal nicotinic "sensory synapse". FASEB J. 2014;28:3064–3074. doi: 10.1096/fj.13-245282. [DOI] [PubMed] [Google Scholar]
- 108.Morita H, Yoshikawa H, Sakata R, Nagata Y, Tanaka H. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of L-arginine by lactobacillus fermentum. J Bacteriol. 1997;179:7812–7815. doi: 10.1128/jb.179.24.7812-7815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yarullina DR, Mikheeva RO, Sabirullina GI, Zelenikhin PV, Ilinskaya ON, Sitdikova GF. Role of nitric oxide produced by lactobacilli in relaxation of intestinal smooth muscles. Bull Exp Biol Med. 2016;160:343–346. doi: 10.1007/s10517-016-3166-z. [DOI] [PubMed] [Google Scholar]
- 110.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, Keller MP, Attie AD, Rey FE, Denu JM. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982–992. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wahba G, Schock SC, Claridge E, Bettolli M, Grynspan D, Humphreys P, Staines WA. MeCP2 in the enteric nervous system. Neurogastroenterol Motil. 2015;27:1156–1161. doi: 10.1111/nmo.12605. [DOI] [PubMed] [Google Scholar]
- 112.Wahba G, Schock SC, Cudd S, Grynspan D, Humphreys P, Staines WA. Activity and MeCP2-dependent regulation of nNOS levels in enteric neurons. Neurogastroenterol Motil. 2016;28:1723–1730. doi: 10.1111/nmo.12873. [DOI] [PubMed] [Google Scholar]
- 113.Vaes, N., Lentjes, M., Gijbels, M.J., Rademakers, G., Daenen, K.L., Boesmans, W., Wouters, K.A.D., Geuzens, A., Qu, X., Steinbusch, H.P.J., et al. (2017). NDRG4, an early detection marker for colorectal cancer, is specifically expressed in enteric neurons. Neurogastroenterol Motil 29. [DOI] [PubMed]
- 114.Litke C, Bading H, Mauceri D. Histone deacetylase 4 shapes neuronal morphology via a mechanism involving regulation of expression of vascular endothelial growth factor D. J Biol Chem. 2018;293:8196–8207. doi: 10.1074/jbc.RA117.001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Helleputte L, Kater M, Cook DP, Eykens C, Rossaert E, Haeck W, Jaspers T, Geens N, Vanden Berghe P, Gysemans C, et al. Inhibition of histone deacetylase 6 (HDAC6) protects against vincristine-induced peripheral neuropathies and inhibits tumor growth. Neurobiol Dis. 2018;111:59–69. doi: 10.1016/j.nbd.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 116.Mahady L, Nadeem M, Malek-Ahmadi M, Chen K, Perez SE, Mufson EJ. HDAC2 dysregulation in the nucleus basalis of Meynert during the progression of Alzheimer's disease. Neuropathol Appl Neurobiol. 2018. [DOI] [PMC free article] [PubMed]
- 117.Schiaffino L, Bonafede R, Scambi I, Parrella E, Pizzi M, Mariotti R. Acetylation state of RelA modulated by epigenetic drugs prolongs survival and induces a neuroprotective effect on ALS murine model. Sci Rep. 2018;8:12875. doi: 10.1038/s41598-018-30659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan Y, Delvaux E, Nolz J, Coleman PD, Chen S, Mastroeni D. Upregulation of histone deacetylase 2 in laser capture nigral microglia in Parkinson's disease. Neurobiol Aging. 2018;68:134–141. doi: 10.1016/j.neurobiolaging.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 119.Duca FA, Sakar Y, Lepage P, Devime F, Langelier B, Dore J, Covasa M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes. 2014;63:1624–1636. doi: 10.2337/db13-1526. [DOI] [PubMed] [Google Scholar]
- 120.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 122.Murphy EF, Cotter PD, Healy S, Marques TM, O'Sullivan O, Fouhy F, Clarke SF, O'Toole PW, Quigley EM, Stanton C, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 123.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 124.Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61:69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 125.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 126.Bodogai Monica, O’Connell Jennifer, Kim Ki, Kim Yoo, Moritoh Kanako, Chen Chen, Gusev Fedor, Vaughan Kelli, Shulzhenko Natalia, Mattison Julie A., Lee-Chang Catalina, Chen Weixuan, Carlson Olga, Becker Kevin G., Gurung Manoj, Morgun Andrey, White James, Meade Theresa, Perdue Kathy, Mack Matthias, Ferrucci Luigi, Trinchieri Giorgio, de Cabo Rafael, Rogaev Evgeny, Egan Josephine, Wu Jiejun, Biragyn Arya. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Science Translational Medicine. 2018;10(467):eaat4271. doi: 10.1126/scitranslmed.aat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Branchereau M, Reichardt F, Loubieres P, Marck P, Waget A, Azalbert V, Colom A, Padmanabhan R, Iacovoni JS, Giry A, et al. Periodontal dysbiosis linked to periodontitis is associated with cardiometabolic adaptation to high-fat diet in mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1091–G1101. doi: 10.1152/ajpgi.00424.2015. [DOI] [PubMed] [Google Scholar]
- 128.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Long J, Cai Q, Steinwandel M, Hargreaves MK, Bordenstein SR, Blot WJ, Zheng W, Shu XO. Association of oral microbiome with type 2 diabetes risk. J Periodontal Res. 2017;52:636–643. doi: 10.1111/jre.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silva-Boghossian CM, Cesario PC, Leao ATT, Colombo APV. Subgingival microbial profile of obese women with periodontal disease. J Periodontol. 2018;89:186–194. doi: 10.1002/JPER.17-0236. [DOI] [PubMed] [Google Scholar]
- 131.Blasco-Baque V, Garidou L, Pomie C, Escoula Q, Loubieres P, Le Gall-David S, Lemaitre M, Nicolas S, Klopp P, Waget A, et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66:872–885. doi: 10.1136/gutjnl-2015-309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 133.Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson's disease. Ageing Res Rev. 2018;45:53–61. doi: 10.1016/j.arr.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 134.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(1469–1480):e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fak F, Jucker M, Lasser T, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects Normal food intake and Glycemia. Diabetes. 2016;65:34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 137.Charpentier J, Waget A, Klopp P, Magnan C, Cruciani-Guglielmacci C, Lee SJ, Burcelin R, Grasset E. Lixisenatide requires a functional gut-vagus nerve-brain axis to trigger insulin secretion in controls and type 2 diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2018;315:G671–G684. doi: 10.1152/ajpgi.00348.2017. [DOI] [PubMed] [Google Scholar]
- 138.Nishizawa M, Nakabayashi H, Uehara K, Nakagawa A, Uchida K, Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am J Physiol Endocrinol Metab. 2013;305:E376–E387. doi: 10.1152/ajpendo.00565.2012. [DOI] [PubMed] [Google Scholar]
- 139.Waget A, Cabou C, Masseboeuf M, Cattan P, Armanet M, Karaca M, Castel J, Garret C, Payros G, Maida A, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152:3018–3029. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
- 140.Kobayashi H, Kusafuka J, Lane GJ, Yamataka A, Satoh K, Hayakawa T, Kase Y, Hatano M. The mechanism of intestinal motility in homozygous mutant Ncx/Hox11L.1-deficient mice--a model for intestinal neuronal dysplasia. J Pediatr Surg. 2007;42:2062–2066. doi: 10.1016/j.jpedsurg.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 141.Viader A, Wright-Jin EC, Vohra BP, Heuckeroth RO, Milbrandt J. Differential regional and subtype-specific vulnerability of enteric neurons to mitochondrial dysfunction. PLoS One. 2011;6:e27727. doi: 10.1371/journal.pone.0027727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Ruhl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G912–G927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 143.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Knop FK, Vilsboll T, et al. Characterisation of oral and i.v. glucose handling in truncally vagotomised subjects with pyloroplasty. Eur J Endocrinol. 2013;169:187–201. doi: 10.1530/EJE-13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trevaskis JL, Sacramento CB, Jouihan H, Ali S, Le Lay J, Oldham S, Bhagroo N, Boland BB, Cann J, Chang Y, et al. Neurturin and a GLP-1 analogue act synergistically to alleviate diabetes in Zucker diabetic fatty rats. Diabetes. 2017;66:2007–2018. doi: 10.2337/db16-0916. [DOI] [PubMed] [Google Scholar]
- 145.Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27:1345–1357. doi: 10.1007/s11695-017-2595-8. [DOI] [PubMed] [Google Scholar]
- 146.Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, le Roux CW. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg. 2010;20:616–622. doi: 10.1007/s11695-010-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ballsmider LA, Vaughn AC, David M, Hajnal A, Di Lorenzo PM, Czaja K. Sleeve gastrectomy and Roux-en-Y gastric bypass alter the gut-brain communication. Neural Plast. 2015;2015:601985. doi: 10.1155/2015/601985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(611–619):e616. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 149.Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 150.Aliasgharzadeh A, Dehghan P, Gargari BP, Asghari-Jafarabadi M. Resistant dextrin, as a prebiotic, improves insulin resistance and inflammation in women with type 2 diabetes: a randomised controlled clinical trial. Br J Nutr. 2015;113:321–330. doi: 10.1017/S0007114514003675. [DOI] [PubMed] [Google Scholar]
- 151.Dehghan P, Pourghassem Gargari B, Asghari Jafar-abadi M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition. 2014;30:418–423. doi: 10.1016/j.nut.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 152.Dehghan P, Gargari BP, Jafar-Abadi MA, Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int J Food Sci Nutr. 2014;65:117–123. doi: 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- 153.Mobini R, Tremaroli V, Stahlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Berteus Forslund H, Perkins R, Backhed F, et al. Metabolic effects of lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 154.Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36:85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 155.Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, Liberti EA, Saad M, Carvalho C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 156.Olivares M, Neyrinck AM, Potgens SA, Beaumont M, Salazar N, Cani PD, Bindels LB, Delzenne NM. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018;61:1838–1848. doi: 10.1007/s00125-018-4647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M, Olsson LM, Serino M, Planas-Felix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]