Abstract
Background/objective
Both polymethylmethacrylate (PMMA) and traditional calcium phosphate–based cements have some deficiencies as augmentation materials for pedicle screw fixation. Here, a novel calcium phosphate–based nanocomposite (CPN) for the augmentation of pedicle screw fixation was developed based on previous study, and the handling properties, biomechanical performance, and biodegradation behaviour of CPN were evaluated and compared with clinical PMMA by means of a cadaver study and animal tests.
Methods
Bone mineral density of the lumbar vertebrae was tested. Pedicle screws were placed into the lumbar vertebrae under the guidance of three dimensionally printed templates; each of which was designed based on computed tomography (CT) reconstruction of each vertebrae and augmented with either PMMA or CPN. X-ray and CT scan were used to evaluate the accuracy of screw placement and dispersion as well as interdigitation of bone cement. The axial pull-out strength and maximum torque were tested using a mechanical testing machine. Degradation behaviour of CPN was evaluated by in vitro immersion tests for 8 weeks and in vivo rabbit femur defect model for up to 6 months, respectively.
Results
Standard mechanical tests revealed that PMMA was much stronger than CPN after setting (compressive strength 95 vs. 49 MPa, respectively, p < 0.001). Results of the projection area and volume distribution of cement along the distal end of the screws revealed that CPN exhibited unique dispersing and interdigitation abilities compared with PMMA. Specifically, CPN dispersed uniformly and symmetrically along the screw, while PMMA was limited to the proximal part of the screw. Axial pull-out test results showed that the axial pull-out strengths of CPN- and PMMA-augmented pedicle screws were similar (1199 ± 225 N vs 1337 ± 483 N, respectively) and not significantly different (p = 0.47), although CPN was an intrinsically weaker material than PMMA. Similarly, CPN showed average torque values of 0.72 ± 0.31 N·m slightly lower than those of PMMA (0.96 ± 0.23 N·m), but statistically there was no significant difference between CPN and PMMA (p = 0.21). In a rabbit model of femoral bone defect, the implanted CPN maintained its clear boundary and there is no disintegration in the cement clump after 20 days and 24 weeks, and there was moderate bioabsorption of CPN and clearly new bone ingrowth at the absorbed sites after 24 weeks.
Conclusion
A new nanocomposite cement CPN, designed for replacing the nondegradable PMMA cement and overcoming the mechanical inferiority of calcium phosphate cement, was evaluated for its biomechanical and biodegradation behaviours in cement-injectable cannulated pedicle screws (CICPS) application. Although CPN is a mechanically weaker material than PMMA, CPN demonstrates similar biomechanical properties to PMMA in the application of augmentation for CICPS fixation in cadaveric vertebrae. This improvement in biomechanical property is attributed to a better dispersion and interdigitation mode of CPN. In addition, the animal study results suggest the in vivo absorption of CPN is slow enough and matches the bone ingrowth.
The translational potential of this article
This work reports a cadaveric and biomechanical study of novel CPN for the application in the augmentation of CICPS. The results suggest that CPN has equivalent or better biomechanical and interdigitation performance compared with PMMA. Together with the biodegradability and ossointegration capability, CPN reveals high translational potential as a new bone cements for load-bearing bone fixation and repair.
Keywords: Calcium phosphate–based cement, Injectable, Osteoporosis, Pedicle screw, PMMA
Introduction
Pedicle screw fixation has been widely used for the stabilization of the thoracolumbar spine in a variety of indications, such as the promotion of bony fusion, the correction of deformity and the fixation of vertebral fractures. But an increasingly ageing population with affiliated increasing instances of osteoporosis presents challenges in spine surgery with pedicle screw fixation because in osteoporotic patients with concomitant reduced bone density, the mechanical force on the screw–bone interface is adversely affected, resulting in loosening, breakage, or back out of the pedicle screws [1], [2], [3], [4], [5], [6]. Therefore, to improve the attachment of pedicle screws in the osteoporotic spine, augmentation with bone cement using cement-injectable cannulated pedicle screws (CICPSs) has been shown to be an effective method [7], [8]. In this method, cement is a key factor that affects the clinical outcomes of augmented pedicle screw procedures. Although only polymethylmethacrylate (PMMA) cement has been approved by the US Food and Drug Administration (FDA) for clinical use in CICPS application, augmentation with PMMA has many drawbacks, including leakage, monomer toxicity, high heat generation, high stiffness, and nondegradability, all of which have in clinical practice been shown to cause serious side effects [9], [10], [11], [12], [13], [14]. Most importantly, owing to its nondegradability and lack of bioactivity, PMMA can neither form a biological bonding with bone nor induce bone ingrowth or osseointegration [15], [16], [17]. PMMA cement, however, forms excessive bonding with screws, and this causes extreme difficulty in retrieving or removing the screws during revision operations. In addition, when considering the cases of postoperation infection, the nondegradable PMMA becomes another high-risk factor for both deep infection and superficial infection [18]. Therefore, biodegradable cement, with biomechanical and handling properties similar to PMMA but with the ability to overcome the disadvantages of PMMA, would be an attractive alternative material for use in the CICPS augmentation.
Our previous studies have focused on this need, and we have developed a new, self-setting cement: calcium phosphate–based nanocomposite (CPN), which is biodegradable, osseointegrative, and injectable [19]. The calcium phosphate cements (CPCs) was mixed with nanoscale network of gelatinized starch and BaSO4 powders to form CPN, with greatly improved injectability and anticollapsibility in water. Moreover, CPN has also improved mechanical properties compared with CPC, resulting from the reinforcing effect of the starch nanonetwork on bone cement matrix. In vivo evaluations of CPN utilizing a rat femoral defect model showed that after 12 weeks of implantation, the surface of the CPN was degraded and had formed a bony interface with bone via new bone ingrowth. In standardized in vitro tests, CPN exhibited fluidity and dispersing ability similar to PMMA cements. These studies conclude that CPN is superior to PMMA in terms of biodegradability and bone growth induction [20], [21]. But as with all other calcium phosphate cements, CPN has an intrinsic mechanical strength much lower than PMMA cement because the compressive strength of CPN is only half that of PMMA after hardening. Our preliminary study, however, revealed that the weaker CPN may demonstrate a pedicle screw fixation augmenting effect similar to the stronger PMMA, which is supported by the results from simulated osteoporotic bone models using the American Society for Testing Materials (ASTM)-approved Sawbones and decalcified sheep vertebrae models [20], [21].
Nevertheless, whether the mechanically inferior CPN can replace the clinically approved PMMA cement in the augmentation of pedicle screw fixation remains a question. The aim of the present work was, therefore, to evaluate the biomechanical properties of CPN for CICPS application in a more rigorous cadaver model by means of a precise method assisted by three dimensionally printed templates. Based on previous results from both simulated osteoporotic models (i.e., ASTM-approved Sawbones and decalcified sheep vertebrae), the hypothesis here was that biodegradable CPN was expected to have a similar augmentation effect on CICPS, although the intrinsic mechanical strength of CPN is much lower than that of PMMA. The present study also attempted to evaluate midterm bioabsorption property of CPN and its osteoconductive ability in vivo.
Materials and methods
Preparation of materials and specimens
Cadaver specimens
This cadaver study is approved by the Human Subjects Institutional Review Board of Peking University First Hospital (No. 2017_16). Four lumbar vertebrae, encompassing L1–L5, were harvested from fresh elderly female cadavers (without tumours, deformities, and fractures) ranging in age from 63 to 92 years and stored at −20 °C until testing. After thawing the specimens at room temperature for 24 h, all extraneous soft tissues were removed (each lumbar vertebra was carefully separated from its neighbours and dissected free of all soft tissues). The bone mineral density of each vertebra was measured using dual-energy radiograph absorptiometry (XR-800; NORLAND, USA) to confirm that all specimens were osteoporotic lumbar vertebrae. The basic data of the specimens are summarized in Table 1.
Table 1.
Information and characterization of vertebra and cement.
| Cadaver sample | Vertebra | Age | BMD (g/cm2)* | Cement type injected on the left side | Cement volume injected on the left side (ml) | Cement type (R) injected on the right side | Cement volume injected on the right side (ml) |
|---|---|---|---|---|---|---|---|
| 1 | L1 | 62 | N | Control | 0 | CPN | 1.5 |
| 1 | L2 | 62 | 0.6912 | CPN | 1.5 | PMMA | 1.5 |
| 1 | L3 | 62 | 0.7894 | PMMA | 1.5 | Control | 0 |
| 1 | L4 | 62 | 0.7778 | Control | 0 | PMMA | 1.5 |
| 1 | L5 | 62 | N | CPN | 1.5 | Control | 0 |
| Average | 0.7551 | ||||||
| 2 | L1 | 65 | N | PMMA | 1.5 | CPN | 1.5 |
| 2 | L2 | 65 | 0.8379 | Control | 0 | PMMA | 1.5 |
| 2 | L3 | 65 | 0.8904 | CPN | 1.5 | PMMA | 1.5 |
| 2 | L4 | 65 | 0.9547 | PMMA | 1.5 | Control | 0 |
| 2 | L5 | 65 | N | Control | 0 | CPN | 1.5 |
| Average | 0.8989 | ||||||
| 3 | L1 | 65 | N | CPN | 1.5 | Control | 0 |
| 3 | L2 | 65 | 0.6554 | PMMA | 1.5 | CPN | 1.5 |
| 3 | L3 | 65 | 0.6628 | Control | 0 | CPN | 1.5 |
| 3 | L4 | 65 | 0.7052 | CPN | 1.5 | PMMA | 1.5 |
| 3 | L5 | 65 | N | PMMA | 1.5 | Control | 0 |
| Average | 0.6767 | ||||||
| 4 | L1 | 94 | N | Control | 0 | CPN | 1.5 |
| 4 | L2 | 94 | 0.7121 | Control | 0 | PMMA | 1.5 |
| 4 | L3 | 94 | 0.7968 | PMMA | 1.5 | CPN | 1.5 |
| 4 | L4 | 94 | 0.7800 | CPN | 1.5 | CPN | 1.5 |
| 4 | L5 | 94 | N | PMMA | 1.5 | PMMA | 1.5 |
| Average | 0.7653 |
*N = not available; BMD = bone mineral density; PMMA = polymethylmethacrylate; CPN = calcium phosphate nanocomposite.
Pedicle screws
In this study, 40 CICPSs (MISpine; Weigao Orthopaedic Device, Weihai, China) were used. The screw is made of Ti6Al4V with an outer diameter of 6.5 mm, a cannula diameter of 1.6 mm, and a length of 45 mm. The cement type for each screw is summarized in Table 1.
Preparation of CPN
As previously reported [20], the base material of CPN is a CPC powder consisting of 90wt% α-tricalcium phosphate (Dingan Science and Technology, Suzhou, China) and 10wt% analytical dicalcium phosphate dihydrate (Sigma–Aldrich, St Louis, MO, USA). For the CPN, 20 wt% starch [Jianjie Industrial (Group) Co., LTD, Zhengzhou, China] and 20 wt% barium sulphate [BaSO4 (BS), Sigma Aldrich, St Louis, MO, USA] were added to 60wt% CPC powder. The starch molecules gelatinized and formed a nanoscale network when added in water, which was confirmed by transmission electron microscopy (HT7700, 200 kV; Hitachi, Tokyo, Japan).The setting liquid was 0.25 mol/L sodium hydrogen phosphate (Sigma Aldrich, St Louis, MO, USA) solution; the CPN was mixed at a liquid-to-powder ratio of 0.45 mL/g. For comparison, clinical PMMA cement (Mendec Spine; Tecres SPA, Sommacampagna, Italy) was mixed at a liquid-to-solid ratio of 0.5 mL/g following the manufacturer's instructions.
Material characterization of CPN
The self-setting ability of the bone cements was demonstrated by injecting the cement paste into coloured water through a syringe with a force of <50 N. The injectability of the cements was tested following a standardized method reported elsewhere [19]. The Gillmore needle method was applied to measure initial and final setting times of the cement. The cements were aged at 37 °C for 3 days before mechanical testing. In vitro degradability of bone cements was measured by the weight loss (%) of the samples, which were soaked in 0.05 M tris–HCl buffer up to 8 weeks. The microstructure of the cements was precoated with an Au–Pd layer and observed by means of scanning electron microscopy (Quanta 250; Thermo Fisher Scientific, Waltham, MA, USA). Chemical and phase composition of CPN were analysed by an X-ray diffractometer (XRD; X'Pert Pro MRD; PANalytical, Almelo, Netherlands). The scan rates were 0.1–120° with step-scan mode of 3° per minute in the Bragg's angle (2θ) range from 10° to 60°.
Surgical technique
Design principles of the template for each vertebra
To ensure that the pedicle screw fixation of each group is as identical as possible, the CICPSs were positioned under the guidance of the template. After collecting human lumbar specimens, high-resolution CT scans (HiSpeed CT/e; GE Healthcare, USA) were performed and an individualized template was designed based on the specimen CT data. The design principle of the template is to ensure that the screws of each segment are parallel on the sagittal plane, the angles to the midline in the coronal plane are the same, and the screws are completely enclosed within the pedicle (Figure 1A). The templates were created using 3D printing technology (C3850, ELSTN, CN).
Figure 1.
Design of three dimensionally printed template and evaluation of the accuracy of CICPS placement. (A) Schematic revealing that design strategy of three dimensionally printed template is to ensure that the (1) screws are parallel on the sagittal plane, (2) the angle to the midline in coronal plane are same, and (3) the screws can be completely placed within the pedicle. (B) X-ray images showing that three points were selected for the transverse section of the pedicle in the CT scan to evaluated the accuracy of screw placement. The point A was a screw into the pedicle, point B was the midpoint of the pedicle, and point C was a screw into the vertebral body (C) The screw was located completely in the transverse section of the point A in pedicle, showing the accuracy of the screw placement guided by three dimensionally printed template. CICPS, cement-injectable cannulated pedicle screw
Process of pedicle screw placement and bone cement augmentation
All screws were placed by means of a standardized procedure under the guidance of three dimensionally printed template (Figure 2). First, soft tissue from the lumbar vertebra specimens was removed, leaving the bony structure intact. The template was placed in the correct position on the lumbar vertebral specimen, and total contact with the vertebral body was ensured. After correctly positioning the template, a cannulated drill bit was inserted along the direction of the template's channel. Then the inner core was removed, and a Kirschner wire was inserted in the needle lumen. Then the cannulated drill bit was pulled out, and a cannulated screw tap was used along the Kirschner wire for tapping. Finally, CICPSs were inserted along the tapping direction. The aforementioned procedure was repeated to insert all the screws into the vertebral body. For the injection test, CPN (or PMMA) powder was mixed with setting solution at a temperature of 21 °C. Then the push rod was filled with the cement paste, and the CPN's doughing period was awaited (after initial setting and about 10 min later), or the drawing period of PMMA (after initial setting and about 8 min later) was awaited. Then the cements were pushed through the cannula of the screw into the vertebrae. Cement type and volume in each screw are summarized in Table 1.
Figure 2.
The CICPSs were inserted into the lumbar vertebra under the guidance of three dimensionally printed template. (A) Screw insertion instruments. ①Screw handle, ②T-handle, ③cannulated screw tap, ④cannulated drill bit, and ⑤Kirschner wire. (B) Insert the cannulated drill bit along the channel of the template. (C) Remove the inner core and insert the Kirschner wire in needle lumen. (D) Remove the cannulated drill bit. (E) Tap along the Kirschner wire. (F) Insert the screw. (G) The specimen with screw placement in all lumbar segments. CICPS, cement-injectable cannulated pedicle screw.
Radiological evaluation
Following the collection of human lumbar specimens, X-ray plain films and CT examination were performed on all specimens to exclude malformations, fractures, and tumours and to provide data for designing and manufacturing the template.
After screw placement in all the lumbar specimens, X-ray plain films and CT scans were performed to evaluate the accuracy of screw position. Three-dimensional reconstruction of the CT scans was performed on the specimens with screws, and we chose 3 points to identify the accuracy of screw position: point A was where the screw penetrated the pedicle; point B was the midpoint of the screw in the pedicle; and point C was where the screw penetrated the vertebral body (Figure 1B and C). After making three sections perpendicular to the long axis of the screw, we observed whether the screw was completely located in the pedicle section to judge the accuracy of screw placement.
After augmentation with different cements, X-ray plain films and CT scans were performed again to compare dispersion between PMMA and CPN (Figure 3A and B). The lumbar spine specimens were split into individual vertebral bodies. Anteroposterior position (A-P) and axial view of X-ray films of each single vertebral body were performed, and the projection area was measured to evaluate dispersion properties of the bone cements (Figure 3C and D).Three-dimensional CT reconstruction was performed on the augmented lumbar samples to measure the dispersive volume of the different bone cements. We chose a point D located in the middle hole of the CICPSs as a demarcation point (Figure 3E). After making a section perpendicular to the long axis of the screw through point D, the bone cement volume was divided into two parts. The volume near the head of the screw was defined as front volume (FV), and the volume near the tail of the screw was defined as rear volume (RV) (Figure 3F).
Figure 3.
Radiological evaluation after augmentation with different cements. (A) A-P and lateral view of X-ray film of the specimen after inserting the screws. (B) A-P and lateral view of X-ray film of the specimen after screws augmentation. (C) A-P view and (D) axial view X-ray film of a single vertebral body after screws augmentation. (E) The dispersion model of CPN and PMMA was different. The CPN bone cement evenly surrounds all side holes of pedicle screw and the PMMA bone cement is before surrounding the proximal side holes. Point D is the location of the hole in the middle of the CICPSs. (F) The total volume (TV) of bone cement was divided into two parts. The yellow part was front volume (FV) and the blue part was rear volume (RV). CPN, calcium phosphate–based nanocomposite; PMMA, polymethylmethacrylate; CICPS, cement-injectable cannulated pedicle screw.
Biomechanical properties
Screw pull-out test
The specimens were wrapped and sealed in denture base resins (Boer Chemical Co., Ltd., Shanghai, China) in a square custom-made mold so that the embedded specimen could be placed in the testing machine and aligned to a required position. Maximum screw pull-out strength was determined using a universal mechanical testing machine (WDW-3020; Ke Xin Precision Instrument Co., Ltd., Changchun, China). The pedicle screw was attached to the testing machine by a rod threaded to the head of the screw, and a laser line marker was used to ensure the screws were placed in the uniaxial pulling direction. After the specimens were fixed, the screw was uniaxially pulled out at a constant displacement rate of 5 mm/min and the force–displacement curve was collected. Peak force reached during the pull-out test (when the screw was completely pulled out or the pedicle was destroyed) was defined as the maximum pull-out strength for comparison.
Torsion test
The specimens were wrapped in denture base resins in a round custom-made mold, and the specimens were embedded, leaving only the screw head exposed. A material testing machine (55 MT; Instron Universal Testing Machine, USA) was used to test maximum torque values. Before the torsion test, prestress was set as 10 N. The head of the screw was connected to the load frame and rotated counter clockwise at a constant rate of 0.5°/second along the direction of the screw axis. Testing continued until the torque value decreased significantly; peak torque value was recorded for each rotation.
Animal studies
To evaluate the bioadsorption and osseointegration capability of the CPN in vivo, ten New Zealand white rabbits (weight 2.5 kg) were used to create a bone defect of 5 mm in diameter and 5 mm in depth. The animal study protocol was approved by the Ethics Committee of Soochow University under the case number 201700031. Anaesthesia was administered by an intramuscular injection of 100 mg/kg ketamine hydrochloride and 12.5 mg/kg xylazine. The CPN powder was mixed with 0.25M Na2HPO4 solution, and then about 0.1 mL cement paste was injected into each bone defect until the defect was fully filled in (Figure 8A and B). The incision was sutured after the cement was hardened after approximately 10 min, and the animals were given antibiotics for 3 days. X-ray plain films were performed to survey the subcutaneous cement samples after the implantation (Figure 8C). The rabbits were sacrificed after 20 days and 24 weeks, and histological analysis on the defect region was performed. The tissue blocks containing cements were retrieved and fixed in 10% neutral formalin for 48 h and then decalcified in 14% ethylenediaminetetraacetic acid solution for 14 days, followed by dehydration using 20% and 30% sucrose solution for 1 day, respectively. The dehydrated tissue samples were embedded by optimal cutting temperature compound embedding medium and sliced to thin sections (8 μm) by using frozen section machine. The slices were stained with haematoxylin and eosin and observed under a fluorescence microscopy (AxioCamHRc; ZEISS).
Figure 8.
Histological analysis of the CPN after implanted in rabbit femur defect. (A) Schematic showing the surgical process and (B) the position of cements implanted into the femoral condyle of the rabbit. (C) X-ray image of the cement. (D)The boundary of the CPN samples kept its original cylindrical shape after 20 days, and (E) CPN almost did not degrade. (F) CPN significantly degraded in vivo after 24 weeks, and (G) bone ingrowth was observed. CPN, calcium phosphate–based nanocomposite.
Statistical analysis
Data were analyzed by means of a statistical package (Version 12, SPSS, Chicago, IL), Origin (Version 8; OriginLab, Massachusetts, USA), and GraphPad Prism (Version 5; GraphPad Software,USA). Data were reported as mean ± standard deviation. Mann–Whitney tests were conducted to compare differences between the different groups. Statistical significance was defined as p < 0.05.
Results
Characterization of CPN
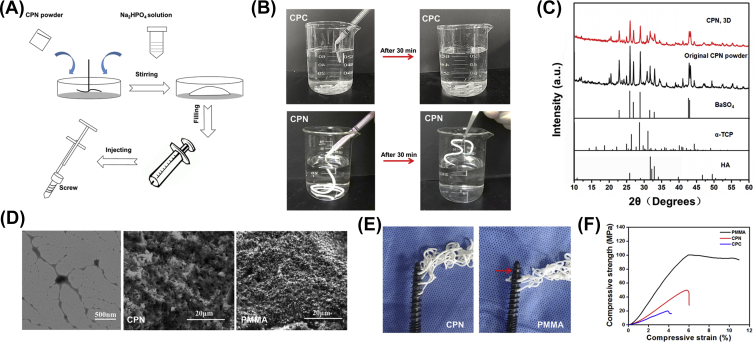
Figure 4A shows the preparation process of the bone cements. CPN paste was both ductile and squeezable, and measured injectability reached 99% similar to PMMA (Table 2). CPN was easily injected through the syringe and remained stable and self-set in deionized water, and CPC revealed low injectability and poor hardening ability and immediately collapsed after injection in water (Figure 4B). After approximately 30 min, the CPN had completely hardened and could be clamped by tweezers (Figure 4B). Interestingly, although both PMMA and CPN could be injected through the CICPSs, CPN could go through all three holes in the screw, whereas PMMA could not go through the distal hole in the screw (Figure 4E). This phenomenon is an important indication for the dispersion and interdigitation behaviour of the two types of cement, which will be discussed later. The initial setting time of CPN in air was about 14 min, and its final setting time was merely 22 min; both metrics were shorter than PMMA (Table 2), indicating a slightly faster hardening property of CPN compared with PMMA. The time difference between initial and setting times, however, was similar for both cements (about 8 min), suggesting that the time window for injecting operation and cement hardening is almost the same between both cements.
Figure 4.
Preparation and properties of calcium phosphate-based nanocomposite. (A) Schematic revealing the preparation of injectable CPN cement; (B) CPN showed good injectability and anticollapsibility in water and self-setting behaviour in the water after 30 min. (C) X-ray diffraction patterns of CPN before and after setting, comparing with hydroxyapatite (HA), alfa-TCP, and BaSO4. (D) TEM image of nanoscale networks of gelatinized starch and SEM images of the microstructure of CPN and PMMA, respectively. (E) CPN could flow out from all the holes of the screw, while PMMA had no cement flowed out from the holes at the far end of the screw. (F) Representative compressive stress–strain curves of CPC, CPN, and PMMA. CPN, calcium phosphate–based nanocomposite; PMMA, polymethylmethacrylate; CPC, calcium phosphate cement; TCP, tricalcium phosphate; TEM, transmission electron microscopy; SEM, scanning electron microscopy.
Table 2.
Mechanical property, injectability, setting times, and in vitro degradability (in tris buffer) of bone cements.
| Cement | Compressive strength (MPa)* | Modulus (GPa) | Injectability (%) | Initial setting time (min) | Final setting time (min) | Weight loss (%) (8 weeks) | |
|---|---|---|---|---|---|---|---|
| CPN | 49.28 ± 2.78 | 1.01 ± 0.29 | ∼99 | 13.68 ± 1.42 | 21.82 ± 1.07 | 12.01 ± 0.80 | |
| PMMA | 95.23 ± 11.21 | 1.91 ± 0.20 | ∼99 | 15.76 ± 1.10 | 23.36 ± 2.10 | 0.00 ± 0.00 | |
PMMA = polymethylmethacrylate; CPN = calcium phosphate nanocomposite.
*p < 0.01.
It is well known that calcium phosphate-based cements are much weaker materials than polymerized acrylic cements and this lower intrinsic mechanical strength limits the application of calcium phosphate–based cements in the repair of load-bearing bones. Indeed, the compressive strength of the CPN in the present study reached 49 MPa (Figure 4F and Table 2), which was only half that of PMMA (95 MPa) but three times than that of CPC (Figure 4F). The compressive stiffness of CPN was also much lower than that of PMMA (Table 2), and the compressive modulus of CPN (1.01 GPa) was also lower than that of PMMA (1.91 GPa). Nevertheless, based on our previous study in synthetic simulated cancellous bone models [22], CPN may be suitable for the augmentation of osteoporotic spine because its mechanical strength is much higher than natural cancellous bone in the vertebral body, but this possibility needs to be verified in the cadaver model.
Figure 4C shows the XRD patterns of the original CPN before solid–liquid reaction and after setting for 3 days. The XRD results revealed that the α-tricalcium phosphate contained in CPN was almost completely converted to hydroxyapatite in 3 days, with remnants of radiopaque BaSO4 that did not affect the reaction of cement. Figure 4D shows the nanoscale networks of starch (leftmost panel), which formed a viscous gel of disentangled amylose and amylopectin because of disintegrations of starch granules by hydration, and the chain-like molecules rearranged into an open network of nanofibers with thickness from tens of nanometres to a few hundred nanometres. The surface morphology of CPN consisted of needle-like hydroxyapatite precipitates after 3 days of hardening (Figure 4D), which also confirmed the XRD results.
Insertion of screws and bone cement augmentation
Under the guidance of three dimensionally printed templates, 40 CICPSs were inserted into lumbar vertebrae; 14 screws were augmented with PMMA and CPN cement, respectively.
In specimens 3 and 4 (the first lumbar spine), CT three-dimensional reconstruction showed that the medial border of lateral cortical bone of the left pedicle of the first lumbar vertebral body was in close contact with the screw (without breaking the lateral border of cortical bone); the remaining 38 pedicle screws were located in the pedicles which were undamaged.
Radiological evaluation
The total volumes of CPN cement and PMMA dispersed in the vertebra were designated to be the same 1.5 mL so that their mechanical reinforcement of CICPSs could be compared. Indeed, Figure 5B reveals that the total volumes of CPN and PMMA dispersion were the same (1310 ± 114 mm3 and 1276 ± 90 mm3, respectively, with no significant difference p = 0.22). Similarly, there was no significant difference in the projected area of CPN and PMMA in all directions (for A-P view, p = 0.089; axial view, p = 0.098) (Figure 5A). CPN was able to evenly wrap and enclose the screw while the PMMA was located only in the proximal part of the screws closer to the spinal canal. Specifically, CPN was more likely to be injected out through all three holes in the CICPSs and disperse along the screw, whereas PMMA was limited to the proximal part of the screw and hardly reached the distal hole of the screw. This observation comports with the aforementioned phenomena observed outside the vertebra. The FV and RV of cement (the forward and rear regions were divided by the medial hole in the screw) were also quantitatively compared, and the front volume of CPN was higher than that of PMMA (p = 0.008).
Figure 5.
Dispersing and interdigitation abilities of cements in the vertebrae. (A) Projected areas of cements in the A-P view and axial view, and there was no significant difference in the projected area of CPN and PMMA in all directions (for A-P view, p = 0.089; axial view, p = 0.098). (B) Dispersion volumes of CPN and PMMA (including total volume, front volume, and rear volume), and the front volume of CPN was higher than that of PMMA (p = 0.008). CPN, calcium phosphate–based nanocomposite; PMMA, polymethylmethacrylate.
Biomechanical properties of cement-augmented pedicle screws
Axial pull-out strength
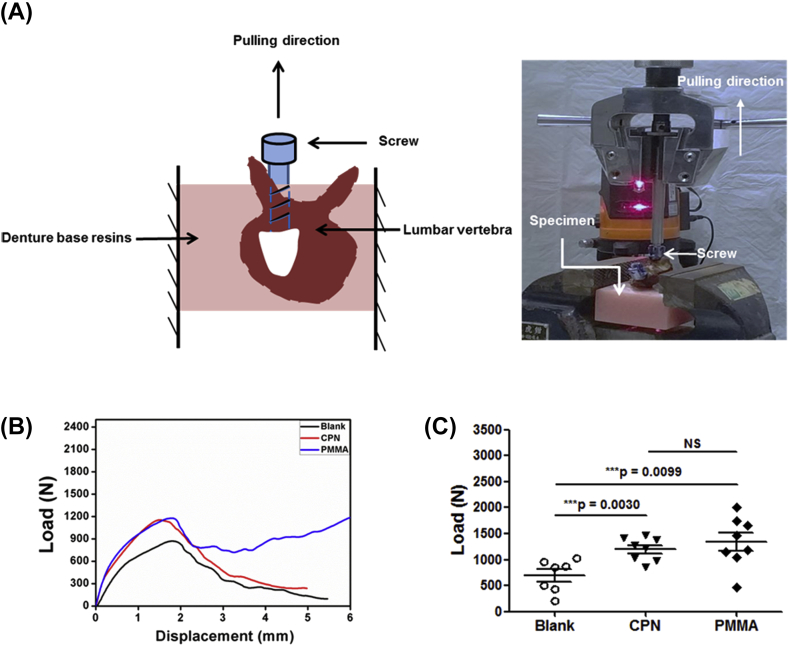
Axial pull-out strengths of cements for the augmentation of cannulated screws in cadaveric specimens were tested by means of a specially designed fixture system as shown in Figure 6A. Figure 6B shows the typical load–displacement curves for cement-augmented pedicle screws in cadaveric specimens. Peak force was defined as the maximum pull-out strength. The experimental results demonstrate that all of the failures occurred at the composite (screw-cement)–bone interface. However, the composite remained well bonded to the screws, indicating that the screw/cement interfacial strength was much higher than the composite/bone interfacial strength. The average ultimate pull-out strengths of cement-augmented pedicle screws are shown in Figure 6C. Pull-out strengths of CPN (1199 ± 225 N) were statistically similar to those of PMMA (1337 ± 483 N), although the average value of CPN was slightly lower but not significantly different (p = 0.47). Both CPN (p = 0.00) and PMMA (p = 0.01) had significantly higher pull-out strengths than those of the screws without cement augmentation (693 ± 312 N).
Figure 6.
Results of axial pull-out tests on cement-augmented CICPS. (A) Schematic and photo showing the setup of axial pull-out tests and vertical alignment of screw in the vertebra. (B) Typical load–displacement curves of the axial pull-out tests of CICPS without cement (Blank) and with CPN and PMMA augmentation, respectively; (C) Statistical results of the axial pull-out strengths of augmented CICPS. The average value of CPN (1199 ± 225N) was slightly lower than that of PMMA (1337 ± 483N), but the difference is not significant (P = 0.47), and both CPN and PMMA had significantly higher pull-out strengths than that of the screws without cement augmentation (693 ± 312 N) (P < 0.01).Data are mean ± standard deviation. CPN, calcium phosphate–based nanocomposite; PMMA, polymethylmethacrylate; CICPS, cement-injectable cannulated pedicle screw.
Maximum torque
To further study the biomechanical properties of cement-augmented pedicle screws, torsion tests were carried out to evaluate the antitorsion ability of bone cements. Figure 7A shows the setup of torsion tests; the method of testing was described in detail. Typical torque–angle curves for cement-augmented pedicle screws in cadaveric specimens are shown in Figure 7B; peak force was defined as the maximum torque, and the results also suggested that bone cements significantly improved the antitorsion ability of pedicle screws. Similar to the results of pull-out force, PMMA showed a slightly higher average torque value than CPN, but the difference has no statistical significance (Figure 7C and 0.96 ± 0.23 N ·m vs. 0.72 ± 0.31 N ·m, p = 0.21). The screws without cement showed maximum torque values (0.29 ± 0.12 N ·m), much lower than those of CPN (0.72 ± 0.31 N·m, p = 0.03) and PMMA (0.96 ± 0.23 N m, p = 0.00). The results of pull-out strength and maximum torque suggest that CPN, which is mechanically inferior to PMMA, achieved a reinforcing effect similar to CICPSs in the cadaver model in comparison to PMMA cement.
Figure 7.
Results of torsion tests on cement-augmented CICPS. (A) Schematic and photo showing the setup of torsion tests and horizontal alignment of screw. (B) Representative torque–angle curves of the torsion tests of CICPS augmented without cement (blank) and with CPN and PMMA augmentation, respectively; (C) Statistical results of the maximum torque of augmented CICPS. PMMA showed a slightly higher average torque value than CPN, but the difference has no statistical significance (P = 0.21). The screws without cement showed maximum torque values (0.29 ± 0.12 N m) much lower than those of CPN (0.72 ± 0.31 N m, p = 0.0276) and PMMA (0.96 ± 0.23 N m, p = 0.0003). Data are mean ± standard deviation. CPN, calcium phosphate–based nanocomposite; PMMA, polymethylmethacrylate; CICPS, cement-injectable cannulated pedicle screw.
Degradation and bioresorption of CPN
In vitro degradability of CPN tested in acellular tris–HCl buffer revealed that CPN degraded ∼12% after 8 weeks of immersion (Table 2), while PMMA was nondegradable. To further evaluate the bioresorption of CPN, a rabbit model of femoral bone defect was used to assess the biodegradation and osseointegration behaviour of CPN for up to 24 weeks (Figure 8). The haematoxylin and eosin–stained histological observations revealed that the implanted CPN maintained its clear boundary, and there is no disintegration in the cement clump after 20 days and 24 weeks (Figure 8D–F), indicating that the integrity of the CPN was conserved after 6 months. Although the integral cement clump was maintained, there was moderate bioabsorption of CPN and clearly observable new bone formation at the absorbed sites after 24 weeks, as shown in Figure 8G. The stained new bone tissue occupied the vacancy because of CPN resorption and formed a tight contact with the existing cement, suggesting a typical bone cell migration and new bone ingrowth into CPN samples (Figure 8G).
Discussion
Many studies have examined the biomechanical characteristics of primary osteoporotic posterior lumbar spinal instrumentation. As a primary approach, or a remedial approach after failure of spinal instrumentation in the osteoporotic spine, cement augmentation with CICPSs is an option to effectively secure screw with adequate robustness in the vertebral body [1], [19], [23], [24], [25], [26]. Although it is widely accepted that PMMA augmentation is effective in improving screw fixation, reports describing its complications and shortcomings have gradually emerged in clinical practice over the past few years [9]. Most drawbacks originate from the excessive mechanical strength or stiffness and nondegradability of PMMA, which are its intrinsic material properties and, as such, difficult to alter. Calcium phosphate–based cement with high biodegradability and similarity to native bone are alternative candidates for PMMA cement but its well-known deficiencies of low mechanical strengths and robustness, making them unsuitable for loading bearing bone applications such as CICPS fixation. This situation requires a new injectable biodegradable material distinct from PMMA but able to prevent the pull-out of screws from the vertebral body. In this particular study, we used cadaver models with standardized template-assisted screw placement to evaluate the biomechanical behaviour of a new material CPN.
The results of compressive strength and modulus (Table 2) show that the intrinsic mechanical strength of CPN is indeed much lower than that of PMMA. When considering the pull-out process of CICPSs, PMMA would have been expected to result in higher pull-out strength because the material is more difficult to fracture by the screw. CPN demonstrates, however, almost similar anti–pull-out strength and maximum toque as PMMA, suggesting that the reinforcing effect of CPN on CICPS fixation in vertebra is almost as good as that of PMMA. In other words, the anti–pull-out and antitorsion abilities of CPN are not inferior to those of PMMA, even though the CPN itself is much weaker than PMMA. Radiological examination further revealed that CPN was able to evenly wrap and enclose the screw in the vertebra, while the PMMA was located only in the proximal part of the screws closer to the spinal canal. In addition, when examining the screws after pull-out tests, fractures were found to have occurred at the interface between the cement and bone (i.e., the screws were pulled out with the PMMA or CPN cement clumps), suggesting that the interface between cement and bone is critical to the pull-out strength of the screw.
Based on these results, we attributed the highly improved anti–pull-out strength and maximum torque of the weaker CPN to its unique dispersion and interdigitation behaviour in the cancellous bone of vertebra, which is very different from that of PMMA and has the distinctive attribute of better distribution of the cement along the distal end of the screw. Notably, the injection tests both in and outside the vertebrae reveal that CPN could be injected through all three holes along the screw, resulting in a better distribution and interdigitation of CPN in the cancellous bone tissue of vertebra, while PMMA hardly reached the distal hole along the screw, leading to localized dispersion and interdigitation of PMMA around the proximal part of the screw. The results of FV/RV quantitatively show that in comparison to PMMA, CPN travelled farther towards the distal end of the screw and thus dispersed more evenly along the screw, indicating a high likelihood that there is more interface formation between CPN and cancellous bone tissue and even distribution along the screw. The increased interface and the even distribution results in a strong interlocking effect between the cement and the bone, leading to a high pull-out force.
The origin of the differentiated dispersion and interdigitation modes between CPN and PMMA cements still needs more investigation. One may argue that the differences in dispersion along the screw and subsequent interdigitation in the cancellous bone tissue result from the disparate setting behaviours of the two cements, because the cement with slower polymerization or setting property (i.e., maintaining the liquid state for a longer time) more easily travels a greater distance down the canal of the screw and is more likely to infiltrate the porous bone tissue. But this is not the case in the present study because the CPN is actually setting faster (initial setting time of CPN is shorter than that of PMMA) and has a setting time similar to PMMA (Table 2).
We also studied the in vitro degradation and in vivo bioresorption property of CPN. CPN was shown to be biodegradable and osteoconductive to host bone tissue, an observation supported by the presence of new bone ingrowth at the sites where resorption occurred. After 6 months of implantation, the CPN cement clump maintained its monolithic shape and with no apparent disintegration in the cement, but bioresorption occurred at the microscale because the new bone ingrowth to the resorption sites were at the scale of tens of micrometres (Figure 8). These results suggested that the bioresorption rate of CPN was moderate and matched the progression of new bone ingrowth and can be used for fixation in the early stages of spinal injury [27]; this bioresorption behaviour is desirable for maintaining the mechanical stability of CICPS fixation during the progress of the degradation of CPN. We, therefore, expect that CPN would not cause loss of the CICPS fixation before the fracture is healed. A further large animal study using CPN-injected CICPSs to evaluate the fixation strength at different times post implantation is currently ongoing.
In addition, the present study also provides well-defined and precisely controlled methods enabling reliable evaluations of the augmentation performance of bone cements, in particular, a standardized method to minimize possible variations caused by screw position. We demonstrated the method of using CT scan and reconstruction of vertebral specimens to design and produce three dimensionally print templates for each vertebra. Under guidance of the template, a standardized insertion process accurately places the screw into a predetermined position and also reduces damage to the pedicle. By using the aforementioned approaches, it is possible to minimize any negative influence associated with the screw insertion procedure on the subsequent biomechanical performance of CICPS in vertebrae.
Conclusion
A new nanocomposite cement CPN, designed for replacing the nondegradable PMMA cement and overcoming the mechanical inferiority of calcium phosphate cement, was evaluated for its biomechanical and biodegradation behaviours in CICPS application. Although CPN is a mechanically weaker material than PMMA, CPN demonstrates similar biomechanical properties to PMMA in the application of augmentation for CICPS fixation in cadaveric vertebrae. Radiological evaluation clearly demonstrated, for the first time, that calcium phosphate–based cement has a different dispersion mode in comparison with PMMA and may result in better interdigitation with cancellous bone. We, therefore, attributed the improvement in biomechanical performance of CPN to its better dispersion and interdigitation mode compared with PMMA. In addition, the animal study results suggested the in vivo absorption of CPN is moderate and matches the bone ingrowth. These results, together with previously reported results in simulated and animal bone models, demonstrate the promise of CPN as a possible replacement for PMMA in CICPS augmentation.
Conflicts of interest
The authors have no conflicts of interest to disclose in relation to this article.
Ethical approval
The cadaver study was approved by the Human Subjects Institutional Review Board of Peking University First Hospital (No. 2017-16). The animal study was approved by the Ethics Committee of Soochow University (No. 201700031).
Acknowledgments
The authors acknowledge the Youth Clinical Research Project of Peking University First Hospital (2017CR06), the National Natural Science Foundation of China (81622032 and 51672184), and Jiangsu Innovation and Entrepreneurship Program. The authors thank Linda Marie Nygaard for her revisions of the manuscript.
Contributor Information
Weiguang Zhang, Email: zhangwg@bjmu.edu.cn.
Chunde Li, Email: chundeli@yeah.net.
Lei Yang, Email: ylei@hebut.edu.cn.
References
- 1.Renner S.M., Lim T.H., Kim W.J., Katolik L., An H.S., Andersson G.B. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine. 2004;29(11):E212–E216. doi: 10.1097/00007632-200406010-00020. Phila Pa 1976. [eng] [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.J., Bridwell K.H., Lenke L.G., Rhim S., Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31(20):2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. Phila Pa 1976. [DOI] [PubMed] [Google Scholar]
- 3.Elder B.D., Lo S.F., Holmes C., Goodwin C.R., Kosztowski T.A., Lina I.A. The biomechanics of pedicle screw augmentation with cement. Spine J. 2015;15(6):1432–1445. doi: 10.1016/j.spinee.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Saracen A., Kotwica Z. Complications of percutaneous vertebroplasty: an analysis of 1100 procedures performed in 616 patients. Medicine (Baltim) 2016;95(24):e3850. doi: 10.1097/MD.0000000000003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mclachlin S.D., Al S.K., Gurr K.R., Bailey S.I., Bailey C.S., Dunning C.E. Comparative assessment of sacral screw loosening augmented with PMMA versus a calcium triglyceride bone cement. Spine. 2010;36(11):699–704. doi: 10.1097/BRS.0b013e3181fb73ea. [DOI] [PubMed] [Google Scholar]
- 6.Choma T.J., Frevert W.F., Carson W.L., Waters N.P., Pfeiffer F.M. Biomechanical analysis of pedicle screws in osteoporotic bone with bioactive cement augmentation using simulated in vivo multicomponent loading. Spine. 2011;36(6):454–462. doi: 10.1097/BRS.0b013e3181d449ec. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi A., Bednar D., Ziada S. Pullout strength of pedicle screws augmented with particulate calcium phosphate: an experimental study. Spine J. 2009;9(5):404–410. doi: 10.1016/j.spinee.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Guler U.O., Derincek A., Hersekli M.A., Ozalay M., Cinar B.M., Acaroglu E. Restoration of pull-out strength of the failed pedicle screw: biomechanical comparison of calcium sulfate vs polymethylmethacrylate augmentation. Acta Orthop Traumatol Turcica. 2014;48(2):202–206. doi: 10.3944/AOTT.2014.3193. [DOI] [PubMed] [Google Scholar]
- 9.Frankel B.M., Jones T., Wang C. Segmental polymethylmethacrylate-augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: a clinical evaluation. Neurosurgery. 2007;61(3):531–537. doi: 10.1227/01.NEU.0000290899.15567.68. discussion 37-8. [DOI] [PubMed] [Google Scholar]
- 10.Waits C., Burton D., McIff T. Cement augmentation of pedicle screw fixation using novel cannulated cement insertion device. Spine. 2009;34(14):E478–E483. doi: 10.1097/BRS.0b013e3181a8f663. Phila Pa 1976. [eng] [DOI] [PubMed] [Google Scholar]
- 11.Cook S.D., Salkeld S.L., Stanley T., Faciane A., Miller S.D. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004;4(4):402–408. doi: 10.1016/j.spinee.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Dai F., Liu Y., Zhang F., Sun D., Luo F., Zhang Z. Surgical treatment of the osteoporotic spine with bone cement-injectable cannulated pedicle screw fixation: technical description and preliminary application in 43 patients. Clinics. 2015;70(2):114–119. doi: 10.6061/clinics/2015(02)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L.H., Tai C.L., Lee D.M., Lai P.L., Lee Y.C., Niu C.C. Pullout strength of pedicle screws with cement augmentation in severe osteoporosis: a comparative study between cannulated screws with cement injection and solid screws with cement pre-filling. BMC Muscoskelet Disord. 2011;12(1) doi: 10.1186/1471-2474-12-33. 33-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar D.E., Clary S.J., Burton D.C., Mciff T.E. Effect of spinous process tether tension for prophylactic treatment of proximal junctional kyphosis in adult spinal deformity surgery. Spine J. 2017;17(10):S190. [Google Scholar]
- 15.Pinera A.R., Duran C., Lopez B., Saez I., Correia E., Alvarez L. Instrumented lumbar arthrodesis in elderly patients: prospective study using cannulated cemented pedicle screw instrumentation. Eur Spine J. 2011;20(Suppl 3):408–414. doi: 10.1007/s00586-011-1907-2. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart R.A., Marshall L.M., Hiratzka S.L., Kane M.S., Volpi J., Hiratzka J.R. Functional limitations due to stiffness as a collateral impact of instrumented arthrodesis of the lumbar spine. Spine. 2014;39(24):1468–1474. doi: 10.1097/BRS.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 17.Albright T.H., Grabel Z., Depasse J.M., Palumbo M.A., Daniels A.H. Sexual and reproductive function in spinal cord injury and spinal surgery patients. Orthop Rev. 2015;7(3) doi: 10.4081/or.2015.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeWald C.J., Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31(19S):S144–S151. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 19.Liu H., Guan Y., Wei D., Gao C., Yang H., Yang L. Reinforcement of injectable calcium phosphate cement by gelatinized starches. J Biomed Mater Res B Appl Biomater. 2016;104(3):615–625. doi: 10.1002/jbm.b.33434. [DOI] [PubMed] [Google Scholar]
- 20.Sun H., Liu C., Liu H., Bai Y., Zhang Z., Li X. A novel injectable calcium phosphate-based nanocomposite for the augmentation of cannulated pedicle-screw fixation. Int J Nanomed. 2017;12:3395–3406. doi: 10.2147/IJN.S131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Liu B., Gao C., Meng B., Yang H., Yu H. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int Orthop. 2018;42(1):125–132. doi: 10.1007/s00264-017-3674-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Liu H.L., Sun H.L., Yang H.L., Yang L. Development and optimization of biodegradable calcium phosphate-based nanocomposite for the application of spinal fixation. Ferroelectrics. 2018;527(1):162–169. [Google Scholar]
- 23.Weiser L., Huber G., Sellenschloh K., Viezens L., Puschel K., Morlock M.M. Insufficient stability of pedicle screws in osteoporotic vertebrae: biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur Spine J. 2017;26(11):2891–2897. doi: 10.1007/s00586-017-5091-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Xu J., Sun D., Luo F., Zhang Z., Dai F. Biomechanical and finite element analyses of bone cement-Injectable cannulated pedicle screw fixation in osteoporotic bone. J Biomed Mater Res B Appl Biomater. 2016;104(5):960–967. doi: 10.1002/jbm.b.33424. [DOI] [PubMed] [Google Scholar]
- 25.Tai C.L., Tsai T.T., Lai P.L., Chen Y.L., Liu M.Y., Chen L.H. A biomechanical comparison of expansive pedicle screws for severe osteoporosis: the effects of screw design and cement augmentation. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0146294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolunay T., Arslan K., Yaman O., Dalbayrak S., Demir T. Biomechanical performance of various cement-augmented cannulated pedicle screw designs for osteoporotic bones. Spine Deform. 2015;3(3):205–210. doi: 10.1016/j.jspd.2014.09.055. [eng] [DOI] [PubMed] [Google Scholar]
- 27.Feng Y., Sun T., Chen L., Xie J., Zhang Z., Huang H. Clinical therapeutic guideline for neurorestoration in spinal cord injury (Chinese version 2016) J Neurorestoratol. 2017;5:73–83. [Google Scholar]