Abstract
A six-year-old domestic shorthair cat was presented for a subcutaneous digital nodular lesion on the right forelimb. On physical examination a similar lesion was identified on the right hindlimb. Disseminated cutaneous phaeohyphomycosis was diagnosed from histopathological changes in representative tissue biopsies and fungal culture. The isolate was identified by sequencing of ITS rDNA as Exophiala spinifera. This is the first report of disseminated cutaneous disease caused by E. spinifera in the cat.
Keywords: Black yeasts, Exophiala, Phaehyphomycosis, Histopathology, Molecular diagnosis
1. Introduction
Multiple genera of melanin-pigmented dematiaceous saprophytic fungi can cause opportunistic fungal infections (phaeohyphomycoses) in humans and animals, usually by traumatic cutaneous inoculation, for example by splinters or thorns [1,2]. Phaeohyphomycoses are uncommon in cats and are typically characterised by cutaneous or subcutaneous lesions affecting the nasal planum or digits, although focal invasive and disseminated infections also occur [1,3,4]. The most common fungal genera implicated in feline phaeohyphomycoses include Alternaria, Cladophialophora, Microsphaeropsis and Exophilia [3,[5], [6], [7]]. Alternaria and Cladophialophora spp. are also the most frequent fungal organisms detected on feline skin [8]. Regional differences in the prevalence of the causal agents of feline phaeohyphomycoses appear to occur, for example in the UK and mainland Europe Alternaria spp. are the most frequent cause, while in Australia Microsphaeropsis arundinis is detected most frequently [[3], [4], [5]].
Due to the traumatic mode of inoculation, cutaneous lesions in phaeohyphomycoses are usually singular. Exophiala spp. have been detected in 10 cases of feline phaeohyphomycosis, of which nine were associated with solitary cutaneous or mucocutaneous lesions affecting a limb, the nasal cavity and/or nasal planum, or face [4,6,7,[9], [10], [11], [12], [13], [14]]. Three species in the genus, E. attenuata (n = 2), E. jeanselmei (n = 4) and E. spinifera (n = 3) are associated with disease in cats.
This is the first report of disseminated cutaneous disease caused by E. spinifera in a cat. Three other cases of phaeohyphomycosis caused by this species have been described in cats but only one of these was definitely identified using molecular sequencing [15].
2. Case
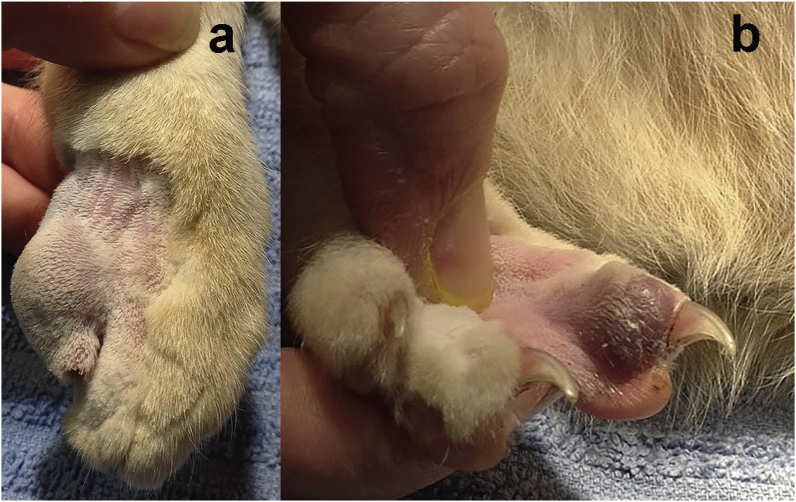
A 6-year old male neutered domestic shorthair cat was presented by its owner for investigation of a nodular digital swelling affecting the right forelimb (RFL) (Day 0). The cat had indoor and outdoor access. It had been presented a year ago for a similar subcutaneous mass on the third distal phalanx (P3) of the right hindlimb (RHL), which had been diagnosed on fine-needle aspiration cytology as a fungal granuloma containing yeast and hyphal forms. The mass had been resected but the infecting fungal species was not identified. On physical examination on Day 0 a 10 mm diameter, discrete, focally extensive, subcutaneous, non-pigmented nodule was identified on the distal P5 of the RFL, which was non-painful on palpation (Fig. 1 a). A second, focally extensive, purplish subcutaneous 5 mm nodule was identified proximal to the nailbed on the distal P3 of the RHL (Fig. 1 b). Physical examination was otherwise unremarkable, and all vital signs were normal.
Fig. 1.
Digital subcutaneous nodules on (a) the distal 5th phalanx (P5) of the right forelimb and (b) the distal 3rd phalanx (P3) of the right hindlimb.
Results of a complete blood count, and total thyroxine measurement were within reference intervals, and serology for Feline immunodeficiency virus (FIV) antibody and Feline leukaemia virus (FeLV) antigen (IDEXX-SNAP, Pty Ltd) was negative. Abnormalities on a serum biochemical profile included a mild azotaemia (11.2 mmol/L; reference range 3.0–10.0mmol/L), mild elevation in creatine kinase (454U/L; reference range <261U/L), and a mild hypertriglyceridaemia (0.8mmol/L; reference range 0.1–0.6mmol/L). Fine-needle aspiration cytology of the lesions showed the presence of neutrophils and numerous macrophages, some containing fungal hyphae and yeast forms. Punch biopsies of the lesions were collected under general anaesthesia on Day 14 for fungal culture and histopathology. Pending results, the cat was commenced on treatment with 50 mg of itraconazole and 62.5 mg of terbinafine orally, once daily. The owner discontinued the terbinafine after four days administration, and then discontinued itraconazole two days later, due to episodes of vomiting. Posaconazole therapy was recommended and declined. Three months after presentation (Day 101) the owner elected to euthanase the cat because the RHL lesion became ulcerated and bled, and the cat was lethargic. A post-mortem examination was not performed.
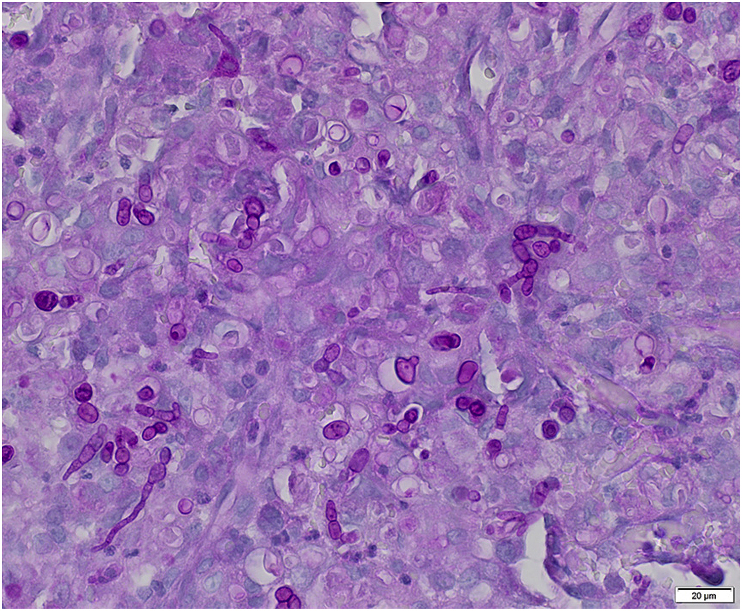
Histopathology sections of the biopsied digital masses, stained with hematoxylin and eosin, revealed diffuse, severe, pyogranulomatous dermal inflammation with intralesional pigmented yeast and hyphal forms. The dermis and deep dermis were expanded by multiple coalescing nodules of inflammation, composed of numerous viable and degenerate neutrophils, epithelioid macrophages, scattered multinucleated giant cells, and rare lymphocytes and plasma cells admixed with moderate numbers of reactive fibroblasts, eosinophilic cellular and necrotic debris and fibrin. Numerous pigmented yeast and hyphal forms were scattered throughout the nodules, including within the cytoplasm of macrophages and giant cells. Fungal elements were markedly positive for Periodic-Acid Schiff (PAS) stain. Yeasts were ovoid, 7–20 μm in diameter, with 2–3 μm-thick golden cell walls, clear to pale cytoplasm with a central basophilic nucleus. Hyphae were 5–10 μm wide, septate with irregular, branched or unbranched with non-parallel pigmented walls (Fig. 2).
Fig. 2.
Ovoid yeast forms and non-parallel hyphae are markedly PAS-positive. Large amounts of pyogranulomatous inflammatory infiltrate efface the dermis. Periodic-Acid Schiff (PAS). Scale bar 20 μ
Black yeast-like colonies were isolated from both digital lesions on malt-extract agar (MEA) at 37 °C. Subcultures of the case isolate were grown on MEA and potato–carrot agar (PCA) at 25 and 37 °C in the dark. The isolate was deposited into the Culture Collection of Fungi (CCF) at the Department of Botany, Charles University, Czech Republic under the number CCF 5820.
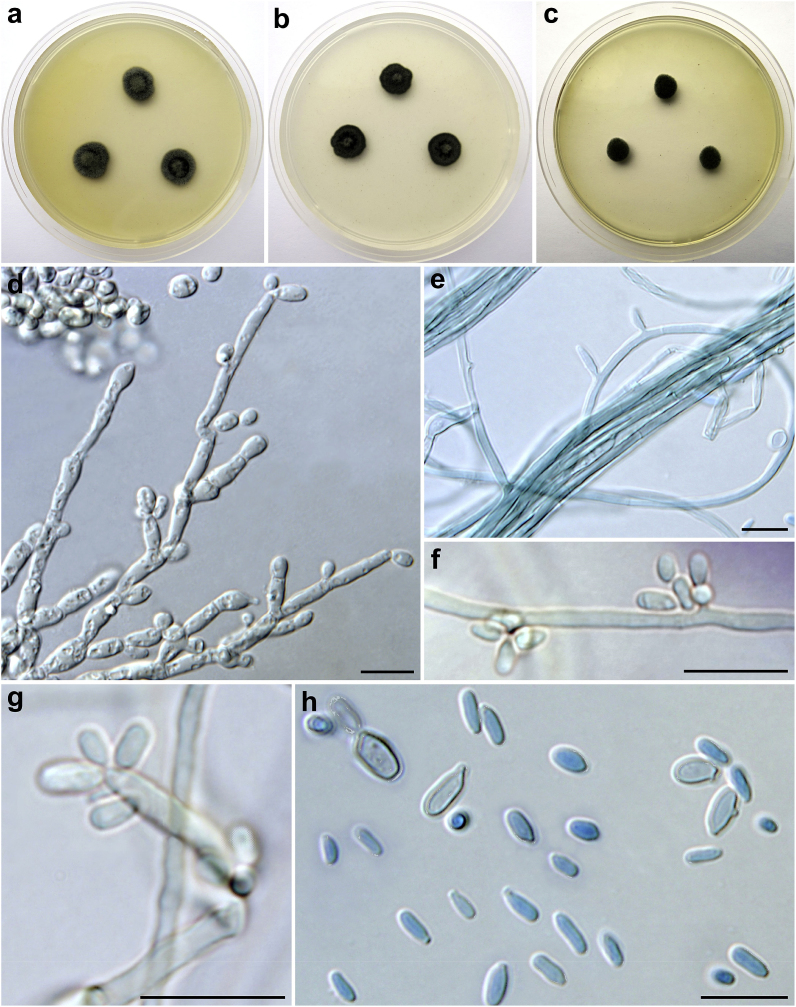
Colonies on MEA at 25 °C (Fig. 3a) attained 10–13 mm diameter after 7 days (20–23 mm after 14 days); lanose; raised; olive (3F5) to olive grey (3F2); reverse dark greyish brown (5F3); at 37 °C 3–5 mm diameter after 7 days (Fig. 3c) (8–10 mm after 14 days). Colonies on PCA at 25 °C (Fig. 2b) attained 10–12 mm diameter after 7 days (17–19 mm after 14 days); velvety to lanose; umbonate; dull green (30E3) to dark green (30F5); reverse dark green (27F3); at 37 °C 12–15 mm diameter after 7 days (17–20 mm after 14 days).
Fig. 3.
Macromorphology and micromorphology of Exophiala spinifera CCF 5820. Colonies incubated 7 days at 25 °C on MEA (a) and PCA (b); colonies on MEA at 37 °C after 7 days (c); poorly differentiated semi-micronematous or micronematous conidiophores producing conidia at apical or lateral conidiogenous loci (d-g); free conidia (h), a budding yeast-like cell is visible in the left corner, two cells with lateral bud scar are present in the right corner of the subfigure. Scale bars: 10 μm. For micromorphology lactic acid with cotton blue was used as a mounting medium. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Microscopic examination revealed the presence of masses of one-celled, smooth-walled, hyaline to mid-brown, ovate or ellipsoidal conidia, mostly 3–5(‒5.5) × 1–2 μm (4 ± 0.6 × 1.5 ± 0.2; mean ± standard deviation), sometimes blastically budding at terminal or lateral positions (Fig. 2h). Conidiophores were usually poorly differentiated from vegetative hyphae (Fig. 3d–g), micronematous to semi-micronematous; conidia arising alongside the hyphae at the intercalary conidiogenous loci or at the apices of poorly differentiated conidiophores.
2.1. Molecular studies
Genomic DNA was extracted from 7-day-old colonies grown on MEA (Oxoid Ltd., Basingstoke, UK) using the ArchivePure DNA yeast and Gram2 + kit (5PRIME Inc., Gaithersburg, MD, U.S.A.) according to the updated manufacturer's instructions [16]. The ITS rDNA region was amplified and sequenced as described previously [16].
Using the BLAST similarity search, the ITS region of the case isolate shared 98.7% identical base pairs (533/540 bp; seven substitutions, one insertion) with the ex-type strain of E. spinifera CBS 899.68 (AY156976); the next most similar species was E. exophialae (ex-type CBS 668.76; AY156973) with 96.3% similarity (522/542 bp; 14 substitutions, six indels); other Exophiala spp. showed similarity lower than 95%. The obtained sequence was deposited into the ENA (European Nucleotide Archive) database under the accession number LR594658.
3. Discussion
Our report is the first to describe more than one cutaneous/subcutaneous lesion in a cat caused by E. spinifera or by any other Exophiala spp. However, in humans with E. spinifera infections multiple cutaneous lesions are common, comprising primarily of a few lesions with regional spread or of few to multiple disseminated lesions [17]. Also, the majority of haematogenously disseminated dematiaceous yeast infections in humans are caused by Exophiala species, which are able to produce yeast-like cells. The ability of these opportunistic pathogens to bud facilitates haematogenous dissemination, whereas with hyphal growth alone, localized infection is more likely [18]. Immunosuppressive therapy is a predisposing factor for the development of multiple skin lesions in humans infected with E. spinifera and CARD9 gene mutations are also suspected, which are associated with defective innate immune recognition of fungi, especially Candida and dermatophytes [17]. The cat in this report did not have any known co-morbidities and tested negative for FIV and FeLV, two common viral causes of immunosuppression in cats.
Including the case we have described here, three of the four cases of feline phaeohyphomycosis caused by E. spinifera were identified in Australia, and the other was from a cat in France [6,7]. Of approximately 40 cases of E. spinifera infection diagnosed in humans, most were also from tropical or subtropical regions and only one case of infection was diagnosed in Europe [17]. In general, members of the genus Exophiala are found commonly in soil, plant materials, extreme environments, hydrocarbon-polluted environments and indoor environments [19]. Species from the E. spinifera complex have a cosmopolitan distribution and are amongst the most aggressive pathogenic species of the genus [15].
Inoculation of one of the limb digits via trauma was the most likely route of infection of the cat described here, given the ubiquitous nature of Exophiala spp, with subsequent haematogenous dissemination. Since a post-mortem examination was not performed, we could not ascertain whether dissemination was more widespread.
The history of a previously excised digital fungal mass involving a different digit of the RFL in the case presented here may have also involved the same organism, although the identity of the isolate was not confirmed. Regional relapse of E. spinifera after excision has been reported in human infections [17].
Since microscopic morphology alone is insufficient to allow routine laboratory identification of black pigmented yeasts, molecular diagnostic techniques are necessary to confirm identification of the infecting agent. Reliable identification to a species level may also be important in understanding progression of the disease and response to treatment [15]. Although molecular characterisation of fungal pathogens is becoming more routine in veterinary medicine, few of the infecting isolates in previous reports of feline phaeohyphomycosis have been definitively identified using molecular techniques. The previous three cases due to E. spinifera were diagnosed by fungal culture and morphology alone [6,7]. One of the isolates was subsequently subjected to DNA sequencing of the ITS rDNA region (GenBank accession AF549450), which, similar to our case, confirmed the species identification [15].
The recommended treatment for localized feline cutaneous phaeohyphomycoses is surgical excision with wide margins, accompanied by systemic therapy with itraconazole or posaconazole pending antifungal susceptibility testing results [1]. The latter is important, since antifungal susceptibility profiles of dematiaceous fungi are variable. For example, high MICs of amphotericin B (>2 mg) have been found among Exophiala species [20]. Itraconazole therapy, alone or in combination with surgical excision, cryotherapy, terbinafine or minocycline was associated with partial or complete response in 16 of 20 cases of E. spinifera infections in humans [17]. Of the three previous E. spinifera infections in cats, outcome was known in one case of mucocutaneous nasal infection, in which surgical resection and therapy with ketoconazole and fluconazole was unsuccessful [7].
Gastrointestinal adverse effects to terbinafine and itraconazole are relatively common in cats. In this case vomiting continued despite discontinuation of terbinafine and may have been a gastrointestinal or hepatotoxic effect of the itraconazole. Itraconazole adverse reactions can be managed in some cases by dose reduction or by changing therapy to posaconazole [1].
Declaration of competing interest
There are none.
Acknowledgements
Contribution of Vit Hubka was supported by the project BIOCEV (CZ.1.05/1.1.00/02.0109) provided by the Ministry of Education, Youth and Sports of CR and ERDF and by the Charles University Research Centre program No. 204069. The authors thank Dr. Adriarne Heton and other staff from East Port Veterinary Hospital, Port Macquarie, NSW who were involved in this case.
References
- 1.Lloret A., Hartmann K., Pennisi M.G., Ferrer L., Addie D., Belák S. Rare opportunistic mycoses in cats: phaeohyphomycosis and hyalohyphomycosis: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013;15(7):628–630. doi: 10.1177/1098612X13489227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caviedes M.P., Torre A.C., Eliceche M.L., Valdivia Monteros D.C., Volonteri V.I., Galimberti R.L. Cutaneous phaeohyphomycosis. Int. J. Dermatol. 2017;56(4):415–420. doi: 10.1111/ijd.13590. [DOI] [PubMed] [Google Scholar]
- 3.Dye C., Johnson E.M., Gruffydd-Jones T.J. Alternaria species infection in nine domestic cats. J. Feline Med. Surg. 2009;11(4):332–336. doi: 10.1016/j.jfms.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhardt A., von Bomhard W., Antweiler E., Tintelnot K. Molecular identification of fungal pathogens in nodular skin lesions of cats. Med. Mycol. 2015;53(2):132–144. doi: 10.1093/mmy/myu082. [DOI] [PubMed] [Google Scholar]
- 5.Reppas G., Gottleib T., Krockenberger M.B., Halliday C., Malik R. Microsphaeropsis arundinis: an emerging cause of phaeohyphomycosis in cats and people. Microbiol. Aust. 2015;36:74–78. [Google Scholar]
- 6.Kettlewell P., McGinnis M., Wilkinson G. Phaeohyphomycosis caused by Exophiala spinifera in two cats. J. Med. Vet. Mycol. 1989;27(4):257–264. [PubMed] [Google Scholar]
- 7.Chermette R., Ferreiro L., De Bievre C., Camadro J., Mialot M., Vauzelle P. Exophiala spinifera nasal infection in a cat and a literature review of feline phaeohyphomycoses. J. Mycol. Med. 1997;7(3):149–158. [Google Scholar]
- 8.Meason-Smith C., Diesel A., Patterson A.P., Older C.E., Johnson T.J., Mansell J.M. Characterization of the cutaneous mycobiota in healthy and allergic cats using next generation sequencing. Vet. Dermatol. 2017;28(1) doi: 10.1111/vde.12373. 71-e17. [DOI] [PubMed] [Google Scholar]
- 9.Overy D.P., Martin C., Muckle A., Lund L., Wood J., Hanna P. Cutaneous phaeohyphomycosis caused by Exophiala attenuata in a domestic cat. Mycopathologia. 2015;180(3-4):281–287. doi: 10.1007/s11046-015-9909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda H., Shibuya H., Yamaguchi Y., Miyoshi T., Irie M., Sato T. Feline digital phaeohyphomycosis due to Exophiala jeanselmei. J. Vet. Med. Sci. 2008;70(12):1395–1397. doi: 10.1292/jvms.70.1395. [DOI] [PubMed] [Google Scholar]
- 11.Helms S.R., McLeod C.G. Systemic Exophiala jeanselmei infection in a cat. J. Am. Vet. Med. Assoc. 2000;217(12):1858–1861. doi: 10.2460/javma.2000.217.1858. [DOI] [PubMed] [Google Scholar]
- 12.Pukay B., Dion W. Feline phaeohyphomycosis: treatment with ketaconazole and 5-fluorocytosine. Can. Vet. J. 1984;25(3):130. [PMC free article] [PubMed] [Google Scholar]
- 13.Bostock D., Coloe P., Castellani A. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat. J. Comp. Pathol. 1982;92(3):479–482. doi: 10.1016/0021-9975(82)90035-4. [DOI] [PubMed] [Google Scholar]
- 14.Nuttall W., Woodgyer A., Butler S. Phaeohyphomycosis caused by Exophiala jeanselmei in a domestic cat. N. Z. Vet. J. 1990;38:123. doi: 10.1080/00480169.1990.35635. [DOI] [PubMed] [Google Scholar]
- 15.Vitale R., Hoog G.D. Molecular diversity, new species and antifungal susceptibilities in the Exophiala spinifera clade. Med. Mycol. 2002;40(6):545–556. doi: 10.1080/mmy.40.6.545.556. [DOI] [PubMed] [Google Scholar]
- 16.Hubka V., Nováková A., Peterson S.W., Frisvad J.C., Sklenář F., Matsuzawa T. A reappraisal of Aspergillus section Nidulantes with descriptions of two new sterigmatocystin producing species. Plant Syst. Evol. 2016;302:1267–1299. [Google Scholar]
- 17.Bohelay G., Robert S., Bouges-Michel C., Gerin M., Levy A., Fain O. Subcutaneous phaeohyphomycosis caused by Exophiala spinifera in a European patient with lymphoma: a rare occurrence case report and literature review. Mycoses. 2016;59(11):691–696. doi: 10.1111/myc.12515. [DOI] [PubMed] [Google Scholar]
- 18.Seyedmousavi S., Netea M.G., Mouton J.W., Melchers W.J., Verweij P.E., de Hoog G.S. Black yeasts and their filamentous relatives: principles of pathogenesis and host defense. Clin. Microbiol. Rev. 2014;27(3):527–542. doi: 10.1128/CMR.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicente V., Attili-Angelis D., Pie M., Queiroz-Telles F., Cruz L., Najafzadeh M. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 2008;61:137–144. doi: 10.3114/sim.2008.61.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L., Wan Z., Li R., Yu J. In vitro activities of nine antifungal agents against rare pathogenic fungi. J. Med. Microbiol. 2019. Sep 25 doi: 10.1099/jmm.0.001083. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]