Abstract
Background
Chronic Cerebral Hypoperfusion (CCH) is a common, crucial and tough problem for old people. It easily leads to Lacunar Infarction and even Vascular Dementia (VD). Western medicine has the advantage to relieve some VD symptoms but fails to cure it. Some classic Chinese medicines have good efficacies to treat and delay the cerebral functional decline resulted from CCH. Among them Modified Dioscorea Pills (MDP) has been proven to have a convincing effect in curing VD. So far the knowledge about neuroplasticity in CCH is little known and the underlying interfered mechanism by MDP on neuroplasticity has not yet been explored. This study explores the changes of neuroplasticity involving neurogenesis, angiogenesis and synaptogenesis in CCH and interfered by MDP.
Methods
40 male SD rats were divided into the Sham operated Group, the Model Group and the MDP Group according to a Random Number Table. Bilateral Common Carotid Arteries Occlusion (BCCAO) was adopted to prepare CCH models. MDP condense decoction had been administered by gavage to rats in the MDP Group (10g·Kg-1·d-1) for 45 days; Rats in the other two groups were accepted normal salts as substitution with same dosage and course. Through Morris Water Maze (MWM) test, pathological observation of hippocampus, ultrastructural study on synapse, Real Time Polymerase Chain Reaction (RT-PCR) and immunohistochemistry detection, the capacities of intelligence of rats, the morphological character of hippocampus CA1 zone and the synapse associated protein and gene such as Growth Associated Protein (GAP-43) mRNA, Vascular Endothelial Growth Factor (VEGF) mRNA, Microtubule-associated Protein (MAP)-2, Synaptophysin (SYP), Postsynaptic Density protein (PSD)-95 and Micro Vessel Density (MVD) were determined. Through one-way ANOVA the data was analyzed and when P<0.05 the result was considered significant.
Results
Compared to the Model Group, rats in the MDP Group achieved much better behavioral performance (P<0.05); more neurons and more synapses regenerated; the expression of SYP, PSD-95and MAP-2 up-regulated (P<0.05); The expressions of GAP-43 mRNA and VEGF mRNA in the Model Group were higher than those in the Sham operated Group (P<0.05), but they reached the highest in the MDP Group (P<0.05); The count of MVD in the Sham operated Group is the lowest, it is higher in the MDP Group and it reaches highest in the Model Group (P<0.05).
Conclusions
Some key genes promoting neuroplasticity such as GAP-43 mRNA and VEGF mRNA remarkably up-regulated in CCH, they only boost angiogenesis but fail to facilitate neurogenesis and synaptogenesis in CCH. However, accompanied by furtherly up-regulation of these two key genes, MDP obviously improves neurogenesis, synaptogenesis and temperate angiogenesis in CCH which may be underlying its good efficacy.
Keywords: Neuroscience, Pathology, Nervous system, Alternative medicine, Internal medicine, Health promotion, Chronic cerebral hypoperfusion, Modified Dioscorea pill, Neurogenesis, Angiogenesis, Synaptogenesis
Neuroscience; Pathology; Nervous System; Alternative Medicine; Internal Medicine; Health Promotion; Chronic Cerebral Hypoperfusion; Modified Dioscorea Pill; Neurogenesis; Angiogenesis; synaptogenesis.
1. Introduction
Chronic Cerebral Hypo-perfusion (CCH) can cause a variety of neurological deficits, it usually leads to Lacunar Infarctions which damage the normal neuronal links [1], in the hippocampus it causes memorial impairment [2]. Many patients suffer from CCH in their long lives, it severely reduces their life quality but they have no way to change that. What pathophysiological changes occurs and how to combat CCH? To answer this tough question, we should firstly know that there are two key pathological changes that took place during CCH: neuroplasticity and neuro-remodeling [3]. According to the theory of neurovascular unit (NVU) [4], the structural and functional unit of brain tissue, neuroplasticity facilitates neuronal compensatory innervation which pathological changes includes neurogenesis, synaptogenesis and angiogenesis as basic conditions for neurological recovery. While neuro-remodeling causes disordered or interrupted nerve connections and leads to neuropsychiatric disorders which pathological changes involves in strong inflammatory response, loss of neurons and gliosis. It is crucial for us to improve neuroplasticity during and after CCH. Medication ameliorating intelligence should utmost boost neurogenesis, angiogenesis and synaptogenesis and inhibit inflammatory response, loss of neurons and gliosis.
Pharmacological compounds can assist recovery from a stroke and other CNS diseases [5], but few have extraordinary positive curative effects. Traditional Chinese medicine has the characteristics of multi-targets intervention and overall regulation. Chinese doctors from different dynasties have unique insights to cerebral vascular disease and accumulated rich experience to cure them. Modified Dioscorea pills (MDP) has a definitive clinical efficacy for vascular cognitive impairment of no dementia (VCIND), post-stroke aphasia and Alzheimer's disease [6, 7]. In our previous studies, we found that MDP can promote the expressions of Brain-derived Neurotrophic Factor (BDNF)mRNA, Nerve Growth Factor (NGF), neutrotrophin-3 (NT-3) in the hippocampus of rats subjected to BCCAO [8,9], which improved VD rats' cognitive function. Neurotrophic factors play very important roles in survival, growth, and function of neurons [10], which partially explained the MDP treating mechanism and suggested that MDP probably promoted neuroplasticity in CCH. In order to explore MDP intervention mechanism on neuroplasticity, this research conducts a series of experiments to measure the key genes and proteins expression level related to neurogenesis, angiogenesis and synaptogenesis and to observe the pathology of the hippocampus and the ultrastructural pathology on synapse in rats subjected to CCH and interfered by MDP.
2. Methods
2.1. Animals
40 male Sprague-Dawley rats, which are specific-pathogen free and purchased from Hubei Provincial Academy of Preventive Medicine [Wuhan, China. License number: SCXK(鄂)2015–0018], weighting 280 ± 20 g, were used in all experiments. Rats were housed at ambient temperature of 23 °C ± 1 °C, relative humidity of 50% ± 10% and 12 h light/dark cycle with free access to food and water. Rats were adaptively accommodated in the Institute of Acupuncture and Moxibustion, Hubei University of Traditional Chinese Medicine (Wuhan, China) for a week before operation. All procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People's Republic of China [11]. The study was approved by the Committee on Ethics of Life Sciences of Hubei University of Traditional Chinese Medicine (NO: 20170112, Wuhan, China).
2.2. Preparation of MDP concentrated decoction
MDP Concentrated decoction was prepared by the pharmacy of Hubei Provincial Traditional Chinese Medicine Hospital. The prescription is composed of 14 kinds of Chinese herbs. 250ml concentrated decoction was extracted from such herbs as follows: Dioscorea,30g; à prepared Rehmannia Root,24g; prepared Polygonum Multiflorum,24g; Codonopsis Pilosula,20g; radices Paeoniae Alba,20g; Codonopsis Pilosula,20g; Polygala Root, 12g; Angelica Sinensis, 20g; Acorus Gramineus Soland,10g; Atractylodes Macrocephala,18g; Poria Cocos,18g; the bark of Eucommia,12g; Lycium Chinensis, 18g and Schisandra Chinensis:,10g. All these herbs were added to 400ml water in a Casserole. Through boiling, concentration, filtration and refining, the herbal extract was condensed to 250 ml (about 1ml decoction contains herbal extract from 1g of these proportionate mixed herbs, 256g/250ml), the average of yield rate was about 38.1%. The bottles of MDP Concentrated decoction were preserved in refrigerator at 4°Cfor use. All the bottles of MDP Concentrated decoction used in this experiment are the same batch and the lot number is Z20150027.
2.3. Preparation of CCH model and intervention measures
40 rats were randomly classified into three groups: Sham operated Group (13 rats), MDP Group (14 rats) and Model Group (13 rats) according to a Random Number Table. BCCAO can definitely prepare CCH model rats whose cognitive impairment were testified by MWM test [12, 13]. The CCH models (including Model Group and MDP Group) were made by a modified BCCAO method which was carried out in two steps: Rats' left and right common carotid artery were ligated separately one week apart. The concrete steps are as follows: After one week from the purchase date, rats were anesthetized with 4% isoflurane inhalation and maintained with 1–2% isoflurane inhalation during the ligation process. A right incision was made in the rat's neck, and the right common carotid artery (CCA) was isolated and double ligated with 1# silk sutures prior to be clipped between two ligated point to be sure the occlusion of blood. After operation rats' skin were sutured and disinfected with penicillin (8000 units per rat), then they were returned to their cages for recovery. After a week of first operation, the left CCA underwent the same process with the right one. The rats in the Sham operated Group underwent the same procedures as the CCH models except for the ligation and clip of the CCA. During surgery, rats' rectal temperature was maintained at 37.0–37.9 °C with a heating pad to prevent brain protection at low temperature. After about 2–4 min, all animals returned to consciousness with free access to food and water. During the second operation, two rats died from hemorrhagic shock. After a week of BCCAO, rats were treated by gastro-gavage. In the Sham operated Group and Model Group, rats were administered by gavage with normal saline by 1ml per 100g weight. Whereas rats in MDP Group were accepted MDP concentrated decoction with the same dosage and course. 25 days after the second operation 1 rats died from pneumonia and 1 rat died from asphyxia for mis-operation in gavage. In the end, there were 36 survived rats distributed in Sham operated Group (13 rats), Model Group (12 rats) and MDP Group (11 rats) respectively.
2.4. Morris Water Maze (MWM) test
The spatial learning and memory capacities of rats was measured by MWM test [14] which began on the 40th day after gavage. The equipment is supplied by Chengdu Taimeng Science and Technology Co., Ltd. in China. The MWM devices consisted of a white painted circular pool (120 cm in diameter and 50 cm in depth) in which the rats were trained to escape from the water by swimming to a hidden platform (11 cm in diameter, 1.5 cm beneath the surface). The location of platform was only hinted at by the curtain surrounding the pool. Rats' behaviors were monitored and recorded by a video camera which was suspended over the pool and connected to a computer. The water was maintained at 22 °C or so and made opaque with milk (about 500g) throughout the training and testing period. The pool was virtually divided into four quadrants by the computer: quadrant I (Platform located), quadrant II (Adjacent to quadrant I), quadrant III (Opposite to quadrant I) and quadrant IV (Between quadrant I and quadrant III). The computer had a computerized tracking system (Chengdu Taimeng Science and Technology Co., Ltd. Chengdu, China) to deal with rats’ swimming traces and compute the values such as escaping latency, crossing platform frequencies and so on.
MWM test was conducted in successive five days, the first 4 days for navigation test and the last day for probing test. In the navigation test, rats were placed into one of the four quadrants facing the wall. Although the starting point was randomly selected, the protocol was fixed at the beginning of each trial and was maintained throughout all trials. Each rat was allowed 120 s to locate and mount the platform; 30 s after the rat mounted the platform, it was rescued and moved to a drying cage and warmed with a heat lamp. The rats that failed to locate the platform within 120 s were gently guided to the platform and required to remain there for 60 s to remember the site by surrounding curtains prior to be transferred to the drying cage. The time spending in locating the platform (escaping latency) were recorded and calculated by computer. On the fifth day, probing tests were performed in which the platform was removed. Rats were placed into water in quadrants III and were allowed to swim freely for 120 s. Frequencies of crossing the platform, swimming time and distance in the target quadrant were recorded. During the MWM test period, the gavage was succeeded as usual.
2.5. Sampling
The day after MWM test, rats were capitalized and brain tissues were obtained through perfusion sampling and fresh sampling. The former is described as follows: 5 rats in each group were deeply anesthetized with2–4% isoflurane inhalation, their hearts were exposed, perfusion needles were inserted from apex into left ventricle and then into the aorta, cut the right atrial appendage fast, 250ml normal saline was perfused through perfusion needles. While clear perfusate flowed out, the rats’ tails straightened, trembling limbs stopped and the livers gradually turned white, 250 ml 4% paraformaldehyde was perfused successively. About 40 min later, rats were decapitated and brain tissues were harvested and fixed in 4% paraformaldehyde buffer solution (PBS) for 12–24 h, which were then dealt with conventional dehydration, paraffin embedding for haematoxylin and eosin (HE) staining and immunohistochemical staining. While fresh sampling is for Transmission Electronic Microscopy (TEM) examination and RT-PCR detection which involves 6 rats in each group. The steps are as follows: Rats were deeply anesthetized with isoflurane inhalation and then decapitated, brain tissues were quickly stripped for TEM and RT-PCR detection.
2.6. Histopathological analysis
Taking the perfusion brain tissue of three rats in each group, the hippocampus forms were exposed, isolated and embedded in paraffin. Coronal sections were cut into approximately 4μm thickness and stained with hematoxylin and eosin for subsequent microscopic observation. Hippocampus histopathological changes were observed under an optical microscope (Olympus biologic microscope, Type BX53). The hippocampal tissue structure, cells arrangement, the damage and proliferation of the white matter were observed at 200 times magnification. The internal structure of the pyramid cells and their nucleus were observed at 400 times magnification.
2.7. Analysis of immunohistochemistry and micro vessel density (MVD)
Three rats’ hippocampus of each group were used for the detection of immunohistochemistry and micro vessel density (MVD). Paraffin sections preparation was the same as histopathological analysis. After the procedures of antigen repairing, blocking endogenous peroxidase, the first antibodies (respectively mice anti-MAP-2,anti-SYP, anti-PSD-95 and anti-CD43)were added to hippocampus paraffin sections, the IgG polymers of horseradish peroxidase labelled second antibody and freshly prepared DAB colour liquid were successively added to these sections, lastly the sections were redyed with Harris hematoxylin; For the analysis of MAP-2, SYP PSD-95, three sections were taken for immunohistochemical analysis for each item in each group, that is, one slice was taken every two slices, and three 400-fold images were extracted from each slice for analysis. The related images were scanned and analyzed with IPP6.0 software, the average optical densities of target proteins were compared among three groups.
For the analysis of MVD, the CD43 labelled images were collected. According to the corrected Weidner method [15], any single endothelial cell or cell clusters stained by anti-CD43, as long as they have clear boundary with the surrounding micro vessels or other connecting tissue, regardless of whether the lumen been formed, can be counted as a micro vessel, excluding the vessels with smooth muscle walls or lumen diameters greater than 8 times erythrocyte’. Each sample is first observed in 100 times magnification to selected 3 spots with most micro vessels as “hot spot”, then the micro vessels were counted from the hot spots with high magnification (400 times magnification) and the average numbers of micro vessels were taken as MVD.
2.8. Transmission electron microscope (TEM) examination
Take 3 6 blocks of the left hippocampus tissue of CA1 zone from two freshly sampled specimens of each group which size was about 1 mm3. By fixation, rinsing, dehydration, infiltration, embedding and other steps to make ultra-thin sections which thickness was about 60–80nm. Then they were double stained with uranium and lead prior to be dried over a night. Select some representative sections to observe by TEM (focusing on the synapse structure at 30000 magnified times).
2.9. Quantitative RT-PCR
Six rats’ hippocampal specimens of freshly sampling from each group were used for the target RNA detection. RNA extraction accorded to the instruction of Reagant Trizol kit. In a gene bank database standard cDNA sequences of related gene were obtained, then in the open reading frame (ORF) conserved regions, primer5 design software, DNA Star primer analysis software and networking blast analysis software were used according to the principle of primer design to design and synthesis primer (supplied by Qingke Co. Ltd.). Select Ratβ-actin as inner reference to compute the expression of Rat GAP-43 mRNA and VEGF mRNA. The sequences are as follows (Table 1):
Table 1.
Primer sequence.
| Name | Primer | Seqμence | Size |
|---|---|---|---|
| Rat β -actin | Forward | 5‘- CACGATGGAGGGGCCGGACTCATC -3’ | 240bp |
| Reverse | 5‘- TAAAGACCTCTATGCCAACACAGT -3 | ||
| Rat VEGF | Forward | 5‘- CGTCTACCAGCGCAGCTATTG -3’ | 145bp |
| Reverse | 5‘- CTCCAGGGCTTCATCATTGC -3 | ||
| Rat GAP43 | Forward | 5‘- GGCTCTGCTACTACCGATGC -3’ | 185bp |
| Reverse | 5‘- TTGGAGGACGGCGAGTTAT -3 |
Fresh sampling hippocampus tissue of 6 rats was taken. 0.05g hippocampal sample from each rat was collected and homogenized in 1mL Trizo reagent (Aidlab, Lot:252250AX) by bead mill (Retsch Tissue Lyzer II, Qiagen, Valencia CA, USA). RNA was isolated from brain homogenates by addition of 10% BCP and standard phase separation, followed by overnight precipitation with isopropanol at -20 °C. RNA was purified using the Qiagen RNeasy Mini kit (Qiagen), Start PCR amplification reaction in PCR instrument. The reaction conditions are as follows: Predenaturation: 95 °C for 5min.; denaturation: 95 °C for 30sec; annealing: 60 °C for 40sec, extension 72 °C for 1min, altogetherfor30 cycles; extension at 7 °C for 10min, preservation at 4 °C. Electrophoresis analysis: taking the product of 5ul PCR and 6 x DNA loading buffer 2ul to mix, then take the mixture into 2% agarose gel (containing EB)to electrophoresis at 150V voltage for 35min prior to being observed under ultraviolet lamp, after focus adjustment, the images were photographed with gel analysis system. With gray scale scanning software existing in the gel imaging system the electrophoresis target zone was analyzed, the intensity ratios of the target gene compared to the reference gene were acquired.
2.10. Statistical analyses
The indexes involved in this experiment were measurement data which was presented as means ± standard deviation (‾X±SD) Statistics was carried out with SPSS19.0 software. Differences among groups were analyzed by One-way analysis of variance (ANOVA), Posthoc Tukey's test was used for intergroup and intragroup comparison. P value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline data
After operation, the amount of water intake and diet of rats in the CCH group significantly decreased, the spirit and activity degrees were low. A week of the second operation later, the mean weight of rats in the CCH group was significantly less than it in the Sham operated Group (F: 19.535, P: 0.000) (Table 2). Following about two weeks of rehabilitation and feeding, rats in the CCH group recovered fast, eating much more food and drinking much more water than the rats in the Sham operated Group. After 45 days of feeding, on the day of sampling there was no significant difference in body weight amongst the three groups (F: 0.010, P: 0.990) (Table 3). There was no adverse event in MDP Group.
Table 2.
Changes in body weight of rats one week after BCCAO.
| n | F- value | P-value | ||
|---|---|---|---|---|
| CCH Group | 25 | 308.85 ± 26.71 | 19.535 | 0.000 |
| Sham operated Group | 13 | 351.00 ± 19.49 |
Table 3.
Comparison of body weight before sampling.
| Group | n | F- value | P-value | |
|---|---|---|---|---|
| Sham-operated Group | 13 | 398.00 ± 41.70 | 0.010 | 0.990 |
| Model Group | 12 | 400.75 ± 39.15 | ||
| MDP Group | 11 | 399.50 ± 41.46 |
3.2. MWM test
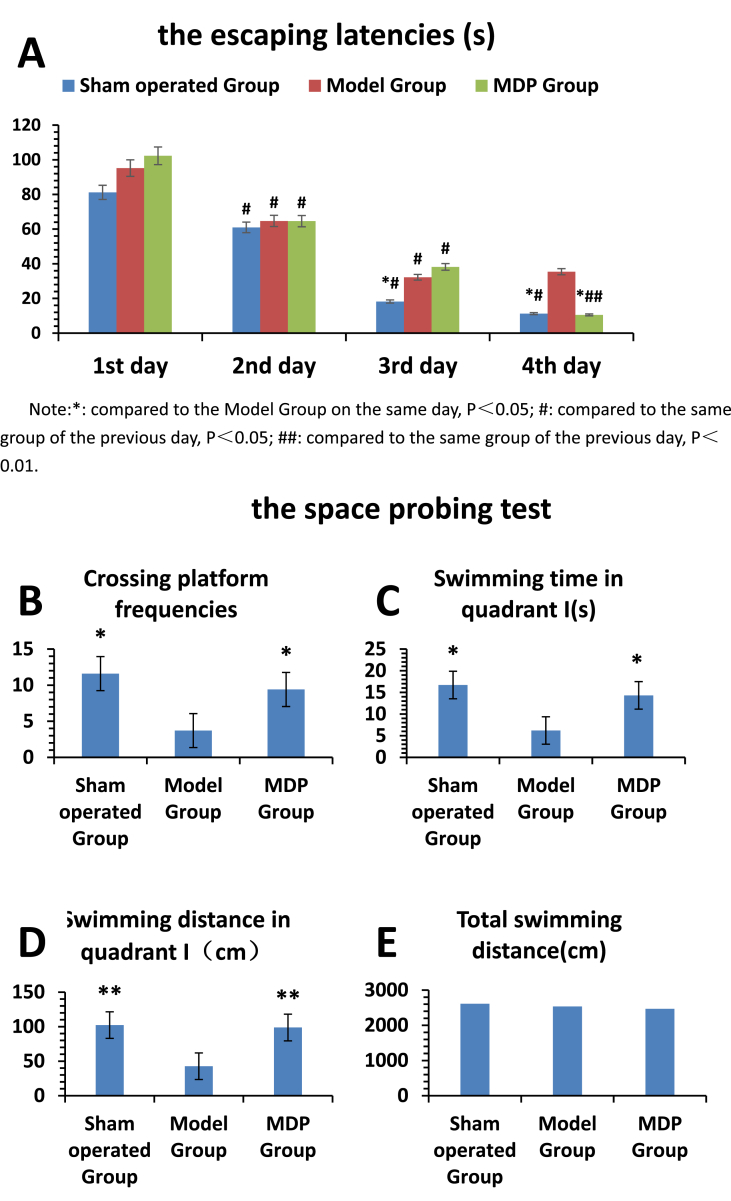
In the navigation test, we adopt vertical and horizontal comparisons to analyze the results. In vertical comparison, in the first three days of experiment, the escaping latency of each group showed a decreasing trend, the results all had significant differences when compared to the previous day in the same group. However, in the fourth day of the experiment, the escaping latencies continued to decline except the Model Group. In horizontal comparison, there was no statistical difference in escaping latency among three groups in the first two days. But from the third day, the escaping latency of the Sham operated Group decreased more significantly compared with it in the Model Group (P < 0.05). On the fourth day, the escaping latency of the MDP Group decreased rapidly, which were close to the Sham operated Group; The escaping latencies of the Sham operated Group and MDP Group were much lower than it in the Model Group (P < 0.05). In the spatial probing test, compared to the rats in the Model Group, rats in the Sham operated Group and the MDP Group crossed over the platform more times (P < 0.05). The time and distance swimming in the platform quadrant in the Sham operated Group and the MDP Group were much more than those in the Model Group (P < 0.05). There was no significant difference among three groups in total swimming distance and swimming speed (Fig. 1).
Fig. 1.
The results of Morris Water Maze. The results were used to evaluate the rats' learning and memorial capacities including navigation test (Part A) and probing test (Part B-E). All the 36 survived rats after operation (including 13 rats in Sham operated Group, 12rats in Model Group and 11 rats in MDP Group) the results were shown in Histogram. were involved in the MWM Test. Part A illustrates the escaping latencies among three groups. The escaping latencies in three groups had declining tendencies except Model Group. On the other hand, we can see that with time elapsing the passage of time, the rats' intelligence in the MDP Group recover fast and in the 4th day of MWM test, it is very close to that in Sham operated Group. Their escaping latencies are much shorter than that in Model Group (P<0.05). Part B-E show the parameters of crossing platform frequencies, swimming time and distance in quadrantⅠ, total swimming time. All those results show the rats in MDP Group and Sham operated Group have much better memorial capacities than that in Model Group P<0.05 or P<0.01). The total swimming distance have no significant difference among three groups.
3.3. Pathology observation
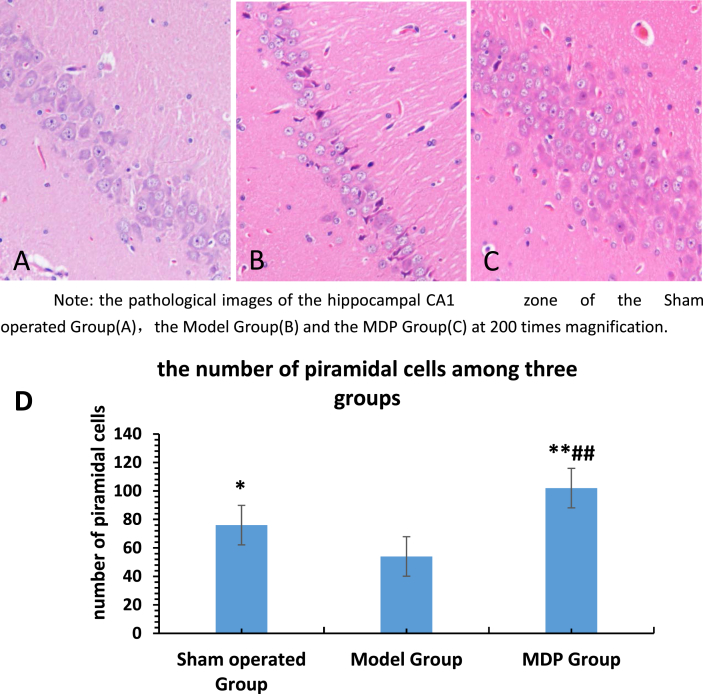
In the Sham operated Group, the pyramidal cells in hippocampal CA1 region remained intact, arranged in 3–5 layers, lined in a close and neat way. Cytoplasm was homogeneously red dyed. The nuclei were large and round. The nucleoli were clear. The radiation layer, molecular layer, lacunar layer were regular and compact.
In the Model Group, the number of pyramidal cells in the hippocampal CA1 region decreased, pyramidal cells were distributed into 1–3 layer in an irregular and sparse way, the cell morphology was incomplete. There were thin cytoplasm with some vacuoles. The boundary between nuclear and cytoplasm was obscure. The nuclei were darkly dyed, pyknosis with a triangular or irregular shape. Some cells’ nucleus disappeared. The nucleoli were vague. The radiation layer, molecular layer and the lacunar layer were loose. Gliosis was obvious and common.
In the MDP Group, the neurons in the CA1 area of the hippocampus arranged in order, the number of pyramidal cells increased obviously which arranged in 5–7 layer. The structure of cells was relatively normal, and the phenomenon of nuclear condensation decreased. The radiation layer, molecular layer and the lacunar layer were dense.
The HE staining images were analyzed with Image J software and the pyramidal cells were calculated, there are 76 ± 14, 54 ± 18 and 102 ± 24 pyramidal neurons respectively in Sham operated Group, Model Group and MDP Group. The results have significant difference (P < 0.05 or P < 0.01).(Fig. 2).
Fig. 2.
The pathological images of the hippocampal CA1 zone of the Sham operated Group(A), the Model Group(B) and the MDP Group(C) at 200 times magnification. Three rats' hippocampus of each group were observed with Optical microscope. The obvious neuronal loss (B) and neuronal proliferation(C) can be seen. Moreover, the white matters are sparse in Model Group (B) and condense in MDP Group(C). Chart D shows that the HE staining images were analyzed with Image J software and the pyramidal cells were calculated, the results have significant difference (P < 0.05 or P < 0.01). there are 76 ± 14, 54 ± 18 and 102 ± 24 pyramidal neurons respectively in Sham operated Group, Model Group and MDP Group.
3.4. Observation of TEM
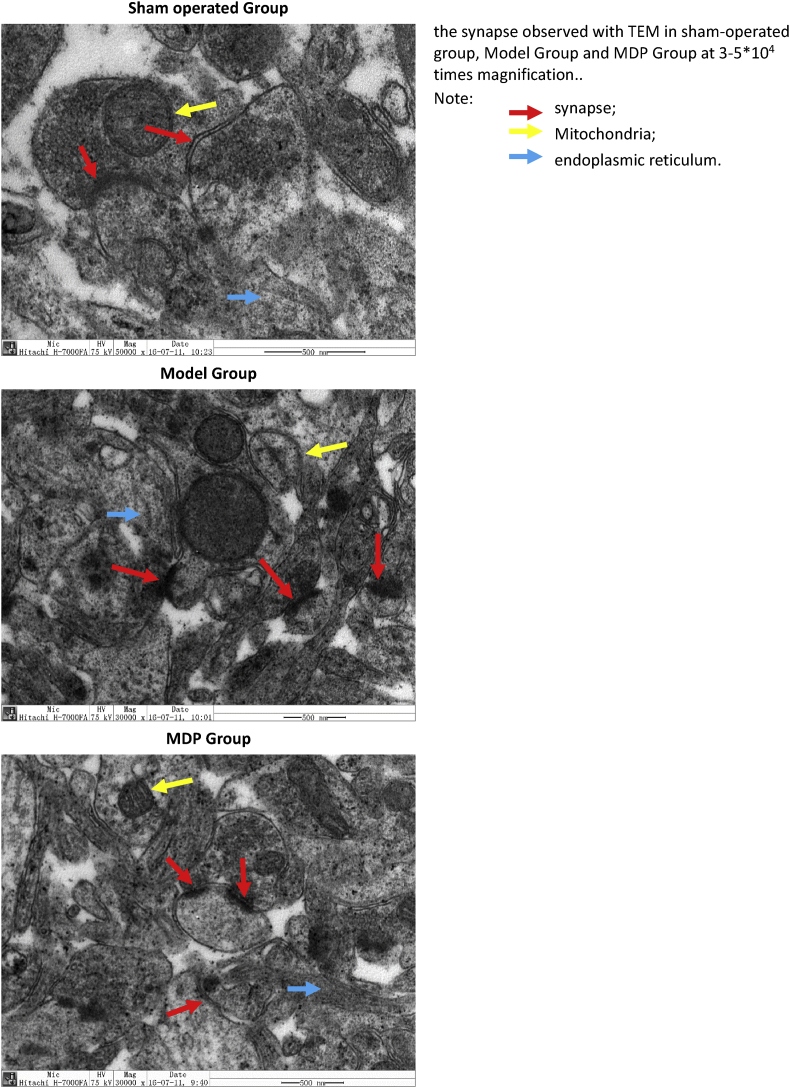
Focus on the ultrastructure of synapse. According to the Gray's Type I ultrastructural analysis standard [16, 17], the observed synapse should be: 1. Synapses must show asymmetry, which is transverse in the plane and is not cut off; 2. The synapse must display a clear postsynaptic density and synaptic gap.
In the Sham operated Group, synaptic cleft was clear, presynaptic and postsynaptic membranes had intact contours. Compared to the Model Group, more synaptic vesicles and more regular curve surfaces were seen; presynaptic and postsynaptic membranes fit to each other very well; the postsynaptic membrane was obviously thickened; there were intact mitochondria and more ribosomes on the endoplasmic reticulum.
In the Model Group, the synaptic cleft was fuzzy, pre and post synaptic membrane were swelling and cavitating, which makes it difficult to distinguish between the presynaptic and postsynaptic membrane; there were fewer synaptic vesicles. Pyknotic mitochondria and Endoplasmic Reticula degranulate were visible.
In the MDP Group, the presynaptic and postsynaptic membrane were structurally complete. The synaptic cleft was clearly visible. There were abundant synaptic vesicles and mitochondria and many particles on the endoplasmic reticula (Fig. 3).
Fig. 3.
The synapse observed with TEM in sham operated group, Model Group and MDP Group. Take two hippocampal specimens of freshly sample from each group and three points (about 1mm3)of each specimen were used to make ultrathin sections for TEM observation. We can see that in the Sham operated Group, two flat synapses are intact, a round mitochondria is clearly seen; In the Model Group, we can see three fuzzy fusion synapses and a remaining mitochondrial shell, the nucleus is intact, the surrounding endoplasmic reticulum is blurred with few particles; In the MDP Group. we can see three regular synapse with larger size and a normal mitochondria, the surrounding endoplasmic reticulum is clear with more particles.
3.5. Immunohistochemical analysis
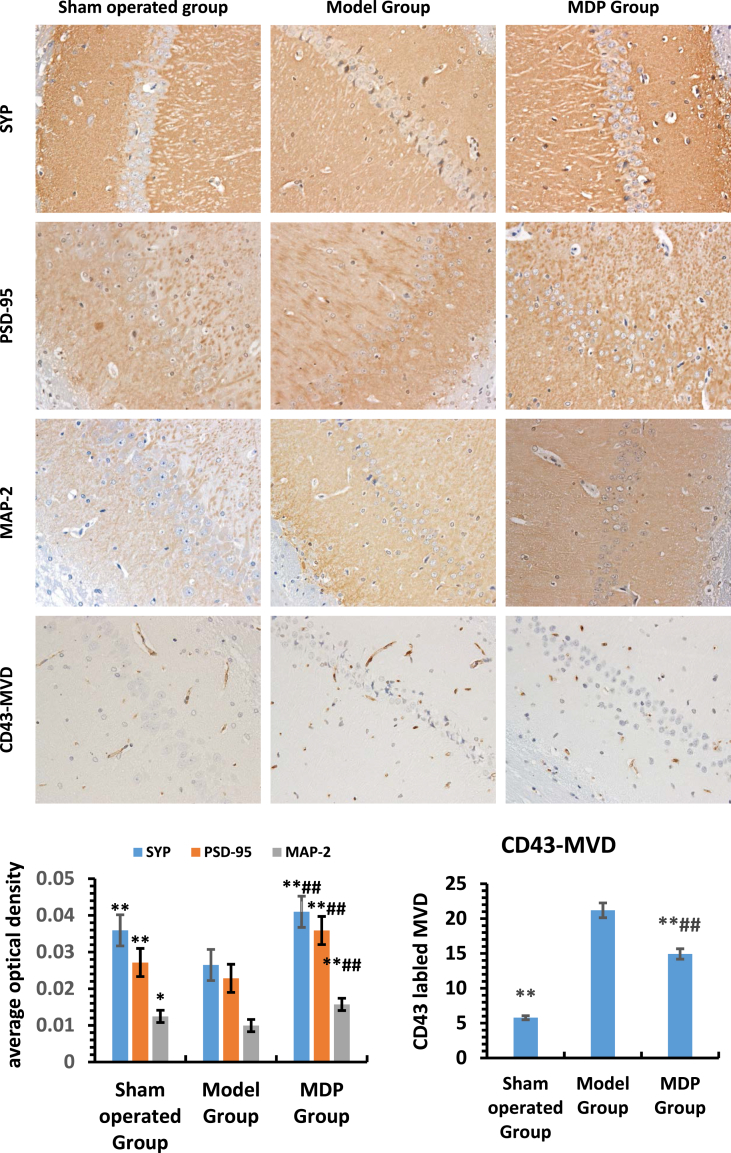
The expression levels of SYP, PSD-95 and MAP-2 were lower in the Model Group than those in the Sham operated Group (P < 0.05), but in the MDP Group, these values all reached highest (P < 0.05). For MVD, there was a contradiction, MVD in the Sham operated group was the least, in the MDP Group it was higher (P < 0.05), it was highest in the Model Group (P < 0.05). (Fig. 4).
Fig. 4.
Expression of SYP, PSD-95, MAP-2 and count of MVD in hippocampus among different groups (400×). Three rats' hippocampal specimens of perfusion sampling from each group were used for the analysis of immunohistochemistry and MVD. The upper images selected from the representative images of Sham operated Group, Model Group and MDP Group and showed the expressions of different synapse related key proteins and CD43-labeled microvessel density. Histogram in bottom left indicates the average optical density of SYP, PSD-95, MAP-2 in different groups; Histogram in bottom right indicates the mean number of MVD in different group. *: compared to the Model Group, P < 0.05; **: compared to the Model Group, P < 0.01; #: compared to the Sham operated Group, P < 0.05; ##: compared to the Sham operated Group, P < 0.01.
3.6. Quantitative RT-PCR
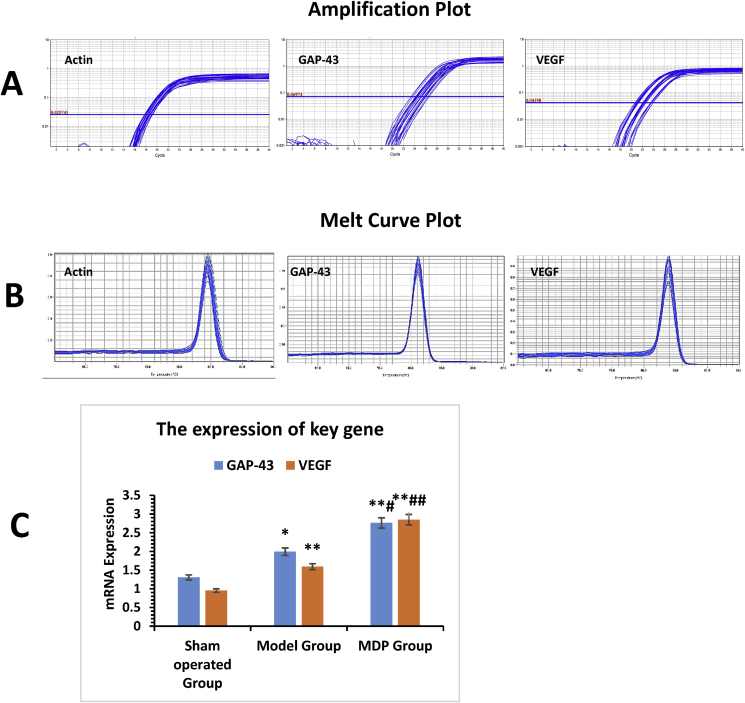
In the Model Group, the expressions of GAP-43mRNA and VEGF mRNA were higher than those in the Sham operated Group (P < 0.05). However, they reached highest in the MDP Group (P < 0.05, P < 0.05). (Fig. 5).
Fig. 5.
Expression of GAP-43mRNA and VEGF-mRNA in different groups. Six rats' hippocampal specimens of freshly sampling from each group were used for the target RNA detection. Part A shows the amplification curves of actin-mRNA, GAP-43- mRNA, VEGF- mRNA; Part B shows the fusion curves of actin-mRNA, GAP-43- mRNA, VEGF- mRNA. The histogram of Part C shows the genes expression of GAP-43 and VEGF. Note: *: compared to Sham operated Group, P < 0.05; **: compared to Sham-operated Group, P < 0.01; #: compared to Model Group, P < 0.05; ##: compared to Model Group, P < 0.01.
4. Discussion
The model of BCCAO is a perfect animal model provided to understand the pathological damage caused by CCH [18]. Diffuse brain injuries occurs after BCCAO in rats, characterized by neurons loss and demyelination of the white matter in hippocampal CA1 zone which lead to impaired cognitive function without movement defects [19], Therefore, the results of behavioral perform in MWM test give us an objective evaluation of intelligence. Similar to our previous researches [8, 9], CCH leads to learning and memorial damage and MDP improves it; But what influence CCH has on neuroplasticity and how MDP interfere it go beyond our knowledge. In this study, we can see that CCH results in losses of neurons, micro vessels and synapses which mean decrease in neuroplasticity; MDP improve neuroplasticity through facilitating neurogenesis, angiogenesis and synaptogenesis in CCH. We can get these answers both from the images of pathology and TEM and from the expressions of key genes and proteins.
In the case of cerebral ischemia, pyramidal neurons in CA1 zone of hippocampus can spontaneously regenerate [20]. GAP-43 is a pleiotropic cytokine, a key axonal and presynaptic component, which serves as a marker for axonal growth, motor neuron regeneration [21, 22] and reconstruction of synapse [23]. It is highly expressed in nerve development and nerve injury [24]. The stimulation of the distal point of mutilation and the sudden lack of existing connecting among neurons can initiate its high expression so as to support the regeneration of axons [25].
In our experiments, compared to the Sham operated Group, the significantly higher expression level of GAP-43mRNA in the Model Group is apparently related to nerve irritability after nerve fibers destroy subject to CCH. MDP can enhance its expression further, suggesting that MDP may boost neurogenesis, axon extension and synaptogenesis. It is confirmed by pathological observation and immunohistochemistry. Observed from HE staining of the hippocampus CA1 zone, neuronal injuries, apoptosis and axons disappearance in the Model Group from CCH were apparent, but these changes were reversed by MDP, which signify that MDP stimulates the expression of GAP-43mRNA so as to initiate neurogenesis, axonal regeneration and sprouting so as to facilitate reconstructing effective neural circuits and restoring the neural information transmitting. MAP-2 express mainly in neuronal cell bodies, dendrites, dendritic spines and postsynaptic dense regions [26]. It is a marker of dendrite and its expression level can reflect neurons density. The lowest expression of MAP-2 in the Model Group and the highest expression in the MDP Group also denote that considerable neurons loss in CCH and MDP can greatly promote neurogenesis.
Vascular endothelial growth factor A (VEGF) is mainly expressed by astrocytes in the human brain and plays an important role in hypoxic induced angiogenesis [27]. In healthy adults, only a small amount of VEGF expresses. As the most powerful cytokine promoting angiogenesis, VEGF promotes the proliferation, migration and angiogenesis of endothelial cells, thereby promoting angiogenesis and improving the blood supply of ischemic brain tissue [28]. With the improvement of blood supply, it can utmost preserve the original structure of NVU, so it is not difficult to understand that it can minimize neuro-remodelling. In our experiments, the expression level of VEGF-mRNA increased obviously in CCH which indicates that adaptive micro vessels formation is aroused by CCH. In addition, VEGF expression arises significantly in the adult rat after transection of nerve fibers [29], so it can be represented as a stress response factor [30]. Recent studies have shown that VEGF can promote neuronal surviving, neuronal and axonal regeneration and neural plasticity [31, 32]. In a word, it is a multi-effects factor for neuroplasticity.
Previous Research shows [33] that therapeutic angiogenesis increases the number of functional cerebral micro vessels, ameliorates cerebral ischemia and improves cognitive function in patients. In this study, the expression of VEGF-mRNA in the Model Group was significantly higher than it in the Sham operated Group, which indicates that the injury and apoptosis of neurons in CCH motivates the nerve stress reaction to increase the expression of VEGF-mRNA. MDP effectively promote the further expression of VEGF-mRNA. However, the elevated VEGF-mRNA did not further promote the formation of micro vessels in the MDP Group. We can see that MVD was significantly less in the MDP Group than that in the Model Group. The reasons may be as follows: Firstly, as we can see from the pathological images, MDP contributes to preserve a certain amount of neurons through anti-apoptosis, boost neuronal regeneration to abate the level of stress response. Angiogenesis is not unlimited, it should be precisely matched to the needs of the neurons. This phenomenon embodies the precise regulation among the elements in NVU. Secondly, studies have shown that BCCAO rats can approximately restore blood supply to brain in about 12 weeks through arteriole regeneration in the vertebral artery system [34], MDP probably increase the blood supply through Vertebro Basilar Artery system and rectify the hypoxia state [6]; Lastly, there are other angiogenic cytokines such as bVGF and so on which were excluded in the study and maybe play a key role in angiogenesis in Model Group. All these cases need to be clarified in the future. We can see that MDP promotes moderate angiogenesis.
The recovery after a CNS injury requires cortical plasticity, including synaptic plasticity [35]. Synaptic pathology, including abnormal density and morphology of dendritic spines, synapse loss, and aberrant synaptic signaling is involved in synaptic plasticity. Therefore, the normal number and morphology of synapses is an important basis for synaptic plasticity [36]. Synaptogenesis and synaptic reconstruction is fundamental to neuroplasticity in CCH. Here we discuss the synaptic plasticity from the synaptic quantity and quality, the latter includes synaptic morphology and synaptic plasticity associated key protein expression. By TEM we noticed that the synapses in Model Group are severely damaged or lost. Whereas the synapses in the MDP Group are significantly normalized and increased in quantity, which may be resulted from the ameliorated blood supply and the increased expression of GAP-43mRNA by MDP. These changes of synapse in the MDP Group facilitate the normal transmission of information. SYP and PSD-95 are markers of synapse structures and their expression levels can represent the roughly numbers of synapse. What's more, they are key proteins mediating in the long-term potentiation (LTP) [37]. From immunohistochemistry, the lowest expression levels of SYP and PSD-95 in Model Group indicate drastic damage and loss of synapse owing to CCH. On the contrary, the highest expression levels of SYP and PSD-95 in MDP Group show most synaptogenesis which corresponds to the results in the TEM depicting. SYP, GAP-43mRNA and MAP-2 are associated with presynaptic and postsynaptic plasticity as well as the neuronal cytoskeletons [32], they play crucial roles in neuroplasticity and recovery from a stroke [38, 39, 40]. The synchronous changes of these three markers reflect the consistency of genes, molecules and cell regulation in NVU.
Our previous study confirmed that MDP can accelerate the expression of BDNF mRNA in the hippocampus of VD rats [8]. Research suggests that the inhibition of BDNF/TrkB (tropomyosin-related kinase B, TrkB) signaling pathway can induce hippocampal dependent cognitive impairment [41] through reducing the expression of SYP, GAP-43 and PSD-95, simultaneously lowering the synapse density and post-synaptic density (PSD) which decrease synaptic transmission efficiency. Therefore, our study once again demonstrates that MDP probably facilitate the expression of these key gene and proteins associated to synaptogenesis through boost the expression of neurotrophic factor such as BDNF. Albeit synaptogenesis triggered by a stroke may compensate for the lost neural circuits [42], synaptogenesis is few in the Model Group because of neuronal loss and apoptosis in CCH. Whereas MDP can preserve abundant qualified synapse through regulation of specific cytokine to promote synaptogenesis and anti-apoptosis of neurons. So, we can see that synaptic destroy and loss in CCH and MDP can promote synaptogenesis through multiple ways.
There are numerous chemical composition in this compound herbal prescriptin, but the mainly active molecules include Yam Polysaccharide, Polygonum Multiflorum Polysaccharide, Catalpol, Lyciumbar Barum Polysaccharide, β-asarone, etc. Yam Polysaccharide and Polygonum Multiflorum Polysaccharide have the functions of immunoregulation and immunopotentiation besides antioxidant, clearing free radicals, anti-aging and anti-apoptosis [43, 44], Catalpol has the neuroprotective function through suppressing LPS-induced secretion of pro-inflammatory mediators [45], Lyciumbar Barum Polysaccharide has anti-inflammatory and antioxidant features [46]. β-asarone significantly decreases the apoptosis rate of hippocampal neuronal cells in rats and enhances CREB, BDNF, Trk-B and Bcl-2 levels [47], Many studies have confirmed that these elements have the functions of antioxidant, clearing free radicals, anti-aging, anti-apoptosis, and promoting nerve regeneration. These studies have fully illustrated that MDP's ingredients protect brain against ischemia and hypoxia, improve inflammation and metabolism in anoxic environment which is conducive to neuroplasticity and functional recovery.
From above studies we can see that MDP can act on multi-targets to promote recovery of nerve function which underlie its definite clinical effect. There are some limitations in this study, for example, the sample of this study is small, more accurate protein assays such as Western Blot were not used in the experiments. Otherwise, there are some problems such as the specific effective ingredients and their acting mechanisms need to be further clarified; some contradiction problems such as the mismatched expression of VEGF mRNA to MVD need to be further studied. Although these issues are suspending, we can see that some traditional Chinese medical prescriptions play very effective roles to prevent dementia and other neurodegenerative diseases resulted from CCH currently.
5. Conclusions
In this study, we know that the components of NVU interplay and precisely regulate each other through specific cytokines and gene expression to adapt to CCH. Neuroplasticity is an important mechanism of neural function's recovery in CCH which involves in angiogenesis, neurogenesis and synaptogenesis. Chinese herbal medication MDP can target multiple hybrids of neuroplasticity to effectively treat CCH. There are some questions such as how the specific gene and protein precisely coordinate neurogenesis, synaptogenesis and angiogenesis to restore the construction and function of NVU remain unknown and need furtherly research.
Declarations
Author contribution statement
H. B. Li: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
W. B. Liang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
L. Zhou: Performed the experiments; Wrote the paper.
Funding statement
This work was supported by Guizhou Provincial Science and Technology Foundation. NO. [2019]1005.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. Y. Cai for her valuable suggestions and help during the course of this work. We thank the Institute of Acupuncture and Moxibustion of Hubei University of Traditional Chinese Medicine for providing facilities and careful guidance.
References
- 1.Jellinger K. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113(4):349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 2.Stasiak A., Mussur M., Unzeta M., Lazewska D., Kiec-Kononowicz K., Fogel W.A. The central histamine level in rat model of vascular dementia. J. Physiol. Pharmacol. 2011;62(5):549–558. [PubMed] [Google Scholar]
- 3.Reines A., Cereseto M., Ferrero A., Sifonios L., Podesta M.F., Wikinski S. Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioral response. Neuropsychopharmacol: off Pub Am Coll Neuropsychopharmacol. 2008;33(8):1896–1908. doi: 10.1038/sj.npp.1301596. [DOI] [PubMed] [Google Scholar]
- 4.Muoio V., Persson P., Sendeski M. The neurovascular unit-concept review. Acta Physiol. 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 5.Floel A., Cohen L.G. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol. Dis. 2010;37(2):243–251. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan Z.H., Lan H.C., Yang Q., Chen J., Mao S.P., Zha Y.F., Xiao S.J. Clinical research of early intervention of modified shuyu pill in vascular cognitive impairment No dementia. Chin. J. Integr. Tradit. West. Med. 2013;33(1):27–30. [PubMed] [Google Scholar]
- 7.Tan Z.H., Liu Y., Li N. Clinical observation of modified shuyu pill combined with acupuncture on aphasia after stroke. J. Emerg. Tradit. Chin. Med. 2013;22(1):34–35. [Google Scholar]
- 8.Tan Z.H., Chen Y. Effects of modified yam pill on the expression of brain-derived neurotropic factor in the Hippocampus of vaxcular dementia rats[J] J. Shanghai Univ.Tradit. Chin. Med. 2016;30(4):69–72. [Google Scholar]
- 9.Tan Z.H., Chen Y. Study on the expression of neurotrophin in the hippocampus of vascular dementia rats and the intervention mechanism of Modified Yam Pill. Modern J. Integrated Chin. Tradit and West Med. 2016;25(18):1939–1941. [Google Scholar]
- 10.Sun G.G., Shih J.H., Chiou S.H., Hong C.J., Lu S.W., Pao L.H. Chinese herbal medicines promote hippocampal neuroproliferation, reduce stress hormone levels, inhibit apoptosis, and improve behavior in chronically stressed mice. J. Ethnopharmacol. 2016;193:159–168. doi: 10.1016/j.jep.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 11.The Ministry of Science and Technology of the People's Republic Laboratory Animals. 2006. [Google Scholar]
- 12.Bhuvanendran S., Bakar S.N.S., Kumari Y., Othman I., Shaikh M.F., Hassan Z. Embelin improves the spatial memory and hippocampal long-term potentiation in a rat model of chronic cerebral hypoperfusion. Sci. Rep. 2019 Oct 10;9(1):14507. doi: 10.1038/s41598-019-50954-y. PubMed PMID: 31601902; PubMed Central PMCID: PMC6787277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang N., He J., Pan C., Wang J., Ma M., Shi X., Xu Z. Resveratrol activates autophagy via the AKT/mTOR signaling pathway to improve cognitive dysfunction in rats with chronic cerebral hypoperfusion. Front. Neurosci. 2019 Aug 20;13:859. doi: 10.3389/fnins.2019.00859. eCollection 2019. PubMed PMID: 31481868; PubMed Central PMCID: PMC6710371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu K.S., Shamprasad B.R., Prasada K.S., Paladhi P., Joshi M.B., Valiathan M.S. Age dependent neuroprotective effects of medhyarasayana prepared from clitoriaternatealinn. in stress induced rat brain. J. Ethnopharmacol. 2017;197:173–183. doi: 10.1016/j.jep.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 15.Feng-Ying L.U., Liao X.H., Zhi-Xian L.I., Gao Y., Tian-Yu L.I. Application of microvascular density in determination of transrectal ultrasound hemodgynamics in differential diagnosis of prostate cancer and chronic prostatitis. J. Jilin Univ. 2014;40(5):1104–1108. [Google Scholar]
- 16.Liu Jiaming, Sun Jing, Wang Fangyan. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed Res. Int. 2015:412946. doi: 10.1155/2015/412946. 2015, Epub 2015 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christoffel D.J., Golden S.A., Dumitriu D. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. Neurosci. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong X.1, Ma M., Fan X., Li M., Liu Q., Liu X., Xu G. Down-regulation of IGF-1/IGF-1R in hippocampus of rats with vascular dementia. Neurosci. Lett. 2012;513(1):20–24. doi: 10.1016/j.neulet.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 19.Jiwa N.S., Garrard P., Hainsworth A.H. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. Neurochem. 2010;115(4):814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 20.Salazar-Colocho P., Lanciego J.L., Del Rio J., Frechilla D. Ischemia induces cell proliferation and neurogenesis in the gerbil hippocampus in response to neuronal death. Neurosci. Res. 2008;61(1):27–37. doi: 10.1016/j.neures.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 21.G1 Morucci, Branca J.J., Gulisano M., Ruggiero M., Paternostro F., Pacini A., Di Cesare Mannelli L., Pacini S. Gc-protein-derived macrophage activating factor counteracts the neuronal damage induced by oxaliplatin. Anti Canccer Drugs. 2015;26(2):197–209. doi: 10.1097/CAD.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 22.Song M.1, Mohamad O., Gu X., Wei L., Yu S.P. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013;22(11):2001–2015. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 23.Gordon T., You S., Cassar S.L., Tetzlaff W. Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp. Neurol. 2015;264:26–32. doi: 10.1016/j.expneurol.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Bulsara K.R., Iskandar B.J., Villavicencio A.T., Skene J.H. A new millenium for spinal cord regeneration: growth-associated genes. Spine. 2002;27:1946–1949. doi: 10.1097/00007632-200209010-00030. [DOI] [PubMed] [Google Scholar]
- 25.Johanson S.O., Crouch M.F., Hendry I.A. Retrograde axonal transport of signal transduction proteins in rat sciatic nerve. Brain Res. 1995;690:55–63. doi: 10.1016/0006-8993(95)00587-g. [DOI] [PubMed] [Google Scholar]
- 26.DA Dawson, JM Hallenbeck. Acute focal ische duced altera-tions in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain inju-ry. J. Cereb. Blood Flow Metab. 1996;16(1):170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Licht T., Keshet E. Delineating multiple functions of VEGF-A in the adult brain. Cell. Mol. Life Sci. 2013;70(10):1727–1737. doi: 10.1007/s00018-013-1280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyoshi S., Sekiguchi M., Konno S., Kikuchi S., Kanaya F. Ncreased expression of vascular endothelial growth factor protein in dorsal root ganglion exposed to nucleus pulposus on the nerve root in rats. Spine (Phila Pa 1976) 2011;36(1):E1–6. [PubMed] [Google Scholar]
- 29.Fu C.Y., Hong G.X., Wang F.B. Expression of vascular endothelial growth factor and its fetal liver kinase-1 receptor in spinal cord and dorsal root ganglia after neurotomy of sciatic nerve in rats. Chin. J. Traumatol. 2005;8(1):17–22. [PubMed] [Google Scholar]
- 30.Gerburg Keilhoff, Benjamin Lucas, Katja Uhde, Hisham Fansa. Selected gene profiles of stressed NSC-34 cells and rat spinal cord following peripheral nerve reconstruction and minocycline treatment. ExpTher Med. 2016;11(5):1685–1699. doi: 10.3892/etm.2016.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg D.A., Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. 2013;70(10):1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song S., Park J.T., Na J.Y., Park M.S., Lee J.K., Lee M.C., Kim H.S. Early expressions of hypoxia-inducible factor 1alpha and vascular endothelial growth factor increase the neuronal plasticity of activated endogenous neural stem cells after focal cerebral ischemia. Neural Regen Res. 2014;9(9):912–918. doi: 10.4103/1673-5374.133136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown W.R., Thore C.R. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37(1):56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soria G., Tudela R., Márquez-Martín A., Camón L., Batalle D., Muñoz-Moreno E., Eixarch E., Puig J., Pedraza S., Vila E., Prats-Galino A., Planas A.M. The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahmani M., Turrigiano G.G. Adult cortical plasticity following injury: recapitulation of critical period mechanisms? Neuroscience. 2014;283:4–16. doi: 10.1016/j.neuroscience.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mmh B., Haque M.N., Mohibbullah M., Kim Y.K., Moon I.S. Radix puerariae modulates glutamatergic synaptic architecture and potentiates functional synaptic plasticity in primary hippocampal neurons. J. Ethnopharmacol. 2017:209. doi: 10.1016/j.jep.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Li H., Xin X. Nitrogen dioxide (NO2) pollution as a potential risk factor for developing vascular dementia and its synaptic mechanisms. Chemosphere. 2013;92(1):52–58. doi: 10.1016/j.chemosphere.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 38.Pendharkar A.V., Levy S.L., Ho A.L., Sussman E.S., Cheng M.Y., Steinberg G.K. Optogenetic modulation in stroke recovery. Neurosurg. Focus. 2016;40(5):E6. doi: 10.3171/2016.2.FOCUS163. [DOI] [PubMed] [Google Scholar]
- 39.Gorup D., Bohacek I., Milicevic T., Pochet R., Mitrecic D., Kriz J., Gajovic S. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci. Lett. 2015;597:176–182. doi: 10.1016/j.neulet.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Kim T., Mehta S.L., Kaimal B., Lyons K., Dempsey R.J., Vemuganti R. Poststroke induction of alpha-Synuclein mediates ischemic brain damage. J. Neurosci. 2016;36(26):7055–7065. doi: 10.1523/JNEUROSCI.1241-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J., Zhang Z., Kang L., Geng D., Wang Y., Cui H. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp. Gerontol. 2014;58:256–268. doi: 10.1016/j.exger.2014.08.011. Epub 2014 Aug 27. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.K., Kim J.E., Sivula M., Strittmatter S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24(27):6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo L., Qin T., Huang Y., Zheng S., Bo R., Liu Z., Xing J., Hu Y., Liu J., Wang D. Exploring the immunopotentiation of Chinese yam polysaccharide poly(lactic-co-glycolic acid) nanoparticles in an ovalbumin vaccine formulation in vivo. Drug Deliv. 2017 Nov;24(1):1099–1111. doi: 10.1080/10717544.2017.1359861. PubMed PMID: 28776443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q., Xu Y., Lv J., Cheng M., Wu Y., Cao K., Zhang X., Mou X., Fan Q. New utilization of Polygonum multiflorum polysaccharide as macromolecular carrier of 5-fluorouracil for controlled release and immunoprotection. Int. J. Biol. Macromol. 2018 Sep;116:1310–1316. doi: 10.1016/j.ijbiomac.2018.02.052. Epub 2018 Feb 10. PubMed PMID: 29432832. [DOI] [PubMed] [Google Scholar]
- 45.Choi Y.H. Catalpol attenuates lipopolysaccharide-induced inflammatory responses in BV2 microglia through inhibiting the TLR4-mediated NF-κB pathway. Gen. Physiol. Biophys. 2019 Mar;38(2):111–122. doi: 10.4149/gpb-2018044. Epub 2019 Feb 26. PubMed PMID: 30806632. [DOI] [PubMed] [Google Scholar]
- 46.Hong C.Y., Zhang H.D., Liu X.Y., Xu Y. Attenuation of hyperoxic acute lung injury by Lycium barbarum polysaccharide via inhibiting NLRP3 inflammasome. Arch Pharm. Res. (Seoul) 2019 Oct;42(10):902–908. doi: 10.1007/s12272-019-01175-4. Epub 2019 Aug 6. PubMed PMID: 31388826. [DOI] [PubMed] [Google Scholar]
- 47.Dong H., Cong W., Guo X., Wang Y., Tong S., Li Q., Li C. β-asarone relieves chronic unpredictable mild stress induced depression by regulating the extracellular signal-regulated kinase signaling pathway. Exp Ther Med. 2019 Nov;18(5):3767–3774. doi: 10.3892/etm.2019.8018. Epub 2019 Sep 18. PubMed PMID: 31616508; PubMed Central PMCID: PMC6781814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]