Abstract
Background
Essential hypertension (EHTN) is emerging as one of the most prevalent disorder with high rate of complications, morbidity and mortality. Brahmi vati, an Ayurvedic medicine is explored for its efficacy in the management of EHTN.
Objective
To evaluate the efficacy of Brahmi vati and sarpagandha Ghana vati in the management of EHTN.
Methods
Total 68 patients meeting the JNC 7 criteria of EHTN of age group 20 to 60 years of either sex participated in the study. They were randomly divided into two groups, group A received capsule Brahmi vati 500 mg and group B capsule Sarpagandha Ghana vati 500 mg respectively twice a day for 30 days. Assessments were done through various variables like systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), lipid profiles, Hamilton anxiety rating scale, 2 weeks sleep diary, serum creatinine, hemoglobin, total leukocyte count and erythrocyte sedimentation rate. Follow up visit was on every 15th day.
Results
Study showed that both Brahmi vati and Sarpagandha Ghanavati produced improvement in most of the variables and were comparable. Improvements were seen in various variables like SBP, DBP, MAP, Hamilton anxiety rating scale, subjective sleep profiles and total cholesterol. However Brahmi vati showed increase in weight and Body Mass Index (BMI). SarpagandhaGhanavati produced reduction in total cholesterol and LDL. Both groups showed good safety profile evaluated through the assessment of serum creatinine levels.
Conclusion
Clinical efficacy of Sarpagandha Ghana vati and Brahmi vati on EHTN showed that both were effective, safe and comparable.
Keywords: Essential hypertension, Sleep profiles, Lipid profile, Sarpagandha Ghana vati, Brahmi vati
1. Introduction
Hypertension (HTN) being a chronic non-communicable disease constitutes an important public health challenge because of its prevalence and concomitant increase in the risk of cardiovascular diseases [1]. In India 14 % of people suffer from HTN and majority of them have essential hypertension (EHTN) [2]. Systolic blood pressure (SBP) above 140 mm of Hg and diastolic blood pressure (DBP) above 90 mm of Hg is the diagnostic criteria of HTN [3]. For every 20 mm Hg systolic or 10 mm Hg diastolic increase in blood pressure (BP), there is a doubling of mortality from both ischemic heart disease and stroke [3]. Even pre-HTN values of 130–139/85–89 mm Hg are associated with more than two-fold increase in cardiovascular disease risk as compared with those with BP levels below 120/80 mmHg [4]. It is responsible for 9.4 million deaths and 7 % of disability adjusted life years in 2010 [5]. The global burden of HTN is anticipated to increase by 60% to affect approximately 29.2 % of adult population that accounts to 1.56 billion adults worldwide by 2025 [6].
The current therapeutic strategy involves use of various pharmacological agents like β blockers, ACE inhibitors, calcium channel blockers, α blockers and diuretics. However use of these drugs is affected because various hindrances like side-effects and cost-effectiveness [7]. In more than 66 % of patients, blood pressure cannot be controlled with one drug and requires two or more anti-hypertensives [8]. Even with these medications, only 34 % of hypertensives have blood pressure controlled below 140/90 mm of Hg [3]. Studies have shown that incidence of use of complementary and alternative system of medicine in chronic disorders is as high as 48 % in patient population [9]. Hence there is a dire need to search for safe and effective medications. Ayurveda is a rich reservoir of knowledge and has huge amount of experience based information documented. However, Ayurveda therapeutics is poorly investigated for its possible role in the management of EHTN. Brahmi (Bacopa monnieri (L.) Pennell) and its products have been widely explored for its medhya (nootropic) effects, used in nidravikara (sleep disorders) and manoroga (psychiatric disorders) [10]. Brahmi vati [11] has various ingredients which have medhya (nootropic), rasayana (rejuvenative), nidrajanana (sleep promoting), shothahara (anti-inflammatory) and hrudya (cardiotrophic) effects. Sarpagandha Ghana vati [12], [13] is one of the widely studied Ayurveda preparation in EHTN. However effective management of EHTN still eludes the medical fraternity [3]. So the present study was designed to study the efficacy of Brahmi vati in the management of EHTN. Also, Comparative study on efficacy was undertaken with a standard drug Sarpagandha Ghana Vati.
2. Materials and methods
The patients attending outpatient department of the institute were recruited for the study. The CONSORT statement guidelines [14] have been followed in reporting the outcomes of the study.
2.1. Subjects
Total 68 patients diagnosed as EHTN as per JNC 7 [3] criteria were recruited from patients visiting outpatient department of KLEU Shri BMK Ayurveda Hospital Belgaum, Karnataka, India.
Inclusion Criteria:
The patients of either sex between 20 and 70 years age were included in the study.
Exclusion Criteria:
The patients with Ischemic heart disease (IHD), Coronary heart disease (CHD) and coarctation of aorta, renal failure; those having any endocrine disease, patient with hypertension complications (e.g. hypertensive encephalopathy, cerebral haemorrhage, convulsive seizure); those with malignant hypertension; pregnant and lactating female patients and patient on treatment for hypertension since 1 month, were excluded from the study.
Screening Methods:
All patients included in this study were examined thoroughly and data was recorded systematically. Various laboratory and Ayurveda variables like Prakriti were assessed. Laboratory investigations were carried out at Clinical Laboratory, KLEU BMK Ayurveda Mahavidyalaya, Belagavi in all patients at baseline and on 30th day of intervention.
2.2. Research design
The study was a randomized, double-blind, parallel group comparative design clinical study. The scholars involved in randomization, distribution and administration of study articles were independent from the investigators. Computer generated random numbers were utilized for the study. Block size was 4. The patients were allocated in control and intervention groups in 1:1 ratio. A pilot study was conducted on 4 patients each from both groups. Mean arterial pressure was calculated at base line. The sample size was 34 in each group under 5 % alpha error and 90 % power of test.
Intervention:
All the patients were randomly divided into two groups: group A and group B. Group A (n = 34) received Brahmi vati [11] capsules 500 mg BD while Group B (n = 34) received Sarpagandha Ghana vati [12] capsules 500 mg BD. Both groups received their respective interventions with water after food intake. Both the interventions are from classical text books of Ayurveda. Dosage of interventions were as per respective classical literature [11], [12]. The ingredients of Brahmi vati were procured from authentic distributors and capsules were prepared in GMP approved KLE Ayurveda Pharmacy, Belgaum as per standard procedures. Sarpagandha Ghana vati were procured from AYU KALP UAP Pharmacy, Ahmedabad, India free of cost and capsules were prepared in GMP approved KLE Ayurveda Pharmacy, Belgaum, Karnataka, India as per standard procedures. Duration of intervention was 30 days with follow-up on every 15th day. The nature and design of the study were explained to patients, and informed consent was obtained. The study was approved by the Institutional Ethics Committee (Protocol Id – BMK/12/PG/KC/03, KLEU BMK Ayurveda Mahavidyalaya Belagavi, Date of Approval – 18.10.2013. CTRI Registration Number – CTRI/2015/08/006120). Data collection was from August 2013 to July 2015. During the study, patients were asked to adhere to the treatment protocol and report any adverse events to the investigators at the earliest. Any manifestations either existing or new during the course of intervention that cause considerable distress were screened for possible adverse events.
2.3. Criteria for assessment
Primary Outcomes:
Systolic blood pressure, diastolic blood pressure, mean arterial pressure were primary outcomes. They were recorded by following standard operating procedures.
Secondary Outcomes:
The secondary outcomes were Hamilton Anxiety Rating Scale [15], Lipid profiles [Total cholesterol, Triglycerides, High Density Lipoproteins (HDL), Low Density Lipoproteins (LDL)], Haemoglobin, WBC-Total Count, Differential count, Erythrocyte Sedimentation Rate (ESR); two weeks sleep dairy [16], sleep onset latency, intermittent awakenings in sleep, sleep duration, day time drowsiness, and serum creatinine.
2.4. Statistical methods
Statistical analysis was carried out using SPSS Version 20.0. Homogeneity of data across the groups was evaluated by the χ2 test. Comparison of groups across different time points was carried out by two way repeated measure Analysis of Variance (rmANOVA) with Bonferroni post-hoc test. Comparison of within groups at two time points was analyzed by paired t-test. Comparison of groups at a time point was through independent sample t-test. Effect size calculated by Partial Eta Square method was used to assess the effect of treatment through the outcome from baseline to 30th and 60th day of treatment. The criteria used for interpreting effect size measures were as follows: 0–0.2 minimal, 0.2–0.5 as small, 0.5–0.8 as medium, and above 0.8 as large effect size [17]. Values are reported as mean ± standard deviation. All tests were considered statistically significant at p < 0.05.
3. Results
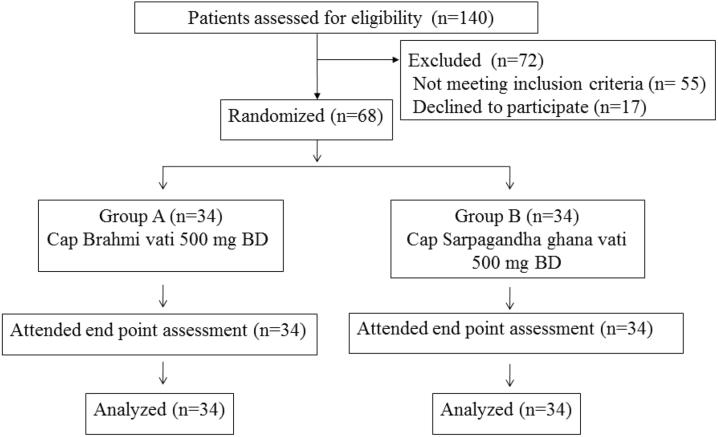
A total of 68 patients participated in the study. No patients in either groups reported any adverse effects. No patient dropped out of our study (Fig. 1).
Fig. 1.
CONSORT flow chart.
3.1. Subject characteristics
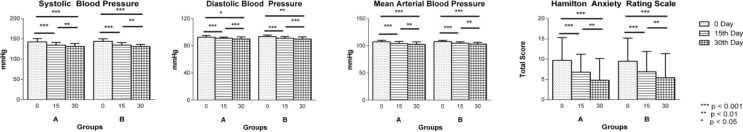
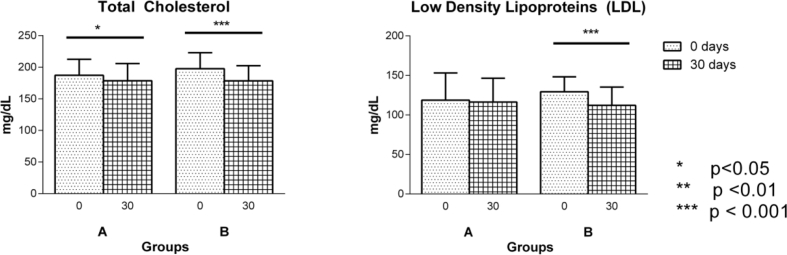
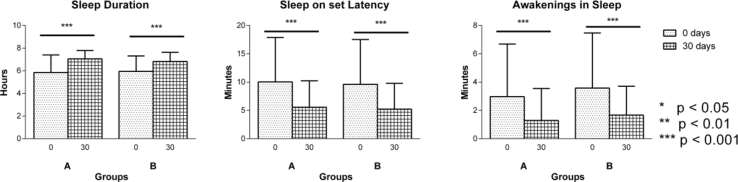
The mean age (p = 0.221), gender (p = 0.947), socio-economic status (p = 0.055), education (p = 0.838), food (p = 0.06) were comparable between groups (Table 1). Clinical variables like Prakriti (p = 0.950), weight (p = 0.583), BMI (p = 0.867), duration of illness (p = 0.221), and history of sleep disturbance (p = 0.203) of the patients were comparable in both the groups (Table 1). Clinical assessments like SBP, DBP, mean arterial pressure, total score of Hamilton Anxiety rating scale (Fig. 2), lipid profiles (Total cholesterol, Triglycerides (Fig. 3), LDL, HDL), sleep variables (duration of sleep, sleep onset latency (Fig. 4), intermittent awakenings in sleep, day time drowsiness) hemoglobin, serum creatinine, ESR, WBC total count at baseline were comparable between the groups (Table 2).
Table 1.
Patient profile: Expressed in Mean, standard deviations (S.D.) and percentage.
| S. No | Clinical Profile | Group A |
Group B |
Total | p value | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| 1 | Age (yrs) | 38.94 ± 11.74 | 42.57 ± 12.63 | 40.78±12.24 | 0.221 | |||
| 2 | Sex | Male | 8 | 23.5% | 9 | 26.4% | 17 (25%) | 0.779 |
| Female | 26 | 76.4% | 25 | 73.5% | 51 (75%) | |||
| 3 | Socio Economic Status | Poor | 0 | 0% | 4 | 11.7% | 4 (5.8%) | 0.055 |
| Middle class | 30 | 88.2% | 29 | 85.2% | 59 (86.7%) | |||
| Higher Middle class | 4 | 11.7% | 1 | 2.9% | 5 (7.3%) | |||
| 4 | Food | Vegetarian | 24 | 68.5% | 30 | 88.2% | 54 (79.4%) | 0.072 |
| Mixed | 10 | 29.4% | 4 | 11.7% | 14 (20.5%) | |||
| 5 | Educational Status | Illiterate | 10 | 29.4% | 15 | 44.1% | 25 (%) | 0.775 |
| Primary | 1 | 2.9 % | 1 | 2.9% | 2 (2.9%) | |||
| Secondary | 9 | 26.4% | 8 | 23.5% | 17 (25%) | |||
| Graduate | 13 | 38.2% | 9 | 26.4% | 22 (32.3%) | |||
| Post Graduate | 1 | 2.9% | 1 | 2.9% | 2 (2.9%) | |||
| 6 | Prakurti (Body constitution) | Vata | 8 | 23.52% | 8 | 23.52% | 16(23.5%) | 0.926 |
| Pitta | 6 | 17.64% | 9 | 26.4% | 15(22.%) | |||
| Kapha | 2 | 5.88% | 2 | 5.88% | 4(5.8%) | |||
| Vata pitta | 1 | 2.94% | 1 | 2.94% | 2(2.9%) | |||
| Vata kapha | 17 | 0.5% | 14 | 41.1% | 31(45.5%) | |||
| 7 | Sleep disturbance | Yes | 27 | 79.4% | 23 | 67.6% | 50(73.5%) | 0.272 |
| No | 7 | 20.5% | 11 | 32.3% | ||||
| 8 | Duration of illness (In Days) | 38.94 ± 11.74 | 42.57 ± 12.63 | 40.74 ± 12.28 | 0.221 | |||
| 9 | Weight | 59.99 ±8.65 | 61.28±10.65 | 0.583 | ||||
| 10 | BMI | 22.52 ±2.86 | 22.68±3.47 | 0.867 | ||||
| 11 | Drop outs | 0 | 0% | 0 | 0% | 0% | ||
| 12 | Study completed | 34 | 100% | 34 | 100% | 68 (100%) | ||
| 13 | Total | 34 | 34 | 68 | ||||
Fig. 2.
Changes in blood pressure parameters in group A (n = 34) and group B (n = 34) as assessed on 0 (baseline), 15th and 30th day of intervention: a. Systolic Blood Pressure, b. Diastolic Blood Pressure, c. Mean Arterial Blood Pressure d. Hamilton Anxiety Rating Scale. Results are expressed in Mean ± standard deviation. Level of significance is *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 3.
Changes in parameters in group A (n = 34) and group B (n = 34) as assessed on 0 (baseline), 15th and 30th day of intervention: a. Total Cholesterol, b. Low Density Lipoproteins (LDL). Results are expressed in Mean ± standard deviation. Level of significance is *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4.
Changes in sleep parameters in group A (n = 34) and group B (n = 34) as assessed on 0 (baseline) and 30th day of intervention: a. Sleep Duration, b. Sleep Onset Latency, c. Awakenings in Sleep. Results are expressed in Mean ± standard deviation. Level of significance is *p < 0.05, **p < 0.01, ***p < 0.001.
Table 2.
Effect of interventions on weight, BMI, lipid profiles, haemoglobin, serum creatinine, ESR, total count. Expressed in Mean and standard deviations (S.D.).
| Sl. No | Parameter | Intervention period | Group A | Group B | p | Effect size (0–30 days) |
|---|---|---|---|---|---|---|
| 1 | Weight in kg | 0 day | 59.99 ± 8.65 | 61.28 ± 10.65 | 0.583 | 0.80 |
| 30 day | 60.16 ± 8.63 | 59.63 ± 14.85 | 0.608 | |||
| p | 0.007 | 0.374 | ||||
| 2 | Body mass index (BMI) | 0 day | 22.52 ± 2.86 | 22.68 ± 3.47 | 0.867 | 0.71 |
| 30 day | 22.58 ± 2.87 | 22.74 ± 3.43 | 0.837 | |||
| p | 0.013 | 0.060 | ||||
| 3 | Total cholesterol (in mg/dL) | 0 day | 187.14 ± 25.47 | 197.55 ± 25.49 | 0.107 | 0.41 |
| 30 day | 178.67 ± 27.26 | 178.35 ± 24.12 | 0.502 | |||
| p | 0.045 | <0.001 | ||||
| 4 | Triglycerides (in mg/dL) | 0 day | 118.52 ± 38.47 | 120.02 ± 41.31 | 0.992 | 0.09 |
| 30 day | 114.94 ± 28.17 | 119.38 ± 30.50 | 0.896 | |||
| p | 0.529 | 0.916 | ||||
| 5 | High density lipoprotein (HDL) (in mg/dL) | 0 day | 38.85 ± 4.06 | 41.50 ± 4.94 | 0.022 | 0.53 |
| 30 day | 39.76 ± 4.04 | 39.73 ± 4.01 | 0.976 | |||
| p | 0.212 | 0.083 | ||||
| 6 | Low density lipoprotein (LDL) (in mg/dL) | 0 day | 118.73 ± 34.40 | 129.26 ± 19.00 | 0.225 | 0.50 |
| 30 day | 116.20 ± 30.28 | 112.14 ± 23.05 | 0.536 | |||
| p | 0.634 | <0.001 | ||||
| 7 | Hemoglobin (in mg/dL) | 0 day | 12.18 ± 1.66 | 12.51 ± 1.85 | 0.530 | 0.38 |
| 30 day | 12.10 ± 1.50 | 11.97 ± 1.93 | 0.748 | |||
| p | 0.654 | 0.037 | ||||
| 8 | Serum creatinine (in mg/dl) | 0 day | 0.84 ± 0.13 | 0.90 ± 0.15 | 0.098 | 0.57 |
| 30 day | 0.86 ± 0.11 | 0.83 ± 0.10 | 0.239 | |||
| p | 0.247 | 0.027 | ||||
| 9 | ESR (mm/h) | 0 day | 22.82 ± 11.83 | 21.20 ± 11.32 | 0.566 | 0.22 |
| 30 day | 20.61 ± 10.20 | 21.02 ± 10.59 | 0.871 | |||
| p | 0.133 | 0.916 | ||||
| 10 | Total count (cells/mm3) | 0 day | 7158.82 ± 1394.82 | 7876.47 ± 1634.85 | 0.085 | 0.04 |
| 30 day | 7058.82 ± 1353.13 | 7850.00 ± 1526.18 | 0.027 | |||
| p | 0.709 | 0.933 |
3.2. Primary outcome
The study showed that improvement in both groups was comparable in SBP, DBP and mean arterial pressure. However, within-group comparison showed significant improvement in both the groups on all these three variables at all the three time points. Both interventions produced significant reduction in SBP at 15th day (p < 0.001), 30th day (p < 0.001) and 15–30th day (p = 0.002) of treatment. DBP showed significant reduction (p < 0.001) at both 0–15th day and 0–30th day; however at 15−30th day, improvement in group A (p = 0.028) and group B (p = 0.018) was different. Mean arterial pressure improvement in both groups was significant (p < 0.001) at both 0–15th and 0–30th day of treatment. Improvements were also noted at 15–30th day of intervention in group A (p = 0.012) and group B (p = 0.005) (Fig. 2).
3.3. Secondary outcomes
Both the interventions were comparable in all secondary outcome variables when compared between the groups. However, within group comparison showed considerable outcomes in both groups. Interventions produced significant linear improvement in Hamilton Anxiety Rating scale scores at all three time points in both groups (p < 0.001); significant changes were noted in pre and post comparison at total cholesterol profiles (group A – p = 0.04, group B – p < 0.001), LDL (group B – p < 0.001), sleep profiles like sleep duration (group A and B – p < 0.001). Non significant improvements were observed in Sleep onset latency (group A, p = 0.05 & group B, p = 0.06). Day time drowsiness showed reduction (group A-73.03%, group B-64.02%). Significant changes in few parameters were noted in individual groups like Haemoglobin reduction in group B (p = 0.037), serum creatinine reduction in group B (p = 0.024), weight gain in group A (p = 0.007), Body Mass Index (BMI) improvement in group A (p = 0.013) (Table 3, Table 4) (Fig. 3, Fig. 4).
Table 3.
Ingredients of Brahmi vati.
| S. No | Sanskrit name | Latin name | Form | Proportion |
|---|---|---|---|---|
| 1. | Brahmi | Bacopa monnieri | Powder | 2 |
| 2. | Shankhpushpi | Convolvulus pluricaulis Choiss | Powder | 2 |
| 3. | Gojihva | Onosma bracteatum Wall. | Powder | 2 |
| 4. | Vaca | Acorus calamus Linn. | Powder | 1 |
| 5. | Swarna Makshika | Copper pyrite and Iron pyrite | Powder | 1 |
| 6. | Rasa Sindoor | Sulphide of mercury | Powder | 1 |
| 7. | Krishna Marich | Piper nigrum Linn | Powder | 1/2 |
| 8. | Jatamansi | Nardostachys jatamansi Dc | Decoction | quantity sufficient for trituration |
Table 4.
Ingredients of Sarpagandha Ghana vati.
| S. No | Sanskrit name | Latin name | Form | Proportion |
|---|---|---|---|---|
| 1. | Sarpagandha | Rauwolfia serpentina Benth Ex. Kurz | Powder | 10 |
| 2. | Jatamansi | Nardostachys jatamansi Dc | Powder | 1 |
| 3. | Parasika yavani | Hyoscyamus niger Linn | Powder | 2 |
| 4. | Pippalimula | Piper longum Linn. | Powder | 1/2 |
| 5. | Bhanga | Cannabis sativa Linn. | Powder | 1 |
Effect size comparison showed that medium size effect in variables like BMI, weight, HDL, LDL, serum creatinine and small effect size was observed in SBP, DBP, Hamilton Anxiety rating scores, sleep duration, hemoglobin, ESR.
4. Discussion
The study showed that Brahmi vati was comparable to Sarpagandha Ghana vati in the management of EHTN in all the aspects, thus failed to reject the null hypothesis. Brahmi vati showed brahmniya effect (increase in weight and increase in BMI) and medohara (decreasing total cholesterol) while Sarpagandha Ghana vati produced apatarpana (debilitating therapy) and medohara effect by decreasing hemoglobin, total cholesterol, LDL levels. Both drugs improved variables like SBP, DBP, mean arterial pressure, decreased Hamilton Anxiety Rating scale score, improved sleep duration, decreased sleep onset latency, decreased intermittent wakefulness in sleep, decreased day time drowsiness (group A – 73.03%, group B – 64.02%) and also decrease in serum creatinine. Both the drugs showed high safety margins as there were no adverse drug reactions/events reported and also serum creatinine levels were in physiological ranges and even showed significant decrease post interventions suggestive of an improved renal function.
Majority of the patients (n = 66) were suffering from stage 1 HTN and few were with stage 2 HTN (n = 2). Patients noticed symptoms which could be related to HTN since 60 days. All the patients were diagnosed with HTN for the first time and had not received treatment for HTN earlier. Co-morbidity of sleep disturbance was in 72.4% patients and day time drowsiness was in 68.11% patients. Sub-components of lipid profile are expressed in desirable, borderline and high risk ranges. Our study revealed that patients in borderline and high risk ranges were 33.3% in total cholesterol, 18.8% in triglycerides, 94.2% in HDL, 43.5% in LDL. Cumulatively patients with all sub-components in desirable range were 2.8%, at least one parameter in border line range were 76.8% and at least one parameter in high risk range were 20.2% suggesting high association of HTN and dyslipidemia.
Brahmi vati showed beneficial effect on variables related blood pressure like SBP, DBP and mean arterial pressure. SBP rises throughout the age where as DBP rises till 50 yrs of age and then it may remain same or fall later [18]. DBP usually refers to peripheral resistance and is a potent risk factor for cardiovascular diseases till 50 yrs of age. However, SBP represents the most common form of HTN and is important [19] as it reduces total mortality, cardiovascular mortality, stroke, and heart failure events [20]. Mean arterial pressure is perfusion pressure seen by organs in the body and has higher importance in patients of essential HTN [21]. Improvement in these variables has a greater consequence in the long term management of EHTN.
Brahmi vati also showed significant decrease in total score of Hamilton anxiety rating scale, total cholesterol levels, intermittent awakenings in sleep, day time drowsiness, serum creatinine levels. Increase in sleep duration, weight and BMI were also observed. However weight and BMI were in normal range (<25 Kg/m2) before & after intervention. Duration of sleep in healthy adults is 7–9 h per night [22], [23], however average sleep in group A was 5.5 hours/night showing sleep deficiency. Brahmi vati intervention showed increase in sleep duration to 7.03 hours/night which was in the normal range. Trends of improvement in Sleep onset latency was observed with decrease from 10 min to 7 min. Normal sleep on set latency is less then 10 min and in the study group it was in the higher range and was reduced to normal range. Awakenings in sleep were significantly decreased from 3 to 1 min. Day time drowsiness was decreased by 73 %. Thus Brahmi vati showed improvement in sleep maintenance and duration of sleep. Grossly it had sleep promotive & restorative effect. Similar results were also seen in Sarpagandha Ghana vati, increased sleep duration from 5.57 h to 6.49 h, trends of decrease in sleep onset latency from 9 to 6 min, decrease in awakening in sleep from 3 to 1 min. Decrease in day time drowsiness was by 64 %.
Sleep disturbance, dyslipidemias and anxiety have closer association with HTN. Short sleep duration is associated with HTN [24]. Short sleep duration (≤5 h/night) was associated with a 60 % higher risk of self-reported incident HTN over an 8 to 10 year follow-up period [25]. Co-morbidity of dyslipidemia in EHTN was 30.7 % and patients with these 2 conditions were found to have 3 to 4 times the prevalence of myocardial infarction than patients with either condition alone, and 2 to 3 times the prevalences of coronary artery disease, peripheral artery disease, and cerebrovascular disease [26]. Meta-analysis study has shown there is association between anxiety and increased risk of HTN [27]. Either these can be co- morbidy or risk factors for development of HTN. Dyslipidemias and HTN form the important criteria for diagnosis of metabolic syndrome as per the NCEP ATP III definition [28]. Activation of the hypothalmic–pituitary–adrenal axis and the sympathetic nervous system as seen in insomnia may predispose to HTN development [29]. Same reasoning is applicable for other components like anxiety and dyslipidemias.
Brahmi vati is a formulation (Table 3) with drugs reportedly having activity on HTN, central nervous system, cardiovascular system, diuretic activity etc. Ingredients like Brahmi (B. monnieri (L.) Pennell) has anxiolytic effects, anticonvulsive action, antioxidant activity, adaptogenic activity cardiac depressive activity on left ventricular contractility, heart rate and coronary flow similar to that of quinidine on heart [30]. Clinical study on Nardostachys jatamansi has shown significant improvement in EHTN [31] which exhibit anti-oxidant [32] anti ischemic [33] and anti-arrhythmic [34] potential. It also increases the HDL levels which are protective lipids [35]. Sankhapushpi (Convolvulus pluricaulis Choisy) has anxiolytic activity [36]. Vacha (Acorus calamus Linn) has calcium inhibitory effect and diuretic activity which may potentiate Na+ excretion in HTN [37]. Krishna maricha (Piper nigrum Linn.) in dose-dependent manner when administrated intravenously decreases pressure in arteries in normotensive anesthetized rats [38]. Rasa sindhoora has augmenting antihypertensive effect [39].
Sarpagandha Ghana vati is a formulation (Table 4) with ingredients like Sarpagandha (Rauvolfia serpentina L. Benth. ex Kurz) having antihypertensive activity due to reserpine and has depressant action on central nervous system and peripheral nervous system by binding to catecholamine storage vesicles present in the nerve cell [40]. Parasika yavani (Hyoscyamus niger) crude extract caused a dose-dependent (10–100 mg/kg) fall in the arterial blood pressure (BP) of rats under anesthesia [41]. Jatamansi (Nardostachys jatamansi DC) with chemical ingredient like jatamansone have reported to possess anti-arrhythmic and antihypertensive activity [42]. Dehydro pipernonaline isolated from Pippali (Piper longum Linn.) has coronary vaso-relaxant activity [43]. Narcotic Drugs and Psychotropic Substances Act of India-1985 [44] allows use of Bhanga for medicinal and research purpose. The main psychoactive cannabinoid is Δ9-tetrahydro cannabinol (THC) and it acts through CB1 receptors that are present in brain, peripheral nerves and autonomic nervous system [45]. Studies have shown beneficial role of Cannabis in various diseases like Alzheimer's disease [46], anorexia and weight loss in Acquired Immuno Deficiency Syndrome [47] and Spasticity due to multiple sclerosis [48]. Bhanga (Cannabis sativa Linn.) has cardioprotective activity and coronary vessels dilation effect mainly due to endocannabinoids [49]. In spite of legal restrictions all over the world, cannabis is the most widely used illicit recreational drug in the world [50]. Long term use is associated with addictions [51], anxiety, depression [52]. However, Ayurveda uses processed bhanga [53] for therapeutic use and it's long term effects needs to be studied.
Various Ayurveda formulations and herbal drugs have shown their efficacy in the management of EHTN. Shankhapushpyadi Ghana vati (Anabhoota yoga) and Sarpagandha Ghana vati (without bhanga) were effective and comparable [13]. Another study showed that both trivrutadi yoga virechana and Dashamoola kwatha kaala basti with add on Arjunadi Ghana vati had antihypertensive effect and were comparable [54]. Brahmyadi churna [55], Shilajatu [55], Chandramaradi yoga [56] were also effective. Other herbals that showed antihypertensive activity are Allium sativum (Garlic) [57], Zingiber officinale (Ginger) [58], Sarpagandha [59], Cassia occidentalis (Kasamarda) [60], Annona muricata [61], Achillea wilhelmsii C [62] and Coleus forskohlii [63]. However, none of these studies have assessed the efficacy of drug in multiple domains as attempted in the current study. Few of these studies have methodological constrains like inadequate sample size etc.
The present study has various merits like it was double blind, randomized study. Variables were from multiple domains like blood pressure, qualitative sleep profile, lipid profiles, creatinine levels, anxiety and blood profiles. Limitations of the study were the lack of use of gold standard control drug mainly from biomedicine. Average level of ambulatory blood pressure assessment would have given better picture of HTN. Patients were predominantly of stage 1 HTN in our study; however drugs effect on stage 2 needs to be evaluated. Sleep assessment through both subjective and objective variables would have been beneficial. Longer duration intervention can throw more light on anti-hypertensive potential of these formulations.
5. Conclusion
The present study showed that both the drugs were comparable in management of EHTN. Ayurveda drugs could demonstrate action on variables of multiple domains and showed to be a comprehensive management strategy for EHTN. Both drugs appeared to be safe as assessed through serum creatinine levels and absence of any adverse drug reactions. Brahmi vati has bhrumhaniya (nourishing), medohara (anti-dyslipedimic), chittodwegahara (anxiolytic), nidrajanana (sleep promoting) effect along with antihypertensive effect. Sarpagandha also has medohara, chittodwegahara, nidrajanana effect and antihypertensive effect. Hence both drugs can be incorporated into comprehensive treatment strategy of HTN.
Sources of funding
None.
Conflict of interest
None.
Acknowledgements
We thank Ayukalpa UAP Pharma Pvt.Ltd. Ahmedabad Gujarat India for providing Sarpagandha Ghana vati free of cost. Otherwise this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Gupta R. Defining hypertension in the Indian population. Natl Med J India. 1997 May–Jun;10(3):139–143. [PubMed] [Google Scholar]
- 2.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Vasan R.S., Larson M.G., Leip E.P., Evans J.C., O’Donnell C.J., Kannel W.B. Impact of high normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 5.Lim Stephen S., Vos Theo, Flaxman Abraham D., Danaei Goodarz, Shibuya Kenji, Adair-Rohani Heather. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1999–2010: a systematic analysis for the global burden of disease studies 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney P.M., Whelton M., Reynolds K., Muntner P., Whelton P.K., He J. Global burden of hypertension: analysis of world wide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor Alka, Kumar Abhimanyu, Mahapatra Arun Kumar, Chauhan Gouri. Open clinical trial of a polyherbal compound M-Sarpagandhamishran in essential hypertension: a pilot study. Int J Res Ayurveda Pharm. 2014;5(5):594–599. [Google Scholar]
- 8.Black H.R., Elliott W.J., Grandits G., Grambsch P., Lucente T., White W.B. Principal results of the controlled onset verapamil investigation of cardiovascular end points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. 28,87,101–103. [DOI] [PubMed] [Google Scholar]
- 9.Phillips A.W., Osborne J.A. Survey of alternative and nonprescription therapy use. Am J Health Syst Pharm. 2000;57:1361–1362. doi: 10.1093/ajhp/57.14.1361. [DOI] [PubMed] [Google Scholar]
- 10.Deole Yogesh S., Chandola H.M. A clinical study on effect of Brahmi ghrita on depression. AYU. 2008;29(4):207–214. [Google Scholar]
- 11.Ayurveda Sara Sangraha. Vattiprakarana, Published by Shri Baidyanath Ayurveda Bhavana Limited, Nani, Allahabada. 2010. p. 455. [Google Scholar]
- 12.Ayurveda Sara Sangraha, Vatiprakarana, Published by Shri Baidyanath Ayurveda Bhavana Limited, Nani, Allahabada. 2010. p. 467. [Google Scholar]
- 13.Mishra Jyoti, Joshi Nayan P., Pandya Dilip M. A comparative study of Shankhapushpyadi Ghana Vati and Sarpagandhadi Ghana Vati in the management of "Essential Hypertension". Ayu. Jan–March 2012;33(1):54–61. doi: 10.4103/0974-8520.100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 16.National Sleep Foundation. Sleep diary. https://sleepfoundation.org/sleep-diary/SleepDiaryv6.pdf [Accessed 18 June 2016].
- 17.Cohen J. 2nd ed. L. Erlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 18.Franklin S.S., Gustin W., Wong N.D., Larson M.G., Weber M.A., Kannel W.B. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 19.Franklin S.S., Larson M.G., Khan S.A., Wong N.D., Leip E.P., Kannel W.B. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 20.Staessen J.A., Thijs L., Fagard R., O'Brien E.T., Clement D., de Leeuw P.W. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 21.Pickering T.G., Ogedebe G. Epidemiology of hypertension. In: Fuster V., O' Rourke R.A., Walsh R.R., Poole Wilson P., editors. Hurst's The Heart. 12th ed. Mc Graw Hill; New York: 2008. pp. 1551–1569. [Google Scholar]
- 22.Carskadon M.A., Dement W.C. Principles and practice of sleep medicine. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and practice of sleep medicine. Elsevier Saunders; Philadelphia: 2005. pp. 13–23. [Google Scholar]
- 23.National Sleep Foundation: How much sleep do we really need? http://www.sleepfoundation.org/article/how-sleep-works/how-much-sleep-do-we-really-need [Accessed 18 June 2016].
- 24.Kuciene R., Dulskiene V. Associations of short sleep duration with prehypertension and hypertension among Lithuanian children and adolescents: a cross-sectional study. BMC Public Health. 2014 Mar 15;14:255. doi: 10.1186/1471-2458-14-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangwisch J.E., Heymsfield S.B., Boden-Albala B. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 26.Johnson M.L., Pietz K., Battleman D.S., Beyth R.J. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004 Dec;10(12):926–932. [PubMed] [Google Scholar]
- 27.Pan Y., Cai W., Cheng Q., Dong W., An T., Yan J. Association between anxiety and hypertension: a systematic review and meta-analysis of epidemiological studies. Neuropsychiatr Dis Treat. 2015 Apr 22;11:1121–1130. doi: 10.2147/NDT.S77710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cholesterol Education Program (NCEP): Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 29.Bonnet M.H. Evidence for the pathophysiology of insomnia. Sleep. 2009;32(4):441–442. doi: 10.1093/sleep/32.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Snafi Ali Esmail. The pharmacology of Bacopa monniera. A review. Int J Pharma Sci Res (IJPSR) Dec 2013;4(12):154–159. [Google Scholar]
- 31.Venkatachalapathy Velpandian, Balakrishnan Sathya, Musthafa Mohammed, Natarajan Anbu. A clinical evaluation of Nardostachys jatamansi in the management of essential hypertension. Int J Pharm Phytopharm Res. 2012;2(2):96–100. [Google Scholar]
- 32.Subashini R., Yogeeta S., Gnanapragasam A., Devaki T. Protective effect of Nardostachys jatamansi on oxidative injury and cellular abnormalities during doxorubicin/induced cardiac damage in rats. J Pharm Pharmacol. 2006;58(2):257–262. doi: 10.1211/jpp.58.2.0014. [DOI] [PubMed] [Google Scholar]
- 33.Salim S., Ahmad M., Zafar K.S., Ahmad A.S., Islam F. Protective effect of Nardostachysjatamansi in rat cerebral ischemia. Pharmacol Biochem Behav. 2003;74(2):481–486. doi: 10.1016/s0091-3057(02)01030-4. [DOI] [PubMed] [Google Scholar]
- 34.Arora R.B., Madan B.R. Antiarrhythmics. III. Antiarrhythmic activity of Nardostachys jatamansi (an Indian indigenous drug) Indian J Med Res. 1956;44(2):259–269. [PubMed] [Google Scholar]
- 35.Dixit V.P., Jain P., Joshi S.C. Hypolipidaemic effects of Curcuma longa and Nardostachysjatamansi, DC in triton/induced hyperlipidaemic rats. Indian J Physiol Pharmacol. 1988;32(4):299–304. [PubMed] [Google Scholar]
- 36.Bhowmik Debjit, Sampath Kumar K.P., Paswan Shravan, Srivatava Shweta, Yadav Akhilesh pd., Dutta Amitsankar. Traditional Indian Herbs Convolvulus pluricaulis and its medicinal importance. J Pharmacogn Phytochem. 2012;1(1):44–51. [Google Scholar]
- 37.Patel Pinal, Vaghasiya Jitendra, Thakor Ashokji, Jariwala Jitesh. Antihypertensive effect of rhizome part of Acoruscalamus on renal artery occlusion induced hypertension in rats. Asian Pac J Trop Dis. 2012;2(Suppl. 1):S6–S10. [Google Scholar]
- 38.Taqvi S.I., Shah A.J., Gilani A.H. Blood pressure lowering and effects of piperine. J Cardiovasc Pharmacol. 2008;52:452–458. doi: 10.1097/FJC.0b013e31818d07c0. [DOI] [PubMed] [Google Scholar]
- 39.Patgiri Biswajyoti, Gokarn Rohit. Research works done on rasasindura (sublimated mercurial preparation) – a critical review. Ayurpharm Int J Ayur Alli Sci. 2014;3(2):41–47. [Google Scholar]
- 40.Kumaria Reeta, Rathib Brijesh, Ranic Anita, Bhatnagar Sonal. Rauvolfia serpentina L. Benth. ex Kurz.: phytochemical, pharmacological and therapeutic aspects. Int J Pharm Sci Rev Res. Nov–Dec 2013;23(2):348–355. no 56. [Google Scholar]
- 41.Aparna K., Joshi Abhishek J., Vyas Mahesh. Phyto-chemical and pharmacological profiles of Hyoscyamus niger Linn (ParasikaYavani) – a review. Pharma Science Monitor. Jan–Mar 2015;6(1):153–158. [Google Scholar]
- 42.Arora R.B., Arora C.K., Sha M.J., Shet U.K. Animal species variation in hypotensive activity of jatamansone with a report in the clinical trial of this drug. Ind J Med Sci. 1967;21:455–460. [PubMed] [Google Scholar]
- 43.Umeyama A., Shoji N., Saito N., Takemoto T., Kajiwara A., Ohizumi Y. Dehydropipernonaline, an amide possessing coronary vasodilating activity isolated from P. longum. J Pharm Sci. 2006;75(12):1188–1189. doi: 10.1002/jps.2600751215. [DOI] [PubMed] [Google Scholar]
- 44.https://india.gov.in/narcotic-drugs-and-psychotropic-substances-act1985. [Accessed 19 March 2017].
- 45.Tsou K., Brown S., Sanudo-Pena M.C., Mackie K., Walker J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 46.Walther S., Mahlberg R., Eichmann U., Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl) 2006;185(4):524–528. doi: 10.1007/s00213-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 47.Beal J.E., Olson R., Laubenstein L., Morales J.O., Bellman P., Yangco B. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manag. 1995;10(2):89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- 48.Vaney C., Heinzel-Gutenbrunner M., Jobin P., Tschopp F., Gattlen B., Hagen U. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10(4):417–424. doi: 10.1191/1352458504ms1048oa. [DOI] [PubMed] [Google Scholar]
- 49.Zubrzycki M., Liebold A., Janecka A., Zubrzycka A. A new face of endocannabinoids in pharmacotherapy. Part I: protective role of endocannabinoids in hypertension and myocardial infarction. J Physiol Pharmacol. 2014 Apr;65(2):171–181. [PubMed] [Google Scholar]
- 50.Maule W.J. Medical uses of marijuana (Cannabis sativa): fact or fallacy? Br J Biomed Sci. 2015;72(2):85–91. doi: 10.1080/09674845.2015.11666802. [DOI] [PubMed] [Google Scholar]
- 51.Huestis M.A., Gorelick D.A., Heishman S.J., Preston K.L., Nelson R.A., Moolchan E.T. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001 Apr;58(4):322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 52.Patton G.C., Coffey C., Carlin J.B., Degenhardt L., Lynskey M., Hall W. Cannabis and mental health in young people: cohort study. BMJ. 2002 Nov 23;325(7374):1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandit Kashinath Shastri, editor. Rasatarangini of Sadanand Sharma, Vishopvisha vidnyaniyam. Ist ed. Motilal Banarsidas Prakashak; Verse 394-398, Delhi: 1994. p. 720. [Chapter 24] [Google Scholar]
- 54.Shukla Gyanendra, Bhatted Santosh K., Dave Alankruta R., Shukla Vageesha Datta. Efficacy of virechan and basti karma with shaman therapy in the management of essential hypertension: a comparative study. AYU. Jan–March 2013;34(1):70–76. doi: 10.4103/0974-8520.115455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali A., Umar D., Farhan M., Basheer B., Baroudi K. Effect of Brahmyadi Churna (Brahmi, Shankhapushpi, Jatamansi, Jyotishmati, Vacha, Ashwagandha) and tablet Shilajatu in essential hypertension: an observational study. J Adv Pharm Technol Res. 2015;6(4):148–153. doi: 10.4103/2231-4040.165015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar Ajaya, Singhal Tina, Upadhyaya B. Clinical evaluation of Chandrmaradi yoga in patients of essential hypertension. AYU. July–Sept. 2009;30(3):249–254. [Google Scholar]
- 57.Ried K., Frank O.R., Stocks N.P., Fakler P., Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008 Jun 16;8:13. doi: 10.1186/1471-2261-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fugh-Berman A. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Prev Cardiol. 2000;3:24–32. doi: 10.1111/j.1520-037x.2000.80355.x. [DOI] [PubMed] [Google Scholar]
- 59.Arora R.B., Roy S., Khan S.U. Role of elements in pathophysiology of hypertension and antihypertensive edrug development. Acta Pharmacol Toxicol (Copenh) 1986;59:344–347. doi: 10.1111/j.1600-0773.1986.tb02776.x. [DOI] [PubMed] [Google Scholar]
- 60.Ajagbonna O.P., Mojiminiyi F.B.O., Sofola O.A. Relaxant effects of the aqueous leaf extract of Cassia occidentalis on rat aortic rings. Afr J Biomed Res. 2001;4:127–129. [Google Scholar]
- 61.Hasrat J.A., Pieters L., Vlietinck A.J. Medicinal plants in Suriname. J Pharm Pharmacol. 2004;56:381–387. doi: 10.1211/0022357022917. [DOI] [PubMed] [Google Scholar]
- 62.Asgary S., Naderi G.H., Sarrafzadegan N., Mohammadifard N., Mostafavi S., Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000;26(3):89–93. [PubMed] [Google Scholar]
- 63.Dubey M.P., Srimal R.C., Nityanand S., Dhawan B.N. Pharmacological studies on coleonol, a hypotensive diterpene from Coleus forskohlii. J Ethnopharmacol. 1981;3:1–13. doi: 10.1016/0378-8741(81)90010-6. [DOI] [PubMed] [Google Scholar]