Highlights
-
•
Cotton fever is a self-limited phenomenon that occurs in IV drug users.
-
•
Colloquially known to IV drug users, cotton shooting can cause cotton fever.
-
•
Enterobacter asburiae endocarditis is a rare complication of cotton fever.
Keywords: Cotton fever, Bacteremia, Enterobacter, Endocarditis, Injection drug use, Withdrawal
Abstract
“Cotton fever” is described as a self-limiting illness following “cotton shooting,” the practice of injecting residual drugs extracted from previously used cotton filters. Cases of related Enterobacter agglomerans bacteremia have been described. We report the first described case of cotton fever in a patient resulting in Enterobacter asburiae endocarditis.
Introduction
Almost three percent of the United States (US) population are persons who inject drugs (PWID) [1]. Twelve percent of injection drug use (IDU)-related hospitalizations in the US are for infective endocarditis (IE) [2]. One of the most common presentations of PWIDs in the Emergency Department (ED) is fever. The etiology of fever in these patients, whether it be infectious or induced by the type of drug injected, can be challenging for physicians to discern [3]. While the ailment of “Cotton Fever” is widely acknowledged amongst PWIDs, it is rarely recognized in the medical community and less than a dozen cases have been presented in peer-reviewed literature [[4], [5], [6], [7]].
Cotton fever occurs after “cotton shooting,” (Fig. 1) which is the process of injecting trace amounts of drugs extracted from previously used cotton filters [8]. It is described as a transient elevation in core body temperature of 2–4℉ that occurs minutes after injection [7]. Other symptoms include rigors, headache, nausea, vomiting, and myalgias, similar to symptoms of opioid withdrawal. The pathophysiology of cotton fever remains unclear, with three proposed theories since its initial description in 1975 [8]. The pharmacologic theory hypothesizes that cotton contains substances with pyrogenic activity, whereas the immunologic theory proposes that people have preformed antibodies to cotton. The endotoxin theory suggests that cotton fever may result from the injection of endotoxins released by Gram negative bacilli such as Enterobacter agglomerans [4].
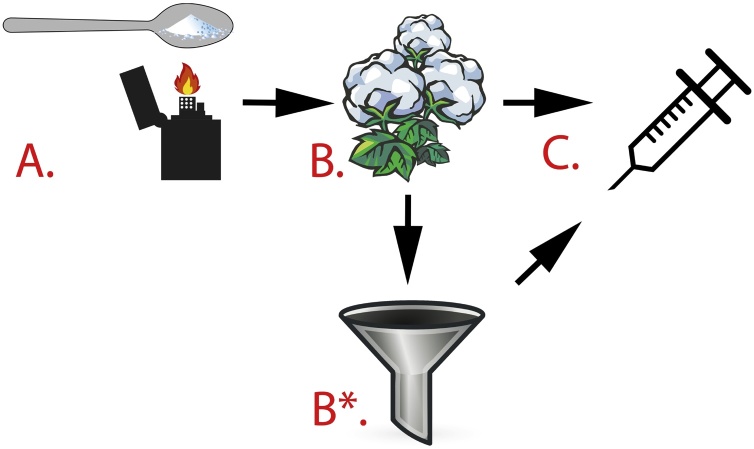
Fig. 1.
Schematic of IV drug use and cotton shooting.
(A) Many IV drugs, such as cocaine and heroin, are heated prior to use.
(B) The resulting product is filtered through cotton balls to sift out any large particles or debris
(C) Syringes are typically used for intravenous injection.
(B*) When drug supply decreases, IVDUs may resort to “cotton shooting”, which is the process of injecting trace amounts of drugs that are extracted from previously used cotton filters. IVDUs have described cotton fever as a transient elevation in core body temperature of 2–4 degrees that occurs within minutes after injection.
Enterobacter agglomerans has been the only bacterium implicated with cotton fever. It has been shown to colonize cotton fibers and has been cultured from blood taken during an episode of cotton fever [9,10]. We present a unique case of cotton fever that is the first to be associated with Enterobacter cloacae complex (ECC) as well as the first to illustrate the complication of infective endocarditis as a potential sequela associated with cotton fever.
Case report
A 32-year-old Caucasian male with a past medical history of intravenous (IV) heroin use and untreated hepatitis C presented to the ED with heroin withdrawal and fever. He reported a two-day history of nausea, vomiting, and palpitations. During this time, he resorted to cotton shooting for relief. Upon injection, he noted that his aforementioned symptoms worsened and were accompanied by fever and rigors. Of note, the patient had a previous admission, 19 months prior, for a lung abscess and a small temporal lobe brain abscess in the setting of negative blood cultures and a negative transesophageal echocardiogram (TEE) which was managed inpatient with 6 weeks of IV antimicrobial therapy of vancomycin and ceftriaxone, and imaging showed improvement in the abscesses.
In the ED, the patient’s vital signs showed a tympanic temperature of 101.6℉, heart rate of 171 beats/minute, and blood pressure of 132/68 mmHg. Physical examination revealed an anxious, thin Caucasian male with moderately dilated pupils and numerous injection site scars and tattoos on his upper extremities bilaterally. Cardiac exam revealed tachycardia. No murmurs were auscultated. The respiratory, abdominal, neurological, and psychiatric exams were unremarkable. Admission labs revealed WBC 4.03 K/uL; Hgb 11.5 g/dL; Hct 36.6 %; Neutrophils 88.2 %, Sodium 136 mmol/L; Potassium 5.3 mmol/L; BUN 14 mg/dL; Creatinine 1 mg/dL; Lactate 1.8, and liver enzymes and coagulation tests were within normal limits. The patient was started on IV vancomycin, cefepime, and fluids. Nonspecific ST depressions and elevations were seen on electrocardiogram, and initial troponin T level was <0.01 ng/mL. Telemetry did not reveal any abnormalities and serial EKGs and cardiac enzymes were unremarkable.
Admission blood cultures grew Enterobacter asburiae (Enterobacter cloacae complex), resistant to ampicillin, amoxicillin, and cefoxitin. All repeat blood cultures were negative. A transthoracic echocardiogram (TTE) revealed a decreased ejection fraction (EF) of 25 %–30 % and mild tricuspid regurgitation. TEE revealed new mitral valve vegetations on the anterior leaflet as well as pulmonic valve thickening/vegetation and an atheroma of the descending aorta. A TEE from his admission the year prior, where he presented with a lung and brain abscess, showed no vegetations and an EF of 35–40 %.
The patient was continued on high dose cefepime with plan for long-term IV antibacterial therapy for infective endocarditis. The patient left against medical advice after a six-day hospital admission and was thereby given a 6-week course of oral ciprofloxacin. Unfortunately, the patient was lost to outpatient follow-up.
Discussion
Classically described by PWIDs, less than a dozen case reports of cotton fever exist in the medical literature. A lack of unique diagnostic criteria outside of patient history and unclear pathogenesis complicate the diagnosis of cotton fever. While the only previously described pathogen associated with cotton fever has been Enterobacter agglomerans, we describe a case of Enterobacter asburiae, a member of the E. cloacae complex, previously described as an endophyte of cotton plants and as was used as a biological seed protectant to control seed-rotting fungi [11].
Our patient had multi-valvular endocarditis secondary to E. asburiae seen only on TEE. Despite not being able to culture the cotton filter to confirm it as the source, this is most plausible given his history of shooting cotton. Gram positive organisms are most commonly causative of IE in PWID [12]. In an article describing non-HACEK gram-negative rod (GNR) endocarditis, non-HACEK GNR accounted for only 49 of the endocarditis cases (2 %) and IDU was uncommon overall (<10 %) [13]. In a 2012 review article of Enterobacter endocarditis, only 2 of the 27 Enterobacter cloacae cases described were associated with IDU [14].
Opioid withdrawal symptoms overlap with those of cotton fever, often mimicking and masking symptoms that would point towards other causes of fever which may be life-threatening. Due to multiple comorbidities associated with IDU, cotton fever is often a diagnosis of exclusion [15]. While early recognition of cotton fever has been shown to decrease the cost of secondary evaluations and minimize prolonged hospital stays, as the clinical course is typically benign and symptoms resolve within the first 12−48 hours of onset, serious infections such as bacteremia and endocarditis must be excluded [4,7]. This case emphasizes the need for clinicians to perform a thorough workup despite the typically benign and self-limited presentation of cotton fever.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethics approval
Not required.
CRediT authorship contribution statement
Munib J. Francis: Conceptualization, Investigation, Data curation, Writing - original draft. Justin Chin: Conceptualization, Investigation, Data curation, Writing - original draft, Writing - review & editing. Christine M. Lomiguen: Conceptualization, Resources, Writing - original draft, Writing - review & editing, Supervision. Allison Glaser: Conceptualization, Methodology, Validation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors disclose that there were no conflicts of interest or financial support in the development of this project.
References
- 1.Lansky A., Finlayson T., Johnson C., Holtzman D., Wejnert C., Mitsch A. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipari R.N., Hedden S.L., Hughes A. 2013. Substance use and mental health estimates from the 2013 national survey on drug use and health: overview of findings. The CBHSQ report. Rockville (MD) pp. 1–10. [PubMed] [Google Scholar]
- 3.Haber P., Demirkol A., Lange K., Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284–1293. doi: 10.1016/S0140-6736(09)61036-9. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D.W., Walls R.M. Cotton fever": a benign febrile syndrome in intravenous drug abusers. J Emerg Med. 1990;8(2):135–139. doi: 10.1016/0736-4679(90)90222-h. [DOI] [PubMed] [Google Scholar]
- 5.Ramik D., Mishriki Y. The other “Cotton fever”. Infect Dis Clin Pract. 2008;16(3):192–193. [Google Scholar]
- 6.Torka P., Gill S. Cotton fever: an evanescent process mimicking sepsis in an intravenous drug abuser. J Emerg Med. 2013;44(6):e385–7. doi: 10.1016/j.jemermed.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y., Pope B.A., Hunter A.J. Cotton fever: does the patient know best? J Gen Intern Med. 2016;31(4):442–444. doi: 10.1007/s11606-015-3424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson B.D. Medical complications following intravenous heroin. Ariz Med. 1975;32(10):798–801. [PubMed] [Google Scholar]
- 9.Dutkiewicz J., Mackiewicz B., Lemieszek M.K., Golec M., Milanowski J. Pantoea agglomerans: a marvelous bacterium of evil and good.PArt I. Deleterious effects: dust-borne endotoxins and allergens - focus on cotton dust. Ann Agric Environ Med. 2015;22(4):576–588. doi: 10.5604/12321966.1185757. [DOI] [PubMed] [Google Scholar]
- 10.Jamison J.P., Lowry R.C. Branchial challenge of normal subjects with the endotoxin of Enterobacter agglomerans isolated from cotton dust. Br J Ind Med. 1986;43(5):327–331. doi: 10.1136/oem.43.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quadt-Hallmann A., Kloepper J., Benhamou N. Bacterial endophytes in cotton: mechanisms of entering the plant. Can J Infect Dis Med Microbiol. 1997;43(6):557–582. [Google Scholar]
- 12.Dworkin R.J., Lee B.L., Sande M.A., Chambers H.F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- 13.Morpeth S., Murdoch D., Cabell C.H., Karchmer A.W., Pappas P., Levine D. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147(12):829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 14.Moon J., Smith T., Sahud A.G., Bhanot N. An unusual etiology of infective endocarditis: enterobacter cloacae. J Infect Chemother. 2012;18(6):925–930. doi: 10.1007/s10156-012-0376-9. [DOI] [PubMed] [Google Scholar]
- 15.Fenton M.C., Keyes K., Geier T., Greenstein E., Skodol A., Krueger B. Psychiatric comorbidity and the persistence of drug use disorders in the United States. Addiction. 2012;107(3):599–609. doi: 10.1111/j.1360-0443.2011.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]