Highlights
-
•
Young patients with cryptogenic strokes have preserved left atrial size.
-
•
Left atrial dysfunction is present in young patients with cryptogenic stroke.
-
•
Left atrial strain is a discriminator of atrial dysfunction in cryptogenic stroke.
-
•
Young paroxysmal AF have abnormal left atrial size and function.
Keywords: Atrial fibrillation, Left atrial function, Stroke
Abbreviations: LVEDD, Left ventricular end diastolic diameter; LVESD, Left ventricular end systolic diameter; LVEF, Left ventricular ejection fraction; LV GLS, Left ventricular global longitudinal strain; RV, Right ventricle; TAPSE, Tricuspid annular plane systolic excursion; RVSP, Right ventricular systolic pressure; RA, Right atrial; LA, Left atrial; LAEF, Left atrial ejection fraction; LA GLS, Left atrial global longitudinal strain
Abstract
Background
Stroke is one of the leading causes of morbidity and mortality with a significant percentage classified as cryptogenic. Left atrial (LA) remodelling, a substrate for atrial fibrillation (AF) and stroke development, may play a role in identification of the aetiology of cryptogenic stroke. We aimed to examine LA function to gain mechanistic insights into the pathophysiology of cryptogenic stroke in young patients otherwise at low risk for cardiovascular disease.
Methods
Patients aged <60 years without traditional cardiovascular risk factors and who were diagnosed with ischaemic cryptogenic stroke or TIA were evaluated and compared to healthy controls and patients with paroxysmal AF with a CHA2DS2-VA score of 0. Conventional and novel left ventricular (LV) and LA echocardiographic parameters between the three groups were assessed.
Results
Each group consisted of thirty patients. There were no significant differences in LV parameters (LVEF, LV endoGLS) between groups. LA strain in stroke patients was significantly lower compared to the controls (median 33%; interquartile range (IQ) [32/39] vs 31 [27/34]; p = 0.008). LA strain was significantly lower in AF patients compared to stroke patients (median 21% [19/22] vs 31% [27/34]; p < 0.0001).
Conclusion
A stepwise reduction in measures of LA function was appreciated between controls, young stroke and paroxysmal AF groups. This may indicate dynamic LA remodelling occurring in the young stroke population and suggest a shared causal mechanism for stroke development in this group. LA strain may further refine the risk for cardioembolic stroke.
1. Introduction
Although the majority of strokes affect the elderly, there has been a rise in incidence of stroke in young adults over the past decade [1], [2]. This presents unique diagnostic challenges as up to 20% of this group lack classical modifiable cardiovascular risk factors which are traditionally associated with stroke development [3]. Many of these strokes are classified as cryptogenic, whereby the exact aetiology and pathophysiology of the stroke remains elusive.
Occult paroxysmal atrial fibrillation (AF) has been proposed to be the cause of a significant proportion of cryptogenic ischaemic strokes [4]. The ASSERT study, which evaluated the incidence of subclinical atrial arrhythmias in stroke in an elder population, demonstrated a relationship between subclinical AF and an increased risk of stroke and embolism. However, interestingly there was a lack of a clear temporal relationship between arrhythmia and stroke development [5]. This suggests that there may be other causative factors which may play a role in the pathogenesis of AF and cryptogenic ischaemic stroke. While shared risk factors such as atherosclerosis and hypertension may be contributive, emerging evidence suggests that left atrial (LA) dysfunction in the absence of captured AF may be associated with thromboembolism and stroke development [6], [7], [8].
Structural and functional changes in the left atrium have been linked to the pathophysiology of incident AF, ischaemic stroke and adverse cardiovascular outcomes [9], [10]. Additionally, there is evidence supporting LA strain as a measure of subclinical LA dysfunction with incremental value in prediction of AF in a cryptogenic stroke cohort over traditional clinical and echocardiographic parameters [11].
Given this intricate and dynamic relationship between LA remodelling, AF and ischaemic stroke development, we propose that measures of LA function such as LA strain may serve as a biomarker for stratification of young patients with cryptogenic stroke with regards to cardioembolic stroke risk. Hence the aim of our study was to evaluate differences in cardiac chamber size and function between a cohort of young cryptogenic stroke patients, young healthy adults and those with paroxysmal AF in a young cohort without cardiovascular risk factors.
2. Methods
2.1. Study population
This a retrospective analysis of a prospectively collected study population. Young patients aged <60 years who were admitted to our institution with diagnosis of cryptogenic ischaemic stroke or transient ischaemic attack (TIA) were evaluated. Only those without traditional cardiovascular risk factors (any history of ischaemic heart disease, heart failure, hypertension, diabetes mellitus, stroke, hypercholesterolaemia, valvular or congenital) and who received transthoracic echocardiography (TTE) during their admission were included. These patients were compared to young (<60 years) healthy controls identified from referrals to our echocardiography laboratory and young (<60 years) patients with paroxysmal AF with a CHA2DS2-VA score of 0 identified from admissions to our service.
All patients in the stroke group received at least 24 hours of cardiac monitoring. As part of their stroke workup, these patients also underwent computed tomography and magnetic resonance imaging of the brain as well as comprehensive vascular imaging of the aortic arch, neck and cerebral vessels. The diagnosis of stroke or TIA was adjudicated by the patient’s treating stroke specialist based on the Causative Classification of Ischaemic Stroke (CCS) system.
Our study was approved by the New South Wales Human Research and Ethics Committee.
2.2. Echocardiography
TTE was performed using commercially available equipment according to recommendations of the American Society of Echocardiography [12]. All echocardiographic images were saved in digital format and analysis was performed offline by two investigators blinded to the clinical history of the patients. All subjects were in sinus rhythm at the time the measurements were obtained with analysis performed using a mean of three cardiac cycles.
Two-dimensional speckle tracking strain analysis was performed offline using vendor independent software (TomTec Arena, Germany v4.6). For LV global longitudinal strain (GLS), the LV endocardium was traced at end-systole in the apical 4-, 3- and 2- chamber views and LV GLS was measured as an average of the 18 LV segments. For LAs, the LA endocardium was manually traced at end-systole in the apical 4- and 2-chamber views and LA reservoir strain was evaluated using R-to-R gating.
Intra- and inter-observer variability were assessed by repeating LV GLS and LA reservoir strain assessment in 10 randomly selected patients from the cohort >4 weeks apart by the same investigator and by a second independent investigator. Reproducibility of these measurements was represented by the intra-class correlation coefficient and coefficient of variation using the logarithmic method.
2.3. Statistical analysis
Categorical variables were presented as numbers and percentages. A Shapiro-Wilk test was performed to evaluate the distribution of the continuous variables, expressed as mean ± standard deviation or interquartile range (Q1–Q3) as appropriate. A Kruskal-Wallis H test was conducted to determine if there were significant differences in continuous variables between the three groups, and the Mann–Whitney U test was used for comparison between two groups. All analyses were performed using SPSS 22.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics
A total of 90 patients (30 controls, 30 cryptogenic stroke patients and 30 patients with paroxysmal AF) were compared (mean age 41.0 ± 11.0 years; 57.8% male). The mean body mass index (BMI) of the study population was 28.6 ± 6.2 kg/m2. There were no significant differences in age or BMI between groups. There were no significant differences between groups with regards to baseline cardiovascular risk factors given the exclusionary criteria for the study.
3.2. Echocardiographic parameters
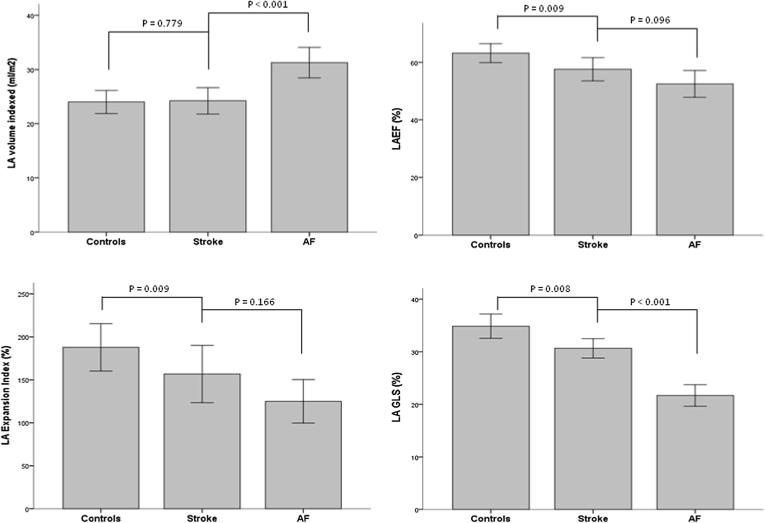
No significant differences were observed in LV parameters between controls and young patients with cryptogenic stroke, including measures of LV size and function. Though there were no significant differences in measures of LA size (LA volume indexed; p = 0.779), a reduction in LA function was appreciated in stroke patients relative to the healthy controls, as reflected by a lower LA emptying fraction (p = 0.009), LA expansion index (p = 0.009) and LA GLS (p = 0.008) (Fig. 1).
Fig. 1.
Comparison of LA parameters between young healthy controls, young cryptogenic stroke and young paroxysmal AF. * Data are expressed as median and interquartile range Error bars: 95% CI.
On evaluation of the three cohorts, a stepwise reduction in functional parameters of the left atrium was appreciated (Table 1) including a reduction in LAEF (p < 0.01), LA expansion index (p < 0.01) and LA GLS (p < 0.01) (Table 1). LAVI was not measurably different between the controls and stroke patients but was significantly larger in patients with paroxysmal AF. Of interest, similar trends were not observed with systolic and diastolic parameters of the left ventricle.
Table 1.
Comparison of echocardiographic parameters between young healthy controls, young cryptogenic stroke and young paroxysmal AF.
| Variables* | Controls | Stroke | AF | p Value† |
|---|---|---|---|---|
| LVEDD (cm) | 4.7 [4.1/4.9] | 4.9 [4.4/5.3] | 4.5 [4.3/5.0] | 0.269 |
| LVESD (cm) | 2.9 [2.7/3.2] | 3.0 [2.6/3.4] | 2.9 [2.7/3.1] | 0.861 |
| LVEF (%) | 63 [58/66] | 59 [58/64] | 63 [61/69] | 0.065 |
| LV GLS (%) | 18.7 [16.9/21.4] | 17.9 [16.5/19.5] | 20.9 [19.4/22.5] | 0.983 |
| LV mass (g/m2) | 68 [56/81] | 72 [65/85] | 75 [62/86] | 0.938 |
| E/e' ratio | 6.4 [5.3/9.3] | 6.7 [5.3/8.6] | 6.8 [5.2/9.0] | 0.872 |
| RV S' vel (cm/s) | 12.8 [11.1/14.3] | 12.4 [11.4/13.8] | 12.0 [11.0/14.0] | 0.421 |
| TAPSE (cm) | 2.4 [2.2/2.5] | 2.5 [2.0/2.8] | 2.6 [2.1/2.8] | 0.601 |
| RVSP (mmHg) | 23 [20/26] | 20 [17/22] | 28 [21/29] | 0.033 |
| RA area (cm2) | 13 [11/16] | 14 [13/17] | 16 [14/19] | 0.046 |
| LA parameters | ||||
| LA max volume (ml/m2) | 24 [19/28] | 24 [18/29] | 31 [26/34] | <0.001 |
| LA min volume (ml/m2) | 9 [7/11] | 10 [7/12] | 13 [12/16] | <0.001 |
| LA EF (%) | 62 [58/69] | 55 [50/63] | 52 [46/61] | 0.001 |
| LA expansion index (%) | 164 [139/222] | 122 [101/174] | 108 [84/156] | 0.001 |
| LA GLS (%) | 33 [32/39] | 31 [27/34] | 21 [19/22] | <0.001 |
Data are expressed as the median [interquartile range].
Kruskal Wallis Test was used to compare the 3 groups.
For the LA GLS measurements, the interclass correlation was 0.97 with a 95% Confidence Interval of 0.90–0.99 and the coefficient of variation was 3.42 with a 95% Confidence Interval of 1.67–5.19.
4. Discussion
Our study findings show a reduction in LA functional metrics between a healthy cohort and young cryptogenic stroke population, despite similar age, comorbidities and traditional echocardiographic profiles within groups. When compared to a comparator group with diagnosed young paroxysmal AF and a CHA2DS2-VA score of 0, a further reduction in measures of LA function and changes to atrial structure was appreciated. This would indicate that there are early subclinical changes to the left atrium in some of the young cryptogenic stroke population without primary causative factors. The changes may originate in the left atrium itself, which contribute to LA remodelling and risk of AF development.
Our findings are in keeping with other studies, which have demonstrated a relationship between markers of atrial pathology and ischaemic stroke development in the absence of captured AF, suggesting that tissue substrate rather than arrhythmia status is the primary driver of stroke risk. Atrial arrhythmias besides AF have been associated with stroke, with studies showing a relationship between atrial ectopy and paroxysmal supraventricular tachycardia with ischaemic stroke independently of AF [13], [14], [15]. Structural changes to the left atrium have also been associated with increased ischaemic stroke risk in the absence of AF; the P-wave terminal force on electrocardiogram, a marker of LA pathology and fibrosis, has been associated with incident ischaemic stroke development [16], [17], [18]. Similarly, LA size as quantified by echocardiography has shown an associated with ischaemic stroke, even after adjusting for AF presence [8], [19], [20].
Of note, in our study unlike the young paroxysmal AF group, patients with cryptogenic ischaemic stroke had no significant change in LA volume when compared to healthy controls. This would indicate that functional impairment precedes structural changes to the left atrium in this population. As enlargement of the left atrium is a reflection of chronic elevation of LA pressures as well as remodelling from AF, LA function is a sensitive marker of early changes in the atrium and useful in a relatively healthy cohort without traditional cardiovascular risk factors.
Our study had a modest sample size given stringent inclusion criterion and included only young patients with no pre-existent cardiovascular disease or traditional risk factors and were considered low cardiovascular risk. Future studies with larger sample sizes are required to better define the associations found in our study.
5. Conclusion
LA functional parameters were impaired in patients with cryptogenic stroke compared with healthy controls with similar LA volumes. In a young cryptogenic stroke population, a reduction in LA function may provide additional insights in the pathophysiology of stroke.
References
- 1.Tibæk M., Dehlendorff C., Jorgensen H.S., Forchhammer H.B., Johnsen S.P., Kammersgaard L.P. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. J. Am. Heart. Assoc. 2016;5 doi: 10.1161/JAHA.115.003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medin J., Nordlund A., Ekberg K. Increasing stroke incidence in Sweden between 1989 and 2000 among persons aged 30 to 65 years: evidence from the Swedish Hospital Discharge Register. Stroke. 2004;35:1047–1051. doi: 10.1161/01.STR.0000125866.78674.96. [DOI] [PubMed] [Google Scholar]
- 3.Aigner A., Grittner U., Rolfs A., Norrving B., Siegerink B., Busch M.A. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017;48:1744–1751. doi: 10.1161/STROKEAHA.117.016599. [DOI] [PubMed] [Google Scholar]
- 4.Healey J.S., Connolly S.J., Gold M.R., Carsten W.I., Gelder I.C., Capucci A., Lau C.P., Fain E., Yang S., Bailleul C., Morillo C.A., Carlson M., Themeles E., Kaufman E.S., Hohnloser S.H. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012;366:120. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 5.Brambatti M., Connolly S.J., Gold M.R., Morillo C.A., Capucci A., Muto C., Lau C.P., Van Gelder I.C., Hohnloser S.H., Carlson M., Fain E., Nakamya J., Mairesse G.H., Halytska M., Deng W.Q., Israel C.W., Healey J.S., ASSERT Investigators Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 6.Kamel H., Bartz T.M., Elkind M.S.V., Okin P.M., Thacker E.L., Patton, Stein K.K., deFilippi C.R., Gottesman R.F., Heckbert S.R., Kronmal R.A., Soliman E.Z., Longstreth W.T. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study) Stroke. 2018;49:980. doi: 10.1161/STROKEAHA.117.020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D., Shim C.Y., Hong G.R., Kim M.H., Seo J., Cho I.J., Kim Y.D., Chang H.J., Ha J.W., Heo J.H., Chung N. Clinical implications and determinants of left atrial mechanical dysfunction in patients with stroke. Stroke. 2016;47:1444–1451. doi: 10.1161/STROKEAHA.115.011656. [DOI] [PubMed] [Google Scholar]
- 8.Barnes M.E., Miyasaka Y., Seward J.B., Gersh B.J., Rosales A.G., Bailey K.R., Petty G.W., Wiebers D.O., Tsang T.S. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo. Clinic. Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 9.Tsang T.S., Abhayaratna W.P., Barnes M.E., Miyasaka Y., Gersh B.J., Bailey K.R., Cha S.S., Seward J.B. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J. Am. College Cardiol. 2006;47:1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 10.Obokata M., Negishi K., Kurosawa K., Tateno R., Tange S., Arai M., Amano M., Kurabayashi M. Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-VASc score and indicates prognostic impact in patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2014;27:709–716. doi: 10.1016/j.echo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Pathan F., Sivaraj E., Negishi K., Rafiudeen R., Pathan S., D'Elia N., Galligan J., Neilson S., Fonseca R., Marwick T.H. Use of atrial strain to predict atrial fibrillation after cerebral ischemia. JACC. Cardiovasc. Imaging. 2018;11:1557–1565. doi: 10.1016/j.jcmg.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Binici Z., Intzilakis T., Nielsen O.W., Kober L., Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;1904–1911 doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 14.Kamel H., Elkind M.S., Bhave P.D., Navi B.B., Okin P.M., Iadecola C., Devereux R.B., Fink M.E. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todo K., Moriwaki H., Saito K., Naritomi H. Frequent premature atrial contractions in stroke of undetermined etiology. Eur. Neurol. 2009;61:285–288. doi: 10.1159/000206853. [DOI] [PubMed] [Google Scholar]
- 16.Kamel H., Soliman E.Z., Heckbert S.R., Kronmal R.A., Longstreth W.T., Nazarian S., Okin P.M. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohsaka S., Sciacca R.R., Sugioka K., Sacco R.L., Homma S., Di Tullio M.R. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamel H., Bartz T.M., Longstreth W.T., Okin P.M., Thacker E.L., Patton K.K., Stein P.K., Gottesman R.F., Heckbert S.R., Kronmal R.A., Elkind M.S., Soliman E.Z. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. doi: 10.1161/STROKEAHA.114.007762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Tullio M.R., Sacco R.L., Sciacca R.R., Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin E.J., D’Agostino R.B., Belanger A.J., Wolf P.A., Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]