Abstract
Herewith, we report on a method that allows to simultaneously protect both the ∆14,15 bond and the carbonyl group of the symmetrical bis-steroidal diketone 2. We found that environmentally friendly and gas-free chlorination is ideally suited to achieve this goal. This method was discovered during our efforts to methoxylate 2 in a solution of dichloromethane and basic methanol in the presence of diacetoxy iodobenzene. Unexpectedly, the ∆14,15 bonds were chlorinated once as well as twice in a statistical manner. Interestingly, the singly dichlorinated desymmetrized product is an ideal precursor for conduction a series of position selective transformations. Importantly, the carbonyl group present in the nonchlorinated hemisphere can be selectively reduced, olefinated or oximated, while the other carbonyl group stays unaltered. A structurally related “monomeric” steroid derivative undergoes ∆14,15 chlorination and 11-position methoxylation under same conditions. These findings represent a powerful entry for preparing new nonsymmetrical cephalostatin derivatives.
Keywords: Natural product chemistry, Organic chemistry, Biochemistry, Cancer research, Cephalostatin 1, Chemo and regioselective transformations, Gas-free chlorination method, Double-functional protection
Natural product chemistry; Organic chemistry; Biochemistry; Cancer research, Cephalostatin 1; Chemo and regioselective transformations.; Gas-free chlorination method; Double-functional protection.
1. Introduction
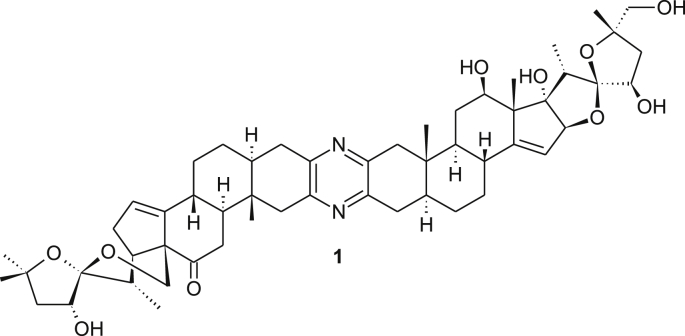
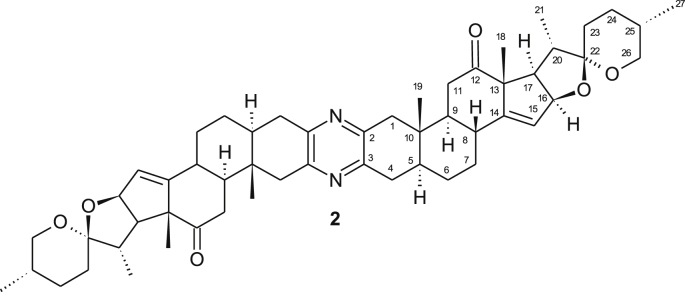
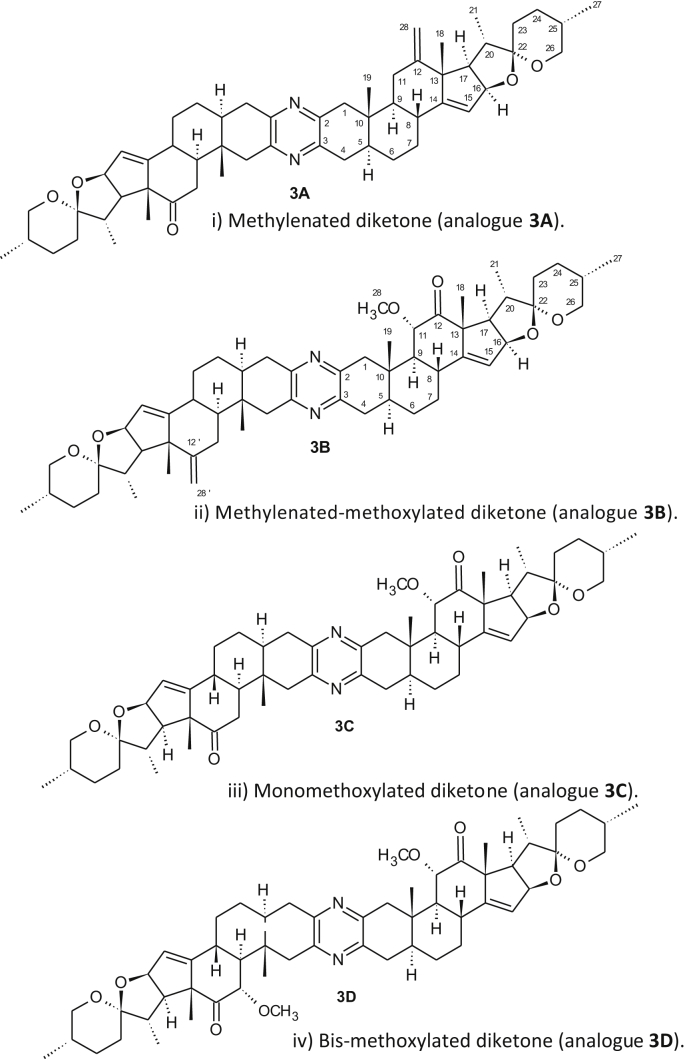
The highly antitumor active natural product cephalostatin 1 1 (Figure 1) which was isolated from the marine worm Cephalodiscus gilchristi among 18 other compounds by the Pettit group has attracted much attention [1, 2, 3, 4]. These metabolites show a unique cytotoxicity profile in in vitro screenings that were conducted at the National Cancer Institute. Indeed, cephalostatin 1 is an extremely powerful agent that acts via a unique apoptosis pathway [5] which was also found by us or an analogue [6]. The first total synthesis of cephalostatin 1 1 and several other natural analogues from the same family were reported by Fuchs et al. [7] Later, Shair and coworkers disclosed the second total synthesis of cephalostatin 1 1 [8]. Both synthetic routes are rather lengthy and relied on the unsymmetrical coupling of both steroidal parts. Winterfeldt and coworkers, however, pursued synthetic approaches based on both symmetrical as well as unsymmetrical approaches [9, 10]. These were based on the synthesis of symmetrical bis-steroidal diketone 2 (Figure 2) followed by selective desymmetrization. The starting material 2 was prepared by our group in gram scale in a straightforward manner [11]. Desymmetrization of the dimer includes three routes: First, selective reduction of the carbonyl group [9, 12]. Secondly, selective ring opening of the spiroketal moiety [13, 14, 15] and thirdly, regioselective hydroboration of both the exocyclic Δ12,28 bond and the ∆14,15 bond of selected dimers (analogues 3A and 3B; Figure 3) [16, 17].
Figure 1.
Structure of cephalostatin 1.
Figure 2.
Structure of diketone 2.
Figure 3.
Selected analogues derived from the diketone 2.
In this work, we disclose the chlorination of the ∆14,15 bond in an unconventional manner in order to mask both the double bond and the carbonyl group. This unprecedented chlorination method of the ∆14,15 bond was discovered when using the mild oxidizing agent diacetoxy iodosobenzene in a solvent system consisting of basic methanol and dichloromethane.
2. Results and discussion
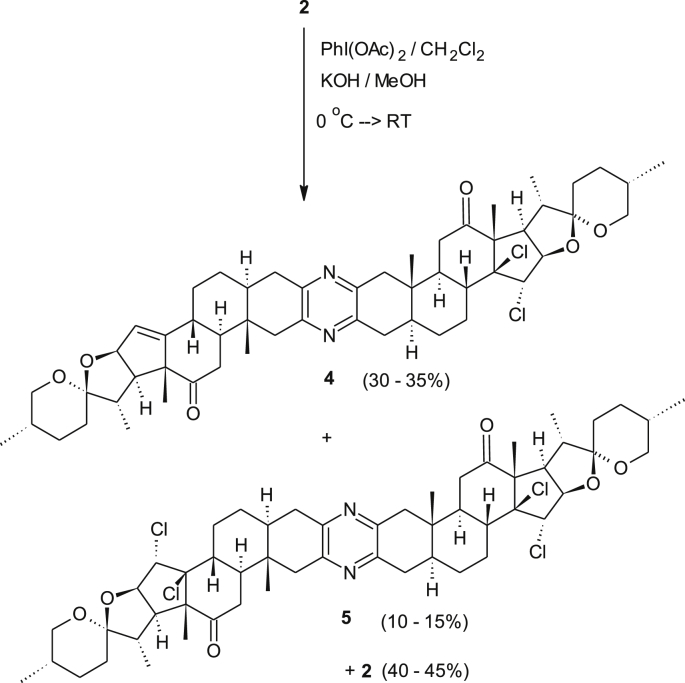
Over the last two decades, we concentrated our efforts on the preparation of new cephalostatin analogues starting from the readily available diketone 2 [9]. The discovery of chemoselective reactions that address only one hemisphere of the symmetrical diketone is of great importance either for the synthesis of new derivatives, that mimic the nonsymmetric cephalostatin 1 1. Therefore, we pursued to utilize multipurpose directing groups. Hence, by treating a solution of 2 in dichloromethane with diacetoxy iodobenzene in a solution of KOH/MeOH at temperatures between 0-10 °C provided the desired 11α-methoxy diketone 3C and its symmetrical byproduct 3D (Figure 3) [12]. However, adding a solution of 2 in undistilled dichloromethane to KOH/MeOH solution at 0 °C followed by the addition of diacetoxy iodobenzene and conducting the reaction at 0 °C for 2 h provided a different major product.
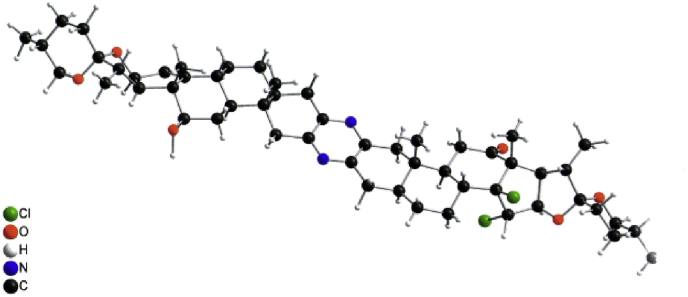
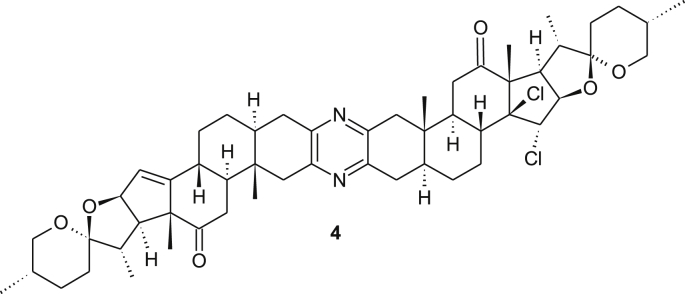
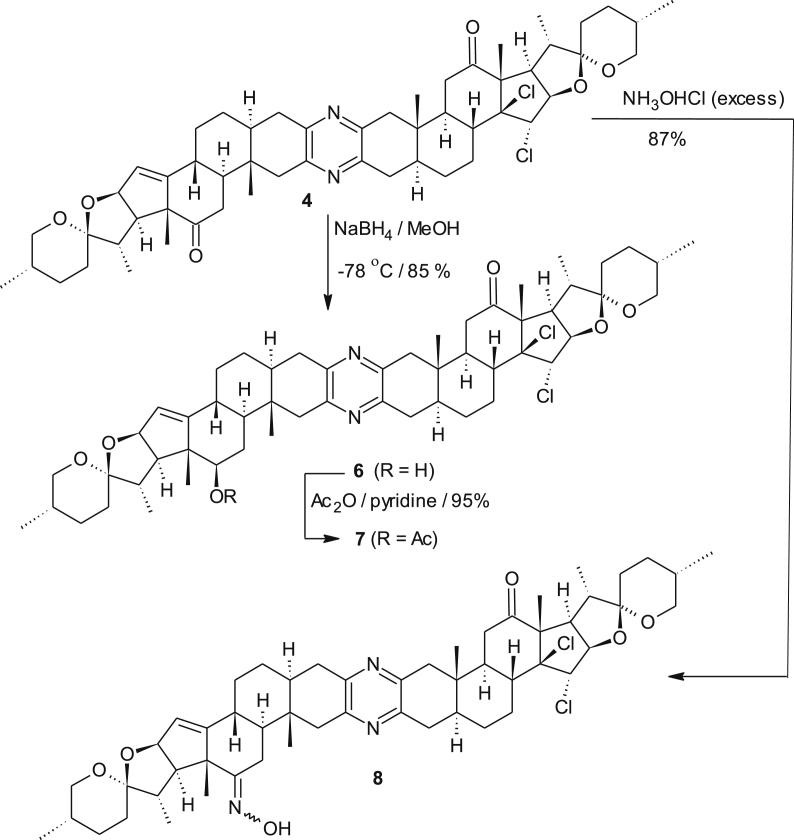
Mass spectrometry (FABS) of the major product showed a main peak at 917 with 71 units heavier compared to the starting material 2. However, analysis of the 1H NMR spectrum of this compound revealed that one signal of the ∆14,15 bonds had disappeared and a new singlet resonating at δ 4.62 ppm besides a doublet resonating at δ 4.46 ppm (J = 6.6 Hz) occurred. The sharp singlet resonating at δ 4.62 ppm indicates no coupling is visible with the neighbouring proton. The 13C-NMR spectrum revealed two signals at δ 210.7 and 209.1 ppm that corresponds to two carbonyl groups. In addition, a strongly deshielded sp [3]-hybridized quaternary carbon atom resonates at δ 90.0 ppm. Despite all these data and 2D-NMR-, IR-, MS- (ESI, HR-ESI) measurements the unequivocal structure elucidation was not possible. Firstly, we reckoned that a multi-oxygenated product was formed which may have resulted from an unknown reaction between the ∆14,15 bond and diacetoxy iodosobenzene. Chemically, this new product showed high resistance towards acetylation and under acidic hydrolysis conditions no transformation was observed. This valuable information suggested the absence of free hydroxyl groups or the presence of an acetal moiety. Moreover, sodium borohydride reduction led to the reduction of only one carbonyl group. This is a very important result, since it suggests that the new functional group in the former double bond region blocks the neighboring keto group. All attempts to collect crystals failed. However, we were satisfied to crystallize product 6 which resulted from the reduction of the new derivative with NaBH4. X-ray crystallographic analysis revealed (Figure 4) that two chlorine atoms were present likely bound at the site of the former ∆14,15 bond and the relative orientation of the 14,15-dichloro derivative 4 (see Figure 5) was trans (Scheme 1). Interestingly, no coupling between 15-H (singlet, δ 4.62 ppm) and 16-H (doublet, δ 4.46 ppm) was observed in the COSY as well as ROESY spectra. Obviously, these protons are oriented towards each other in a dihedral angle around 90° which agrees with the crystallographic data. These results raise the question of a possible mechanism (see Scheme 2).
Figure 4.
X-Ray molecular structure of 6.
Figure 5.
Structure of dichlorodiketone.
Scheme 1.
Chlorination of the symmetrical diketone 2.
Scheme 2.
Suggested mechanism of chlorination.
In Scheme 2, the possible mechanistic routes of chlorination are summarized. To gain information on the source of chlorine in the resulting product, two hypotheses were examined. Either, the solvent dichloromethane could be the source. The second hypothesis is based on the fact that dichloromethane contains traces of HCl which could be an alternative source for chlorine under the given conditions. We conducted several experiments to verify which hypotheses/route is the reasonable one (Scheme 2).
The first hypothesis follows the idea that the chlorinating agent is generated by the reaction of dichloromethane with diacetoxy iodobenzene in the presence of methanol under basic conditions. The possible substitution of one chloride with methoxy leads to intermediate A and Cl-ion which in turn can substitute the acetate group(s) in PhI(OAc)2. As a consequence, PhI(OAc)Cl (reagent A1, Scheme 2), which may be the reagent of chlorination (Route 1), and finally PhICl2 (reagent A2) would be formed. Still, PhICl2 can undergo reductive elimination to form iodobenzene and Cl2 which then adds to the olefinic double bond to yield the dichloro adduct (Scheme 2, Route 2). Moreover, PhICl2 may be added to the alkene forming the intermediate B and then decomposes to PhI and Cl-ion which in turn attacks the chloronium intermediate C (Scheme 2, Route 3). The other possibility is the formation of Cl2 from the oxidation of Cl-ion with PhI(OAc)2 (Scheme 2, Route 4).
Following an alternative procedure, a solution of diketone 2 at 0 °C was treated with 1.5 eq of a freshly prepared PhICl2 at rt which was prepared from PhI and 5% of a bleach solution [18]. This initiated a chlorination reaction in less than 30 min yielding compounds 4 and 5 in a statistical ratio along with some other byproducts. This relatively fast reaction can be followed by TLC. Moreover, one cannot exclude the possibility of Cl− oxidation by PhI(OAc)2 to form Cl2 as reported before [19, 20]. Noteworthy, treating the diketone with 15 eq CaCl2 in methanol and traces of water yielded compound 5 and traces of 4. In contrast, repeating the original reaction with distilled instead of crude dichloromethane did not give any chlorinated products, but instead a methoxylated product in low yield was obtained. The above experiments led us to exclude hypothesis 1 and conclude that the reported chlorination reaction mechanism proceeds via a different mechanism in which the less reactive PhI(OAc)Cl intermediate is the possible chlorinating agent which may only react at high concentrations. To verify the most reasonable mechanistic pathway, extra ideas and experiments were tested. The trapping method for the chloronium ion was one of the ideas. But, applying this idea was not helpful, since the chloronium ion will be formed in several suggested routes. Alternatively, the substitution reaction was tested against the oxidation reaction of the Cl− ions. However, treating Cl− ions from CaCl2 source in MeOH and distilled CH2Cl2 with PhI(OAc)2 led to the formation of PhICl2 (approved by comparison with authentic sample) which then reacts directly with the substrate 2 in an open system leading to the desired products 4 as a major. This makes route 3 (Scheme 2) as the mechanism of choice.
Due to its symmetry, the tetrachloro derivative 5 only shows half the set of NMR signals, while the assignment of the unsymmetrical compounds 4 provide individual signals with distinct chemical shifts for all protons.
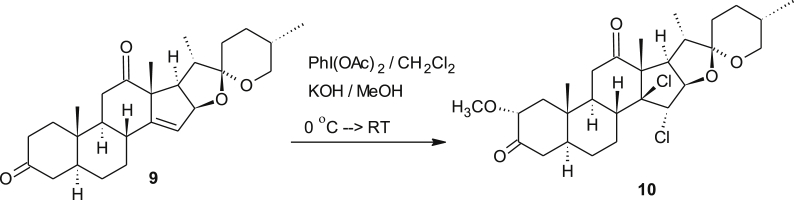
As this method of chlorination does not require chlorine gas, it may become a useful method for chlorination reactions. Moreover, the generation of defined amounts of chlorine is much easier. Chemically, compound 5 failed to undergo oxime formation or reduction mediated by sodium borohydride. These results led us to conclude that, the presence of one chloro group prevents nucleophilic attack on the carbonyl group and consequently these findings pave the way to conduct important and useful regioselective transformations with 4. For instance, derivative 4 regioselectively reacts with sodium borohydride to give 6 as a single product. This derivative also underwent oxime formation to furnish new cephalostatin derivative 8 in good yields (Scheme 3). In both cases, only the carbonyl group reacted that is located in the “chlorine-free hemisphere. However, upon the exposure of excess ylid to a solution of compound 4, Wittig-reaction seems to be problematic, since it led to multicomponent products and hence it does not lead to a reproducible yield of the desired methylenated product. But, still the bis-olefine product was absent. This was concluded basing on the mass analysis of the resulted products-mixture. In contrast, using a stoichiometric amount of the ylid yielded the desired methylenated product in fair to good yield. Additionally, we studied the reactivity of the “monomeric” precursor of cephalostatin 9 under the chlorinating conditions described above. Gratifyingly again a dichlorinated adduct 10 was formed as a single isomer (Scheme 4). In this case, the reagent diacetoxy iodobenzene played a twofold role. It serves as a source for chlorine as described above and it promotes the established α-methoxylation of a carbonyl group, leaving position-11 unaltered. The α-configuration of the methoxy group was determined from the NOESY spectrum which showed a cross peak between the 2-H proton resonating at 2.95 ppm and the methyl protons of C-19 resonating at 0.77 ppm.
Scheme 3.
Regioselective reactions in the chlorine-free hemisphere of the cephalostatin derivative 4.
Scheme 4.
Application of the chlorination method to a monosteroidal precursor of the cephalostatins.
As an additional support, we were able to successfully dechlorinate compound 4 and collect cephalostatin derivative 2 in about 65% yield upon refluxing of 4 in a solution of dichloromethane and acetic acid (1:1) for 1.5 h in the presence of Zn powder. This is an important finding as the introduction of the two chlorine atoms serve to desymmetrize symmetric cephalostatin and after selectively altering the other hemisphere one can remove them again. Hence, the chlorination can be regarded as protecting group strategy for asymmetric cephalostatins. The structure of the product has been approved from spectroscopic data and by comparison with authentic sample. This reaction broadened the margin of freedom by enabling us to deprotect the double bond keeping the carbonyl protected by its neighboring methoxy group.
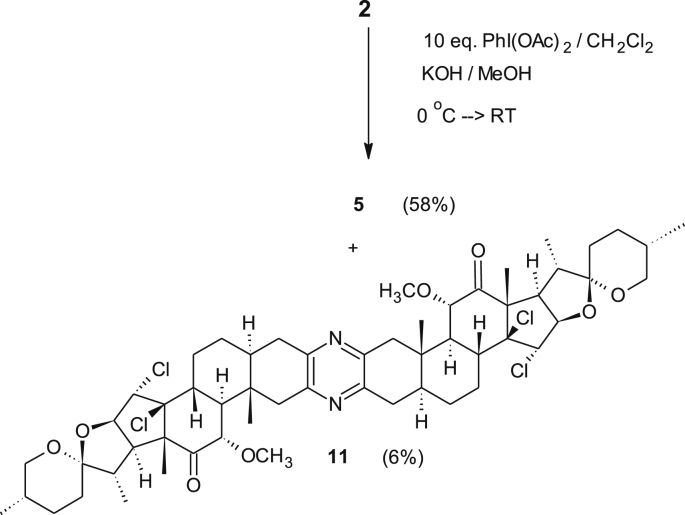
Furthermore, to understand the effect of chloro groups on the chemistry of the steroidal backbone especially the chemo selectivity of the upcoming F-ring opening reaction, an attempt aimed at the synthesis of symmetrical bis-steroid 5 from 2 found to give in addition to the desired product a small amount of a less polar side-product, when excess reagents (10 eq) was used (Scheme 5). Spectral inspection of the side-product 11 indicated that both chlorination and α-methoxylation took place.
Scheme 5.
Chlorination and methoxylation of the diketone 2.
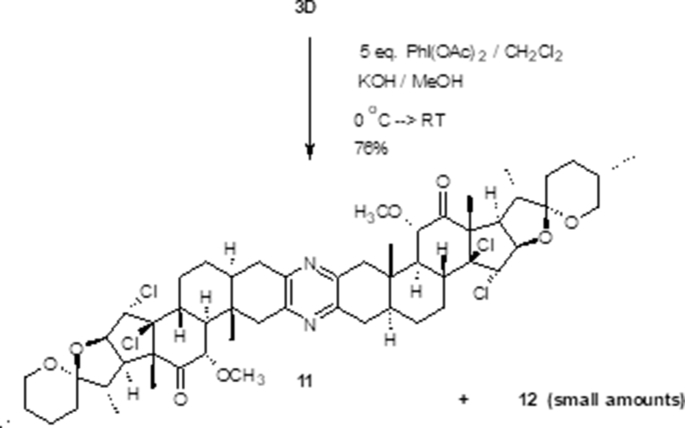
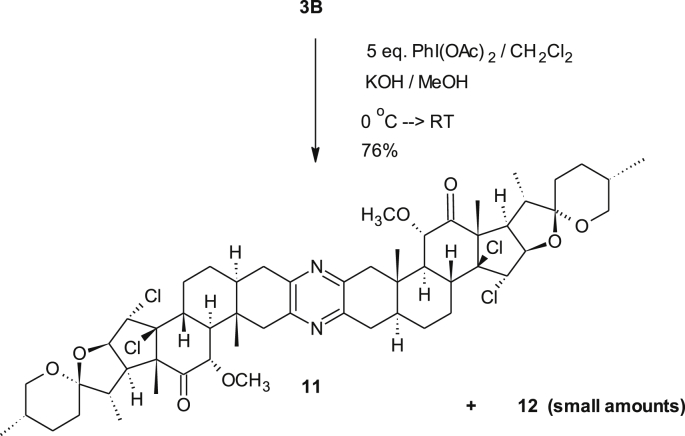
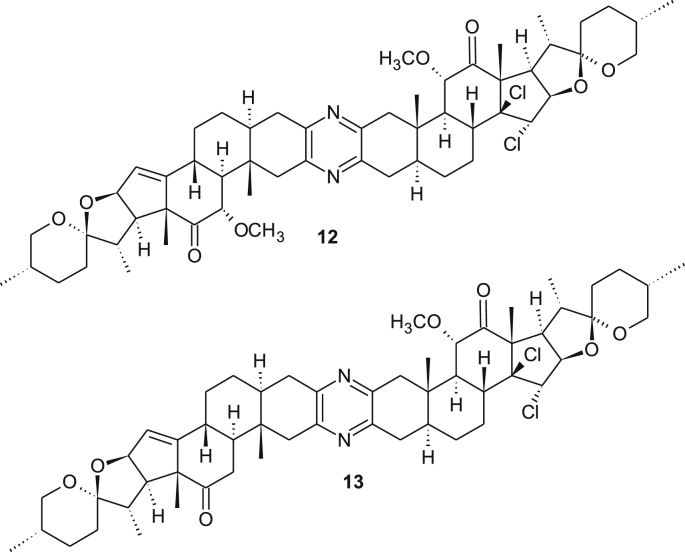
To explain the formation of the side-product 11 and to understand more about controlling aspects and favorability/priority of both processes, two attempts were designed. In the first, the dimer 5 was tested for methoxylation process however, in the second compound 3D was tested for chlorination. Compound 5 failed to give 11 as a major upon exerting a methoxylation conditions using excess reagents (Scheme 6) and instead a multi-component product was obtained. In contrast, the bis-methoxy derivative 3D, which was synthesized before using a straightforward procedure [12], underwent chlorination reaction easily and compound 11 was obtained as a major product in addition to little amount of the unsymmetrical derivative 12 along with small amount of the unconverted starting material (Scheme 7). This led us to a conclusion that the methoxylation process occurs prior the chlorination process. Additionally, the masked carbonyl with α-methoxy group may not be involved in the mechanism of chlorination and hence the reagent attacks from β-face rather than α-face. Moreover, the double bond in the methoxylated analogue is more labeled toward electrophile than the unmethoxylated dimer. This was also noticed before toward borane electrophile in which a hydroboration of ∆14,15 bond in the methoxy-half was predominant over the unmethoxylated half [17]. This result may be devised to synthesize an analogue as 13 selectively. Therefore, these directing groups (methoxy and chloro groups) are used in the designed strategy.
Scheme 6.
Methoxylation test of compound 5.
Scheme 7.
Chlorination of the bis-methoxy derivative 3B.
Even the same reagents were used in both processes, the difference between chlorination/methoxylation reaction depends on technical and tactical aspects like temperature-time variations. For the methoxylation reaction, the reaction temperature should not exceed 10–15 min at 0 °C after addition of PhI(OAc)2. However, for the chlorination reaction the temperature should be kept at 0 °C not less than 5 h after the exposure of the reagent. Noteworthy, the TLC follow-up showed no change on the starting material during the chlorination reaction until solvent removal. However, after solvent evaporation under light vacuum and final drying in high vacuum, TLC-analysis revealed that the product spots could only be detected at this stage of workup, which contrasts the methoxylation reaction, that could easily be monitored. Noteworthy, it was noticed that the chlorination reaction was succeeded when chloroform was used instead of dichloromethane.
Efforts will be directed towards discovering chemo- and regioselective transformations of analogues such as 4, 12 and 13 (Figures 5 and 6). Ultimately, we hope to unfold numerous opportunities to further develop the symmetrical concept towards at least one of the cephalostatins, or very close analogue(s).
Figure 6.
Some interested chlorinated-methoxylated analogues.
Noteworthy, compounds 4, 5, 6, 8, and 10 were tested against three cancer cell lines HMO2 (stomach cancer), MCF 7 (breast cancer) and HEGP (hepatocellular adenocarcinoma) but they didn't show any significant activity (GI50 ≥ 10 μM). This insignificant biological activity may be rationalized basing on the following suggested reasons. First, the negative role played by the chloro groups on blocking the neighbouring carbonyl from certain enzymatic nucleophilic attacks. Second, the chloro groups reduce the polarity of the substrate resulting in solubility difficulties. Third, the geometrical shape of the molecule will be changed due to the crucial role played by the double bond. Moreover, and according to previous studies, the double bond itself played an important positive role in biological activity of cephalostatins [21].
3. Conclusion
Having demonstrated the ability of using the symmetrical coupling method as alternative to the unsymmetrical one used in total synthesis, we are report on two new important aspects in the chemistry of the cephalostatins. First, we demonstrated that it is possible to protect both the carbonyl and ∆14,15 double groups in one step and which allows to flexibly derivatize the unmasked hemisphere. The regioselective desymmetrization can be utilized to design synthetic strategies towards important analogs or the natural product that initially rely on symmetrical precursors. Secondly, the reagent system for chlorination is new and the chlorination method is a remarkable, since chlorination of olefinic double bonds can be conducted without the use of toxic chlorine gas. Moreover, one can use the easily prepared dichloroiodobenzene to achieve the desired chlorinated products as 4 and 5. Introduction of a multi-purpose directing groups as methoxy and chloro groups, which in some cases is/are more useful than reagents, serves our new methodology to mimic total synthesis of cephalostatins.
4. Experimental
Notice that, selected spectral data of the following compounds is attached as a supplementary material.
4.1. General
1H-NMR and 13C-NMR spectra were carried out using the instruments Brucker AVS 400 (400 MHz, Avance) and AVS 500 (500 MHz, Avance). The measurements were taken using deuterated solvents and tetramethylsilane or solvent peak as a reference. Peaks in the region (0.7–2.0 ppm), which corresponds to the aliphatic region, will not be assigned except for the methyl groups and in few cases 9-H.
FAB-MS spectra were carried out using the VG-Autospec in a low resolution measurement in which the Nitrobenzyl Alcohol Matrix (NBA-Matrix) was used.
HR-FABMS spectral were carried out using the VG-Autospec in which the peak-matching method and the NBA-Matrix were used.
Thin Layer Chromatography (TLC) was carried out using aluminum TLC coated with the silica gel 60F254 from (Merck) combined with the polygram® Alox N/UV254 Macherey-Nagel. The detection of spots in TLC was done with the help of a UV-lamp (λ = 254 nm) and development in Ce(IV) sulphate reagent.
Preparative Column Chromatography was carried out using the flash chromatography principle.
Silica gel used was from Baker (0.03–0.06 mm). Elution was carried out under a light pressure achieved by the use of a hand pump.
Solvents used are commercial solvents after fractional distillation. In other cases, dry solvents were used.
Chemicals used have not been further purified. The organometallic reagents were handled under absolute conditions.
4.2. Preparation methods and spectral data
4.2.1. Preparation of 14β,15α-dichlorodiketone 4 (C54H72N2O6Cl2)
A solution of KOH (0.115 g, 2.0536 mmol) in 4.0 ml MeOH in a well dried round bottom flask was cooled to 0 °C, before a solution of diketone 2 (0.456 g, 0.5396 mmol, 1 eq dissolved in 3 ml of undistilled CH2Cl2) was added dropwise under argon. The reaction mixture was stirred for 20 min at 0 °C followed by a portion-wise addition of diacetoxy iodobenzene (0.273 g, 0.848 mmol, 1.6 eq). The solution was stirred for additional 4 h at 0 °C and then for 24 h at RT. After the removal of the solvent and drying under vacuum the crude yellowish material was subjected to silica gel column chromatography (eluant: EtOAc/hexane = 1:4) yielding 14β,15α-dichlorodiketone 4 as a light-yellow solid (155 mg, 31%) the symmetrical tetrachloro derivative 5 (52 mg) as amorphous and 190 mg of the starting material.
IR: CHCl3/vmax/cm−1: 2960 s (C–H), 2932 s (C–H), 1712 s (C=O), 1644 w (C=C), 1456 m (C–H), 1400 m (pyrazine), 1292 m (C–O), 612 m (C–Cl). LC-MS (ESI): 915.4753 (M+1, 35%). HR-MS: calculated: [M + H]+ = 915.48402, found: 915.4846. 1H-NMR: (400 MHz, CDCl3); δ = 5.45 (br s, 1H, 15′-H), 4.75 (dd, J16´-17´ = 8.1 Hz, J16´-15´ = 2.0 Hz, 1H, 16′-H), 4.62 (s, 1H, 15-H), 4.46 (d, J16-17 = 6.6 Hz, 1H, 16-H), 3.67 (tr, J17-16 = 6.8 Hz, 1H, 17-H), 3.52 (m, 2H, 26a/26′a-H), 3.33–3.45 (m, 3H, 26b/26′b/17′-H), 2.58–2.92 (m, 7H, 1a/1′a/4a/4′a/4b/4′b/11′a-H), 2.42–2.56 (m, 7H, 11a/1b/1′b/11b/11′b/5/5′-H), 2.31 (m, 2H, 8/20-H), 2.06 (m, 1H, 8′-H), 1.41 (s, 3H, 18-H), 1.30 (s, 3H, 18′-H), 1.01 (d, J21-20 = 6.8 Hz, 3H, 21-H), 0.97 (d, J21´-20´ = 6.7 Hz, 3H, 21′-H), 0.89 (s, 3H, 19′-H), 0.84 (s, 3H, 19-H), 0.77 (2d, J27-25 = J27´-25´ = 6.2 Hz, 6H, 27/27′-H). 13C-NMR: (100 MHz, CDCl3); δ = 210.69 (C, 12′-C), 209.07 (C, 12-C), 154.25 (C, 14′-C), 148.27,148.25, 148.19, 148.12 (all C, pyrazine-C), 121.56 (CH, 15′-C), 107.01 (2C, 22/22′-C), 89.91 (C, 14-C), 88.07 (CH, 16-C), 83.87 (CH, 16′-C), 68.2 (CH, 15-C), 67.10 (2xCH2, 26/26′-C), 62.26 (C, 13′-C), 61.83 (C, 13-C), 53.03 (CH, 9′-C), 50.32 (CH, 17-C), 49.68 (CH, 17′-C), 48.55 (CH, 11-C), 45.21 (CH), 45.06 (2xCH2, 1/1′-C), 44.34 (CH), 44.15 (CH), 41.41 (CH, 20-C), 41.15 (CH, 20′-C), 38.68 (CH), 37.14 (CH2, 11′-C), 37.14 (C, 10-C), 36.29 (C, 10′-C)), 35.35 (CH2, 4-C), 35.02 (CH2, 4′-C), 33.89 (CH, 5′-C), 31.20 (CH2), 31.03 (CH2), 30.31 (C) 30.26 (CH), 30.06 (CH), 29.07 (CH2), 28.68 (2xCH2), 28.53 (CH2), 27.77 (CH2), 26.54 (CH2), 20.75 (CH3, 18′-C), 19.21 (CH3, 18-C), 17.00, 17.07 (2xCH3, 27/27′-C), 14.27 (CH3, 21-C), 13.70 (CH3, 21′-C), 11.51 (CH3, 19′-C), 10.63 (CH3, 19-C).
4.2.2. Tetrachlorodiketone 5 (C54H72N2O6Cl4)
FAB-MS (NBA-matrix): m/z (%): 988 [MH]+ (100%). HR-MS [M + H]+ calculated: 985.4223 found 985.4227. 1H-NMR (400 MHz, CDCl3); δ = 4.65 (s, 1H, 15-H), 4.49 (d, J16-17 = 8.3 Hz, 1H, 16-H), 3.37 (pseudo tr, J17-16 ≈ J17-20 = 8.6, 1H, 17-H), 3.51 (dd, J26a-26b = 11.4 Hz, J26a-25 = 3.8 Hz, 1H, 26a-H), 3.37 (pseudo tr, J26b-26a ≈ J26b-25 = 11.2 Hz, 1H, 26b-H), 2.85 (dd, J4a-4b = 17.6 Hz, J4a-5 = 5.1 Hz, 1H, 4a-H), 2.76 (br d, J1a-1b = 16.4 Hz, 1H, 1a-H), 2.51–2.68 (m, 5H, 1b/4b/7a/7b/8-H), 2.47 (br d, J11a-9 = 11.2, 1H, 11a-H), 2.29–2.39 (m, 2H, 20/9-H), 1.43 (s, 3H, 18-H), 0.99 (d, J21-20 = 6.8 Hz, 3H, 21-H), 0.86 (s, 3H, 19-H), 0.79 (d, J27-25 = 6.4 Hz, 3H, 27-H). 13C-NMR (100MHz, CDCl3); δ = 209.1 (C, 12-C), 148.2, 148.1 (both C, pyrazine-C), 107.1 (C, 22-C), 90.0 (C, 14-C), 88.1 (CH, 16-C), 68.2 (CH, 15-C), 67.1 (CH2, 26-C), 61.9 (C, 13-C), 50.4 (CH, 17-C), 48.6 (CH, 8-C), 45.2 (CH2, 1-C), 45.3 (CH), 44.4 (CH), 41.4 (CH, 5-C), 37.1 (C, 10-C), 35.4 (CH2), 35.1 (CH2, 4-C), 31.1 (CH2), 30.1 (CH), 28.7 (CH2), 28.6 (CH2), 26.6 (CH2, 11-C), 19.2 (CH3, 18-C), 17.1 (CH3, 27-C), 14.1 (CH3, 21-C), 10.7 (CH3, 19-C).
4.2.3. Preparation of 12′β-hydroxy-14β,15α-dichloroketone 6 (C54H74N2O6Cl2)
To a solution of the dichlorodiketone 4 (130 mg, 0.142 mmol; 1 eq), dissolved in 4 ml abs. MeOH and 4 ml abs. CH2Cl2 at -78 °C, NaBH4 (30 mg, 1.111 mmol, 8 eq.) was added in three portions under argon atmosphere. The reaction mixture was stirred for 30 min to give a single, more polar product as judged by tlc. The reaction was terminated by addition of 1 ml of acetaldehyde before extraction was conducted using a saturated ammonium chloride solution. The aqueous phase was further extracted with chloroform and the the combined organic extracts were removed under reduced pressure. The resulting crude material was purified by column chromatography using a silica gel column chromatography which yielded dichlorohydroxy ketone 6 (110 mg, 85%) as a pale-yellow solid material.
HR-MS: [M + H]+ calculated: 917.49967, found: 917.5159. 1H-NMR: (400 MHz, CDCl3); δ = 5.45 (br s, 1H, 15′-H), 4.85 (dd, J16´-17´ = 8.3 Hz, J16´-15´ = 1.4 Hz, 1H, 16′-H), 4.62 (s, 1H, 15-H), 4.46 (d, J16-17 = 6.6 Hz, 1H, 16-H), 3.67 (tr, J17-16 = 6.8 Hz, 1H, 17-H), 3.31–3.51 (m, 4H, 26a/26′a/26b/26′b-H), 3.24 (dd, J12´-11´a = 11.2 Hz, J12´-11´b = 4.6 Hz, 1H, 12′-H), 2.71–2.90 (m, 4H, 1a/1′a/4a/4′a-H), 2.42–2.65 (m, 8H, 1b/1′b/4b/4′b/5/11a/11b/17′-H), 2.33 (m, 2H, 8/20-H), 1.41 (s, 3H, 18-H), 1.03 (d, J21´-20´ = 6.8 Hz, 3H, 21′-H), 1.01 (s, 3H, 18′-H), 0.97 (d, J21-20 = 6.8 Hz, 3H, 21-H), 0.85, 0.84 (both s, 6H, 19/19′-H) 0.77, 0.78 (2d, J27-25 = J27´-25´ = 6.2 Hz, 6H, 27/27′-H). 13C-NMR: (100 MHz, CDCl3); δ = 209.07 (C, 12-C), 157.22 (C, 14′-C), 148.61, 148.62, 147.92, 147.93, 147.98 (4C, pyrazine-C), 119.68 (CH, 15′-C), 107.01 (C, 22-C), 106.75 (C, 22′-C), 89.94 (C, 14-C), 88.07 (CH, 16-C), 84.55 (CH, 16′-C), 78.84 (CH, 12′-C), 68.2 (CH, 15-C), 67.11 (2xCH2, 26/26′-C), 61.83 (C, 13-C), 55.92 (CH, 17′-C), 52.71 (C, 13′-C), 50.29 (CH, 17-C), 52.12 (CH, 9′-C), 50.03 (CH, 17-C), 48.55 (CH) 45.47, 45.05 (2xCH2, 1/1′-C), 45.19 (CH), 44.38, 44.32 (2xCH), 41.41, 41.38 (2xCH), 37.03 (CH), 35.95 (C, 10′-C), 35.34 (CH2), 35.22 (CH2, 4′-C), 35.03 (CH2, 4-C), 33.62 (CH), 31.08 (CH2), 31.03 (CH2), 30.31 (CH), 30.04 (CH), 29.88 (CH2), 29.09 (CH2), 28.74 (CH2), 28.68 (CH2), 27.96 (CH2), 26.54 (CH2), 19.15 (CH3, 18-C), 17.09 (2xCH3, 27/27′-C), 14.27 (CH3, 21-C), 13.81 (CH3, 21′-C), 13.28 CH3, 18′-C), 11.78 (CH3, 19′-C), 10.63 (CH3, 19-C).
4.2.4. Preparation and spectral data of monoxime derivative 8
In a clean and dry 10-mL RBF, 8 mg (8.7 μmol, 1 eq) of compound 4 and 5 mg (71.9 μmol, 8.3 eq) were dissolved in 2 mL of Pyridine/Ethanol (1:1) mixture and stirred at rt overnight. After the reaction completion (TLC follow) which showed a complete conversion of the starting material, the turbid solution was quenched by the addition of 2 mL dis. water. The reaction solution was extracted with dichloromethane twice and the combined organic phase was extracted with 2 M solution of oxalic acid. The organic phase was dried in the fumehood at 50 °C to remove the pyridine rest. The resulted dry crude material was chromatographed on silica-gel column using EtOAc/PE (1:2) eluent yielded 7 mg of 8 as a pure pale-yellow solid material (87% yield).
IR: (KBr/vmax/cm−1); 3600 br (O–H), 2948 s (C–H), 1656 w (C=C), 1636 w (C=N), 1448 s (C–H), 1396 s (pyrazine), 1240 s (C–O), 618 m (C–Cl). HR-MS: [M + H]+ calculated: 930.494919, found: 930.4955.
1H-NMR: (600 MHz, CDCl3); δ = 5.45 (br s, 1H, 15′-H), 4.85 (dd, J16'-15' = 8.3 Hz, J16'-17' = 1.6 Hz, 1H, 16′-H), 4.68 (s, 1H, 15-H), 4.52 (d, J16-17 = 8.3 Hz, 1H, 16-H), 3.35–3.85 (m, 8H, 17/17'/11′a/11′b/26a/26b/26′a/26′b-H), 1.46 (s, 3H, 18-H), 1.29 (s, 3H, 18′-H), 1.09 (d, J21'-20' = 6.8 Hz, 3H, 21′-H), 1.01 (d, J21-20 = 6.8 Hz, 3H, 21-H), 0.83 (2d, 6H, 27/27′-H).
13C-NMR: (150 MHz, CDCl3); δ = 209.1 (C, 12-C), 162.2 (C, 12′-C), 157.3 (C, 14′-C), 148.8, 148.4, 147.2, 146.8 (all C, pyrazine-C), 119.9 (CH, 15′-C), 107.2, 107.1 (both C, 22/22′-C), 90.0 (C, 14-C), 88.1 (CH, 16-C), 84.3 (CH, 16′-C), 68.3 (CH, 15-C), 67.1 (2xCH2, 26-C), 61.9 (C, 13-C), 53.5 (C, 13′-C), 52.7 (CH, 9′-C), 50.9, 50.4 (both CH, 17/17′-C), 48.6 (CH), 45.3, 44.4 (both CH2, 1/1′-C), 44.3 (CH), 41.5 (CH), 41.4 (CH), 37.1, 36.1 (both C, 10/10′-C), 35.4, 35.2 (both CH2, 4/4′-C), 35.0 (CH2), 34.2 (CH) 31.9 (CH), 31.5(CH2), 31.4 (CH2), 31.1 (CH2), 30.4 (CH), 30.2 (CH), 30.1 (CH), 29.4 (CH2), 29.2 (CH2), 28.8 (CH2), 28.5 (CH2), 27.8 (CH2), 26.6 (CH2), 22.7 (CH3,18′-C), 19.2 (CH3, 18-C), 17.2 (CH3, 27-C), 14.4 (CH3, 27′-C), 14.1,13.9 (both CH3, 21/21′-C), 11.5, 10.8 (both CH3, 19/19′-C).
4.2.5. Preparation of 2α-methoxy-14β,15α-dichlorodione 10 (C28H40O5Cl2)
To an icy cold solution of KOH (0.63 g, 11.4286 mmol, 9.7 eq.) dissolved in 5 ml MeOH in a dry 100 ml round bottom flask under argon atmosphere a solution of the dione 10 (0.5 g, 1.174 mmol, 1 eq.) dissolved in 5 ml of undistilled CH2Cl2 was added dropwise and the reaction mixture was stirred for 20 min. Diacetoxy iodosobenzene (2.55 g, 7.919 mmol, 6.8 eq) was added portion-wise to the reaction mixture and stirring was continued for 2 h at 0 °C and for 24 h at RT. After removal of the solvent under reduced pressure and complete drying under high vacuum the resulting crude material was subjected to a silica gel column chromatography (eluted with 10% EtOAc:PE) which yielded 3α-methoxy-14β,15α-dichlorodione 11 (170 mg), 27.5%) as a brownish yellow solid (27.5 %) along with 95 mg of the starting material.
IR: CHCl3/vmax/cm−1: 2980, 2869 s (C–H), 1717 s (C=O), 1454 m (C–H), 1170 s (C–O), 620 m (C–Cl). EI-MS: m/z (%); 526 (7%) [M]+, 491 (10%) [M-Cl]+, 458 (17%), 419 (9%), 377 (23%), 341 (52%). 126 (100%). HR-MS: [M + H]+ calculated: 527.232556, found 527.2331. 1H-NMR: (400 MHz, CDCl3); δ = 4.64 (s, 1H, 15-H), 4.49 (d, J = 8.3 Hz, 1H, 16-H), 3.68 (tr, J = 5.9 Hz, 1H, 17-H), 3.67 (s, 3H, 28-H), 3.49 (m, 1H, 26a-H), 3.36 (m, 1H, 26b-H), 2.95 (m, 1H, 2-H), 1.41 (s, 3H, 18-H), 0.97 (d, J = 6.9 Hz, 3H, 21-H), 0.78 (d, J = 6.4 Hz, 3H, 27-H), 0.77 (s, 3H, 19-H).13C-NMR: (100 MHz, CDCl3); δ = 208.94 (C, 12-C), 177.23 (C, 3-C), 106.92 (C, 22-C), 90.30 (C, 14-C), 88.35 (CH, 16-C), 68.08 (CH, 15-C), 67.02 (CH2, 26-C), 62.28 (C, 13-C), 51.79 (CH3, 28-C), 50.08 (CH, 17-C), 49.52 (CH), 48.77 (CH), 45.31 (CH), 45.18 (C, 10-C), 44.78 (CH), 43.03 (CH2), 39.98 (CH, 2-C), 37.33 (CH2), 31.56 (CH2), 31.02 (CH2), 30.07 (CH), 28.69 (CH2), 26.99 (CH2), 26.87 (CH2), 19.53 (CH3, 18-C), 17.10 (CH3, 27-C), 14.30 (CH3, 21-C), 11.73 (CH3, 19-C).
4.2.6. Spectral data of compound 11 (C56H76N2O8Cl4)
IR: (CHCl3/vmax/cm−1); 2956, 2932 both s (C–H), 1732 s (C=O), 1456 s (C–H), 1400 m (pyrazine), 1320, 1280 both s (C–O), 860, 896 both m(C–Cl). FAB-MS: (NBA-Matrix): m/z (%); 1048 [MH+] (100 %). HR-FAB-MS: calculated: 1047.02324, found: 1047.02308. 1H-NMR: (400 MHz, CDCl3): δ = 4.27 (m, 1H, 16-H), 4.13 (m, 1H, 15-H), 3.78 (s, 3H, 28-H), 4.23–4.31 (m, 1H, 26a-H), 4.08–4.16 (m, 1H, 26b-H), 3.21 (m, 1H, 17-H), 2.75–2.90 (m, 2H, 1a/4a-H), 2.40–2.65 (m, 5H, 1b/4b/5/8/11-H), 1.96 (tr, J9-11 = J9-8 = 12.3 Hz,1H, 9-H), 1.31 (s, 3H, 18-H), 0.85 (d, J21-20 = 6.4 Hz, 3H, 21-H),0.80 (d, J27-25 = 5.7 Hz, 27-H), 0.79 (s, 3H, 19-H). 13C-NMR: (100MHz, CDCl3) δ = 172.70 (C, 12-C), 148.31, 147.88 (both C, pyrazin-C), 108.33 (C, 22-C), 85.25 (C-14-C), 81.52 (CH, 16-C), 72.28 (CH, 15-C), 67.38 (CH2, 26-C), 55.06 (C, 13-C), 54.70 (CH), 54.36 (CH, 9-C), 53.85 (CH), 52.02 (CH3, 28-C), 48.97 (CH, 17-C), 46.02 (CH2, 1-C), 45.00 (CH), 40.67 (CH), 37.04 (C, 10-C), 34.67 (CH2, 4-C), 31.71 (CH2), 30.33(CH), 29.92 (CH2), 28.26 (CH2), 27.95 (CH2), 21.09 (CH3, 18-C), 17.04 (CH3, 27-C), 13.65 (CH3, 21-C), 11.65(CH3, 19-C).
Declarations
Author contribution statement
Mansour Nawasreh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Andreas Kirschning: Analyzed and interpreted the data.
Helmut Duddeck, Gerald Dräger, Dieter Fenske: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The corresponding author would like to acknowledge Professor W. Beil from MHH at Leibniz Hannover University for testing the synthesized compounds against selected cancer cell lines.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Pettit G.R., Xu J., Ichihara Y., Williams M., Boyd M. Antineoplastic agents 285. Isolation and structures of cephalostatins. Can. J. Chem. 1994;72:2260–2267. [Google Scholar]
- 2.Pettit G.R., Xu J.P., Schmidt J.M., Boyd M.R. Isolation and structure of the exceptional Pterobranchia human cancer inhibitors cephalostatins 16 and 17. Bioorg. Med. Chem. Lett. 1995;5(17):2027–2032. [Google Scholar]
- 3.Pettit G.R., Inoue M., Kamano Y., Dufresne C., Christie M., Niven M., Herald D.L. Isolation and structure of the hemichordate cell growth inhibitors cephalostatins 2, 3, and 4. J. Chem. Soc., Chem. Commun. 1988:865–867. [Google Scholar]
- 4.Pettit G.R., Tau R., Xu J.P., Ichihara Y., Williams M.D., Boyd M.R. Antineoplastic agents. 398. Isolation and structure elucidation of cephalostatins 18 and 19. J. Nat. Prod. 1998;61:955–958. doi: 10.1021/np9800405. [DOI] [PubMed] [Google Scholar]
- 5.Rudy A., López-Antón N., Dirsch V.M., Vollmar A.M. The cephalostatin way of apoptosis. J. Nat. Prod. 2008;71(3):482–486. doi: 10.1021/np070534e. [DOI] [PubMed] [Google Scholar]
- 6.Tahtamouni L.H., Nawasreh M.M., Al-Mazaydeh Z.A., Al-Khateeb R.A., Abdellatif R.N., Bawadi R.M., Bamburg J.R., Yasin S.R. Cephalostatin 1 analogues activate apoptosis via the endoplasmic reticulum stress signaling pathway. Eur. J. Pharmacol. 2018;818:400–409. doi: 10.1016/j.ejphar.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 7.LaCcour T.G., Guo C., Bhandaru S., Boyd M.R., Fuchs P.L. Interphylal product splicing: the first total syntheses of cephalostatin 1, the North Hemisphere of Ritterazine G, and the highly active hybrid analogue, Ritterostatin GN1N1. J. Am. Chem. Soc. 1998;120:692–707. [Google Scholar]
- 8.Fortner K.C., Kato D., Tanaka Y., Shair M.D. Enantioselective synthesis of (+)-cephalostatin 1. J. Am. Chem. Soc. 2010;132:275–280. doi: 10.1021/ja906996c. [DOI] [PubMed] [Google Scholar]
- 9.Drögemüller M., Jautelat R., Winterfeldt E. Directed synthesis of nonsymmetrical bis-steroidal pyrazines and the first biologically active cephalostatin analogues. Angew. Chem. Int. Ed. 1996;35:1572–1574. [Google Scholar]
- 10.Drögemüller M., Flessner T., Jautelat R., Scholz U., Winterfeldt E. Synthesis of cephalostatin analogues by symmetrical and non-symmetrical routes. Eur. J. Org. Chem. 1998:2811–2831. [Google Scholar]
- 11.Kramer A., Ullmann U., Winterfeldt E. A short route to cephalostatin analogues. J. Chem. Soc. Perk. Trans. 1993;1:2865–2867. [Google Scholar]
- 12.Nawasreh M., Winterfeldt E. Novel routes to nonsymmetric cephalostatin analogous. Curr. Org. Chem. 2003;7(7):649–658. [Google Scholar]
- 13.Nawasreh M. Chemo-, regio-, and stereoselectivity of F-ring opening reactions in the cephalostatin series. Bioorg. Med. Chem. 2008;16(1):255–265. doi: 10.1016/j.bmc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Nawasreh M. Selective transformations of cephalostatin analogues. Pure Appl. Chem. 2011;83(3):699–707. [Google Scholar]
- 15.Nawasreh M.M. Progress in chemo- and regioselective transformations of symmetrical cephalostatin analogues. Lett. Org. Chem. 2018;15:155–161. [Google Scholar]
- 16.Nawasreh M. Stereoselective synthesis of bis-steroidal pyrazine derivatives. J. Nat. Prod. Res. 2007;21(2):91–99. doi: 10.1080/14786410500059243. [DOI] [PubMed] [Google Scholar]
- 17.Nawasreh M. Chemo- and regioselective hydroboration of Δ14,15 in certain cephalostatin analogue. Chin. Chem. Lett. 2008;19(12):1391–1394. [Google Scholar]
- 18.Zhao X.F., Zhang C. Iodobenzene dichloride as a stoichiometric oxidant for the conversion of alcohols into carbonyl compounds; two facile methods for its preparation. Synthesis. 2007:551–557. [Google Scholar]
- 19.Yoshimura A., Zhdankin V.V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 2016;116(5):3328–3435. doi: 10.1021/acs.chemrev.5b00547. [DOI] [PubMed] [Google Scholar]
- 20.Cresswell A.J., Eey S.T.-C., Denmark S.E. Catalytic, stereoselective dihalogenation of alkenes: challenges and opportunities. Angew Chem. Int. Ed. Engl. 2015;54(52):15642–15682. doi: 10.1002/anie.201507152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias-Arteaga M.A., Morzycki J.W. Chapter two – Cephalostatins and ritterazines, alkaloids. Alkaloids Chem. Biol. 2013;72:153–279. doi: 10.1016/b978-0-12-407774-4.00002-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.