Introduction
Rituximab is a human/murine chimeric immunoglobulin monoclonal antibody that binds to CD20, a B-cell lymphocyte transmembrane protein. Rituximab been used in the treatment of various hematologic malignancies.1 More recently, rituximab was found to have utility in treating pemphigus vulgaris (PV). The US Food and Drug Administration approved its use in 2018 for treatment of moderate-to-severe pemphigus vulgaris following a randomized trial that demonstrated superior efficacy and fewer adverse events when used in conjunction with prednisone compared with prednisone alone.2,3 As an immunomodulator, rituximab carries a black box warning for potentially fatal infusion reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy (PML).2 Rarely, rituximab has been associated with cerebral toxoplasmosis infection.4, 5, 6, 7 Here we present a case of cerebral toxoplasmosis infection in a patient treated with rituximab for pemphigus vulgaris, along with a brief review of central nervous system (CNS) toxoplasmosis in the setting of rituximab therapy.
Case report
A 52-year-old woman with a history of PV, treated with mycophenolate mofetil (MMF), 2 g/day, presented for follow-up. Prior treatment with prednisone, up to 80 mg daily, was complicated by osteonecrosis of the bilateral hips, and azathioprine was ineffective. Persistent oral mucosal erosions correlated with antidesmoglein 3 indices by enzyme-linked immunosorbent assay of 168 U/mL (normal, <9 U/mL). Following 2 cycles of rituximab (1 g IV day 0 and day 14), enzyme-linked immunosorbent assay indices for antidesmoglein 3 remained elevated (129 U/mL), and persistent mucosal-limited disease was distressful, impairing activities of daily living.
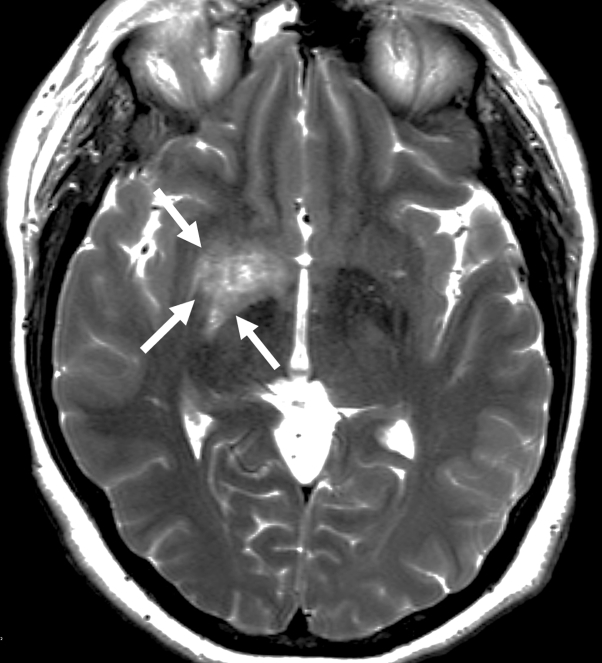
Six weeks after the third cycle of rituximab (375 mg/m2 weekly for 4 weeks), the patient had persistent rhinorrhea and concern for pseudotumor cerebri. She underwent a computed tomography scan and was found to have an encephalocele and cerebrospinal fluid (CSF) leak. She underwent repair with lumbar drain placement. Magnetic resonance imaging (MRI) found an ill-defined, round, increased signal intensity within the right globus pallidus and the lentiform nuclei on axial T2-weighted series (Fig 1). There was also a smaller volume of ring-like enhancement centrally, suggestive of atypical infection or infiltrative tumor.
Fig 1.
Axial T2-weighted MRI shows ill-delineated T2 signal increase within the right basal ganglia involving the lentiform nucleus and globus pallidus (arrows).
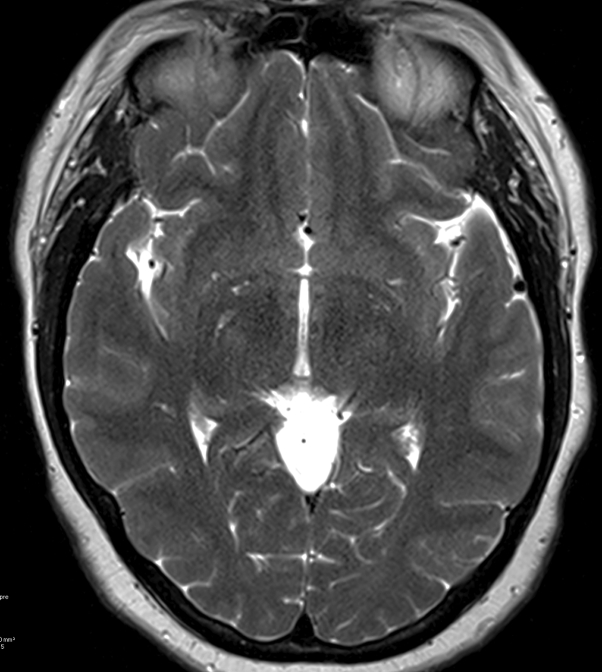
The patient underwent lumbar puncture for CSF analysis, which was negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus 1 and 2, and varicella zoster virus DNA. Two weeks prior to symptom onset, the patient's pet cat gave birth to kittens and was using a litter box. Serology found a toxoplasmosis IgG of less than 3.0 AU/mL (normal, <7.9 AU/mL) and toxoplasmosis IgM of 35.9 AU/mL (normal, <7.9 AU/mL). Polymerase chain reaction of CSF for Toxoplasma was negative, but a plasma-based next-generation sequencing assay (Karius test) detected circulating cell-free Toxoplasma DNA. A diagnosis of acute toxoplasmosis was made. Treatment with pyrimethamine and leucovorin was initiated; after 2 weeks of therapy, repeat MRI found complete resolution of abnormal signal changes in the right basal ganglia (Fig 2). Pyrimethamine and leucovorin were continued for 6 weeks, followed by trimethoprim-sulfamethoxazole (TMP-SMX), 3 times daily for 6 weeks, then TMP-SMX, once daily as maintenance.
Fig 2.
Two weeks later, repeat axial T2-weighted follow-up MRI shows complete resolution of abnormal signal changes in the right basal ganglia.
MMF was tapered to discontinuation given ongoing treatment for active infection. Approximately 6 months after the third cycle of rituximab, the patient's mucosal PV was in remission. Additional treatment with rituximab was deferred.
Discussion
Rituximab has been associated with PML in cases of lymphoproliferative disorders, systemic lupus erythematosus, rheumatoid arthritis, immune thrombocytopenia, and immune pancytopenia.2,8 Only 4 cases of cerebral toxoplasmosis following rituximab therapy have previously been reported (Table I).4, 5, 6, 7 Neither PML nor cerebral toxoplasmosis has been described in the context of rituximab treatment for dermatologic indications. In all cases of cerebral toxoplasmosis following rituximab therapy, patients were taking another immunosuppressant medication in addition to rituximab.
Table I.
Cerebral toxoplasmosis in patients treated with rituximab
| Study | Patient descriptor | Relevant medications | Onset | Presentation | Outcome |
|---|---|---|---|---|---|
| Desmond et al, 20094 | 63 M with CLL | Fludarabine, cyclophosphamide, and rituximab | 8 mo following rituximab treatment | Apraxia, forgetfulness, and cognitive impairment | Incidental finding of toxoplasmosis along with PML; died 4 wk after diagnosis |
| Safa and Darrieux, 20135 | 71 F with cutaneous necrotizing vasculitis associated with essential cryoglobulinemia | Prednisone, methylprednisolone, azathioprine rituximab (1 cycle, 375 mg/m2 weekly for 4 weeks) | 4 mo following rituximab treatment | Speech disturbance, behavioral changes, weight loss | No improvement of mental status following treatment with pyrimethamine, sulfadiazine sodium, and folinic acid |
| Savsek and Opaskar, 20166 | 62 F with DLBCL | 2 cycles of rituximab and chlorambucil 8 cycles of R-CHOP |
5 d following 8 cycles of R-CHOP | High fever, headache, altered mental status, pancytopenia | Mild cognitive defects persistent after 6 wk of treatment with sulfadiazine, pyrimethamine, and folic acid Maintenance therapy with rituximab was subsequently restarted |
| Gharamti et al, 20187 | 65 F with pseudolymphoma | Abatacept 6 cycles of bendamustine and rituximab Trametinib |
5 wk after trametinib therapy | Gait incoordination, fine motor skill difficulties | Substantial clinical improvement and reduction in CNS lesions following 16-wk treatment with TMP-SMX |
| Current study | 52 F with PV | MMF 3 cycles of rituximab |
6 wk after 3rd cycle of rituximab | Rhinorrhea (CSF leak with encephalocele) | Clinical improvement and reduction in CNS lesions following treatment with pyrimethamine, sulfadiazine, and folinic acid, followed by prophylaxis. Follow-up brain MRI after treatment showed the lesion decreasing in size, most recently at 5 mm. Previously measured to be 7 mm prior to treatment. |
CLL, Chronic lymphocytic leukemia; DLBCL, diffuse large B cell lymphoma; R-CHOP, rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone.
Usually, toxoplasma encephalitis is chronic, because of reactivation of Toxoplasma gondii. This patient presented with elevated IgM but normal IgG against Toxoplasma, consistent with acute cerebral toxoplasmosis. Given that toxoplasma encephalitis is a chronic manifestation in most affected patients, this case describes a unique presentation. A single prior case of acute disseminated toxoplasmosis with encephalitis in an immunocompromised patient has been described.7
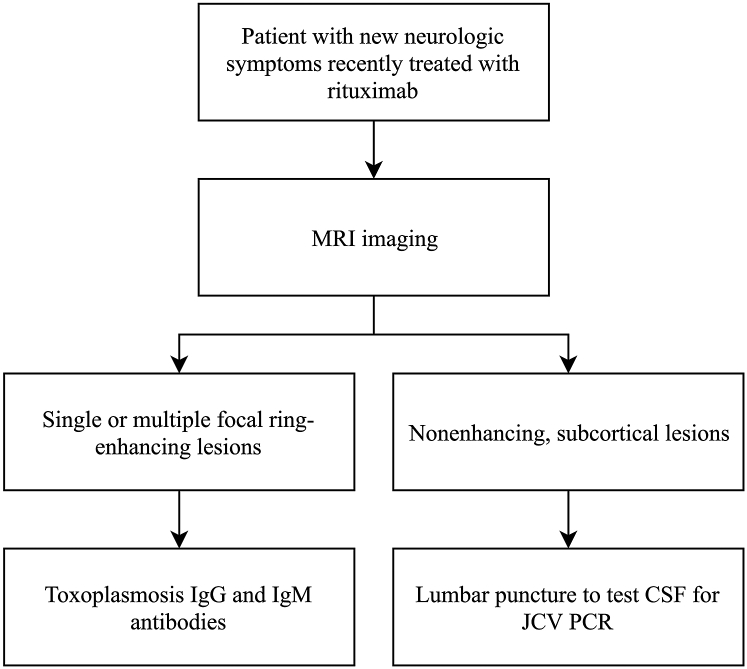
Although rare, dermatologists should consider cerebral toxoplasmosis and PML when rituximab-treated patients present with neurologic symptoms, to prompt appropriate referral and evaluation. A diagnostic algorithm is included in Fig 3.
Fig 3.
Suggested diagnostic algorithm for patients on rituximab presenting with new neurologic symptoms. JCV, John Cunningham virus; PCR, polymerase chain reaction.
Patients with cerebral toxoplasmosis respond well to treatment with pyrimethamine and sulfadiazine for at least 6 weeks. Immunocompromised patients should receive continual prophylaxis. However, little data exist to guide the duration of prophylaxis in patients with cerebral toxoplasmosis due to immunosuppressant therapy. For patients with HIV, the Centers for Disease Control and Prevention indicates that patients who are at low risk for recurrence, successfully complete initial therapy, remain asymptomatic, and have sustained CD4+ T-cell counts of greater than 200 cells/μL may reasonably discontinue prophylaxis.9 Similar to Pneumocystis jirovecii pneumonia, cerebral toxoplasmosis is uncommon but possible in patients with autoimmune bullous diseases, occurs in the context of multiple immunosuppressant therapies and may very rarely be observed in patients treated with rituximab. Thus, routine prerituximab prophylaxis for cerebral toxoplasmosis is not indicated in patients with autoimmune bullous diseases including pemphigus.
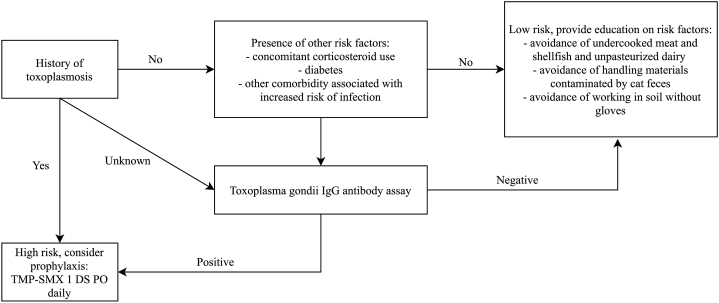
However, screening for risk factors prior to rituximab therapy is recommended to identify patients who may benefit from prophylaxis (Fig 4). Because Toxoplasma encephalitis is typically caused by reactivation, a thorough history to elicit previous toxoplasmosis or prolonged use of other immunosuppressants is helpful. Patients on concomitant systemic corticosteroids, with history of diabetes, or with other comorbidities associated with immunocompromise may be at higher risk for toxoplasmosis.9 In these more specific contexts, serologic evaluation for T gondii IgG and prophylaxis in seropositive individuals should be considered. The antibiotic of choice for prophylaxis is TMP-SMX (1 double strength tablet) once daily; in patients with sulfonamide allergy, dapsone 50 mg daily with pyrimethamine 50 mg, and leucovorin, 25 mg weekly may be used.10 In seronegative patients without history of toxoplasmosis, counseling to avoid exposure during rituximab therapy is sufficient. Patients should avoid undercooked meat, shellfish, unpasteurized dairy products, materials contaminated by cat feces (such as litter boxes), and direct contact with soil.
Fig 4.
Algorithm for screening and prophylaxis for toxoplasmosis prior to rituximab therapy. DS, double strength; PO, by mouth.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Salles G., Barrett M., Foà R. Rituximab in B-Cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rituxan (ritxuimab) [package insert] Genentech, Inc; South San Francisco, CA: 2018. [Google Scholar]
- 3.Joly P., Maho-Vaillant M., Prost-Squarcioni C. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 4.Desmond R., Lynch K., Gleeson M., Farrell M., Murphy P. Progressive multifocal leukencephalopathy and cerebral toxoplasmosis in a patient with CLL. Am J Hematol. 2009;85(8):607. doi: 10.1002/ajh.21589. [DOI] [PubMed] [Google Scholar]
- 5.Safa G., Darrieux L. Cerebral toxoplasmosis after rituximab therapy. JAMA Intern Med. 2013;173(10):924. doi: 10.1001/jamainternmed.2013.374. [DOI] [PubMed] [Google Scholar]
- 6.Savsek L., Opaskar T.R. Cerebral toxoplasmosis in a diffuse large B cell lymphoma patient. Radiol Oncol. 2016;50(1):87. doi: 10.1515/raon-2014-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gharamti A.A., Rao A., Pecen P.E., Henao-Martínez A.F., Franco-Paredes C., Montoya J.G. Acute Toxoplasma dissemination with encephalitis in the era of biological therapies. Open Forum Infect Dis. 2018;5(11):ofy259. doi: 10.1093/ofid/ofy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson K.R., Evens A.M., Richey E.A. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams R. June 2015. Role of trimethoprim-sulfamethoxazole prophylaxis against the infectious complications of rituximab treatment in autoimmune blistering diseases – a retrospective descriptive study. Available at: https://dash.harvard.edu/handle/1/17295871. Accessed December 17, 2019. [Google Scholar]
- 10.Brief | Toxoplasma gondii | Adult and Adolescent Opportunistic Infection | AIDSinfo. https://aidsinfo.nih.gov/guidelines/brief-html/4/adult-and-adolescent-opportunistic-infection/322/toxoplasma-gondii