Abstract
Background
Fast, effective, and rapid processing of central nervous system (CNS) tissue with good preservation of myelin, especially in tissue from diseased mice, is important to many laboratories studying neurosciences.
New method
In this paper, we describe a new method to process and embed CNS tissue from mice. Spinal cords and optic nerves from naive C57BL/6 mice were used to standardize the microwave protocol following perfusion with fixative. The CNS tissue was processed and embedded using the microwave embedding protocol.
Results
We observed that the tissue is well preserved and good quality light and electron microscope images were obtained after using the microwave embedding protocol.
Comparison with existing methods
Traditional way of embedding CNS tissue in resin is challenging and time consuming. The microwave technology offers an efficient way to quickly embed CNS tissue while preserving morphology and retaining the integrity of the myelin.
Conclusions
This new method is fast, reliable and an effective way to embed CNS tissue in resin.
Keywords: Cell biology, Neuroscience, Pathophysiology, Pathology, Neurology, Ophthalmology, Spinal cord, Optic nerve, Plastic embedding, Electron microscopy, Microwave
Cell biology; Neuroscience; Pathophysiology; Pathology; Neurology; Ophthalmology; Spinal cord; Optic nerve; Plastic embedding; Electron microscopy; Microwave
1. Introduction
Many experimental murine models have been developed that use central nervous system (CNS) tissue to study different neurodegenerative diseases. Preservation of cells, organelles, axoplasm and myelin integrity are important for understanding pathology. Conventional fixation, processing and resin embedding of tissue for light and transmission electron microscopic examination is a slow, time consuming process that requires days to complete (Spurr, 1969; Newman and Hobot, 1999). In recent years, use of microwave technology for processing tissue has gained popularity due to ease, superior visualization of morphology and shortening tissue processing time to a few hours (Giberson and Demaree, 1999; Giberson et al., 1997, 2003). The microwave technology also allows flexibility where parts of the protocol can be done using the microwave and the final resin infiltration can be in a conventional oven. In this paper, we detail a protocol for processing, dehydrating, and embedding of CNS tissue for light and electron microscopy (EM) using the PELCO Biowave® Pro + microwave. This protocol preserves ultrastructure, myelin, and tissue organelles, thereby saving many days of processing compared to the conventional method.
2. Materials and methods
At least 29 small Petri dishes per tissue holder (Ted Pella, Redding, CA; Cat. No. 36135-5)
3 large Petri dishes, polypropylene, 150mm (ProSciTech, Kirwan, QLD, Australia; Cat. No. H636-150)
3400ml beakers (Electron Microscopy Sciences (EMS), Hatfield, PA; Cat. No. 60974)
31000ml beakers (EMS, Hatfield, PA; Cat. No. 60978)
320ml scintillation vials (EMS, Hatfield, PA; Cat. No. 72632)
Ampoule cracker (EMS, Hatfield, PA; Cat. No. 60600)
5 fl. oz. staccup beaker (EMS, Hatfield, PA; Cat. No. 60950)
Acetone 500ml, Thermo Fisher Scientific, Pittsburgh, PA; Cat. No. S25120B
PELCO Prep-Eze specimen holders (Ted Pella, Inc., Redding, CA; Cat. No. 36158-1)
PELCO Prep-Eze numbered mat (Ted Pella Inc., Redding, CA; Cat. No. 36158-3)
Embedding Capsule Holder (EMS, Hatfield, PA; Cat. No. 70022-04)
Capsule Press (EMS, Hatfield, PA; Cat. No. 69920-00)
Acetone Wash Bottle, 250 ml (EMS, Hatfield, PA; Cat. No. 64100)
PELCO 21 Cavity EM Embedding Mold (TedPella Inc., Redding, CA; Cat. No. 10505)
Flat Bottom Embedding Capsules (EMS, Hatfield, PA; Cat. No. 70021)
NaOH pellets EMS; Hatfield, PA; Cat. No. 21160
PELCO® Tabbed Center-Marked Grids, 200 mesh, 3.0mm O.D., Nickel grids for EM (Ted Pella Inc., Redding, CA; Cat. No. 3HGN200)
Toluidine blue (EMS, Hatfield, PA; Cat. No. 22050)
Sodium borate (EMS, Hatfield, PA; Cat. No. 21130)
Slide mountant Cytoseal XYL (Thermo Fisher Scientific, Pittsburgh, PA; Cat. No. 8312-4)
Weighing Balance
Cotton swab applicator or wooden applicator sticks
Wooden tongue depressor
5-10 transfer pipettes
Graduated cylinder 50 ml
Print-out with tissue ID labels
Kim-wipes
5 Bottles to collect osmium, uranyl acetate, resin, acetone, fixatives, and cacodylate buffer waste
Wash bottle for distilled water
Glass bottles for various acetone dilutions (25, 30, 50, 75, 95, 100%)
2 - glass Petri dishes (10 cm)
Lab coat, protective glasses and extended cuff gloves (Kimberly Clark, Roswell, GA; Cat. No. 50603)
2.1. Solutions
Sodium cacodylate buffer, 0.2M, pH 7.4 (EMS, Hatfield, PA; Cat. No. 11653)
Embed-812 kit (EMS, Hatfield, PA; Cat. No. 14120)
Low viscosity kit (Dr. Spurr, EMS, Hatfield, PA; Cat. No. 14300)
Osmium tetroxide, 4% aqueous solution (EMS, Hatfield, PA; Cat. No. 19170)
Potassium ferrocyanide, 5% aqueous solution (EMS, Hatfield, PA; Cat. No. 25154-5)
Uranyl acetate, 2% solution (EMS, Hatfield, PA; Cat. No. 22400-2)
Sucrose (EMS, Hatfield, PA; Cat. No. 21600)
Calcium chloride (EMS, Hatfield, PA; Cat. No. 12340)
Paraformaldehyde, 16% solution (Ted Pella, Inc., Redding, CA; Cat. No. 18505-100)
Glutaraldehyde, 50% solution (EMS, Hatfield, PA; Cat. No. 16310)
Sorensen's Phosphate buffer (0.2M, EMS, Hatfield, PA; Cat. No. 11600-10)
2.2. Preparation of solutions
-
1.
To make 1000ml of perfusion buffer containing 2% paraformaldehyde and 2% glutaraldehyde, combine 335ml distilled water with 500ml 0.2M of phosphate buffer, 125ml of 16% paraformaldehyde solution and 40ml of 50% glutaraldehyde solution.
-
2.
Prepare 500mM stock of sucrose solution by adding 17.12g sucrose to 100ml of distilled water.
-
3.
Prepare 0.1M stock of sodium cacodylate buffer by diluting 0.2M sodium cacodylate buffer 1:1 with distilled water.
-
4.
Prepare 100ml EM fixative by mixing 10ml of 500mM sucrose solution, 0.25g CaCl2, 50ml 0.1M sodium cacodylate buffer, 9.5ml 16% paraformaldehyde (final concentration 1.5%), 3ml 50% glutaraldehyde (final concentration 1.5%), and finally make the volume to 100ml (about 27.5ml) with distilled water.
-
5.
Prepare 3% potassium ferrocyanide solution by diluting 5% potassium ferrocyanide 3:2 with distilled water.
-
6.
Prepare 0.5% uranyl acetate solution by diluting 2% uranyl acetate 1:3 with distilled water.
-
7.
For staining EM grids- Prepare 5% uranyl acetate (EMS, Hatfield, PA; Cat. No. 22400) by weighing 1.6g and dissolve in 30ml of distilled water in a 50ml Falcon tube. Wrap in foil, vortex and let sit for about 10min until everything is dissolved. Centrifuge briefly and transfer to a 10ml syringe. Store at 4°C and protect solution from light. Reynold's Lead citrate solution- Dissolve 1.33g lead nitrate (EMS Hatfield, PA; Cat. No. 17900) and 1.76g sodium citrate (EMS Hatfield, PA; Cat. No. 21140) in 30ml of freshly boiled and cooled distilled water. Shake the solution intermittently for about 30 min (it should become milky). Add 5ml of 1N NaOH solution to the solution (the milkiness- will clear). pH the solution to 12 using a pH meter. Bring the total volume to 50ml with distilled water. Transfer to a syringe (Fisher Scientific, Waltham, MA). Use after filtering through a syringe filter (Fisher Scientific, Waltham, MA; Cat. No. 09-719-000). This solution can be stored at 4°C. The pH of this solution should be 12 for good staining results. The detailed method is provided at www.lecia-microsystems.com.
2.3. Equipment
PELCO Biowave® Pro + Microwave Processing System (Ted Pella, Inc., Redding, CA; Cat. No. 36700) along with ColdSpot® Pro, temperature probe, and vacuum chamber.
General Notes
-
1.
All procedures on mice were approved by the Institutional Animal Care and Use Committee (IACUC) committee. Storage of chemicals and disposal of waste reported in this paper are according to the institutional guidelines. Before beginning, check institutional requirements for storage of chemicals, get permission for uranyl acetate use, waste collection and disposal.
-
2.
Perform all steps in a chemical fume hood and use a common laboratory disposable surface protector. Small Petri dishes can be put inside large Petri dishes to avoid spills on the counter surface.
-
3.
Use acetone from the squirt bottle to remove resin from gloves or other surfaces.
-
4.
Wear lab coat, eye protection and two layers of gloves.
-
5.
Put all waste (i.e. dirty gloves, Kim-wipes, etc.) in one of the 1000ml beakers, except when noted.
-
6.
The day before processing, the tissue is transferred to EM fixative and left to sit overnight at room temperature. Additional fixation times may be required depending on tissue type.
-
7.
Avoid bubbles in the solution or in Prep-Eze wells at all times during tissue processing.
-
8.
Spurr's resin and other components should be used fresh as traces of moisture will hamper good results.
-
9.
Print out specimen ids using appropriate font, cut them so that they fit into embedding molds and capsules.
2.4. Pre-processing instructions
-
1.
Just prior to beginning the embedding procedure, the reagents can be mixed. Place one large Petri dish (150mm) on the weighing scale inside the chemical fume hood (reset scale to zero) and carefully add the resin ingredients to the beaker in the order specified in Table 1. Use transfer pipettes to measure out DER736, DMAE, and DMP-30; the other ingredients can be poured slowly. Wet a Kim-wipe with acetone and use it to wipe off the mouth of each bottle after pouring. Once all of the ingredients have been added, stir the resin mix with the tongue depressor and cover the beaker with parafilm.
-
2.
Prepare the acetone/resin mixes in the glass vials. Add resin to the vials using a transfer pipette and pour acetone from the graduated cylinder. The resin and acetone can be added in either order. For the 3:1 mix, weigh out 5g of resin and measure 15ml of acetone using a graduated cylinder. For the 2:1 mix, use 6g of resin and 12ml of acetone. For the 1:1 mix, use 9g of resin and 9ml of acetone. Cap the scintillation vials and shake them. Remove the weigh balance from the fume hood when done.
-
3.
Break the cotton swab applicator in half and shape one of the broken ends into a flat surface using a razor blade. Use the flat end of the stick to transfer tissue sections into the tissue holder, starting at one o'clock and ending at twelve o'clock or use a numbered mat. Note down the well number for each tissue ID. Use the stick to ensure that each section is pushed to the bottom of its well.
-
4.
Put the small Petri dish containing the tissue holder into a large Petri dish and put the large Petri dish inside the vacuum chamber. Follow these instructions for each step of the processing.
-
5.
Seal the lid tightly for each step of the processing for which there is a vacuum cycle (refer to Table 3).
-
6.
Place the vacuum chamber on the cold plate and check that the vacuum tube is hooked up to the vacuum nozzle. Confirm that the water in the ColdSpot® Pro has no air pockets and add water to the cold plate if necessary.
-
7.
During each step throughout the processing, fill a small Petri dish with the solution for the following step. Before placing the tissue holder in the vacuum chamber, make sure that all of the tissue sections are pushed to the bottom of their wells. After each step throughout the processing, place the used Petri dishes in one of the 1000ml beakers, unless otherwise noted.
Table 1.
Amounts of reagents needed for embedding.
| 1 specimen holder | 2 specimen holders | |
|---|---|---|
| SPURR | Amt. (g) | Amt. (g) |
| ERL 4221 | 20 | 30 |
| DER 736 | 8 | 12 |
| NSA | 52 | 78 |
| DMAE | 0.80 | 1.20 |
| TOTAL | 80.80 | 121.20 |
| Mix with wooden spatula | ||
| EPON | Amt. (g) | Amt. (g) |
| EPON 812 | 28 | 42 |
| DDSA | 13 | 19.5 |
| NMA | 13.20 | 19.80 |
| DMP-30 | 2.14 | 3.21 |
| TOTAL | 56.34 | 84.51 |
| Mix with wooden spatula | ||
| TOTAL SPURR + EPON | 137.14 | 205.71 |
ERL 4221 is a cycloaliphatic expoxide resin; DER 736; NSA Nonenyl Succinic Anhydride; DMAE 2-Dimethylaminoethanol.
Epon 812; DDSA Dodecenyl Succinic Anhydride; NMA Methyl-5-Norbornene-2,3-Dicarboxylic Anhydride; DMP-30 2,4,6-Tris(dimethylaminomethyl)phenol.
Table 3.
Embedding protocol for the microwave.
| Steps | Description | user prompt | time (hr:min:sec) | Power (W) | Temp (oC) | Load Cooler | Vacuum |
|---|---|---|---|---|---|---|---|
| 1* | Cacodylate buffer rinse | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 2 | Osmium fixation | ON | 0:03:00 | 100 | 60 | AUTO | CYCLE |
| 3 | Osmium fixation | OFF | 0:02:00 | 0 | 60 | AUTO | CYCLE |
| 4 | Osmium fixation | OFF | 0:03:00 | 100 | 60 | AUTO | CYCLE |
| 5 | Osmium fixation | OFF | 0:02:00 | 0 | 60 | AUTO | CYCLE |
| 6 | Osmium fixation | OFF | 0:03:00 | 100 | 60 | AUTO | CYCLE |
| 7* | Distilled water rinse | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 8 | Uranyl acetate solution | ON | 0:02:00 | 100 | 60 | AUTO | CYCLE |
| 9 | Uranyl acetate solution | OFF | 0:02:00 | 0 | 60 | AUTO | CYCLE |
| 10 | Uranyl acetate solution | OFF | 0:02:00 | 100 | 60 | AUTO | CYCLE |
| 11 | 20% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 12 | 35% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 13 | 50% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 14 | 75% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 15 | 95% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 16* | 100% acetone | ON | 0:00:40 | 150 | 60 | AUTO | OFF |
| 17 | 3:1 acetone/resin | ON | 0:03:00 | 150 | 60 | AUTO | CYCLE |
| 18 | 2:1 acetone/resin | ON | 0:03:00 | 150 | 60 | AUTO | CYCLE |
| 19 | 1:1 acetone/resin | ON | 0:03:00 | 150 | 60 | AUTO | CYCLE |
| 20# | 100% Resin mixture -infiltration | ON | 0:03:00 | 150 | 60 | AUTO | CYCLE |
Please note: Steps 1,7,16* run 3 times; step 20# repeat 5–8 times as this may vary according to tissue size; steps 2–6 and 8–10 can be done in one dish thus no transferring in between steps. All steps with vacuum cycle will have the vacuum chamber shut tightly with the lid. The final resin polymerization step is done at 60–63 °C in a regular vacuum oven (VWR).
2.5. Tissue fixation
-
1.
Fill one of the small Petri dishes about two-thirds with EM fixative. Wet the bottom of a tissue holder with water (in order to break the surface tension of the fixative) before lowering it into the Petri dish.
-
2.
On the microwave display, select the EM Fix Protocol, then “ready system”, then “start” (Table 2).
-
3.
When the cycle is finished, transfer the tissue holder to another Petri dish filled with EM fixative and repeat the EM fix step. Dispose of the EM fixative in the waste container.
-
4.
After running the EM fix step for the second time, transfer the tissue holder to a small Petri dish filled with cacodylate buffer.
Table 2.
Fixation protocol for the microwave.
| Step | Time (min) | Power | Temp (oC) | Vacuum |
|---|---|---|---|---|
| 1 | 1 | 150 | 50 | Auto |
| 2 | 1 | 0 | 50 | Auto |
| 3 | 1 | 150 | 50 | Auto |
Repeat steps 1–3 twice.
2.6. Tissue processing
-
1.
Set up and run the Processing Protocol. Please refer to steps in Table 3.
-
2.
After each of the cacodylate buffer steps, transfer the tissue holder to a Petri dish filled with fresh buffer and dispose of the old buffer in the waste bottle.
-
3.
Use the ampoule cracker to break open an ampoule of osmium tetroxide. Pour its contents into a Petri dish. Place the balance inside the fume hood and use a transfer pipette to weigh out 5g of potassium ferrocyanide solution into the osmium tetroxide. Swirl the mixture, transfer the tissue holder to the Petri dish, place it in the vacuum chamber, seal the chamber, and continue the protocol.
-
4.
After the osmium steps, transfer the tissue holder to distilled water and continue the protocol. Repeat the distilled water step twice (for a total of three times), using fresh distilled water each time.
-
5.
Pour/pipette the osmium tetroxide/potassium ferrocyanide mixture into its waste bottle. Wash out the Petri dish three times with distilled water, dumping the water into the waste bottle each time.
-
6.
After each of the distilled water wash steps, pour the water into the osmium waste bottle and use distilled water to wash out the Petri dishes three times, collecting the water in the waste bottle.
-
7.
After the distilled water steps, transfer the tissue holder to uranyl acetate solution and continue the protocol. Transfer the used uranyl acetate solution into its waste bottle. Record the use in the uranyl acetate log when the dilution is made in accordance with institutional regulations.
-
8.
Transfer the tissue holder to the acetone dilutions and run their steps in the following order: 20, 35, 50, 75, 95, and 100%. Repeat the 100% step twice (for a total of three times). Use a freshly opened bottle of acetone for the 100% acetone step.
-
9.
Pour the used acetone dilutions into the acetone waste bottle after each step.
-
10.
Transfer the tissue holder to the acetone/resin mixtures and run their steps in the following order: 3:1, 2:1, and 1:1. Take special care to make sure that each section is pushed to the bottom of its well on each step.
-
11.
Fill the Petri dish up to two-thirds for the first resin step. Fill up the remaining (5–7) dishes at the beginning to ensure that you have enough resin mixture for all steps. Less amount of resin is needed for the subsequent steps. All the sections must be submerged in resin however avoid overfilling the resin as it will spill out after immersing the Prep-Eze. Transfer the tissue holder to the pure resin mixture and run the first resin step. Repeat the step for 5–8 times, using fresh resin mixture for each step. Between steps, lift the tissue holder and let the resin run off the holder into the dish from the previous step before transferring the holder to the next dish. After each step, stack the Petri dishes containing the resin mixture in a large Petri dish in the fume hood. The acetone/resin mixtures can be poured into their waste bottle for collection or polymerized before disposal.
-
12.
During the resin steps, place capsules inside the capsule holder. Cut out ID labels from the printout and put them inside the capsules (for spinal cords) or PELCO embedding molds (for optic nerves) using forceps. Fill the capsules and molds about two-thirds with resin using a transfer pipette.
-
13.
When the last step of the microwave program is finished, transfer the sections to their corresponding capsules using the stick. Use the stick to gently position each section so that it is lying flat in the middle of the bottom of its capsule or against the end of the embedding mold. After all of the sections have been properly positioned, use a transfer pipette to top off each capsule with resin.
-
14.
Place the capsule holder containing all of the capsules in the oven at 60–63 °C. The next day, check whether polymerization is complete, by checking whether resin has hardened, before removing the capsules and molds. Allow at least 18 h for polymerization.
2.7. Cleanup
-
1.
Pour about 100ml of acetone into a 400ml beaker, place the tissue holder inside, and swirl the acetone to clean the tissue holder. After a few minutes, transfer the tissue holder to 100ml of acetone in a different 400ml beaker and, again, swirl the acetone to clean the holder. Once the tissue holder is clean, place it on a paper towel in the fume hood to dry. Transfer the acetone/resin waste to its waste container, washing out the beaker a couple of times with acetone.
-
2.
Used Petri dishes (except for the ones used in osmium, post-osmium wash - and uranyl acetate steps) can be washed and reused.
2.8. Sectioning of blocks
Remove the blocks from capsules using the capsule press. The blocks from PELCO embedding molds can be removed by twisting the mold. The blocks and molds are trimmed. Sections (500μm) are collected on slides for light microscopy. Sections for EM (70 nm) are collected on Nickel grids using Leica Ultramicrotome (EM UC7).
2.9. Toluidine blue staining for light microscopy
Prepare the toluidine blue solution by weighing out 2.5g toluidine blue, 2.5g sodium borate and combine with distilled water (make the volume up to 500ml). For staining, place a nickel-sized droplet of water on a slide and transfer about 5–6 tissue sections (500μm) to it and place the slide on a hot plate set at 165°C. Once the water droplet has completely evaporated, use a syringe to apply enough toluidine blue solution to cover the tissue sections. After 30–40s, use distilled water to rinse the toluidine blue off into a waste beaker. Place slide back on hot plate for at least 10 min to dry. Once the slide is completely dry, apply mountant (Cytoseal) and cover slip.
2.10. Grid staining for electron microscopy
Place a small piece of parafilm in two clean glass Petri dishes. In the first Petri dish, place one drop of 5% uranyl acetate for each grid to be stained on the parafilm piece. In the second Petri dish, place 3–4 NaOH pellets on the side and a drop of Reynold's lead citrate for each grid to be stained on the parafilm and cover with the Petri dish lid. Note down the orientation and IDs of the grids that are stained. Place the grid tissue-face down in 5% uranyl acetate solution droplets for 10min, then drip-wash grid with distilled water and dry on filter paper. Then place grids tissue face down on 3% Reynold's lead citrate for 5min. Drip-wash grids with distilled water, use filter paper to wick the water off without touching the section and dry on filter paper. Let grids dry for at least 30min face-up on filter paper before imaging. Collect and dispose solutions in accordance to guidelines.
3. Results
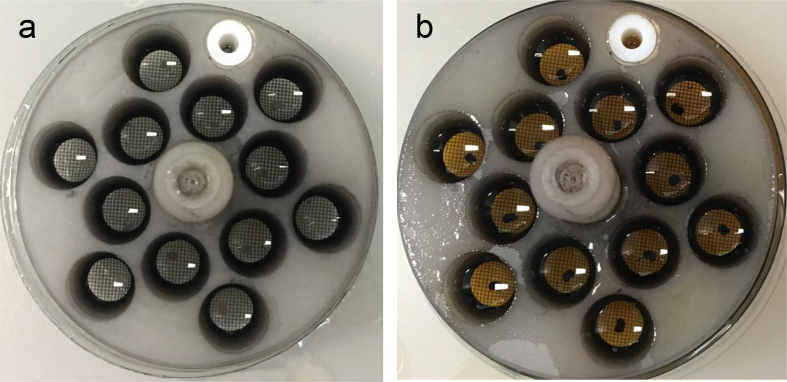
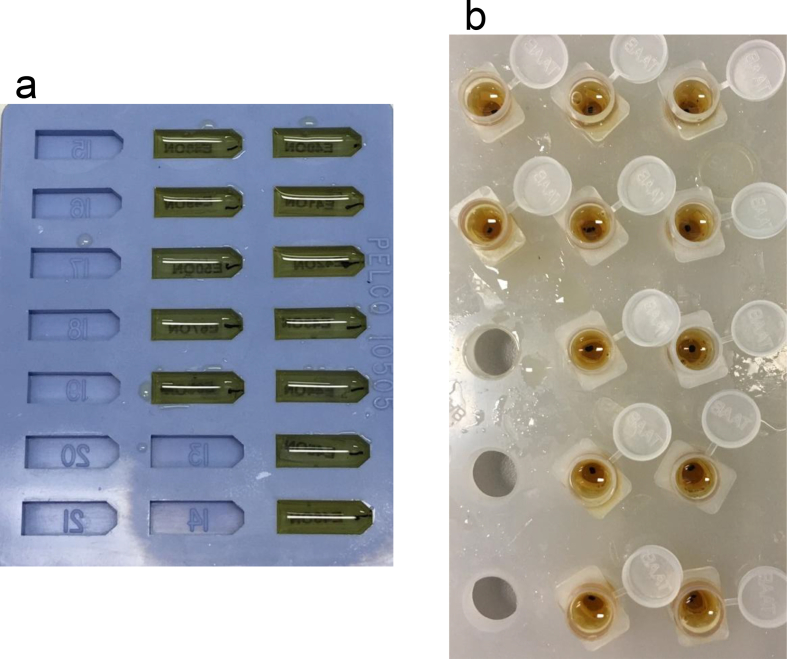
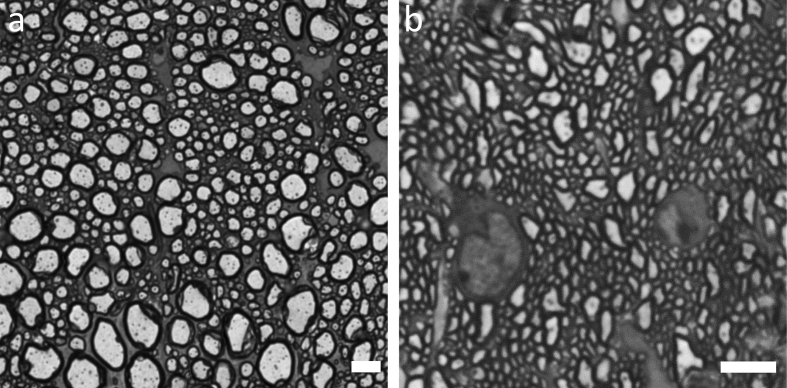
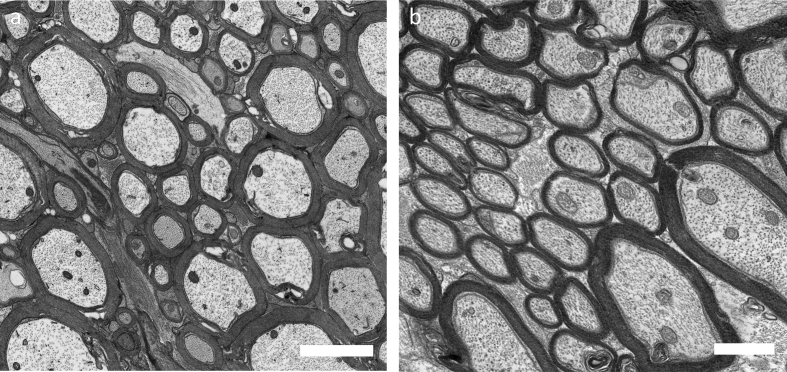
Nervous tissue needs special procedures due to high myelin content. In this paper, we provide a detailed protocol for embedding of murine CNS tissue samples using the Biowave® Pro +. The C57BL/6 mice were deeply anesthesized and perfused with 2% glutaraldehyde and 2% paraformaldehyde in phosphate buffer saline (Login and Dvorak, 1993, 1994). The spinal cord and optic nerves were isolated around 24 hours post-perfusion. The optic nerves were dissected about 2mm behind the optic nerve head. Lumbar spinal cord sections (300–500 microns) were cut and used for embedding. The tissue was fixed and embedded using the microwave (Table 2, Figure 1). Resin mixture was prepared as described in Table 2 and the protocol used for microwave embedding is listed in Table 3. Figure 2a shows spinal cord sections before beginning of the procedure and Figure 2b shows how the samples look after osmium fixation and while resin is being infiltrated. Finally, the resin is polymerized in an oven using flat-bed molds (optic nerves, Figure 3a) and flat embedding capsules (spinal cord, Figure 3b). After polymerization, the blocks are removed and sectioned for light and EM. ApoTome (Zeiss) was used for light microscopy (Figure 4). FEI Tecnai T12 transmission electron microscope with a 16Mpixel camera using AMT software (Advanced Microscopy Techniques Corp.) was used for EM imaging. Figure 5a, b shows EM images of spinal cord and optic nerves processed using the protocol described. Myelin sheath, cytoskeleton and organelles inside the axons are well preserved. The method described here in detail has also been used in other publications from our laboratory (Benedek et al., 2017; Hartley et al., 2019).
Figure 1.
Microwave Pro + arrangement near the chemical hood. Notice that the exhaust in vented into the chemical hood.
Figure 2.
Spinal cord sections in 12 well Prep-Eze specimen holders a. before beginning embedding process; b. at the end of embedding process.
Figure 3.
Polymerized plastic blocks. a. optic nerves embedded in flat embedding mold; b. embedded spinal cord sections in embedding capsule.
Figure 4.
Light microscope images of spinal cord and optic nerve sections. Zeiss microscope with 63X objective was used for imaging. a. spinal cord b. optic nerve. Scale bar 5μm.
Figure 5.
EM images of spinal cord and optic nerve sections. The myelin is well preserved after using the fast and efficient microwave plastic embedding process. a. spinal cord section at 2900X, scale bar 2 μm; b. optic nerve section at 4800X, scale bar 1μm.
4. Discussion
Microwave technology has been used for fixation, resin embedding, bone demineralization and immunolabeling of tissue (Boon and Kok, 1994; van Dorp et al., 1995; Leong and Sormunen, 1998; Tinling et al., 2004; Webster, 2007, 2014). The preservation of tissue along with speed of processing are the two main hurdles in resin embedding. Microwave processors address both these problems and improve efficiency. These machines are designed for tissue processing in research and diagnostic laboratories. Microwave processors have been used to process a variety of tissue including mouse liver (Webster, 2007), hair cells from inner ear (Webster, 2014), retina (Wendt et al., 2004), clinical specimens like skeletal muscle and sural nerve (Austin, 2001), and plant samples (Zechmann and Zellnig, 2009; Kuo, 2014). CNS tissue is especially challenging to work with because of excessive amounts of myelin. We and other laboratories have used the traditional bench processing method of plastic embedding in the past (Chaudhary et al., 2011; Aharoni et al., 2011; Su et al., 2012; Recks et al., 2013); however the microwave technology assists in getting better preservation of fine structure and ultrastructural morphology while retaining myelin integrity (Figure 5, Benedek et al., 2017; Hartley et al., 2019). Neurofilaments, the most abundant cytoskeletal component in axons, are well maintained in tissue processed using the microwave. This preservation of neurofilament is important to understand various neuropathologies. To our knowledge, this is the first detailed protocol for CNS tissue embedding using microwave processing (Figure 5). The Ted Pella Biowave® Pro + microwave allows processing of multiple samples efficiently and decreases both the time to process and the amount of chemicals used. This equipment will soon become an indispensable part of all histology laboratories as it can be modified to meet the unique needs of any biological samples. The use of this novel technology is rapidly growing and this will provide critical knowledge in understanding cell biology processes.
4.1. Important steps
-
-
Fixation preserves the tissue in its natural in vivo form
-
-
Tissue blocks should be cut to less than 1mm3 in order to ensure good Spurr/Epon penetration
-
-
All reagents need to be measured accurately
5. Conclusion
We describe an efficient, fast, reproducible and simplified protocol to embed CNS tissue using the Biowave® Pro +. We have tested this protocol for embedding spinal cord, optic nerves and corpus callosum. This detailed protocol allows for preparation of resin embedded tissue from multiple samples in less time than conventional processing. Importantly, this protocol results in superior ultrastructural preservation and allows tissues to be used for visualization by both light and electron microscopy.
Declarations
Author contribution statement
Evan Calkins: Performed the experiments; Wrote the paper.
Edvinas Pocius: Performed the experiments.
Gail Marracci: Contributed reagents, materials, analysis tools or data.
Priya Chaudhary: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work is supported by the National Multiple Sclerosis Society, United States (grant #RG-1607-25053), Department of Veterans Affairs Biomedical Laboratory Research and Development, United States and the Laura Fund for Innovation in MS Research, United States. We would like to express our thanks to the Advanced Light Microscopy Core and OHSU EM core supported by National Institutes of Health, United states (grant #P30 NS061800).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Dr. Robert Kayton and Lisa Dirling Vecchiarelli for their invaluable discussions, help with preparation of staining solutions and protocol optimization. We appreciate Drs. Gregory Duncan and Ben Emery for providing the tissue.
References
- Aharoni R., Vainshtein A., Stock A., Eilam R., From R., Shinder V., Arnon R. Distinct pathological patterns in relapsing-remitting and chronic models of experimental autoimmune enchephalomyelitis and the neuroprotective effect of glatiramer acetate. J. Autoimmun. 2011;37(3):228–241. doi: 10.1016/j.jaut.2011.06.003. Epub 2011 Jul 14. PubMed PMID: 21752599. [DOI] [PubMed] [Google Scholar]
- Austin R.L. Basic procedure for electron microscopy processing and staining in clinical laboratory using microwave oven. In: Giberson R.T., Demaree R.S., editors. Microwave Techniques and Protocols. Humana Press; 2001. pp. 37–48. [Google Scholar]
- Benedek G., Chaudhary P., Meza-Romero R., Calkins E., Kent G., Offner H., Bourdette D., Vandenbark A.A. Sex-dependent treatment of chronic EAE with partial MHC class II constructs. J. Neuroinflammation. 2017;14(1):100. doi: 10.1186/s12974-017-0873-y. PubMed PMID: 28477623; PubMed Central PMCID: PMC5420407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon M.E., Kok L.P. Microwaves for immunohistochemistry. Micron. 1994;25(2):151–170. doi: 10.1016/0968-4328(94)90040-x. Review. PubMed PMID: 8055246. [DOI] [PubMed] [Google Scholar]
- Chaudhary P., Marracci G., Yu X., Galipeau D., Morris B., Bourdette D. Lipoic acid decreases inflammation and confers neuroprotection in experimental autoimmune optic neuritis. J. Neuroimmunol. 2011;233(1-2):90–96. doi: 10.1016/j.jneuroim.2010.12.002. Epub 2011 Jan 7. PubMed PMID: 21215462; PubMed Central PMCID: PMC4987082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giberson R.T., Demaree R.S., Jr., Nordhausen R.W. Four-hour processing of clinical/diagnostic specimens for electron microscopy using microwave technique. J. Vet. Diagn. Investig. 1997;9(1):61–67. doi: 10.1177/104063879700900111. PubMed PMID: 9087927. [DOI] [PubMed] [Google Scholar]
- Giberson R.T., Demaree R.S., Jr. Microwave processing techniques for electron microscopy: a four-hour protocol. Methods Mol. Biol. 1999;117:145–158. doi: 10.1385/1-59259-201-5:145. Review. PubMed PMID: 10327404. [DOI] [PubMed] [Google Scholar]
- Giberson R.T., Austin R.L., Charlesworth J., Adamson G., Herrera G.A. Microwave and digital imaging technology reduce turnaround times for diagnostic electron microscopy. Ultrastruct. Pathol. 2003;27(3):187–196. doi: 10.1080/01913120309937. PubMed PMID: 12775508. [DOI] [PubMed] [Google Scholar]
- Hartley M.D., Banerji T., Tagge I.J., Kirkemo L.L., Chaudhary P., Calkins E., Galipeau D., Shokat M.D., DeBell M.J., Van Leuven S., Miller H., Marracci G., Pocius E., Banerji T., Ferrara S.J., Meinig J.M., Emery B., Bourdette D., Scanlan T.S. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight. 2019;4(8) doi: 10.1172/jci.insight.126329. pii: 126329. PubMed PMID:30996143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. Processing plant tissues for ultrastructural study. Methods Mol. Biol. 2014;1117:39–55. doi: 10.1007/978-1-62703-776-1_3. PubMed PMID: 24357358. [DOI] [PubMed] [Google Scholar]
- Login G.R., Dvorak A.M. A review of rapid microwave fixation technology: its expanding niche in morphologic studies. Scanning. 1993;15(2):58–66. doi: 10.1002/sca.4950150202. Review. PubMed PMID: 8287206. [DOI] [PubMed] [Google Scholar]
- Login G.R., Dvorak A.M. Methods of microwave fixation for microscopy. A review of research and clinical applications: 1970-1992. Prog. Histochem. Cytochem. 1994;27(4):1–127. Review. PubMed PMID: 8159803. [PubMed] [Google Scholar]
- Leong A.S., Sormunen R.T. Microwave procedures for electron microscopy and resin-embedded sections. Micron. 1998;29(5):397–409. doi: 10.1016/s0968-4328(98)00018-3. Review. PubMed PMID:9842723. [DOI] [PubMed] [Google Scholar]
- Newman G.R., Hobot J.A. Resins for combined light and electron microscopy: a half century of development. Histochem. J. 1999;31(8):495–505. doi: 10.1023/a:1003850921869. Review. PubMed PMID:10507456. [DOI] [PubMed] [Google Scholar]
- Recks M.S., Stormanns E.R., Bader J., Arnhold S., Addicks K., Kuerten S. Early axonal damage and progressive myelin pathology define the kinetics of CNS histopathology in a mouse model of multiple sclerosis. Clin. Immunol. 2013;149(1):32–45. doi: 10.1016/j.clim.2013.06.004. Epub 2013 Jun 18. PubMed PMID: 23899992. [DOI] [PubMed] [Google Scholar]
- Spurr A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. PubMed PMID: 4887011. [DOI] [PubMed] [Google Scholar]
- Su K.G., Savino C., Marracci G., Chaudhary P., Yu X., Morris B., Galipeau D., Giorgio M., Forte M., Bourdette D. Genetic inactivation of the p66 isoform of ShcA is neuroprotective in a murine model of multiple sclerosis. Eur. J. Neurosci. 2012;35(4):562–571. doi: 10.1111/j.1460-9568.2011.07972.x. PubMed PMID: 22277070; PubMed Central PMCID: PMC3279590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinling S.P., Giberson R.T., Kullar R.S. Microwave exposure increases bone demineralization rate independent of temperature. J. Microsc. 2004;215(Pt3):230–235. doi: 10.1111/j.0022-2720.2004.01382.x. PubMed PMID: 15312187. [DOI] [PubMed] [Google Scholar]
- van Dorp R., Boon M.E., Kok P.G., Marani E. Combining microwave stabilization and microwave-stimulated fixation of brain tissue with microwave-stimulated staining. Eur. J. Morphol. 1995;33(2):164–173. PubMed PMID: 7488521. [PubMed] [Google Scholar]
- Webster P. Microwave-assisted processing and embedding for transmission electron microscopy. Methods Mol. Biol. 2007;369:47–65. doi: 10.1007/978-1-59745-294-6_4. PubMed PMID: 17656746. [DOI] [PubMed] [Google Scholar]
- Webster P. Microwave-assisted processing and embedding for transmission electron microscopy. Methods Mol. Biol. 2014;1117:21–37. doi: 10.1007/978-1-62703-776-1_2. PubMed PMID: 24357357. [DOI] [PubMed] [Google Scholar]
- Wendt K.D., Jensen C.A., Tindall R., Katz M.L. Comparison of conventional and microwave-assisted processing of mouse retinas for transmission electron microscopy. J. Microsc. 2004;214(Pt 1):80–88. doi: 10.1111/j.0022-2720.2004.01310.x. PubMed PMID: 15049872. [DOI] [PubMed] [Google Scholar]
- Zechmann B., Zellnig G. Microwave-assisted rapid plant sample preparation for transmission electron microscopy. J. Microsc. 2009;233(2):258–268. doi: 10.1111/j.1365-2818.2009.03116.x. PubMed PMID: 19220692. [DOI] [PubMed] [Google Scholar]