Introduction
With their long-term exposure to immunosuppressive medications, solid organ transplant recipients are known to be prone to skin cancers, necessitating regular skin surveillance. They are also prone to various opportunistic skin infections. Here we describe a rare case of recurrent thigh nodules in a renal transplant recipient caused by deep fungal infection secondary to a rare fungus, Pleurostomophora richardsiae.
Case report
A 74-year-old Chinese man, a retiree who is an avid gardener and golfer, received a deceased donor renal transplant in 1994, and had been on long-term azathioprine, everolimus, and prednisolone since his transplant. There were no changes in his medications since 1994. In 2017, he presented with a painless enlarging flesh-colored nodule of 1 cm diameter on his left thigh, for which he underwent an excisional biopsy. Histology of the skin biopsy sample showed the presence of fungal hyphae, and fungal culture grew unidentifiable molds. He declined further workup or further treatment then.
One year later, he presented again, with flesh-colored nodules at the site of the previous skin excision. On examination, there was a 1-cm flesh-colored, nontender, mobile nodule on the lateral aspect of the left thigh adjacent to the knee (Fig 1). Clinically well, the patient decided to proceed with a repeat excision of the thigh nodule.
Fig 1.
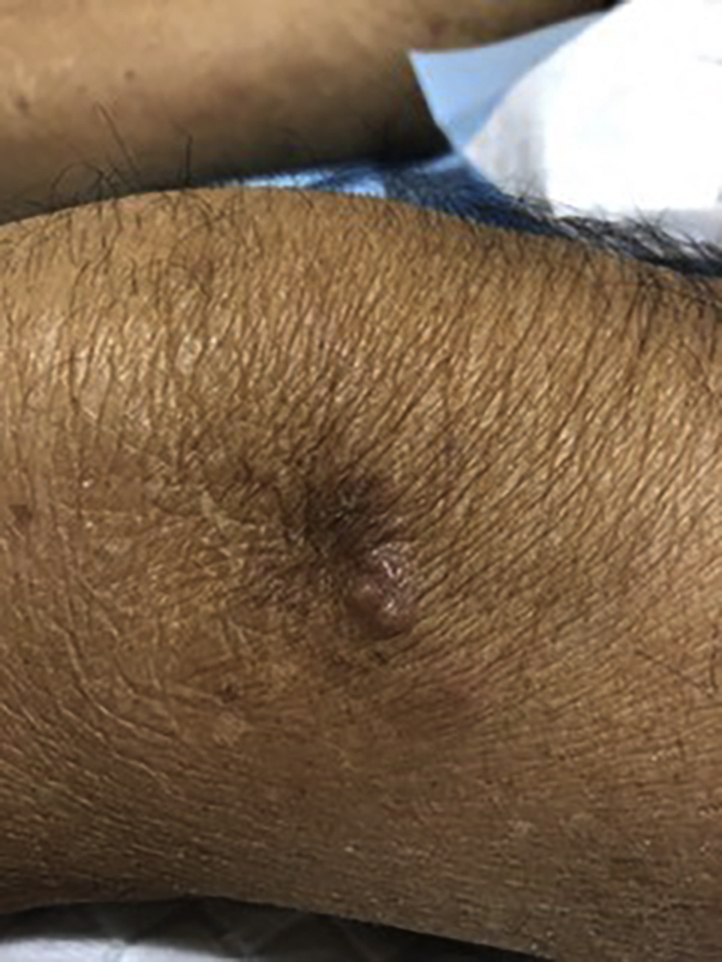
One-centimeter flesh-colored nodule at the left thigh adjacent to the knee.
Histopathologic examination of the nodule found necrotizing granulomatous inflammation within the dermis and subcutis associated with formation of irregular convoluted central cystic spaces (Fig 2, A). Sheets of histiocytes with prominent interspersed multinucleated giant cells and lymphocytes surrounded the cystic spaces, and aggregates of neutrophils formed microabscesses within some of the cystic spaces (Fig 2, B). Gomori methenamine silver stain showed fungal hyphae within the granulomatous areas (Fig 3). The pus from the left thigh nodule grew a dark-colored mold in 3 days, which matured in 6 days. The microscopic morphology showed hyphae with phialides that ended in wide-angled collarettes, bearing conidia suggestive of P richardsiae (Fig 4). An internal transcribed spacer sequencing was done, and the result best matched P richardsiae based on the Westerdijk Fungal Biodiversity Institute database.
Fig 2.
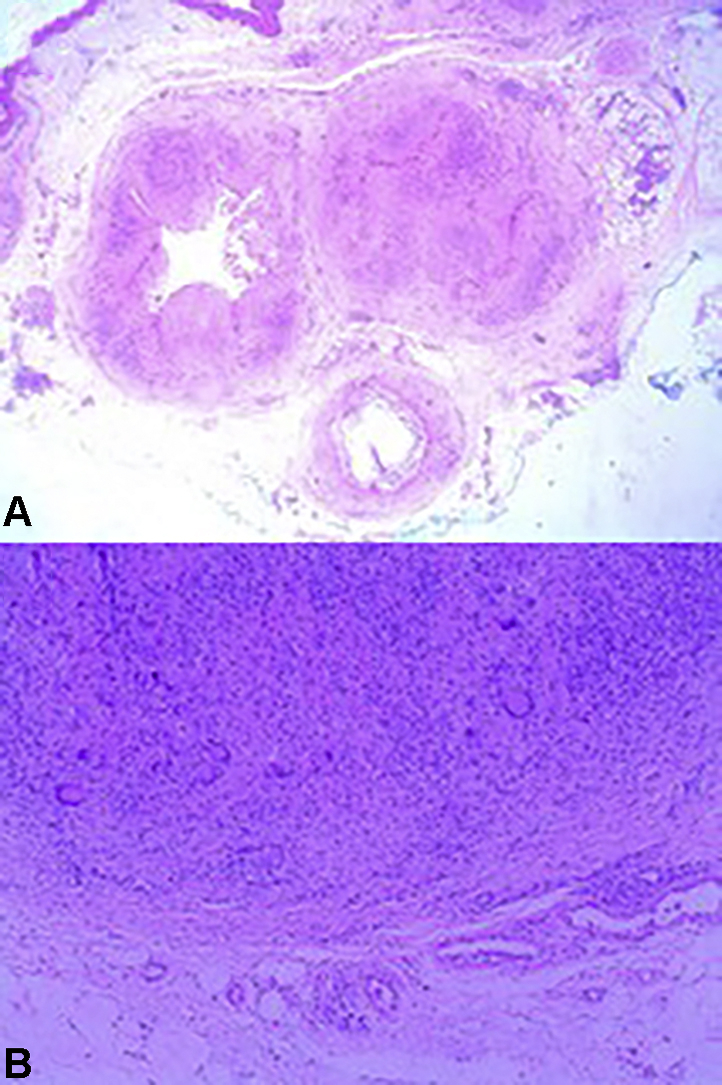
Histopathologic images from the excision biopsy of a P richardsiae nodule. A and B, Necrotizing granulomatous inflammation within the dermis and subdermis associated with irregular convoluted cystic spaces. (A and B, Hematoxylin-eosin-stain. Original magnifications: A, ×40; B, ×100.)
Fig 3.
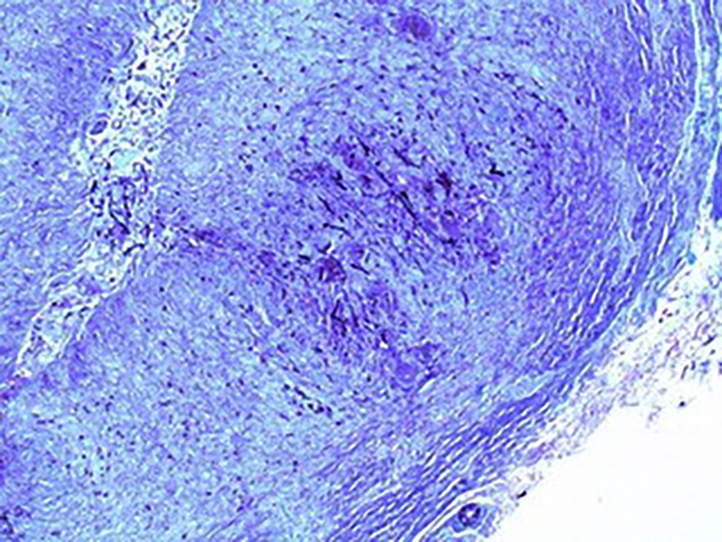
Gomori methenamine silver staining shows scattered hyphae situated within the granulomatous areas. (Original magnification: ×200.)
Fig 4.
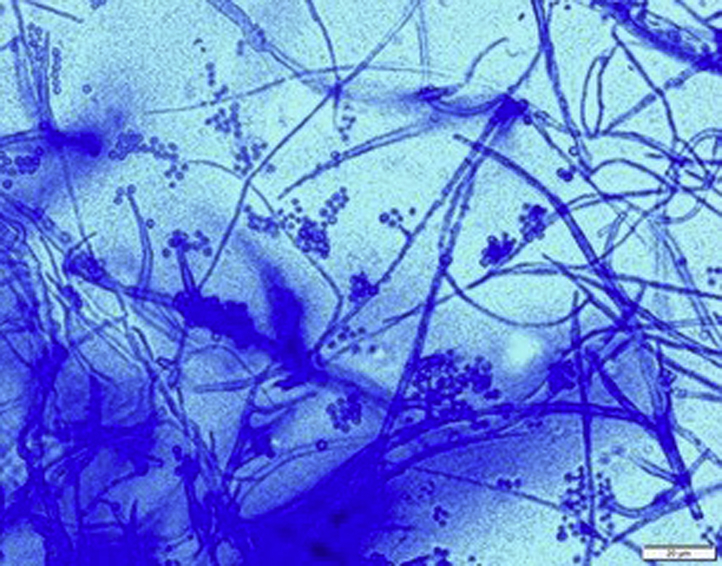
Microscopic morphology of P richardsiae on lactophenol cotton blue. (Original magnification: ×40.)
Ultrasound scan of the knee and thigh showed no invasion of the infection to the muscle, and computed tomography of the thorax incidentally showed mild bronchiectasis and a benign subpleural nodule but no evidence of fungal infection. Itraconazole was started for the treatment of the P richardsiae infection for 10 months at doses ranging from 400 mg to 900 mg daily, with serum itraconazole levels measured regularly to ensure adequate dosing of the drug. Because of the interaction of itraconazole with everolimus, the serum level of everolimus was monitored, and the dose of everolimus was reduced accordingly. One year after surgical excision, the patient reports no recurrence of his knee nodules.
Discussion
With only 25 cases of human infections reported to date since its identification in 1968,1, 2, 3 P richardsiae is a rare dematiaceous (dark-colored) fungus found in woody plants that most commonly presents as subcutaneous phaeohyphomycosis, as seen in our patient. It is believed to be more common in the tropics and subtropics.4 Even though the patient resides in a tropical climate and is an enthusiastic gardener and golfer, the patient could not recall any recent skin trauma involving plants. Yet given the insidious and indolent nature of the infection, with case reports describing 12-year, 30-year, and even 44-year lag periods between exposure and symptoms,1 it is possible that the patient was inoculated with the fungus in the distant past but did not attribute any significance to the exposure.
In this case, the uncommon diagnosis was clinched by histopathology and fungal culture of the excisional biopsy specimen. Therefore, this case highlights the importance of obtaining a deep biopsy specimen (an incisional or excisional biopsy including subcutaneous tissue) instead of a punch biopsy specimen for histopathologic examination to identify usually deep-seated fungal organisms. In addition, a piece of the biopsy specimen should always be sent for fungal culture to evaluate for a possible fungal etiology. Even though this patient showed no signs or symptoms of systemic fungal infections, cutaneous involvement may be the first sign of systemic fungal infections in solid organ transplant recipients; hence, timely diagnosis and treatment is essential.
Until 1989, surgical therapy was the treatment of choice for dematiaceous fungi because it was thought that this group of fungi was resistant to systemic antifungals.1,3 Nevertheless, recent reports have shown that systemic antifungal treatments such as itraconazole are effective.5,6 The patient's infection recurred after excision, suggesting that combined surgical and medical therapy may be required to completely eradicate P richardsiae. Importantly, itraconazole, a direct cytochrome P450 3A4 inhibitor, can increase serum levels of calcineurin inhibitors and mammalian target of rapamycin inhibitors, such as everolimus in this patient.7 Therefore, initiation of antifungal therapy often necessitates the reduction in the dosages of immunosuppressant therapies in transplant recipients.
To our knowledge, dematiaceous fungus infections, such as P richardsiae infections, are extremely rare.6 Given the vulnerability of organ transplant recipients to chronic infections and the indolent nature of P richardsiae infection, the differential diagnosis of deep fungal infection by an insidious fungus such as P richardsiae should be considered in a transplant recipient with chronic subcutaneous nodules or abscesses. Notably, it is difficult to identify P richardsiae using morphology and culture characteristics alone.4 Therefore, modern molecular techniques such as internal transcribed spacer sequencing may be required for the identification of this rare mold. Furthermore, a deep excisional or incisional biopsy for histopathologic examination and fungal culture is key to obtaining the diagnosis of a deep fungus infection, and because skin manifestations may be the first sign of systemic fungal infection in an organ transplant recipient, prompt diagnosis and treatment are crucial.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Levenstadt J.S., Poutanem S.M., Mohan S. Pleurostomophora richardsiae – an insidious fungus presenting in a man 44 years after initial inoculation: A case report and review of the literature. Can J Infect Dis Med Microbiol. 2012;23(3):110–113. doi: 10.1155/2012/406982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam M.S., Vaidehi D., Therese K.L. Rare Pleurostomophora richardsiae mass causing transient nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 2017;33(6):e154–e156. doi: 10.1097/IOP.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 3.Cuenca-Barrales C., De Salazar A., Chueca N. Phaeohyphomycosis due to Pleurostomophora richardsiae: an uncommon cutaneous fungal infection. J Eur Acad Dermatol Venereol. 2018;32(10):e376–e377. doi: 10.1111/jdv.14943. [DOI] [PubMed] [Google Scholar]
- 4.Mostert l., Groenewald J., Summerbell R. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol. 2005;43:1752–1767. doi: 10.1128/JCM.43.4.1752-1767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam M., Freeman S. Phaeohyphomycosis due to Phialophora richardsiae. Australas J Dermatol. 1989;30:37–40. doi: 10.1111/j.1440-0960.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Yehia M., Thomas M., Helen P., Walter M., Dittmer I. Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: Three cases. Transplantation. 2004;77:140–142. doi: 10.1097/01.TP.0000107287.70512.E7. [DOI] [PubMed] [Google Scholar]
- 7.Yokomasu A., Yano I., Sato E. Effect of itraconazole on the pharmacokinetics of everolimus administered by different routes in rats. Biopharm Drug Dispos. 2009;30:517–523. doi: 10.1002/bdd.687. [DOI] [PubMed] [Google Scholar]


