Abstract
Background
Diabetic nephropathy (DN) is the foremost cause of morbidity and has become the most recurrent cause of end-stage renal disease among diabetic patients. Thus, agents having antidiabetic effect along with safety potential in the kidneys would have a higher remedial value.
Objective
The present study aimed to investigate possible protective effect of homeopathic preparation of Cephalandra indica Mother tincture, 6C and 30 C potencies on DN in Wistar rats.
Materials and methods
DN was induced by intraperitoneal injection of STZ (60 mg/kg) 15 min after Nicotinamide (230 mg/kg, i.p.) administration. Rats were divided into six groups (n = 6). Group 1 and 2 was kept normal control and diabetic control respectively whereas Groups 3–5 consist of diabetic nephropathy rats treated with different doses of C. indica Mother tincture, 6C and 30 C potencies for 45 days. Glimepride (10 mg/kg) was used as standard. DN was assessed by determining serum glucose, urea, uric acid, creatinine level and tissue histological examination. Tissue antioxidant enzymes (SOD, GSH, LPO) level was measured to assess the oxidative stress. Also, the level of advanced glycation end products in kidney was determined.
Results
Mother tincture, 6C and 30 C potencies of C. indica produced significant attenuation in the biochemical parameters used to assess diabetic nephropathy. Moreover, oxidative stress and AGE's level in kidney was also found to be significantly reduced.
Conclusion
We conclude that Mother tincture, 6C and 30 C potencies of C. indica confers protective effect against diabetic nephropathy via inhibition of Oxidative stress and AGE's.
Keywords: Diabetic nephropathy, Hyperglycemia, Cephalandra indica, Oxidative stress, AGE's
1. Introduction
Cephalandra indica belongs to family Cucurbitaceae, also known as Kundru in Hindi and Ivy Gourd in English, is a perennial climbing herb with tuberous roots that grows in the wild in abundance in major parts of India. The plant has been used since ancient times for treating diabetes mellitus according to the science of Ayurveda.C.indica has been reported to have an insulin stimulating effect on β cells [1]. The usefulness of C. indica for diabetes mellitus in mother tincture in Homeopathy has also been documented through case reports [2]. A study conducted by Central Council for Research in Homoeopathy on Albino rat model showed pancreatic β cell-regeneration with the mother tincture of C. indica [3]. The plant also has been reported as many reported scientific values as anti-diabetic medicine [4], [5]. Fresh juice of roots is used to treat diabetes; tincture of leaves is used to treat gonorrhea, paste of leaves is applied to the skin diseases. Various phytoconstituents reported in C. indica are cephalandrol, tritriacontane, lupeol, β-sitosterol, cephalandrine A, cephalandrine B, stigma-7-en-3-one, taraxerone and taraxerol. Terpenoids are found to be responsible for antidiabetic activity. Dried bark is a good cathartic. Leaves and stem are antispasmodic and expectorant. The fleshy green fruit is very bitter. Green fruit is chewed to cure sores on the tongue [6], [7].
Diabetic complications are now a global health problem and the foremost cause of morbidity and mortality in diabetic patients without effective therapeutic approach. Diabetic nephropathy (DN) is a common and a major complication in both type 1 and 2 diabetes mellitus [8], [9], [10] and a prominent cause of end stage renal disease (ESRD) worldwide. Progression of DN is characterized by renal structural abnormalities, including glomerular hypertrophy, mesangial matrix expansion, thickening of tubular glomerular basement membrane and occurrence of pathological values of urine albumin, creatinine and protein excretion, and abnormal glomerular filtration rate (GFR) [11], [12].
Oxidative stress is the prompt and serious event caused by an imbalance between the production of reactive oxygen species (ROS) and a biological system's ability to detoxify the reactive intermediates or restore the resulting destruction [13], [14], [15]. The principal effect of oxidative stress is the production of ROS, which includes free radicals. These free radicals attack polyunsaturated fatty acids and cause their oxidation in physiological systems (lipid peroxidation). By-products of lipid peroxidation such as conjugated dienes and malondialdehyde (MDA) are increased in the patients with obesity, metabolic syndrome and type 2 diabetes mellitus (T2DM) [16], [17]. These consequences of oxidative stress can uphold the development of complications of diabetes mellitus [18].
The process of diluting and vigorous shaking of the remedies known as potentization in Homeopathy is believed to often render the remedy more potent in terms of clinical response. In homeopathic potentization procedure, the mother tincture (e.g. original extract of the plant if the drug is derived from the plant) is generally diluted with 99 ml of rectified spirit (90% ethyl alcohol) and given 10 ‘successions’ or ‘jerks’ to produce the potency 1 C. Similarly, 1 ml of the drug solution at potency 1 C is again added with 99 ml of 90% ethanol and 10 jerks given to produce the potency 2 C and in this way by successive dilutions and successions, further potencies like 6 C, 30 C, 200 C and beyond are produced. Therefore, at high dilutions, say beyond potency 12 C (i.e. beyond Avogadro's limit, i.e. 10–23) the solution is unlikely to contain even a single molecule of the original drug material (i.e. the mother tincture).
Considering the literature gap in exploring the therapeutic potential, the present study was carried out to investigate beneficial effects of homeopathic preparation (mother tincture, 6 C and 30 C potencies) of C. indica in diabetic nephropathy.
2. Materials and methods
2.1. Chemicals
Streptozotocin (STZ) was obtained from Sigma Aldrich, USA and Nicotinamide from Finar, India Ltd. and diagnostic kits for the biochemical estimations were obtained from Reckon Diagnostics Pvt. Ltd., India. Mother tincture, 6 C and 30 C potencies of C. indica was procured from Dr. Willmar Schwabe, India Pvt. Ltd. All the other chemicals used were of analytical grade.
2.2. Experimental design and animals
Male Wistar rats (260–280 g) were obtained from Central Animal Facility, NIPER, Mohali and kept under standard conditions (24 ± 2 °C, relative humidity 45 ± 5% and 12 h light/dark cycle). The animals were fed with standard pellet diet (Ashirwad Industries, Mohali, Punjab, India) and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC no. MMCP/IAEC/13/07) and by the regulatory body of the Government (Reg. No. 585/05/A/CPCSEA).
Diabetes mellitus was induced by single intraperitoneal injection of freshly prepared STZ (single dose of 65 mg/kg) in 0.1 M citrate buffers (pH 4.5) 15 min after nicotinamide (230 mg/kg, i.p.). Seven days after STZ administration, blood was withdrawn from retro-orbital plexus of rats under mild anesthesia to determine fasting blood glucose level. Rats with fasting blood glucose level 330–380 mg/dL were selected for the study. Diabetic nephropathy symptoms typically develop after 30–45 days. At the end of 30 days, the levels of serum urea, uric acid, creatinine and BUN (Blood Urea Nitrogen) were significantly high suggesting development of diabetic nephropathy.
Animals were divided into six groups consisting of six rats each. Group 1 was normal control; Group 2 was diabetic nephropathy control; Group 3 to 5 consisted diabetic nephropathy rats receiving 2 ml/kg of C. indica Mother tincture, 6 C and 30 C; Group 6 consisted of diabetic nephropathy rats receiving 10 mg/kg Glimepride. All the doses were administered orally. Doses were selected on the basis of previous studies on homeopathic preparations [19]. Gymnema sylvestre Mother tincture, 6 C and 30 C were diluted 5 times with double distilled water and then administered in divided doses thrice daily (six hourly; at 6 a.m., 12 noon and 6 p.m.).
Blood glucose level and body weight were estimated on 30th, 45th, 60th and 75th day of STZ induction. All biochemical estimations was carried out using commercially available kits of Reckon Diagnostics Pvt. Ltd. Animals were sacrificed at the end of the study and liver, kidney and pancreas were removed and stored at −70 °C until use for histopathological and biochemical studies.
Body weight of each animal was measured at the start of study and animals with similar weight range were chosen and grouped. Body weight of each group was measured periodically till the end of the study. Fasting blood glucose level was estimated at interval of 15 days by using commercial enzymatic kits purchased from Reckon Diagnostics Pvt. Ltd. India throughout the study. Plasma insulin was determined using Insulin ELISA kit (DRG, Germany) in blood collected into tubes with anticoagulant. Serum total cholesterol (TC), triglycerides (TG), low density lipoproteins (LDL), very low density lipoproteins (VLDL) and high density lipoproteins (HDL) were measured by the commercial enzymatic kits purchased from Reckon Diagnostics Pvt. Ltd. India. Serum creatinine, urea and uric acid were measured by using commercial enzymatic kits purchased from Reckon Diagnostics Pvt. Ltd. India. Kidney homogenate was used to estimate thiobarbituric acid reactive substances (TBARS) [20] and level of antioxidant enzymes, viz. superoxide dismutase (SOD) and reduced glutathione (GSH) [21].
2.2.1. AGEs estimation in kidneys
AGEs levels in the kidneys were determined by a method as previously described by Ref. [22]. Perfused kidneys were homogenized in 2 mL of 0.25 M sucrose followed by centrifugation at 900 g at 5 °C and the supernatant was separated. The pellet was re suspended in 2 mL sucrose solution and centrifuged and the supernatant obtained was mixed with the previous one. The proteins present were precipitated by adding equal volume of trichloroacetic acid (TCA). Following centrifugation at 4 °C with 900 g, the protein pellet obtained was mixed with 1 mL methanol twice to remove the lipid fraction. The insoluble protein, after washing with 10% cooled TCA was centrifuged and the residue was solubilized in 1 ml of 1 N NaOH and the protein concentration was estimated by measuring the absorbance at 280 nm against BSA standard curve. The AGEs content was then measured fluorometrically with an emission at 440 nm and excitation at 370 nm, and the results were expressed as relative fluorescence units (RFU)/mg protein.
2.2.2. Histopathology
Kidney was harvested from the animals and fixed in 10% neutral buffered formalin solution, dehydrated in ethanol and embedded in paraffin. Sections of 5 μm thickness were prepared using a rotary microtome and stained with hematoxylin and eosin (H & E) dye for histopathological examination.
2.3. Statistical analysis
Statistical analysis was performed using Graphpad Prism 6. Values were expressed as mean ± SEM and one-way analysis of variance (ANOVA) was used for statistical analysis. ANOVA was followed by Tukey's as post hoc multiple comparison test. The results were considered significant if p ≤ 0.05.
3. Results
3.1. Effect of C. indica on body weight
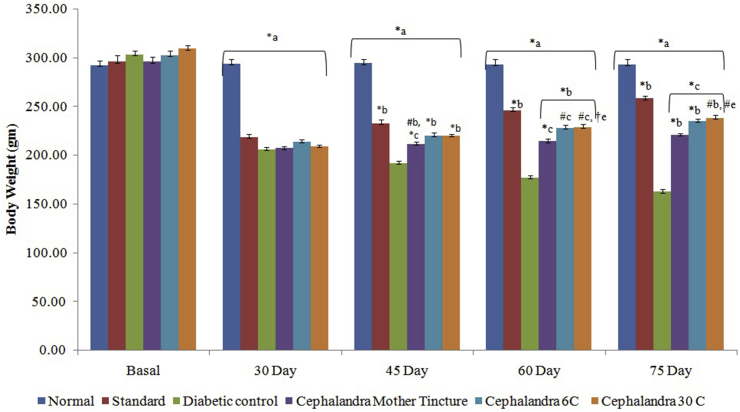
Body weight of diabetic nephropathy control rats decreased by 44.09% at end of study as compared to the initial body weight. Administration of C. indica MT, 6 C and 30 C increased the body weight significantly by 5.31, 11.16 and 17.96% respectively. Glimepride also increased the body weight by 18.34% in diabetic nephropathy rats (Fig. 1).
Fig. 1.
Effect of C. indica on body weight (gms)in diabetic-nephropathy Wistar rats. Each group (n = 6) represents Mean± SEM. Data was analyzed by using One way ANOVA followed by Tukey’s multiple test; a vs control; b vs Diabetic control; c vs Cephalendra Mother tincture; d vs Cephalendra 6C; e vs Standard. ∗p < 0.001, #p< 0.01, †p < 0.05.
3.2. Effect of C. indica on blood glucose and serum insulin level
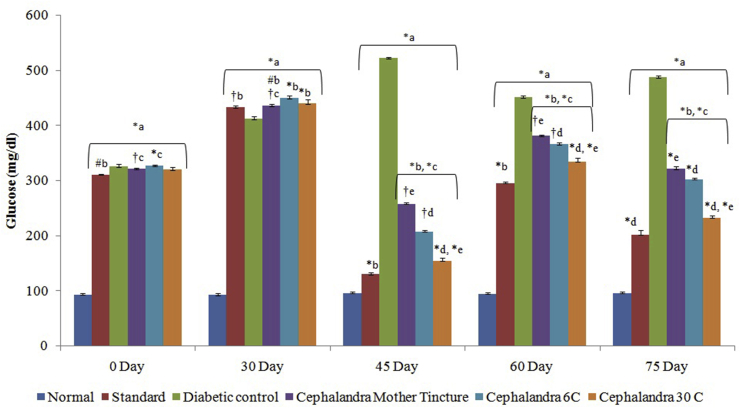
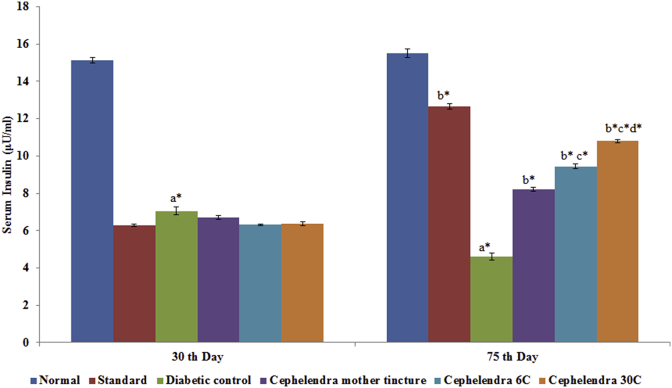
Fasting blood glucose level of all animals was within the normal range initially and STZ induction increased blood glucose level significantly with time in respect to control group. A significant reduction in fasting blood glucose level was observed in diabetic nephropathy rats treated with C. indica MT, 6 C and 30 C from 427.67 to 255.81 mg/dl, 436.17 to 207.49 and 432.73 to 152.56 mg/dL respectively at the end of study (Fig. 2). A significant decrease in fasting insulin level (7.04 ± 0.21 μIU/ml) was observed in STZ-treated diabetic nephropathy rats in comparison to normal control rats (15.13 ± 0.13 μIU/mL). Administration of C. indica MT, 6 C and 30 C for 30 days produced significant increase in serum insulin level (Fig. 3).
Fig. 2.
Effect of C. indica on fasting blood glucose level (mg/dL) in diabetic-nephropathy Wistar rats. Each group (n = 6) represents Mean ± SEM. Data was analyzed by using One way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; c vs Cephalendra Mother tincture; d vs Cephalendra 6 C; e vs Standard. ∗ p < 0.001, #p < 0.01, †p < 0.05.
Fig. 3.
Effect of C. indica on fasting serum insulin level (μIU/ml) in diabetic-nephropathy Wistar rats. Each group (n = 6) represents Mean ± SEM. Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; c vs Cephalendra Mother tincture; d vs Cephalendra 6 C; e vs Standard. ∗ p < 0.001, #p < 0.01, †p < 0.05.
3.3. Effect of C. indica on renal function
In diabetic nephropathy control rats a significant increase in urea (97.96 mg/dl), uric acid (16.04 mg/dl) and creatinine (4.11 mg/dl) levels was observed in STZ induced diabetic nephropathy rats over the period of study. Treatment from C. indica MT, 6 C and 30 C produced the effect in accordance with the dilutions, i.e. higher the dilution more was the effect observed (Table 1).
Table 1.
Effect of C. indica on renal function estimation in diabetic-nephropathy Wistar rats.
| Parameters | Urea (mg/dL) | Uric Acid (mg/dL) | Creatinine (mg/dL) | |||
|---|---|---|---|---|---|---|
| Groups | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day |
| Normal | 33.15 ± 0.789 | 33.28 ± 0.629 | 5.22 ± 0.216 | 5.27 ± 0.198 | 0.72 ± 0.009 | 0.81 ± 0.009 |
| Diabetic control | 82.13 ± 1.64a∗ | 97.96 ± 0.518a∗ | 12.59 ± 0.854a∗ | 16.04 ± 0.612a∗ | 2.83 ± 0.046a∗ | 4.22 ± 0.062a∗ |
|
C. indica MT |
85.29 ± 1.57 | 60.42 ± 0.730b∗c∗e∗ | 15.02 ± 0.238 | 8.28 ± 0.117b∗c∗ | 4.01 ± 0.127 | 2.10 ± 0.040b∗c∗e† |
|
C. indica 6 C |
82.48 ± 1.18 | 52.48 ± 0.514b∗c∗d∗ | 12.74 ± 0.716 | 7.39 ± 0.160b∗d† | 4.37 ± 0.079 | 1.71 ± 0.115b∗c∗d† |
|
C. indica 30 C |
84.99 ± 1.40 | 44.72 ± 0.795b∗c∗d∗e∗ | 11.42 ± 0.210 | 6.36 ± 0.171b∗d#e† | 4.20 ± 0.170 | 1.24 ± 0.058b∗d∗e# |
| Glimepride 10 mg/kg | 83.87 ± 1.098 | 34.35 ± 0.933b∗ | 12.29 ± 0.634 | 6.01 ± 0.167 b∗ | 3.00 ± 0.223 | 0.96 ± 0.052 b∗ |
Each group (n = 6) represents Mean ± SEM.
3.4. Effect of C. indica on lipid profile
After 30 days of STZ induction, Serum total cholesterol (TC), triglyceride (TG), low density lipoproteins (LDL) and very low density lipoproteins (VLDL) significantly increased in diabetic nephropathy rats. At 75th day of study C. indica MT, 6 C and 30 C and Glimepride ameliorating TC level by 37.15, 49.01, 53.80 and 59.52% respectively. TG levels were reduced by 24.83, 39.47, 49.00 and 55.06% with administration of C. indica MT, 6 C, 30 C and Glimepride respectively. Similarly levels of LDL and VLDL were also reduced significantly by administration of C. indica MT, 6 C and 30 C and Glimepride in diabetic nephropathy rats. HDL-c significantly decreased in diabetic nephropathy rats and the treatment with C. indica MT, 6 C and 30 C and Glimepride significantly increased the level of HDL-c (Table 2).
Table 2.
Effect of C. indica on Lipid Profile in diabetic-nephropathy Wistar rats.
| Parameters | Groups | Normal | Diabetic control | C. indica MT | C. indica 6C | C. indica 30C | Glimepride10 mg/kg |
|---|---|---|---|---|---|---|---|
| TC (mg/dl) | 30th day | 103.5 ± 2.75 | 250.4 ± 2.54a∗ | 253.77 ± 5.29 | 255.51 ± 4.17 | 245.36 ± 2.42 | 252.2 ± 2.91 |
| 75th day | 103.6 ± 2.02 | 295.8 ± 2.49a∗ | 159.98 ± 1.84b∗c∗e∗ | 130.51 ± 1.78b∗c∗d∗ | 113.91 ± 2.104b∗c∗d∗e∗ | 102.5 ± 1.33b∗ | |
| TG (mg/dl) | 30th day | 72.44 ± 2.06 | 161.72 ± 3.27a∗ | 152.12 ± 1.85 | 153.99 ± 1.36 | 151.05 ± 1.01 | 158.89 ± 2.24 |
| 75th day | 71.38 ± 1.46 | 203.26 ± 1.12a∗ | 115.46 ± 1.51b∗c∗e∗ | 92.64 ± 1.2b∗c∗d∗ | 77.31 ± 1.42b∗d∗e∗ | 71.68 ± 2.02b∗ | |
| LDL (mg/dl) | 30th day | 14.41 ± 2.07 | 145.76 ± 2.16a∗ | 158.77 ± 4.60 | 159.79 ± 3.12 | 150.98 ± 2.60 | 148.97 ± 2.89 |
| 75th day | 14.64 ± 2.73 | 176.97 ± 2.79a∗ | 72.42 ± 2.44b∗c∗e∗ | 47.63 ± 2.89b∗c∗d∗ | 33.67 ± 2.64b∗c∗d∗e# | 16.00 ± 1.90b∗ | |
| VLDL (mg/dl) | 30th day | 33.07 ± 0.94 | 73.84 ± 1.49a∗ | 69.91 ± 0.614 | 70.62 ± 0.788 | 69.27 ± 0.645 | 72.55 ± 1.02 |
| 75th day | 32.59 ± 0.66 | 92.81 ± 0.51a∗ | 53.10 ± 0.684b∗c∗e∗ | 42.30 ± 0.436b∗c∗d∗ | 35.52 ± 0.529b∗c†d∗e∗ | 32.73 ± 0.92b∗ | |
| HDL (mg/dl) | 30th day | 56.09 ± 0.63 | 30.86 ± 0.424a∗ | 26.75 ± 0.547 | 27.09 ± 0.426 | 26.93 ± 0.314 | 30.68 ± 0.703 |
| 75th day | 56.44 ± 0.54 | 26.07 ± 0.329a∗ | 36.12 ± 0.503b∗c∗e∗ | 40.40 ± 0.917b∗c∗d∗ | 45.04 ± 0.406b∗c∗d∗e∗ | 53.81 ± 0.295b∗ |
Each group (n = 6) represents Mean ± SEM.
3.5. Effect of C. indica on antioxidant enzymes and lipid peroxidation
The levels of reduced glutathione (GSH) and superoxide dismutase (SOD) was found significantly high after 30 days of STZ induction in diabetic nephropathy animals as compare to normal control group. Continuous administration of C. indica MT, 6 C and 30 C and Glimepride significantly reduced the levels at end of study i.e. at 75th day.
Moreover, the level of TBARS, marker of lipid peroxidation, significantly increases in the STZ-induced diabetic nephropathy rats. But, treatment with C. indica MT, 6 C and 30 C and Glimepride significantly reduced the level of TBARS by the end of the study (Table 3).
Table 3.
Effect of C. indica on level of antioxidant enzymes and lipid peroxidation (TBARS) in kidney of diabetic-nephropathy Wistar rats.
| Parameters | SOD (U/mg protein) |
GSH (μM/mg protein) |
TBARS (nmol/mg protein) |
|---|---|---|---|
| Groups | Kidney | Kidney | Kidney |
| Normal | 4.87 ± 0.083 | 74.93 ± 0.621 | 0.55 ± 0.01 |
| Diabetic control | 1.23 ± 0.049a∗ | 38.60 ± 0.481a∗ | 2.98 ± 0.017a∗ |
|
C. indica MT |
2.05 ± 0.017b∗c∗e∗ | 43.46 ± 0.520b∗c∗e∗ | 2.51 ± 0.015c∗e∗ |
|
C. indica 6 C |
2.80 ± 0.027b∗c∗d | 54.25 ± 0.477b∗c∗d∗ | 1.95 ± 0.009b∗c∗d∗ |
|
C. indica 30 C |
3.73 ± 0.040b∗d∗e∗ | 66.04 ± 0.668b∗c∗e∗ | 1.32 ± 0.014b∗c∗d∗e∗ |
| Glimepride 10 mg/kg | 3.86 ± 0.018 b∗ | 67.83 ± 0.535 b∗ | 1.19 ± 0.03 b∗ |
Each group (n = 6) represents Mean ± SEM.
3.6. Effect of C. indica on AGEs in kidneys
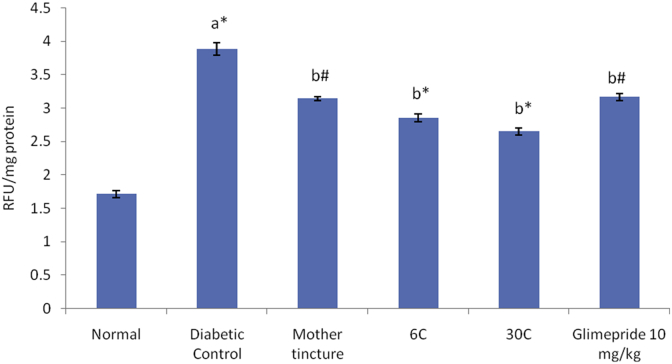
Induction of DN in rats led to a significant increase in kidney AGEs levels when compared to normal animals. Administration of C. indica MT, 6 C and 30 C significantly reduced the AGEs levels in kidneys when compared to control group. However, administration of Glimepride (10 mg/kg) also produced significant change (p < 0.05) in AGEs levels when compared to control group (Fig. 3).
3.7. Histopathology
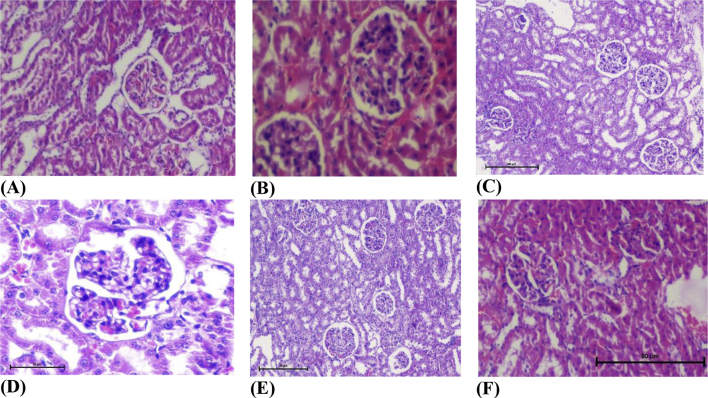
The renal tissue of diabetic rats showed glomeruli with mesangio capillary proliferation. Administration of STZ produced significant structural changes in the nephron. Treatment with C. indica significantly improved the structural changes in kidney (Fig. 4).
Fig. 4.
Effect of C. indica on formation of AGEs in kidney (RFU/mg protein) in diabetic-nephropathy Wistar rats. One way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; c vs Cephalendra Mother tincture; d vs Cephalendra 6 C; e vs Standard. ∗ p< 0.001, #p< 0.01, †p < 0.05.
4. Discussion
Diabetes is a multifactorial disease that has a considerable adverse impact on health. In support of management of diabetes as well as of complications related to it [23], [24], [25], [26]. There is urgent need of critical pharmacological research to establish new agents with high therapeutic value and fewer side effects. Nowadays, herbal drugs are gaining popularity in the treatment of diabetes and its related complications. Therefore, we have made an attempt to investigate potential of C. indica (homeopathic preparation) in management of diabetic nephropathy (see Fig. 5).
Fig. 5.
Histopathological changes in Kidney of normal and treated rats (hematoxylin and eosin 40×). (A) Normal, (B) diabetic nephropathy control, (C) standard, (D) C. indica mother tincture, (E) C. indica 6 C, (F) C. indica 30 C.
So far, there are many types of methods that can be used for the establishment of the diabetic animal model. In the present study, diabetic nephropathy was induced by chemical drugs STZ. STZ, a cytotoxic glucose analog, is the most prominent diabetogenic chemicals in diabetes research [27], [28], [29]. Because of its structural features, STZ gets selective entry into the β-cells of the islets of Langerhans via the low affinity glucose transporter GLUT-2 in its plasma membrane and causes destruction of β-cells, which leads to a reduction in insulin release, which in turn results in a rise in blood glucose concentration, i.e. hyperglycemia [30] and chronic hyperglycemia has been considered to be the precondition for development of diabetic complications [31], [32], [33]. NAD is an antioxidant which exerts protective effect on the cytotoxic action of STZ by scavenging free radicals and causes only minor damage to pancreatic β-cell mass producing type 2 diabetes. STZ and NAD provide good opportunity to investigate diabetes in much closely similar pathophysiological situation as in humans [34]. The earliest clinical manifestation for incipient diabetic nephropathy is the development of the persistent microalbuminuria (urinary albumin excretion rate; 20–200 μg/min). In type 2 diabetes, if no treatment is initiated, up to 20–40% of patients will progress to overt albuminuria and 20% of those with overt albuminuria will develop ESRD over the next 20 years [35].
Diabetic nephropathy is characterized by a series of renal structure abnormality including glomerular basement membrane thickening, mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis, podocytes and renal cell death, increased albuminuria, glomerular hypertrophy, creatinine and protein excretion, and abnormal glomerular filtration rate (GFR) and renal dysfunction [36].
Administration of STZ resulted in increase in blood glucose level of experimental animals. Oral administration of homeopathic preparation of C. indica (MT, 6 C and 30 C) treatment restored the blood glucose level to significant extent. Due to hyperglycemia, the catabolic reactions increases, leading to muscle wasting and ultimately results to reduction in body weight [37]. Oral administration of C. indica improved the body weight in diabetic rats. Hyperglycemia is accompanied with abnormalities in the lipoprotein metabolism therefore leads to increase in TC, TG, LDL, VLDL and decrease in HDL levels which is attributable to excess mobilization of fat from the adipose due to under peripheral utilization of glucose [38]. The results revealed that in diabetic rats levels of TC, TG, LDL, VLDL levels were significantly decreased and HDL level was increased with dosage intervention of C. indica as compared to diabetic control.
Increased urea, uric acid and creatinine levels are indications of the development of diabetic nephropathy in diabetic rats [39], [40], [41]. In the present study, these biochemical variables were found be high in diabetic rats as compared to the normal rats. With treatment by homeopathic preparation of C. indica, these variables were found to be closer to those of normal rats and were high in the diabetic controls. These results suggest that C. indica plays a role, either directly or indirectly, in providing protection against diabetic nephropathy or delay in its development.
Oxidative stress and hyperglycemia are interlinked factors which plays important role in onset of diabetes. Hyperglycemia is the single most important factor in the generation of early and sustained oxidative stress [33]. GSH and SOD (antioxidant enzyme) have long been reported to protect rats against the development of diabetes [42], [43]. GSH and SOD enzymes were depleted in diabetic rats as compared to non-diabetic rats. The increase in thiobarbituric acid-reactive substances (TBARS), an index of lipid peroxidation in the diabetic rats might be due to increased levels of oxygen free radicals. In the present study, homeopathic preparation of C. indica administration lead to decrease TBARS level and increase GSH and SOD in kidney due to its potential antioxidant activity.
AGEs are a group of heterogeneous compounds formed from the non-enzymatic glycation of proteins, lipids and nucleic acid and are known to contribute to diabetes, insulin resistance etc. AGEs induce direct injury to the mesangial cells leading to mesangial expansion and basement membrane thickening [44], [45] Therefore, inhibition of AGEs formation could be crucial therapeutic approach for management of diabetic nephropathy. The present study demonstrates that C. indica MT, 6 C and 30 C exhibits potential inhibitory effect against the formation of AGEs.
Histopathological observations in the present study showed glomeruli with mesangio capillary proliferation in kidney of diabetic rats along with amelioration of hemodynamic parameters of kidney due to increased formation of AGEs. C. indica MT, 6 C and 30 C administered diabetic nephropathy rats showed amelioration in structural improvement in kidney of diabetic rats. The antioxidant activity of C. indica might play a significant role in the recovery of damaged cells of kidney which in turn may be due to the presence of flavonoid and phenolic compounds in C. indica.
5. Conclusion
We conclude that homeopathic preparation of C. indica has potential to attenuate diabetic nephropathy in STZ induced diabetic rats. The different potencies (MT, 6 C, 30 C) of C. indica exerts its beneficial effects by the reducing FBG, improvement in lipid profile and renal functions, restoration of antioxidant enzymes and inhibition of AGEs formation in kidney. However, further histopathological and biochemical studies are needed to elucidate the exact mechanism of action of C. indica in diabetic nephropathic rats.
Conflicts of interest
Authors do not have any conflict of interest.
Sources of funding
CCRH, Department AYUSH, New Delhi, Government of India (Z.28015/68/2013-HPC (EMR)-AYUSH dated 29.03.2014)
Acknowledgment
Financial assistance from CCRH, Department AYUSH, New Delhi, Government of India is highly acknowledged.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Chopra R.N., Chopra I.C., Handa K.L., Kapur L.D. Medicinal plants in diabetes. In: Gupta P., editor. Indigenous drugs of India. 2nd ed. U.N. Dhar & Sons Ltd; Calcutta, India: 1958. pp. 314–316. [Google Scholar]
- 2.Chandra Ghose Sarat. LXVII, No.6. CD Rom; 1952. (Cephalandra India; homoeopathic recorder.). [Google Scholar]
- 3.Rastogi D.P., Saxena A.C., Kumar S. Pancreatic beta-cell regeneration-A novel anti-diabetic action of Cephalandra indica mother tincture. Br Homoeopath J. 1988;77:147–151. [Google Scholar]
- 4.Mukerjee K., Ghosh N.C., Datta T. Coccinia indica as a potential hypoglycemic agent. Indian J Exp Biol. 1972;5:347–349. [PubMed] [Google Scholar]
- 5.Venkateswaran S., Pari L. Effect of Coccinia indica leaves on antioxidant status in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2003;84:163–168. doi: 10.1016/s0378-8741(02)00294-5. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar B., Mukherjee B., Mukherjee S.K. Blood sugar lowering potentiality of selected Cucurbitaceae plants of Indian origin. Indian J Med Res. 1989;90:300–305. [PubMed] [Google Scholar]
- 7.Khan A.K., Akhtar S., Mahtab H. Treatment of diabetes mellitus with Coccinia indica. Brit Med J. 1980;280:1044. doi: 10.1136/bmj.280.6220.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur N., Kishore L., Singh R. Attenuating diabetes: what really works? Curr Diabetes Rev. 2016;12:259–278. doi: 10.2174/1573399811666150826115410. [DOI] [PubMed] [Google Scholar]
- 9.Kaur N., Kishore L., Singh R. Diabetic autonomic neuropathy: pathogenesis to pharmacological management. J Diabetes Metabol. 2014;5:402. [Google Scholar]
- 10.Satirapoj B. Nephropathy in diabetes. Adv Exp Med Biol. 2012;771:107–122. doi: 10.1007/978-1-4614-5441-0_11. [DOI] [PubMed] [Google Scholar]
- 11.Alsaad K.O., Herzenberg A.M. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vleming L.J., Baelde J.J., Westendorp R.G., Daha M.R., van Es L.A., Bruijn J.A. The glomerular deposition of PAS positive material correlates with renal function in human kidney diseases. Clin Nephrol. 1997;47:158–167. [PubMed] [Google Scholar]
- 13.Kaur N., Kishore L., Singh R. Antidiabetic effect of new chromane isolated from Dillenia indica L. leaves in streptozotocin induced diabetic rats. J Func Foods. 2016;22:547–555. [Google Scholar]
- 14.Ratnam D.V., Ankola D.D., Bhardwaj V., Sahana V.D., Kumar M.N. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Singh R., Kishore L., Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Freeman B.A., Crapo J.D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 17.Slater T.F. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pazdro R., Burgess J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131:276–286. doi: 10.1016/j.mad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Rakshit G., Singh J.P., Pathak S.D., Banoth C.K., Chadra P.K., Rajpal A multi-centric double-blind randomized homeopathic pathogenetic trial of Gymnema sylvestre. Indian J Res Homeopath. 2013;7:9–21. [Google Scholar]
- 20.Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Owen O.G. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 22.Sensi M., Pricci F., Pugliese G. Role of advanced glycation end-products (AGE) in late diabetic complications. Diabetes Res Clin Pract. 1996;28:9–17. doi: 10.1016/0168-8227(94)01061-4. [DOI] [PubMed] [Google Scholar]
- 23.Goth A. 9th ed. CV Mosby Company; St. Louis: 1978. Medical pharmacology: principles and concepts. [Google Scholar]
- 24.Kaur N., Kishore L., Singh R. Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE's and oxidative stress. J Food Sci Technol. 2017;54:408–421. doi: 10.1007/s13197-016-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishore L., Kaur N., Singh R. Distinct biomarkers for early diagnosis of diabetic nephropathy. Curr Diabetes Rev. 2017;13(6):598–605. doi: 10.2174/1573399812666161207123007. [DOI] [PubMed] [Google Scholar]
- 26.Szkudelski T. The Mechanism of alloxan and streptozotocin action in β-cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 27.Accili D., Drago J., Lee E.J., Johnson M.D., Cool M.H., Salvatore P. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 28.Gajdosík A., Gajdosíkova A., Stefek M., Navarova J., Hozova R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18:54–62. [PubMed] [Google Scholar]
- 29.Kishore L., Kaur N., Singh R. Bacosine isolated from aerial parts of Bacopa monnieri improves the neuronal dysfunction in Streptozotocin-induced diabetic neuropathy. J Func Foods. 2017;34:237–247. [Google Scholar]
- 30.Dufrane D., Van S.M., Guiot Y., Goebbels R.M., Saliez A., Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81:36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 31.Kishore L., Kaur N., Singh R. Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. J Food Drug Anal. 2017;25:576–588. doi: 10.1016/j.jfda.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishore L., Kaur N., Singh R. Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren Fail. 2016;38:1528–1544. doi: 10.1080/0886022X.2016.1227920. [DOI] [PubMed] [Google Scholar]
- 33.Singh R., Kaur N., Kishore L., Kumar G.K. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150:51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 35.Yamagishi S.I., Nakamura K., Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93–106. doi: 10.2174/1573399052952631. [DOI] [PubMed] [Google Scholar]
- 36.Kolset S.O., Reinholt F.P., Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60:976–986. doi: 10.1369/0022155412465073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajkumar L., Srinivasan N., Balasubramanian K., Govindarajulu P. Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol. 1997;29:1081–1083. [PubMed] [Google Scholar]
- 38.Krishna Kumar K., August K.T., Vijayammal P.L. Hypolipidemic effect of Salacia oblonga Wall. Root bark in Streptozotocin diabetic rats. Med Sci Res. 2000;28:65–67. [Google Scholar]
- 39.Breyer M.D., Bottinger E., Brosius F.C., Coffman T.M., Harris R.C., Heilig C.W. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 40.Makino H., Tanaka I., Mukoyama M., Sugawara A., Mori K., Muro S. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–1765. doi: 10.1097/01.asn.0000019782.37851.bf. [DOI] [PubMed] [Google Scholar]
- 41.Sun S.Z., Wang Y., Li Q., Tian Y.J., Liu M.H., Yu Y.H. Effects of benazepril on renal function and kidney expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in diabetic rats. Chin Med J. 2006;119:814–821. [PubMed] [Google Scholar]
- 42.Lazarow A., Patterson J.W., Levey S. The mechanism of cysteine and glutathione protection against alloxan diabetes. Science. 1948;108:308–309. doi: 10.1126/science.108.2803.308. [DOI] [PubMed] [Google Scholar]
- 43.Winterbourn C.C., Munday R. Glutathione-mediated redox cycling of alloxan. Mechanisms of superoxide dismutase inhibition and of metal-catalyzed OH formation. Biochem Pharmacol. 1989;38:271–277. doi: 10.1016/0006-2952(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 44.Lee H.B., Yu M.R., Yang Y., Jiang Z., Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 45.Uribarri J., del Castillo M.D., de la Maza M.P., Filip R., Gugliucci A., Luevano-Contreras C. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461–473. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]