Abstract
Knowledge with regard to the pathogenesis of lupus erythematosus has progressed rapidly over the past decade, and with it has come promising new agents for the treatment of cutaneous lupus erythematous (CLE). Classification of CLE is performed using clinical features and histopathologic findings, and is crucial for determining prognosis and choosing therapeutic options. Preventative therapy is critical in achieving optimal disease control, and patients should be counseled on sun-safe behavior and smoking cessation. First-line therapy includes topical corticosteroids and calcineurin inhibitors, with antimalarial therapy. Traditionally, refractory disease was treated with oral retinoids, dapsone, and other oral immunosuppressive drugs, but new therapies are emerging with improved side effect profiles and efficacy. Biologic agents, such as belimumab and ustekinumab, have been promising in case studies but will require larger trials to establish their role in routine therapy. Other novel therapies that have been trialed successfully include spleen tyrosine kinase inhibitors and fumaric acid esters. Finally, new evidence has been published recently that describes safer dosing regimens in thalidomide and lenalidomide, both effective medications for CLE. Given the chronic disease course of CLE, long-term treatment-related side effects must be minimized, and the introduction of new steroid-sparing agents is encouraging in this regard.
Introduction
Lupus erythematosus (LE) is a common autoimmune disease that manifests in systemic and cutaneous forms. The disease is caused by a complex interplay of genetics, environmental factors, hormones, and ethnicity. The resultant morbidity and mortality has a significant impact on patients’ quality of life.
Epidemiology
Women have a higher incidence of cutaneous LE (CLE) and systemic LE (SLE) across all age brackets and ethnicities. Peak incidence is typically seen mid-adulthood, but initial onset occurs later in affected men (Rees et al., 2017b, Vera-Recabarren et al., 2010). In Australia, the highest incidence of SLE is seen in Indigenous Australians and Australians with a South-Asian background (Grennan and Bossingham, 1995, Segasothy and Phillips, 2001). In Central Australia, Indigenous Australians are 3.8 times more likely to develop SLE than their Caucasian counterparts (Segasothy and Phillips, 2001). Indigenous Australians are reported to have reduced incidence of CLE, but this may be due to difficulties diagnosing the condition in patients with darker skin types (Segasothy and Phillips, 2001).
Pathogenesis of lupus erythematosus
LE is driven by dysfunction within the adaptive and innate immune system, beginning with a loss of self-tolerance in the adaptive immune system through the production of autoantibodies. These antibodies inappropriately react to the self-antigens present in cellular debris after apoptosis, resulting in activation and recruitment of T and B cells and production of immune complexes, which cause direct tissue injury. A number of proinflammatory signaling pathways are upregulated in patients with LE, which results in increased cytokine activity. The innate immune and complement systems are also pivotal for pathogen clearance, recognition of foreign antigens, and removal of apoptotic cells. Dysfunction in these two systems further drives LE manifestations.
In addition to the roles of genetics and sex in CLE, a number of potential environmental triggers have been identified. Many common medications have been linked to SLE and CLE (Table 1). Smoking is linked with increased CLE disease activity and poorer response to antimalarial treatment (Bockle and Sepp, 2015, Chasset et al., 2015). There is also a strong association between CLE disease activity and ultraviolet (UV) radiation. Exposure to UV light induces and exacerbates cutaneous lesions and can potentiate the symptoms of arthralgia and fatigue in patients with systemic symptoms (Kuhn et al., 2005).
Table 1.
Drugs linked to drug induced lupus erythematosus
| Drug-induced SCLE and drug induced chronic cutaneous lupus |
| • Antihypertensives – hydralazine, captopril, acebutol |
| • Antiarrhythmics – procainamide, quinidine |
| • Antibiotics – minocycline, isoniazid |
| • Antipsychotics – chlorpromazine, lithium |
| • Chemotherapy – doxorubicin, taxanes, anastrazole, 5-fluorouracil, bortezomib |
| • Biologics – etanercept, infliximab, adalimumab, IL-2, IFN-alpha, IFN-1b |
| Drug-induced SCLE |
| • Chemotherapy agents - nab-paclitaxel, docetaxel, tamoxifen, capecitabine, gemcitabine, 5- fluorouracil, carboplatin/pemetrexed, doxorubicin |
| • Proton pump inhibitors – omeprazole, esomeprazole, lansoprazole, pantoprazole |
| • Statin – simvastatin and pravastatin |
| • Thiazide diuretics – hydrochlorothiazide and chlorthiazide |
| • Angiotensin-converting enzyme inhibitor (ACEIs) – enalapril, captopril, lisnopril, captopril |
| • Calcium channel blockers – diltiazem, verapamil, nifedipine |
| • Beta blockers – oxprenolol and acebutolol |
| • Antifungals – terbinafine and griseofulvin |
| • Carbamazepine |
| • Leflunomide |
| • Antihistamines – ranitidine and brompheniramine |
| • Biologics – etanercept, efalizumab, golimumab, ranibizumab |
| • Nonsteroidal anti-inflammatory drugs – naproxen and piroxicam |
| • Hormonal agents – leuprorelin and anastrazole |
| • Antibiotics – doxycycline, norfloxacin, minocycline |
| • Other – rivaroxaban, imiquimod, citalopram, lamotrigine |
|
Drug-induced chronic cutaneous lupus |
| • Fluorouracil |
| • Nonsteroidal anti-inflammatory drugs |
| • Methimazole |
| • Dapsone, isoniazid, gold and penicillamine |
| • Hydroxyurea |
| • Pantoprazole |
| • Biologics – infliximab, adalimumab, bortezomib |
| TNF-alpha–induced CLE |
| • Adalimumab |
| • Infliximab |
| • Etanercept |
CLE has occasionally been linked to malignancy. Discoid LE (DLE) can be associated with scar carcinomas. Subacute LE (SCLE) in particular can be a paraneoplastic phenomenon and is associated with solid and nonsolid organ tumors (Kuhn et al., 2017a). A Swedish study of 3663 patients with CLE demonstrated an increased risk of lymphoma, nonmelanoma skin cancer, buccal cancer, and lung cancer and an increased risk of malignancy overall (hazard ratio: 1.8; Gronhagen et al., 2012). Diagnosis should prompt a malignancy screen in older patients.
Classification of cutaneous lupus erythematosus
Cutaneous features, which occur in 75% to 80% of all patients with LE (Vera-Recabarren et al., 2010), are classified as specific or nonspecific cutaneous manifestations. Specific cutaneous lupus can be further classified into acute cutaneous LE (ACLE), SCLE, chronic cutaneous LE (CCLE), and bullous LE. Within CCLE are further subtypes, including discoid LE (DLE), lupus profoundus (LP), lupus tumidus (LET), and chilblain lupus. Similar histology findings are seen in ACLE, SCLE, and CCLE, and differentiation among these subtypes is not possible with histology alone. These findings are also commonly seen in dermatomyositis, and a careful clinical examination is required to make a diagnosis.
The clinical manifestations and prognosis differ between the different categories of CLE. Approximately 25% of all patients with CLE progress to systemic disease; however, almost 100% of patients with ACLE progress to systemic disease, compared with only 5% of patients with CCLE and 30% of patients with SCLE (Vera-Recabarren et al., 2010). Patients with generalized DLE are at a higher risk of progressing to systemic disease than those with a localized variant (Lee, 2008).
Acute cutaneous lupus erythematosus
ACLE is typically confined to the face with malar erythema but can extend to a generalized distribution. The classical malar rash manifests as symmetrical erythematous plaques across the malar eminences and nasal bridge, with sparing of the nasolabial folds. The generalized variant involves a morbiliform or exanthematous eruption, which is worst on the extensor surfaces of the arms with sparing of the knuckles. When the trunk is involved, a triangular pattern of lesions is often observed.
There is also a rare subtype of ACLE that presents with bullous lesions and mucosal involvement, mimicking toxic epidermal necrolysis. The lesions associated with ACLE are nonscarring but can develop postinflammatory pigment changes. The cutaneous disease activity tends to flare in parallel with the systemic manifestations of the disease.
ACLE’s histopathology demonstrates an interface dermatitis, with mild focal vacuolar alteration of the basal layer. There is usually mucin in the upper dermis with follicular plugging, but epidermal thickening is uncommon.
Subacute cutaneous lupus erythematosus
SCLE is characterized by a nonscarring photosensitive eruption with psoriasiform or annular lesions on the face, V area of the neck, or extensor surfaces of the arms and upper back (Fig. 1). Less commonly, SCLE can present in an acral distribution as exfoliative erythroderma or with vesiculobullous lesions at the periphery of plaques. SCLE is associated with positive Ro/SSA antibodies and can overlap with other autoimmune disorders, such as Sjögren syndrome, rheumatoid arthritis, and Hashimoto’s thyroiditis. Histopathology is similar to that seen in other CLE subtypes and demonstrates interface dermatitis with vacuolar alteration of basal keratinocytes with areas of lichenoid dermatitis. Mucin deposition is seen in conjunction with perivascular and periadnexal mononuclear cell infiltrate. The classic features of follicular plugging, dermal melanophages, and hyperkeratosis seen with DLE may be present, but to a lesser extent.
Fig. 1.
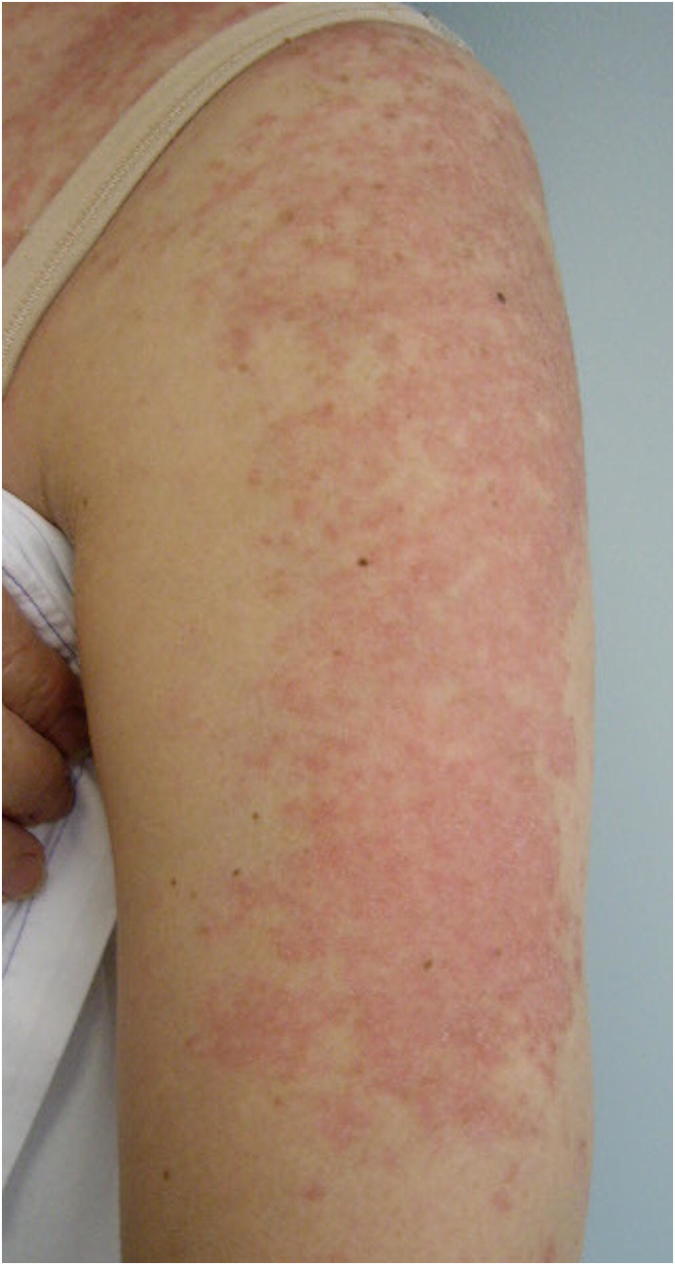
Subacute cutaneous lupus erythematosus.
Patients with SCLE who develop systemic disease tend to have milder symptoms, limited to joint involvement. Severe disease (e.g., lupus nephritis or central nervous system manifestations) is seen in < 10% of SCLE cases (Okon and Werth, 2013).
Chronic cutaneous lupus erythematosus
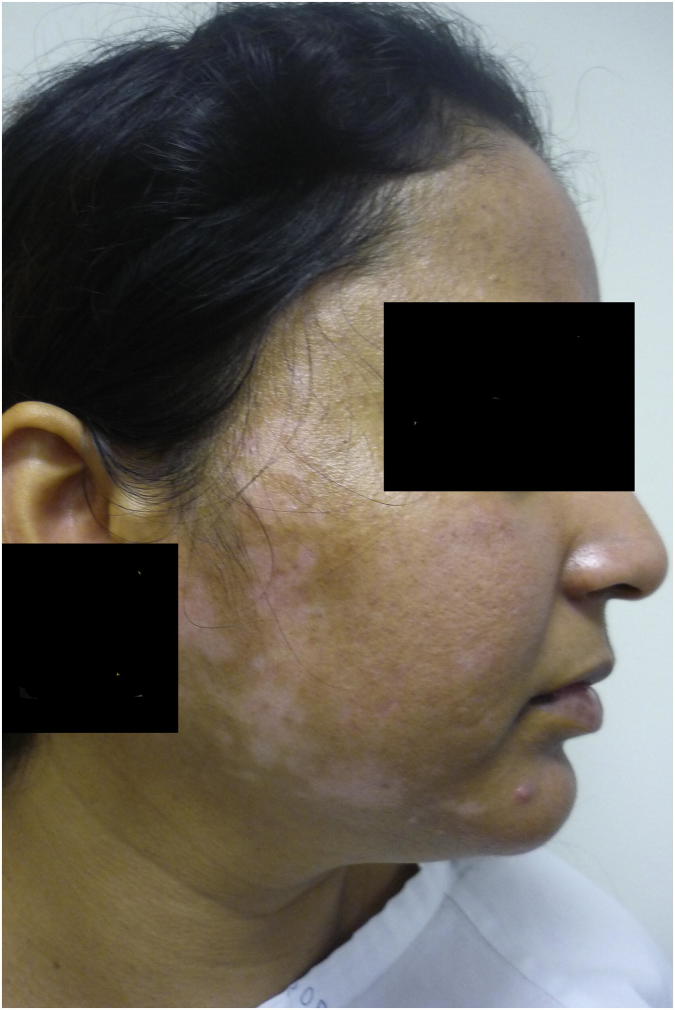
DLE is the most common subtype of CCLE and presents with characteristic indurated discoid lesions with an overlying scale, predominantly on the face and scalp (Fig. 2). Scarring alopecia associated with DLE should be distinguished from nonscarring alopecia associated with SLE. Less common variants include hypertrophic DLE, which affects the extensor surface of the arms, and acral and mucosal variants.
Fig. 2.
Discoid lupus erythematosus.
The classic carpet tack sign (or tin tack sign) is commonly present in DLE lesions, whereby keratotic spikes, similar in appearance to carpet tacks, are seen on the underside of the adherent scale when it is lifted. DLE has the potential for significant scarring and postinflammatory hypo- and hyperpigmentation. Longstanding lesions can develop squamous cell carcinoma.
The histopathology of CCLE is variable, with marked hyperkeratosis, interface dermatitis, thickening of the epidermal basement membrane, mucin deposition, perivascular and periappendageal lymphocytic infiltrate, and the presence of melanophages. The inflammatory infiltrate is denser than that seen in SCLE or ACLE, and scarring lesions may show dermal fibroplasia.
LP is a subtype of CCLE in which the inflammatory infiltrate lies in the lower dermis and subcutaneous tissue and presents as nodular lesions that are approximately 1 to 3 cm in diameter, with tethering of the overlying skin. If confined to subcutaneous tissue only, the disease is referred to as lupus panniculitis.
Chillblain lupus, another subtype of CCLE, presents as purpuric patches and plaques on acral surfaces and face that are aggravated by cold. They also often have concurrent DLE lesions and develop scarred atrophic plaques with telangiectasia as the lesions progress. Another subtype of CCLE is LET, which presents with edematous urticarial plaques that affect the face and trunk. Histology reveals periadnexal and perivascular inflammation and mucin deposition. LET is not typically associated with interface dermatitis and is clinically more photosensitive than other subtypes of CLE. LET resolves without scarring and is less likely to progress to systemic disease, with affected patients often having a normal autoantibody profile. Generally, LET has a better prognosis than other CLE subtypes, and lesions can spontaneously resolve within days or weeks of onset (Kuhn et al., 2005).
Nonspecific manifestations of lupus erythematosus
Nonspecific cutaneous manifestations are clinical signs that are secondary to the LE disease process but do not involve interface dermatitis. These include vascular lesions, such as leucocytoclastic vasculitis, Raynaud’s, and livedo reticularis. These lesions are not specific to LE and can be seen in other autoimmune conditions. Nail changes, such as nailfold erythema, telangiectasia, clubbing, paronychia, pitting, leukonychia striata, and oncholysis, can be seen in all variants of CLE as well as SLE.
Drug-induced cutaneous lupus erythematosus
Approximately 10% of SLE cases are related to drug-induced lupus, where symptoms begin after exposure to a triggering medication (Chang and Gerschwin, 2011). The identification of the drug precipitant can be difficult due to delayed disease onset occurring months to years after initial exposure, but it is clinically very useful because symptoms will resolve after cessation of the medication (Chang and Gerschwin, 2011).
Drug-induced LE can be subdivided into drug-induced SLE, drug-induced SCLE, and drug-induced CCLE. Drug-induced LE is associated with > 80 medications, and this number is increasing with the advent of biologics and other immunological therapies (Dalle Vedove et al., 2012a). The incubation period for onset of disease varies widely and is drug dependent.
The most common subtype of drug-induced LE is drug-induced SCLE, which commonly presents with lesions similar to idiopathic SCLE (erythematous plaques with or without scale in a photosensitive distribution). However, these lesions tend to be more limited in number and more likely to affect the legs (Lowe et al., 2010). The histologic findings are also similar between idiopathic and drug-induced SCLE, and it is associated with positive ANA and Anti-Ro/Anti-La antibodies. Drug-induced SCLE is associated with a wide number of medications (Table 1) but is most commonly seen with antifungals, antihypertensive drugs, diuretics, statins, and proton pump inhibitors (Dalle Vedove et al., 2012a, Lowe et al., 2010).
Patients with drug-induced SLE do not develop typical CLE skin manifestations or interface dermatitis and often have nonspecific LE cutaneous manifestations instead. Affected patients usually have concurrent systemic symptoms, such as fever, arthralgia, and myalgia (Chang and Gerschwin, 2011). Drug-induced SLE is strongly associated with a positive ANA and antihistone antibodies, but the characteristic serological findings of SLE, such as hypocomplementemia and positive anti-dsDNA antibodies, are less common. Chemotherapy and biologic agents, antiarrhythmics, antihypertensive medications, antipsychotics, and antibiotics are the most common precipitating medications (Table 1; Dalle Vedove et al., 2012a).
Drug-induced CCLE can be further divided into DLE and LET subtypes, both of which are rare. These subtypes are associated with nonsteroidal antiinflammatory drugs, chemotherapy agents, and antitumor necrosis factor (TNF) alpha agents (Dalle Vedove et al., 2012a). Drug-induced LE induced by anti-TNF alpha agents is distinct from classic drug-induced LE and occurs in an older, predominantly female population. These patients frequently have systemic symptoms, such as fever, myalgia, arthralgia, and serositis, and more commonly experience cutaneous manifestations (Chang and Gerschwin, 2011). Anti-dsDNA antibodies are often present in this group (Chang and Gerschwin, 2011, Dalle Vedove et al., 2012a).
Neonatal lupus
Babies of mothers with anti SSA/Ro antibodies may develop neonatal lupus, which presents with lesions similar to SCLE in a photosensitive distribution that mainly affect the face in the periorbital region. Despite the classic photosensitive distribution, the lesions do not necessarily have preceding sun exposure and lesions may be present at birth. These lesions are nonscarring but may heal with dyspigmentation and telangiectasia. Neonatal lupus should be recognized early given the associated risk of congenital heart block, cardiomyopathy, hepatobiliary disease, and cytopenias. Heart block is the most common of these complications, and two thirds of affected babies go on to require a pacemaker (Lee, 2004). Hepatobiliary disease and cytopenias are both present in approximately 10% of cases (Lee, 2004). These comorbidities may be present at birth or develop during the first months of life.
Coexisting conditions
Patients with LE often have concurrent chronic urticaria, but the pathogenesis behind this is poorly understood (Costner and Sontheimer, 2008). LE is also associated with cutaneous mucinosis, lichen planus, acanthosis nigricans, acquired ichthyosis, cutis laxa, and interstitial granulomatous dermatitis.
Clinical scoring tools
The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) has been developed to assist physicians in monitoring patients with CLE and their response to treatment (Jolly et al., 2013). CLASI score is calculated based on the scoring of erythema, scale, and the presence of mucous membrane lesions or nonscarring alopecia, ranging from 0 to 70. Affected areas of the skin that are routinely visible, such as the face, are given higher scores with the CLASI. The CLASI has been validated against the Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index, Systemic Lupus International Collaboration Clinics-American College of Rheumatology Damage Index, and LupusPRO scoring systems. Jolly et al. (2013) demonstrated a strong correlation between the CLASI and these existing LE indices.
The CLASI is useful in clinical practice as an objective measure of clinical response and is used in clinical trials. The CLASI has been validated for all subtypes of CLE, except for bullous lupus and lupus panniculitis, both of which are rare (Bonilla-Martinez et al., 2008, Jolly et al., 2013)
Diagnosis of cutaneous lupus erythematosus
The diagnosis of CLE is made using a combination of clinical assessments, serologic testing, and histopathologic findings. Histologic findings in each subtype of specific CLE lesions are explored in Table 2, but histology alone cannot differentiate among the subtypes.
Table 2.
Subclassification of cutaneous lupus erythematosus disease types
| Clinical appearance | Scarring | Histology | |
|---|---|---|---|
|
Acute cutaneous lupus erythematosus • Localized ACLE • Generalized ACLE • Toxic epidermolysis necrolysis-like ACLE |
Localized: Classical malar rash, or erythematous macules and papules that tend to become confluent, typically in a photosensitive distribution with nasolabial fold sparing. May develop vesicobullous changes if severe Generalized: Maculo-urticarial or papular rash in photosensitive distribution. May develop nail changes including periungal erythema, splinter hemorrhages, or nailfold telangiectasia Toxic epidermolysis necrolysis-like ACLE: Widespread desquamation of the skin and mucous membranes, mimicking toxic epidermolysis necrolysis |
No | Interface dermatitis with basal layer vacuolization with superficial perivascular lymphocytic infiltrate in upper half to deep dermis and mucin deposits in the reticular dermis (Obermoser et al., 2010). Toxic epidermolysis necrolysis-like ACLE: Epidermal necrosis with basal vacuolization, and necrotic keratinocytes. Sparse lymphohistiocytic infiltrate (Boontaveeyuwat et al., 2012). |
|
Subacute lupus erythematosus • Annular • Papulosquamous • Mixed |
Annular: Annular lesions in a photosensitive distribution Papulosquamous: Psoriasiform lesions in a photosensitive distribution Mixed: Both annular and psoriasform lesions in a photosensitive distribution May develop vesiculobullous lesions in severe cases, especially in the periphery of annular lesions |
No | Dense perivascular inflammatory infiltrate, with thinning and atrophy of the epidermis and vacuolar changes across the dermoepidermal junction. May see basement membrane thickening (Obermoser et al., 2010). |
|
Chronic cutaneous lupus erythematosus • Discoid lupus • Verrucous DLE • Chillblain LE • Mucosal LE • Lupus panniculitis • Lupus tumidus |
Discoid: Classical erythematous discoid plaques with central hyperkeratosis, typically affecting the face, V region of the neck, and extensor surface of the arms, but can also affect scalp, trunk and mucosal areas. Can have associated nail changes including dystrophy, clubbing, and leukonychia striata Verrucous: Hyperkeratotic lesions, typically affecting the extensor surfaces of the arms, upper back, and face. Chillblain: Erythematous to violaceous plaques on cold-exposed acral skin (fingers, toes, heels, knees, elbows). Can progress to become hyperkeratotic or ulcerative at severe stages. Mucosal: Erythematous macule, macules, blisters, and erosions on the mucosal surfaces. Most commonly affects mouth, but can involve any mucosal area. Lupus panniculitis: Subcutaneous nodules that become atrophic, causing localized depressions in the skin. May also be associated with erythema or hyperkeratosis. These nodules may proceed to secondary infection, calcification, or ulceration in the severe stages. The lesions typically affect the face, upper arms, buttocks, and thighs. Lupus tumidus: Erythematous or violaceous plaques on the face and can involve upper limbs and trunk in a photosensitive distribution. Nonscarring lesions that appear swollen macroscopically. |
Yes | Discoid: Inflammatory infiltrate becomes less prominent over time, and scarring often features in DLE biopsies. Basal membrane is thickened and periodic acid–Schiff positive, with thinning of the epidermis and sclerotic changes of the upper dermis. Mucin deposits are seen in the reticular dermis (Obermoser et al., 2010). Chillblain: Interface dermatitis with superficial and deep invasion. Lymphocytic vasculitis and fibrin deposition within the walls of dermal blood vessels in chilblain LE, which can assist with differentiating between this and other CLEs. Lupus panniculitis: Lymphocytic panniculitis seen in subcutaneous fat, with vessel wall thickening and polymorphonuclear leukocyte infiltration Lupus tumidus: Lymphocytic infiltrate and interstitial mucin deposition is seen, and edema in the upper dermis can be seen in a majority of cases (Schmitt et al., 2010). There is minimal basal cell involvement. |
| Nonspecific lupus lesions | |||
| • Livedo reticularis | |||
| • Leucocytoclastic vasculitis | |||
| • Thrombophlebitis | |||
| • Raynaud’s syndrome | |||
| • Extravascular necrotizing palisaded granulomatous dermatitis | |||
| • Neutrophilic urticarial dermatosis | |||
| • Erythromelagia | |||
| • Cutaneous mucinosis | |||
The American College of Rheumatology classification system includes the universally accepted criteria for the diagnosis of LE (Hochberg, 1997). The system consists of 11 clinical and laboratory criteria and assesses all potential manifestations of LE. However, this is a general score for all manifestations of LE and is not always useful in assessing patients with CLE without systemic involvement.
Researchers have since proposed alternative classification systems for the diagnosis of CLE. Gilliam and Sontheimer initially proposed the classification of LE lesions into specific lesions, which are further categorized into ACLE, SCLE, CCLE (and its variants), and bullous LE, and nonspecific lesions, such as urticaria or vasculitis (Kuhn et al., 2005, Sontheimer et al., 1979). From there, further classification systems have been described that expand on the work of Gilliam and Sontheimers. The most recent suggested classification system is the Dusseldorf classification created by Kuhn, which made small changes to already classified subsets (Kuhn and Landmann, 2014).
Autoantibody testing is useful in both the diagnosis and monitoring of CLE. ANA is typically positive in SLE at moderate-to-high titers and positive in 60% to 80% of patients with CLE (Costner and Sontheimer, 2008). Patients with ACLE often have serologic profiles similar to those of patients with SLE, including positive ANA, dsDNA, and hypocomplementaemia test results. Patients with SCLE have anti-SSA/Ro antibodies (70%-90% of patients), anti-SSB/La in (30%-50% of patients; Costner and Sontheimer, 2008), and anti-dsDNA (5% of patients; Okon and Werth, 2013). Anti-histone antibodies are typically elevated in drug-induced CLE and may be useful in differentiating between drug hypersensitivity and classic CLE. Patients with CCLE have a lower incidence of positive ANA and other autoantibodies compared with other subtypes of CLE (Okon and Werth, 2013, Vasquez et al., 2012).
Differential diagnoses
The differential diagnosis for CLE varies depending on the appearance of the lesions and other systemic symptoms that may be present. In annular lesions of SCLE, granuloma annulare, erythema annulare centrifugum, and erythema gyratum repens should be considered. For papulosquamous eruptions of SCLE, the differential includes psoriasis and its photosensitive variant or photoallergic drug eruption. The generalized lesions of ACLE may be confused with drug hypersensitivity, phototoxic or photoallergic drug eruptions, viral exanthems, or dermatomyositis. Localized LE lesions include a differential of rosacea, dermatomyositis, polymorphic light eruption, photoallergic contact dermatitis, or seborrheic dermatitis. The clinical presentation and histologic findings of DM and LE are often identical, requiring other elements to aid in the diagnosis (e.g., history of muscle weakness), subtle clinical signs (e.g., involvement of nasolabial fold or a heliotrope rash), antibodies, and further investigations (e.g. magnetic resonance imaging or muscle biopsy). The correct diagnosis is important because dermatomyositis has potential medical co-morbidities and can be associated with malignancies that require screening.
Early in the onset, DLE lesions can appear similar to polymorphic light eruptions, sarcoidosis, granuloma faciale, lymphoma cutis, SCLE, and pseudolymphoma. In the later stages, discoid lesions of DLE can be mistaken for actinic damage or nonmelanoma skin cancers, such as squamous cell carcinoma and keratoacanthomas. Fully developed DLE lesions can also mimic hypertrophic lichen planus. Biopsy testing can help differentiate and should especially be performed in asymmetric longstanding discoid lesions due to the risk of nonmelanoma skin cancer masquerading as CLE.
Treatment options
A multidisciplinary team consisting of dermatologists, rheumatologists, nephrologists, and general practitioners is key for the optimal management of patients with systemic symptoms. Patients with cutaneous findings alone can be treated and monitored by their dermatologist.
Preventative measures
Photoprotection including broad-spectrum sunscreens, a broad brimmed hat, and long-sleeved clothes are essential for patients with CLE. In a study of 128 patients with cutaneous LE, 43% of participants developed lesions after exposure to UV-B and UV-A radiation (Lehmann et al., 1993). Broad-spectrum sunscreen, ideally with a physical blocker, can prevent the development of CLE lesions (Okon et al., 2014) and should be applied 20 to 30 minutes prior to sun exposure daily. Vitamin D reduction due to sun avoidance can be restored with oral replacement. Vitamin D replacement may improve disease severity in addition to its benefits for bone health (Gronhagen et al., 2016).
Patients should be encouraged to stop smoking because smoking increases disease activity associated with CLE and SLE (Bockle and Sepp, 2015) and reduces the efficacy of antimalarial therapy (Chasset et al., 2015). In drug-induced cases of LE, the causative drug should be promptly identified and ceased.
Topical therapy
In mild cases of CLE, topical treatment and preventative measures alone may be sufficient for disease control. Topical corticosteroids are traditionally first-line treatment for CLE and are very effective at minimizing the erythema and scales associated with the disease (Sigges et al., 2013). However, the long-term application of potent topical corticosteroids on the face has potential side effects, including atrophy and telangiectasia, which limits their use.
An alternative treatment for CLE is topical calcineurin inhibitors, such as tacrolimus and pimecrolimus. A randomized controlled trial of 38 patients with CLE demonstrated that tacrolimus 0.1% is effective in this group (Kuhn et al., 2011, Kuhn et al., 2011). These agents can be combined with topical steroids, and tacrolimus with clobetasol propionate has been shown to be successful (62% of participants) in achieving good or excellent improvement of lesions in previously treatment-resistant CLE and reduced the incidence of telangiectasia (Madan et al., 2009). Unfortunately, DLE may be refractory to topical steroids and calcineurin inhibitors, given the thick and scaly nature of these lesions.
Intralesional steroid injections can be used for localized disease, particularly the DLE subtype (Chang and Werth, 2016). Intramuscular corticosteroid injections can also be used. In a cohort of 50 patients, intramuscular corticosteroid injections were as efficacious as oral corticosteroid therapy and achieved a more rapid initial response rate (Conti et al., 2016, Danowski et al., 2006).
Antimalarials
Antimalarials such as hydroxychloroquine, quinacrine, and chloroquine are the first-line systemic therapy of choice in CLE. Responsiveness to antimalarial therapy has been shown to vary across disease subtypes. A meta-analysis of 1990 treatment courses between 1965 and 2005 showed an overall response rate of 63%, but a 91% response rate in ACLE and only 31% in chilblain lupus (Chasset et al., 2017).
Patients can cycle from one antimalarial to another due to poor tolerance of the treatment or ineffectiveness. A recent study of 64 patients with CLE examined the effectiveness of switching from hydroxychloroquine to chloroquine due to intolerable side effects or ineffectiveness (Chasset et al., 2018). Of the participants who switched secondary to inadequate treatment response, 56% were responders to the second antimalarial agent at 3 months (Chasset et al., 2018). This effect was not maintained long term, however, with 42% responding at 1 year and 22% at 2 years (Chasset et al., 2018). Participants who had discontinued treatment due to adverse events had a higher incidence of adverse events with the second antimalarial agent (31% vs. 12%). Combinations of antimalarials can also be used, except for hydroxychloroquine and chloroquine, which have an additive risk of retinopathy when combined (Ang and Werth, 2005). A study of 11 patients demonstrated a 67% response rate to hydroxychloroquine and quinacrine in patients who failed on hydroxychloroquine monotherapy (Chang et al., 2011).
Common adverse events associated with antimalarial therapy include cutaneous eruptions, gastrointestinal upset, mucocutaneous pigment changes, neurological events (dizziness and headache), peripheral neuropathy, and ototoxicity. Retinopathy was seen with hydroxychloroquine and chloroquine, and the incidence of retinopathy is approximately 1%. The incidence increases with duration of therapy and is dose-dependent. Chasset et al have suggested a daily dose of 5 mg/kg or less to balance therapeutic efficacy and risk of retinopathy, and this group showed a 2% incidence of retinopathy after 10 years of therapy (Chasset et al., 2018). Baseline ophthalmological examinations should be performed prior to the commencement of therapy and then annually after 5 years.
Treatment-refractory disease
A number of immunosuppressive medications can be useful after failure of topical therapies and antimalarial therapy in CLE, including dapsone, oral vitamin A derivatives, oral corticosteroids, methotrexate, mycophenolate mofteil (MMF), azathioprine, cyclophosphamide, and cyclosporine. These drugs can be used as monotherapy, in combination with antimalarial therapy, or in combination with each other.
Regular monitoring for drug-specific side effects, such as myelosuppression and hepatotoxicity, needs to be performed while patients are receiving these agents. Given the risk of adverse effects associated with these agents, current European guidelines for the treatment of CLE only recommend methotrexate and MMF and advise against the use of cyclosporine, cyclophosphamide, and azathioprine in patients with CLE without systemic involvement (Kuhn et al., 2017a).
Oral vitamin A derivatives
Oral retinoids have been used successfully as treatment for refractory CLE. Acitretin was shown to be comparably effective to hydroxychloroquine in a double-blinded randomized controlled trial (RCT; Ruzicka et al., 1992), and both isotretinoin and alitretinoin have been used successfully in small case series (Kuhn et al., 2012, Vena et al., 1989). Patients on retinoid therapy need to have regular blood testing due to the risk of hepatotoxicity and deranged lipids. Counseling and contraception must be given to women of childbearing age given the teratogenic effects of the therapy.
Dapsone
Dapsone possesses antimicrobial and antiinflammatory properties and was shown to induce remission or significant clinical improvement in > 50% of patients when used in combination with antimalarial therapy or alone (Klebes et al., 2016). Of note, dapsone was effective in 60% of participants with DLE, a clinical subtype that is classically resistant to therapy (Klebes et al., 2016).
Systemic corticosteroid therapy
Systemic corticosteroid therapy can be useful in the treatment of CLE, particularly for flares of disease. This therapy can also be useful as a bridging therapy for patients with severe disease while waiting for steroid-sparing agents to take effect. When used for flare treatment or bridging therapy, clinicians should aim to taper the dose to cessation and introduce steroid-sparing therapies. In a prospective cohort study, oral corticosteroids had the highest response rate of all systemic therapies (94.3%) and were the most successful in the ACLE subtype (Sigges et al., 2013). Long-term use is limited by the side effect profile, which includes weight gain, insulin resistance, and osteoporosis.
Methotrexate
A retrospective study of 43 patients with CLE was performed using methotrexate (15-25 mg weekly), and 98% of participants showed a clinical response (Wenzel et al., 2005). However, 16 of the 43 participants (37%) had to discontinue methotrexate prior to study completion due to treatment-related side effects. Cyclosporine has been used in combination with methotrexate to good effect and may allow lower dosing when used in combination (Klein et al., 2011).
Mycophenolate mofetil
MMF has been shown in small case studies to be effective in SCLE, chilblain LE, and discoid LE (Okon and Werth, 2013). A retrospective analysis of 24 patients with treatment-resistant CLE showed some clinical response in all patients and resolution or near resolution of disease activity in 62% of patients (Gammon et al., 2011). MMF in combination with hydroxychloroquine has been trialed successfully in a small case series of three cases (Sadlier et al., 2012).
Azathioprine
Case reports have shown successful treatment of DLE lesions with azathioprine (Okon and Werth, 2013); however, there are no controlled trials to support routine use in CLE. Azathioprine has been used successfully during pregnancy in patients with SLE (Murase et al., 2014, Saavedra et al., 2015).
Cyclosporine
Cyclosporine with hydroxychloroquine has been used successfully in a patient with refractory SCLE with concurrent lichen planus (Grabbe and Kolde, 1995) and another patient with refractory tumid lupus (Saeki et al., 2000). However, cyclosporine was shown to be ineffective in six refractory DLE cases (Kuhn et al., 2011, Kuhn et al., 2011).
Cyclophosphamide
Cyclophosphamide has been used successfully in six patients with refractory CLE, achieving complete remission in four patients and partial remission in two patients (Raptoopoulou et al., 2010). Cyclophosphamide achieved an excellent clinical response in five of nine patients with DLE in a prospective trial (Schulz and Menter, 1971).
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) has been described in case reports and a single case series as effective for treatment refractory CLE (Espírito Santo et al., 2010, Genereau et al., 1999, Goodfield et al., 2004, Kreuter et al., 2005, Ky et al., 2015, Lampropoulos et al., 2007, Piette et al., 1995). The most frequently observed adverse events are headache and fever, which can be mitigated with premedication and trials of different formulations of IVIG (e.g., privigen, flebogamma, and intragam; Bonilla, 2008). There is significant cost involved in the administration of IVIG as an infusion as well as the drug itself, which limits its widespread use.
B cell–targeted therapies
B cells play a significant role in the pathogenesis of LE, and rituximab has been identified as a potential treatment for refractory disease. Rituximab is a monoclonal anti-CD20 antibody and induces B-cell suppression. In a study of rituximab in 26 patients with CLE, only 9 (35%) had a response to treatment at the 6-month follow up (Vital et al., 2015). The highest response rates were seen in ACLE (43%) compared with CCLE (0%). Unfortunately, 12 patients had a flare of the disease after treatment, and 9 of the 26 patients developed new SCLE or CCLE lesions after treatment with rituximab.
Another cohort study was performed with 17 patients with SLE who had cutaneous disease and demonstrated a 53% response rate in previously refractory patients at 6 months (Hofmann et al., 2013). This cohort also showed more favorable results in ACLE and SCLE subtypes, and less than one third of patients with CCLE responded to treatment. Two large RCTs have been performed using rituximab in patients with SLE (EXPLORER and LUNAR trials), and both failed to reach their primary endpoint (Hofmann et al., 2013, Merrill et al., 2010, Rovin et al., 2012). At this stage, the role for rituximab in CLE in unclear, and further clinical trials with long-term follow up are needed to assess the efficacy and quantify the risk of flares or induction of new CLE lesions with rituximab.
Belimumab is a monoclonal antibody that blocks B lymphocyte stimulators to suppress the activity of B cells. Belimumab was studied in five patients with SLE and cutaneous manifestations, and each patient had a marked clinical response to treatment, with a 14-point reduction in CLASI score (Vashisht et al., 2016). A steroid-sparing effect was also observed with a reduction in mean prednisone dose from 31 mg to 3 mg.
Cytokine modulators
A phase II RCT assessing ustekinumab, an interleukin (IL)-12 and IL-23 inhibitor, in patients with SLE demonstrated a statistically significant reduction in CLASI score (van Vollenhoven et al., 2018). A phase I trial was completed to evaluate the safety of sirukumab, an anti-IL-6 antibody, and demonstrated a reasonable safety profile (Vashisht et al., 2016). The promising results of Syk inhibitors in SLE have suggested a role for them in refractory CLE (Deng and Tsokos, 2016).
Fumaric acid esters
Fumaric acid esters (monoethylfumrate and dimethylfumrate) are effective in CLE (Kuhn et al., 2016, Saracino and Orteu, 2017). A recent open-label phase II study demonstrated an improvement in disease activity in 11 patients treated with monoethylfumrate and dimethylfumrate for 9 weeks but did not meet the primary endpoint of 50% reduction in RCLASI score (Kuhn et al., 2016). The most frequent adverse events with fumaric acid esters are gastrointestinal side effects (e.g., abdominal cramping, nausea, and diarrhea).
Thalidomide
Thalidomide has been used to treat CLE in severe refractory cases. However, thalidomide is associated with a significant risk of peripheral neuropathy, and this requires close monitoring. A Chinese study of 69 patients was performed to identify the lowest effective dose of thalidomide and demonstrated an optimal response rate (71%) at 50 mg daily (Wang et al., 2016). In a prospective study of 60 patients with refractory CLE treated with 100 mg thalidomide daily, 98% achieved a clinical response. The rate of relapse after cessation was high in this group (70%). Eighteen percent of the participants developed paraesthesia, and 45% were found to have a sensory neuropathy on nerve conduction studies (Cortes-Hernandez et al., 2012).
A recent meta-analysis of 21 studies of thalidomide in CLE showed a pooled response rate of 90% but a high relapse rate of 71% (Chasset et al., 2018). Peripheral neuropathy was seen in 16% of pooled participants; however, only 4% had persistent symptoms after cessation of thalidomide (Chasset et al., 2018).
Lenalidomide
Lenalidomide has also been shown to be effective in patients with CLE (Okon et al., 2014) and is promising because lower rates of adverse effects were observed than with thalidomide. In a prospective open-label trial of five participants with refractory CLE, a clinically important difference in CLASI was seen. Lenalidomide was well tolerated in four patients; however, one patient developed arthralgia and new-onset proteinuria (Okon et al., 2014). Given the risk of significant adverse events, in particular the risk of permanent peripheral neuropathy, thalidomide and lenalidomide should be reserved for severe refractory cases.
Nonpharmacological management
Pulsed dye laser has shown promising results in small retrospective studies of CLE. Two prospective studies of 26 patients demonstrated an improvement in CLASI scores in patients with mild disease (Erceg et al., 2009) and improvement in flare activity with photodynamic therapy in 10 patients with LET (Truchuelo et al., 2012). Given the photosensitive nature of CLE, there is a risk of flare in disease activity after treatment with pulsed dye laser. As such, pulsed dye laser is not currently recommended for the treatment of active CLE lesions, and European guidelines recommend its use be limited to the management of vascular lesions associated with LE (Kuhn et al., 2017a).
Conclusion
Knowledge with regard to the pathogenesis of LE has progressed rapidly over the past decade, and with it has come promising new biologic agents for the treatment of CLE. Given the chronic disease course of CLE, long-term treatment-related side effects must be minimized, and the introduction of new steroid-sparing agents are encouraging in this regard. It is also pivotal that each subtype of CLE be considered individually, given their variance in clinical presentation, prognosis for systemic disease, and response to treatment. CLE, and CCLE in particular, can be a challenge for clinicians, but new insights are promising for the introduction of novel therapies for refractory patients.
Acknowledgments
Acknowledgments
The authors thank Professor Dedee Murrell for the use of her clinical photographs in this review.
Conflict of Interest
None.
Funding
None.
Study Approval
NA.
Footnotes
No human subjects were included in this study. No animals were used in this study.
References
- Ang G.C., Werth V.P. Combination anti-malarials in the treatment of cutaneous dermatomyositis. JAMA Dermatology. 2005;141(7):855–859. doi: 10.1001/archderm.141.7.855. [DOI] [PubMed] [Google Scholar]
- Bockle B., Sepp N. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: Analysis of 405 patients. Lupus. 2015;24(7):669–674. doi: 10.1177/0961203314559630. [DOI] [PubMed] [Google Scholar]
- Bonilla F. Intravenous immunoglobulin: Adverse reactions and management. J Allergy Clin Immunol. 2008;122(6):1238–1239. doi: 10.1016/j.jaci.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Bonilla-Martinez Z.L., Albercht J., Troxel A., Taylor L., Okawa J., Dulay S. The Cutaneous Lupus Erythematosus Disease Area and Severity Index - A responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol. 2008;144(2):173–180. doi: 10.1001/archderm.144.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boontaveeyuwat E., Silpa-Archa N., Kulthanan K. Toxic epidermal necrolyisis-like acute cutaneous lupus erythematosus (TEN-like ACLE) in SLE patients: A report of two cases. Asian Pac J Allergy Immunol. 2012;30:83–87. [PubMed] [Google Scholar]
- Chang C., Gerschwin M.E. Drug-induced lupus erythematosus. Drug Saf. 2011;34(5):357–374. doi: 10.2165/11588500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Chang A., Piette E., Foering K., Tenhave T., Okawa J., Werth V. Response to antimalarial agents in cutaneous lupus erythematosus: A prospective analysis. Arch Dermatol. 2011;147(11):1261–1267. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Werth V. Therapeutic options for cutaneous lupus erythematosus: Recent advances and future prospects. Expert Rev Clin Immunol. 2016;12(10):1109–1121. doi: 10.1080/1744666X.2016.1188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasset F., Arnaud L., Jachiet M., Monfort J.B., Bouaziz J.D., Cordoliani F. Changing antimalarial agents after inefficacy or intolerance in patients with cutaneous lupus erythematosus: A multicenter observational study. J Am Acad Dermatol. 2018;78(1):107–114. doi: 10.1016/j.jaad.2017.08.045. [DOI] [PubMed] [Google Scholar]
- Chasset F., Bouaziz J.D., Costedoat-Chalumeau N., Frances C., Arnaud L. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: A systematic review and meta-analysis. Br J Dermatology. 2017;1771(1):188–196. doi: 10.1111/bjd.15312. [DOI] [PubMed] [Google Scholar]
- Chasset F., Frances C., Barete S., Amoura Z., Arnaud L. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: A meta analysis of the literature. J Am Acad Dermatol. 2015;72(4):634–639. doi: 10.1016/j.jaad.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Chasset F., Tounsi T., Cesbron E., Barbaud A., Frances C., Arnaud L. Efficacy and tolerance profile of thalidomide in cutaneous lupus erythematosus: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;78(2):342–350. doi: 10.1016/j.jaad.2017.09.059. [DOI] [PubMed] [Google Scholar]
- Conti F., Ceccarelli F., Iaiani G., Perricone C., Giordano A., Amori L. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res Ther. 2016;18:177. doi: 10.1186/s13075-016-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Hernandez J., Torres-Salido M., Castro-Marrero J., Vilardell-Tarres M., Ordi-Ros J. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: Prognostic factors of clinical outcome. Br J Dermatol. 2012;166(3):616–623. doi: 10.1111/j.1365-2133.2011.10693.x. [DOI] [PubMed] [Google Scholar]
- Costner M., Sontheimer R. Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill; New York, NY: 2008. Lupus erythematosus; pp. 1515–1532. [Google Scholar]
- Dalle Vedove C., Simon J., Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNF alpha agents. J Dtsch Dermatol Ges. 2012;10(12):889–897. doi: 10.1111/j.1610-0387.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Vedove C., Simon J., Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents. J Dtsch Dermatol Ges. 2012;10(12):889–897. doi: 10.1111/j.1610-0387.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danowski A., Magder L., Petri M. Flares in lupus: outcome assessment trial (FLOAT), a comparison of oral methylprednisolone and intramuscular triamcinolone. J Rheumatol. 2006;33(1):57–60. [PubMed] [Google Scholar]
- Deng G., Tsokos G. The role of Syk in cutaneous lupus erythematosus. Exp Dermatol. 2016;25:674–675. doi: 10.1111/exd.13018. [DOI] [PubMed] [Google Scholar]
- Erceg A., Bovenschen H., van de Kerkhof P., de Jong E., Seyger M. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60(4):626–632. doi: 10.1016/j.jaad.2008.11.904. [DOI] [PubMed] [Google Scholar]
- Espírito Santo J., Gomes M.F., Gomes M.J., Peixoto L., Pereira S., Acabado A. Intravenous immunoglobulin in lupus panniculitis. Clin Rev Allergy Immunol. 2010;38(2–3):307–318. doi: 10.1007/s12016-009-8162-x. [DOI] [PubMed] [Google Scholar]
- Gammon B., Hansen C., Costner M. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;65(4):717–721. doi: 10.1016/j.jaad.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Genereau T., Chosidow O., Danel C., Cherin P., Herson S. High-dose intravenous immunoglobulin in cutaneous lupus erythematosus. Arch Dermatol. 1999;135:1124–1125. doi: 10.1001/archderm.135.9.1124. [DOI] [PubMed] [Google Scholar]
- Goodfield M., Davison K., Bowden K. Intravenous immunoglobulin (IVIg) for therapy-resistant cutaneous lupus erythematosus (LE) J Dermatol Treat. 2004;15:46–50. doi: 10.1080/09541440042000269. [DOI] [PubMed] [Google Scholar]
- Grabbe S., Kolde G. Coexisting lichen planus and subacute cutaneous lupus erythematosus. Clin Exp Dermatol. 1995;20:249–254. doi: 10.1111/j.1365-2230.1995.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Grennan D.M., Bossingham D. Systemic lupus erythematosus (SLE): Different prevalences in different populations of Australian aboriginals. Aust NZ J Med. 1995;25:182–183. doi: 10.1111/j.1445-5994.1995.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Gronhagen C.M., Fored C.M., Linder M., Granath F., Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: A population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167:296–305. doi: 10.1111/j.1365-2133.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- Gronhagen C., Tang M., Tan V., Tan K., Lim Y. Vitamin D levels in 87 Asian patients with cutaneous lupus erythematosus: A case-control study. Clin Exp Dermatol. 2016;41(7):723–729. doi: 10.1111/ced.12884. [DOI] [PubMed] [Google Scholar]
- Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Hofmann S., Leandro M., Morris S., Isenberg D. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus – report of 17 cases and review of the literature. Lupus. 2013;22(9):932–999. doi: 10.1177/0961203313497115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly M., Kazmi N., Mikolaitis R., Sequeira W., Block J. Validation of the Cutaneous Lupus Disease Area and Severity Index (CLASI) using physician- and patient-assessed health outcome measures. J Am Acad Dermatol. 2013;68(4):618–623. doi: 10.1016/j.jaad.2012.08.035. [DOI] [PubMed] [Google Scholar]
- Klebes M., Wutte N., Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? A retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232(1):91–96. doi: 10.1159/000441054. [DOI] [PubMed] [Google Scholar]
- Klein A., Vogt T., Wenzel S., Fleck M., Landthaler M. Cyclosporin combined with methotrexate in two patients with recalcitrant subacute cutaneous lupus erythematosus. Australas J Dermatol. 2011;52(1):43–47. doi: 10.1111/j.1440-0960.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- Kreuter A., Hyun J., Altmeyer P., Gambichler T. Intravenous immunoglobulin for recalcitrant subacute cutaneous lupus erythematosus. Acta Derm Venereol. 2005;85:545–547. doi: 10.1080/00015550510037071. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Aberer E., Bata-Csorgo Z., Caproni M., Dreher A., Frances C. S2k guideline for treatment of cutaneous lupus erythematosus - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venerology (JEADV) J Eur Acad Dermatol Venerol. 2017;31:389–404. doi: 10.1111/jdv.14053. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Gensch K., Haust M., Schneider S.W., Bonsmann G., Gaebelein-Wissing N. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: A multicenter, randomized, double-blind, vehicle-controlled trial. J Am Acad Dermatol. 2011;65(1):54–64. doi: 10.1016/j.jaad.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Landmann A. The classification and diagnosis of lupus erythematosus. J Autoimmun. 2014;48–9:14–19. doi: 10.1016/j.jaut.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Landmann A., Patsinakidis N., Ruland V., Nozinic S., Perusquia Ortiz A. Fumaric acid ester treatment in cutaneous lupus erythematosus (CLE): A prospective, open-label, phase II pilot study. Lupus. 2016;25(12):1357–1364. doi: 10.1177/0961203316644335. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Lehmann P., Ruzicka T. Classification of cutaneous lupus erythematosus. In: Phillip M., editor. Cutaneous lupus erythematosus. Springer-Verlag; Berlin: 2005. pp. 53–77. [Google Scholar]
- Kuhn A., Patsinakidis N., Luger T.A. Alitretinoin for cutaneous lupus erythematosus. J Am Acad Dermatol. 2012;67:e123–e126. doi: 10.1016/j.jaad.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Ruland V., Bonsmann G. Cutaneous lupus erythematosus: Update of therapeutic options: Part II. J Am Acad Dermatol. 2011;65(6):e195–e213. doi: 10.1016/j.jaad.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Ky C., Swasdibutra B., Khademi S., Desai S., Laquer V., Grando S.A. Efficacy of intravenous immunoglobulin monotherapy in patients with cutaneous lupus erythematosus: Results of proof-of-concept study. Dermatol Reports. 2015;7(1):5804. doi: 10.4081/dr.2015.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulos C.E., Hughes G.R., Cruz D.P.D. Intravenous immunoglobulin in the treatment of resistant subacute cutaneous lupus erythematosus: A possible alternative. Clin Rheumatol. 2007;26:981–983. doi: 10.1007/s10067-006-0222-5. [DOI] [PubMed] [Google Scholar]
- Lee L. Neonatal lupus: Clinical features and management. Paediatr Drugs. 2004;6(2):71–78. doi: 10.2165/00148581-200406020-00001. [DOI] [PubMed] [Google Scholar]
- Lee L.A. Lupus erythematosus. In: Bolognia J., editor. Dermatology. 2nd ed. Mosby; London: 2008. [Google Scholar]
- Lehmann P., Holzle E., Kind P., Goerz G., Plewig G. Phototesting in lupus erythematosus. J Invest Dermatol. 1993;100:53S–57S. doi: 10.1111/1523-1747.ep12355594. [DOI] [PubMed] [Google Scholar]
- Lowe G., Henderson C., Grau R., Hansen C.B., Sontheimer R.D. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2010;164(3):465–472. doi: 10.1111/j.1365-2133.2010.10110.x. [DOI] [PubMed] [Google Scholar]
- Madan V., August P., Chalmers R. Efficacy of topical tacrolimus 0.3% in cloebetasol propionate 0.05% ointment in therapy-resistant cutaneous lupus erythematosus: A cohort study. Clin Exp Dermatol. 2009;35(1):27–30. doi: 10.1111/j.1365-2230.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- Merrill J.T., Neuwelt C.M., Wallace D.J., Shanahan J.C., Latinis K.M., Oates J.C. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomised, double-blind, phase II/III systemic lupus erythematosus evaluation of rituxumab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: Part 1. Pregnany. J Am Acad Dermatol 2014;70(3):401.e401–14. [DOI] [PubMed]
- Obermoser G., Sontheimer R., Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19(9):1050–1070. doi: 10.1177/0961203310370048. [DOI] [PubMed] [Google Scholar]
- Okon L., Rosenbach M., Krathen M., Rose M., Propert K., Okawa J. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: Efficacy and safety in a 52 week trial. J Am Acad Dermatol. 2014;70(3):583–584. doi: 10.1016/j.jaad.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon L., Werth V. Cutaneous lupus erythematosus: Diagnosis and treatment. Clin Rheum. 2013;27(3):391–404. doi: 10.1016/j.berh.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Frances C., Roy S., Papo T., Godeau P. High-dose immunoglobulins in the treatment of refractory cutaneous lupus erythematosus. Open trial in 5 cases. Arthritis Rheum. 1995;38:S304. [Google Scholar]
- Raptoopoulou A., Linardakis C., Sidiropoulos P., Kritikos H., Boumpas D. Pulse cyclophosphamide treatment for severe refractory cutaneous lupus erythematosus. Lupus. 2010;19(6):744–747. doi: 10.1177/0961203309358601. [DOI] [PubMed] [Google Scholar]
- Rees F., Doherty M., Grainge M., Lanyon P., Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- Rovin B.H., Furie R., Latinis K.M., Looney R.J., Fervenza F.C., Sanchez-Guerrero J. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- Ruzicka T., Sommerburg C., Goerz G., Kind P., Mensing H. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br J Dermatol. 1992;127:513–518. doi: 10.1111/j.1365-2133.1992.tb14851.x. [DOI] [PubMed] [Google Scholar]
- Saavedra M.A., Sánchez A., Morales S., Ángeles U., Jara L.J. Azathioprine during pregnancy in systemic lupus erythematosus patients is not associated with poor fetal outcome. Clin Rheumatol. 2015;34(7):1211–1216. doi: 10.1007/s10067-015-2987-x. [DOI] [PubMed] [Google Scholar]
- Sadlier M., Kirby B., Lally A. Mycophenolate mofetil and hydroxychloroquine: An effective treatment for recalcitrant cutaneous lupus erythematosus. J Am Acad Dermatol. 2012;66(1):160–161. doi: 10.1016/j.jaad.2011.08.036. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Ohshima S., Kurimoto I., Miura H., Suemura M. Maintaining remission of lupus erythematosus profundus (LEP) with cyclosporin A. Lupus. 2000;9:390–392. doi: 10.1191/096120300678828406. [DOI] [PubMed] [Google Scholar]
- Saracino A., Orteu C. Severe recalcitrant cutaneous manifestations in systemic lupus erythematosus successfully treated with fumaric acid esters. Br J Dermatol. 2017;176(2):472–480. doi: 10.1111/bjd.14698. [DOI] [PubMed] [Google Scholar]
- Schmitt V., Meuth A.M., Amler S., Kuehn E., Haust M., Messer G. Lupus erythematosus tumidus is a seperate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162(1):64–73. doi: 10.1111/j.1365-2133.2009.09401.x. [DOI] [PubMed] [Google Scholar]
- Schulz E., Menter M. Treatment of discoid and subacute lupus erythematosus with cyclophosphamide. Br J Dermatol. 1971;85:60–65. [Google Scholar]
- Segasothy M., Phillips P. Systemic lupus erythematosus in Aborigines and Caucasians in central Australia: A comparative study. Lupus. 2001;10(6):439–444. doi: 10.1191/096120301678646191. [DOI] [PubMed] [Google Scholar]
- Sigges J., Bizar C., Landmann A., Ruland V., Patsinakidis N., Amler S. Therapeutic strategies evaluated by the European Society of Cutaneous Lupus Erythematosus (EUSCLE) Core Set Questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun Rev. 2013;12(7):694–702. doi: 10.1016/j.autrev.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Sontheimer R.D., Thomas J.R., Gilliam J.N. Subacute cutaneous lupus erythematosus: A cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115(12):1409–1415. [PubMed] [Google Scholar]
- Truchuelo M., Boixeda P., Alcantara J., Moreno C., de las Heras E., Olasolo P. Pulsed dye laser as an excellent choice of treatment for lupus tumidus: A prospective study. J Eur Acad Dermatol Venerol. 2012;26(10):1272–1279. doi: 10.1111/j.1468-3083.2011.04281.x. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven R., Hahn B., Tsokos G., Wagner C., Lipsky P., Hsu B., Chevrier M., Gordon R., Triebel M., Rose S. Presented at: 2017 American College of Rheumatology/Association of Rheumatology Health Professionals. San Diego; California: 2018. Efficacy and Safety of Ustekinumab, an Interleukin 12/23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: Results of a Phase 2, Randomized Placebo-Controlled Study 2017. [Google Scholar]
- Vashisht P., Borghoff K., O’Dell J., Hearth-Holmes M. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2016;26(8):857–864. doi: 10.1177/0961203316682097. [DOI] [PubMed] [Google Scholar]
- Vasquez R., Tseng L., Victor S., Zhang S., Chong B. Autoantibody and clinical profiles in patients with discoid lupus and borderline systemic lupus. Arch Dermatol. 2012;148(5):651–655. doi: 10.1001/archdermatol.2011.3249. [DOI] [PubMed] [Google Scholar]
- Vena G.A., Coviello C., Angelini G. Use of oral isotretinoin in the treatment of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 1989;124(6):311–315. [PubMed] [Google Scholar]
- Vera-Recabarren M., Garcia-Carrasco G., Ramos-Casals M., Herrero C. Comparative analysis of subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus: clinical and immunological study of 270 patients. Br J Dermatol. 2010;162(1):91–101. doi: 10.1111/j.1365-2133.2009.09472.x. [DOI] [PubMed] [Google Scholar]
- Vital E., Wittmann M., Edward S., Md Yusof M., MacIver H., Pease C. Brief report: Responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol. 2015;67(6):1586–1591. doi: 10.1002/art.39085. [DOI] [PubMed] [Google Scholar]
- Wang D., Chen H., Wang S., Zou Y., Li J., Pan J. Thalidomide treatment in cutaneous lesions of systemic lupus erythematosus: A multicenter study in China. Clin Rheumatol. 2016;35(6):1521–1527. doi: 10.1007/s10067-016-3256-3. [DOI] [PubMed] [Google Scholar]
- Wenzel J., Brahler S., Bauer R., Bieber T., Tuting T. Efficacy and safety of methotrexate in recalcitrant cutaneous lupus erythematosus: Results of a retrospective study in 43 patients. Br J Dermatol. 2005;153(1):157–162. doi: 10.1111/j.1365-2133.2005.06552.x. [DOI] [PubMed] [Google Scholar]