Abstract
Peripheral pulmonary artery stenosis (PPAS) is a rare pulmonary vasculopathy characterized by multiple stenoses and obstructions in the peripheral pulmonary arteries. PPAS often develops in children with congenital diseases such as Williams syndrome and Alagille syndrome; however, recent studies have reported PPAS cases in adults with Moyamoya disease (MMD). Recent genetic studies have demonstrated that ring finger protein 213 (RNF213) is a susceptibility gene for MMD. However, the pathophysiology of combined PPAS and MMD and the relationship between the two diseases remain largely unknown. Here we report a case of PPAS in a 16-year-old male, with a history of MMD, who died suddenly at 24. An autopsy was performed, and remarkable pathological changes were identified in the pulmonary arteries and in other arteries. Furthermore, genetic analysis revealed that the patient had a homozygous c.14576G > A (p.R4859K) mutation in RNF213. This is the first report to demonstrate the histopathology of systemic arteriopathy in a case with MMD and PPAS with a confirmed homozygous RNF213 mutation. We also review immunohistochemical data from the case and discuss how RNF213 mutation could have resulted in the observed vascular abnormalities.
Keywords: Ring finger protein 213, Homozygote, Peripheral pulmonary artery stenosis, Moyamoya disease, Pulmonary hypertension
1. Introduction
Peripheral pulmonary artery stenosis (PPAS) is characterized by the presence of one or more stenoses in the main and lobar pulmonary arteries, and can lead to pulmonary hypertension (PH) and right-sided heart failure [1]. Childhood PPAS is often associated with congenital heart disease and genetic syndromes such as Williams syndrome and Alagille syndrome [2]. PPAS in adults is rarer [3], and it its etiology is not well understood.
Moyamoya disease (MMD) is a progressive cerebral vascular disease caused by stenosis of the Circle of Willis, leading to the development of the numerous collateral arteries on both sides of the brain [4]. The Japanese word ‘moyamoya’, meaning ‘puff of smoke’, describes these collateral vessels.
The RNF213 (Ring Finger Protein 213) has been identified as a susceptibility gene for MMD [5,6]. RNF213 is located on chromosome 17q25.3 and encodes a really interesting new gene finger protein that possesses two AAA + ATPase domains and one E3 ligase domain. A founder missense mutation in RNF213, c.14576G > A (p.R4859K) is found in about 80% of East Asian MMD patients [[5], [6], [17]]. Importantly, a recent report documented two cases with PH and MMD, both of whom exhibited homozygous RNF213 mutation [7]. However, the role and pathological impacts of mutated RNF213 in diseased arterial walls are poorly understood. Here, we report a case of PPAS in a 16-year-old male with a history of MMD, on whom we performed autopsy as well as genetic and histopathological analyses.
2. Case report
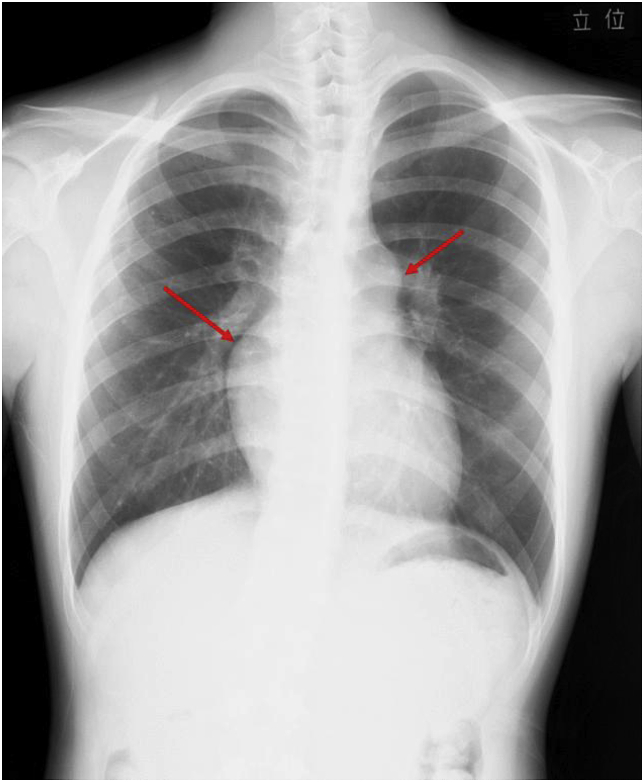
A 16-year-old male with a history of MMD and electrocardiogram (ECG) abnormalities was referred to our department for an evaluation following his father's death due to heart failure. Additionally, his brother had a medical history of cerebral infarction at the age of 18. There were no known consanguineous marriages within the family. The patient was diagnosed with MMD and intellectual disabilities at the age of four and subsequently underwent bilateral cerebral vascular bypass surgeries. He had not experienced any symptoms or signs suggestive of acute venous thrombosis. He had a complaint of shortness of breath upon exertion since the age of 10, and at 13, an ECG showed signs of increased right heart load, which prompted the use of echocardiography. As a result, mild PH was suspected. He was put under observation, and referred to our department at 16 for further evaluation. His chest X-ray displayed protrusions of the right and left second arches of the heart, and cardiothoracic ratio (CTR) was 47% (Fig. 1).
Fig. 1.
Chest X-ray shows protrusions of the right and left second arches of the heart (arrows).
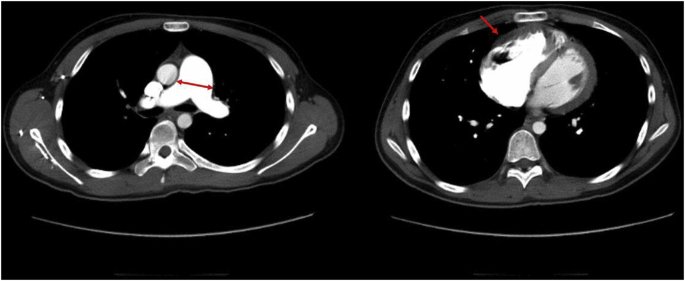
The ECG showed a sinus rhythm with a heart rate of 60 beats per minute, an axis of +120°, normal P waves, elevated R waves in lead V1, and inverted T waves in leads V1–V4, indicating the presence of right ventricular hypertrophy. The computed tomography (CT) revealed dilation of the main trunk of the pulmonary artery, right arterial and ventricular dilation, and thickening of the right ventricular wall (Fig. 2). No thrombi were detected in the pulmonary arteries. The lung window images showed mosaic attenuation, depicting heterogeneous perfusion differences.
Fig. 2.
Chest CT imaging of the mediastinum.
The left panel indicates dilation of the main trunk of the pulmonary artery (arrows), and the right panel shows dilatation and wall thickening of the right ventricle (arrow). No thrombi were detected in the pulmonary arteries.
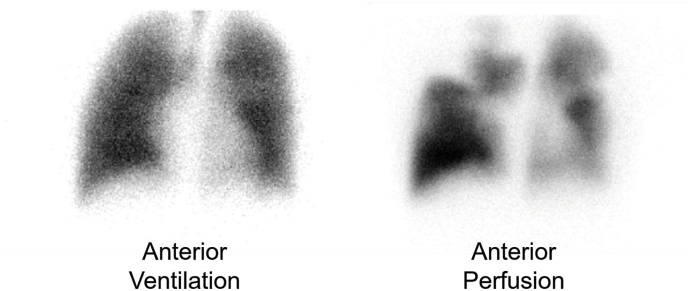
A lung ventilation-perfusion scan revealed segmental perfusion defects with homogeneous ventilation (Fig. 3).
Fig. 3.
Lung ventilation-perfusion scan showing ventilation-perfusion mismatch.
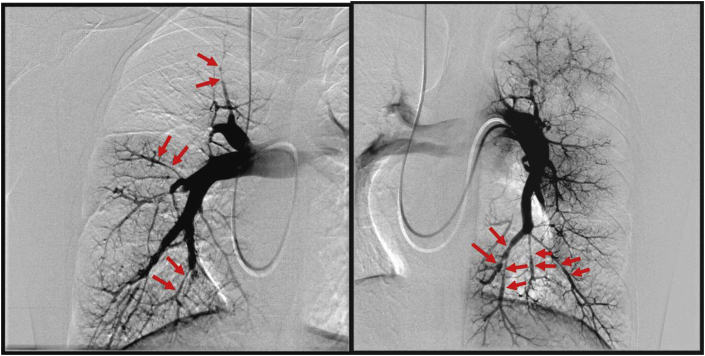
Right heart catheterization revealed a mean pulmonary arterial pressure of 63 mmHg, pulmonary artery wedge pressure of 10 mmHg, cardiac output of 3.99 L/min, a cardiac index of 2.48 L·min−1·m−2, and 13.3 Wood units of pulmonary vascular resistance. Pulmonary angiography (PAG) revealed pouching on the right A2, and abrupt narrowing and poststenotic dilatation of bilateral segmental pulmonary arteries (Fig. 4).
Fig. 4.
Pulmonary angiography (left: right pulmonary artery; right: left pulmonary artery). Abrupt narrowing and poststenotic dilatation were observed in bilateral segmental pulmonary arteries (arrows).
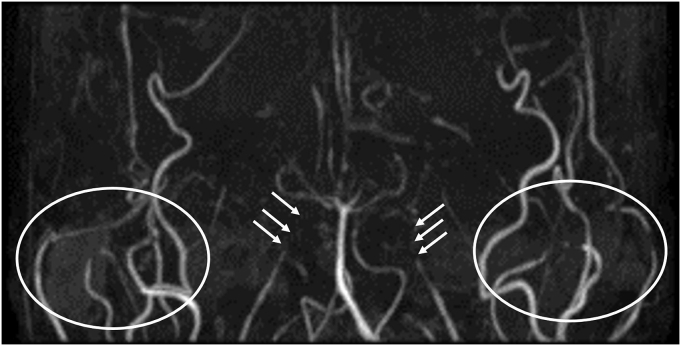
Chronic thromboembolic pulmonary hypertension (CTEPH) was suspected, although the multiple poststenotic dilatations of the segmental pulmonary arteries, relatively young age of onset, and the lack of coagulation abnormalities led us to a diagnosis of PPAS. Brain magnetic resonance imaging acquired at 16 years old were reviewed, and displayed diffuse narrowing and obstruction of the bilateral internal carotid arteries with compensatory dilatation of external carotid arteries, consistent with MMD (Fig. 5).
Fig. 5.
Brain magnetic resonance angiography.
The image shows bilateral severe narrowing of the internal carotid arteries (arrows). The external carotid arteries are dilated in compensation (white circles).
Because of the patient's mental condition, invasive treatment was avoided and he was treated with an anti-coagulant (warfarin 1.5 mg/day) and vasodilators (beraprost, 240 μg/day; ambrisentan, 10 mg/day) and kept under surveillance. The prothrombin time-international normalized ratio was maintained between 2.0 and 3.0, and while no obvious improvement was observed, the condition was relatively stable with drug therapy until one morning when he was 24 years old, when he suddenly collapsed, was brought to our hospital by ambulance, and was admitted. He was in a cardiac pulmonary arrest on arrival, and was not responsive to resuscitation. The cause of death was not specified by digital autopsy using CT. Therefore, with the family's written consent for pathological and genetic investigation, an autopsy was performed. We examined the organs in the chest and abdominal cavities, but were not allowed to examine the brain.
3. Autopsy study
3.1. Gross findings
The autopsy was conducted 3 hours after death. There were trivial amounts of pleural effusion in the right and left pleural cavities, and mild pulmonary congestion was observed. The right and left lungs weighted 230 g and 204 g, respectively. The proximal pulmonary artery was dilated (the diameter of the pulmonary valve was 65 mm). There were multiple stenosis in the branch pulmonary arteries with poststenotic dilation. The weight of the heart had increased to 516 g, compared to standard male heart weight (342g ± 89.9 [8]). His right atrium and ventricle had dilated, and the right ventricular free wall had substantially thickened (18 mm). There were no septal defects between the atria nor between the ventricles, and both atria were free of thrombi. The coronary arteries arose normally and displayed no evidence of atherosclerotic changes. There was no evidence of bleeding or infarction within the myocardium. All four pulmonary veins flowed into left atrium. There was a 13 × 10 mm stenotic area in the ascending aorta.
3.2. Histopathology
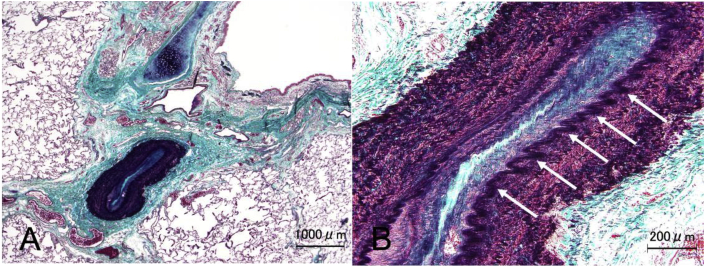
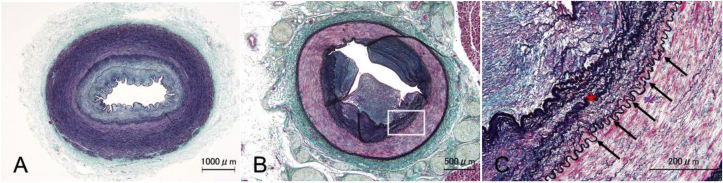
Pathological findings in the stenotic areas of the pulmonary arteries showed thickening of the intima and the media, which accounted for the reduction of the luminal area (Fig. 6A). Moreover, irregular waving of the internal elastic lamina was observed (Fig. 6B, arrows), which is not a common pathological characteristic of PPAS or CTEPH.
Fig. 6.
(A, B). Histopathology of pulmonary artery stenosis
A. Masson trichrome staining with elastic staining (modified Masson trichrome staining) shows a narrowed pulmonary artery with intimal and medial thickening. B. Irregular waving of the internal elastic lamina is indicated by arrows.
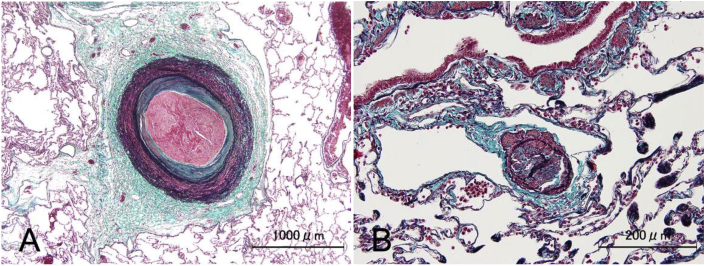
In addition, both old and recently formed thrombi were observed within the peripheral pulmonary arteries (Fig. 7A and B) but these lacked fibrous web/band formation, which is characteristic to CTEPH.
Fig. 7.
(A, B). Thrombus formation in the peripheral pulmonary artery
A, B. Modified Masson trichrome staining of peripheral pulmonary arteries. A. Recently formed thrombus. B. Old organized thrombus.
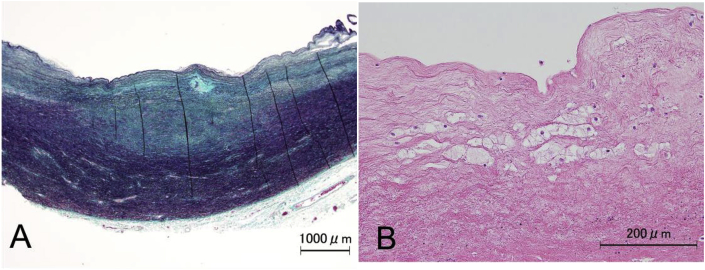
In the walls of the aorta, there was intimal thickening (Fig. 8A) with partial infiltration of macrophages (Fig. 8B).
Fig. 8.
Intimal thickening and macrophage infiltration in the aortic wall
A. Modified Masson trichrome staining shows intimal thickening of the aorta. B. Macrophage infiltration was detected by hematoxylin and eosin staining.
In the renal and splenic arteries and branches of the subclavian arteries, observed reductions in luminal area were due to intimal and medial thickening (Fig. 9A and B), similar to the pulmonary arteries. Irregular waving of the internal elastic lamina was also observed in the stenotic segment of the splenic artery (Fig. 9C).
Fig. 9.
Histopathology of distal arteries.
Modified Masson trichrome staining of branches of the subclavian (A) and splenic (B) arteries displays intimal and medial thickening. (C) High magnification image of the square in B, displaying intimal and medial thickening (asterisk) with irregular waving of the internal elastic lamina (arrows).
For immunohistochemical analysis, we used antibodies against RNF213 (HPA003347, Atlas Antibodies, Bromma, Sweden; 1:250), caveolin-1 (ab2910, Abcam, Cambridge, UK; 1:10,000), α smooth muscle actin (SMA; 1A4, Dako, Santa Clara, CA; 1:400), and desmin (DE-R-11, Roche, Basel, Switzerland, 1:1) to study the distribution of RNF213 and caveolin-1 in the vascular walls. We also examined healthy pulmonary artery specimens as controls.
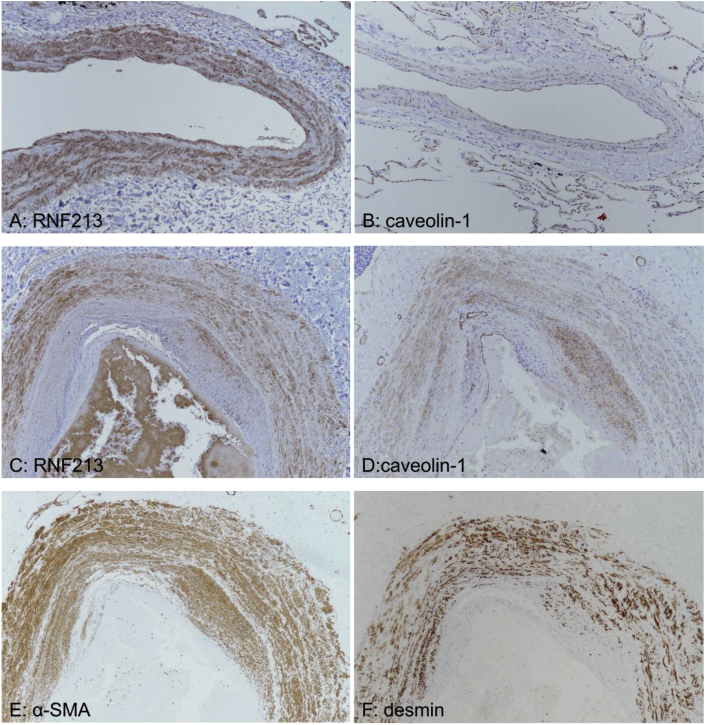
In the control samples, RNF213 and caveolin-1 expression was detected in the endothelium and the medial vascular smooth muscle cells; however, caveolin-1 expression in vascular smooth muscle cells was weaker than RNF213 expression (Fig. 10 (A, B)). In the patient samples, irregular thickening of the intima and the media was observed, which was positive for RNF213 and caveolin-1 expression (Fig. 10 (C, D)). In addition, α-SMA and desmin were observed within the thickened intima (Fig. 10E and F), indicating that the excess cells in the intima harbor the characteristics of fibromuscular or smooth muscle cells.
Fig. 10.
(A-F, 100×magnification). Immunohistochemical comparison of the pulmonary arteries of the present case and a control subject
(A) and (B) show RNF213 and caveolin-1 staining, respectively, in control specimens, while (C) and (D) show RNF213 and caveolin-1 staining, respectively, in patient specimens. (E) α-SMA and (F) desmin staining of patient specimens.
3.3. Genetic analysis
We extracted DNA samples from frozen tissues of liver and skeletal muscle, and analyzed the genome sequence by pyrosequencing. A homozygous mutation in RNF213, c.14576G > A (p.R4859K) was identified. This homozygous mutation is observed in 7–8% of MMD patients [9,10].
4. Discussion
4.1. Summary
We report the pathological and genetic findings of a postmortem case of PPAS with MMD. Histopathological findings in the arteries revealed distinctive features compared to PPAS or MMD alone, with overlapping characteristics of both diseases. Genetic analysis identified a homozygous c.14576G > A (p.R4859K) mutation in RNF213. These findings suggest that RNF213, c.14576G > A (p.R4859K) homozygosity may play an important role not only in PPAS, but also in systemic vascular abnormalities.
Although this case report exhibits significant findings in a case of PPAS and MMD, and supports recent reports describing the characteristics of combined PPAS and MMD, the facts are limited to a sole case. Future studies with more samples are awaited to strengthen and verify the findings demonstrated here.
4.2. PPAS (clinical)
Although there are no articulated diagnostic criteria for PPAS to date, diagnosis is usually made based on characteristic PAG features and medical history, in addition to diagnosis of PH using right heart catheterization. The observation of unmatched segmental lung perfusion defects can make it difficult to distinguish PPAS with CTEPH [3]. In our case, we first diagnosed CTEPH based on the right heart catheterization and ventilation perfusion scan results, but after discovering the poststenotic dilatation of segmental pulmonary arteries by PAG, PPAS was raised as a differential diagnosis. In conclusion, we ultimately diagnosed the case as PPAS, based on the PAG pattern, patient's age, MMD comorbidity, and lack of evidence of coagulopathy and other risk factors for CTEPH.
4.3. PPAS (pathological)
Histopathological reports on PPAS are limited, due to rarity of the disease and the difficulty in obtaining patient lung tissues. However, Kreutzer et al. reported that the pulmonary arteries of children with PPAS displayed narrowing of the vascular lumen due to thickening of the media with increased elastic fibers, and invasion of the intima by proliferative smooth muscle cells [3]. In addition, unlike in CTEPH, Kreutzer et al. reported that PPAS cases do not display web or band formation, nor recanalization of the thrombus that occludes the pulmonary arteries. Importantly, these pathological features were consistently observed in our case. However, the present case exhibited wavy internal elastic lamina, which has not been reported in PPAS. This inconsistency between prior reports and our case will be discussed later.
4.4. MMD (clinical)
MMD was diagnosed by the neurosurgery department of another hospital when the patient was four years old. The diagnosis was made based on imaging studies, and he subsequently underwent bypass surgery at the same hospital. Although the original images prior to the bypass surgeries were not available, the results of an MRI acquired at 16 were consistent with MMD, exhibiting stenosis and arterial occlusions around the Circle of Willis, and compensatory dilatations of the external carotid arteries. There were no inconsistencies in the course of the illness, and the diagnosis was definitely confirmed.
4.5. Co-occurrence of PPAS and MMD
Previously, a few reports have described the co-occurrence of PH and MMD, although the etiology remains unknown [11,12]. In 2016, two patients with PH and MMD were reported, and interestingly, these patients were found to have a homozygous p.Arg4810Lys mutation in RNF213 [7]. In 2018, five cases of PPAS were reported, who were also homozygous for this change [13]. Notably, three of these five patients had MMD, and these three patients displayed multiple extracranial arterial stenoses in arteries other than the pulmonary artery. Taken together, these recent reports suggest that the co-occurrence of PPAS and MMD may be associated with mutation of RNF213, a susceptibility gene for MMD. Although the etiology of PPAS/MMD co-occurrence is not fully understood, the present case report further highlights the subset of patients with co-occurring PPAS and MMD, and the possible contribution of RNF213 mutation as an underlying cause of the two diseases.
4.6. Histopathological findings
The vascular changes observed through histopathological analysis were hyperplasia of the intima and the media, presumably caused by the proliferation of smooth muscle cells, and abnormal waving of the internal elastic lamina. A significant discovery in this case was that the histopathological findings of PPAS combined with MMD are distinctive compared to those of PPAS and MMD individually. For example, hyperplasia of the intima and the media was consistent with reported descriptions of PPAS [3,14]; however, the irregular waving of the internal elastic lamina seen was not. Conversely, hypertrophy of the intima and irregular waving of the internal elastic lamina are hallmarks of MMD [15], both of which were observed in our patient. In addition, the involvement of extrapulmonary arteries is often observed in MMD, and this was pathologically confirmed in our patient. However, thinning of the media, another histopathological feature of MMD [16] was not observed. Thus, the histopathological findings in this report revealed overlapping characteristics of both PPAS and MMD, which were not exactly consistent with either (Table 1).
Table 1.
Typical histopathological findings of PPAS and MMD and pathological changes observed in the present case.
| Portion of vessel | Typical findings |
Present case | |
|---|---|---|---|
| PPAS | MMD | ||
| Intima | Hyperplasia | Hyperplasia | Hyperplasia |
| Internal elastic lamina | No remarkable change | Abnormal waving | Abnormal waving |
| Media | Hyperplasia | Thinning | Hyperplasia |
4.7. Genetic analysis
A homozygous mutation was identified in RNF213 by pyrosequencing. RNF213 encodes a ring finger protein with an AAA ATPase domain, and functions as both an E3 ubiquitin ligase and an ATPase. The c.14576G > A (p.R4859K) variant of RNF213 is a susceptibility variant for MMD, which is expressed by approximately 74–84% of Japanese patients with MMD [11,18]. The majority of c.14576G > A (p.R4859K) mutations observed in MMD are heterozygous, with homozygosity occurring in an estimated 7–8% of all patients with MMD [9,10]. Recent reports have suggested that the phenotypes observed after RNF213 mutation are gene dosage-dependent, with the p.Arg4810lys variant causing MMD when heterozygous, and both MMD and systemic vascular disease, including that of the pulmonary artery, when homozygous [7]. In the present case, confirmation of homozygosity for c.14576G > A (p.R4859K) suggests that homozygous c.14576G > A (p.R4859K) mutation contributes to the development of PPAS, MMD, and systemic vasculopathy.
4.8. Caveolin-1
Intriguingly, recent reports have shown that caveolin-1 may be a possible factor associated with vascular remodeling in MMD [[18], [19], [20]]. Moreover, reports indicate that presence of RNF213 variant is associated with serum caveolin-1 level [18,19].
Caveolae are small (50–100 nm), invaginated membrane structures that are abundant in endothelial cells and play major roles in the regulation of endothelial vesicular trafficking and signal transduction [[20], [21]]. Caveolin-1 is a caveolae scaffolding protein that is involved in the pathogenesis of cancer and vascular diseases [20]. Recent studies have reported that, negative serum caveolin-1 expressions may be associated with suppressed angiogenesis in the endothelial cells and smooth muscle cells in MMD patients. Furthermore, a RNF213 mutation could cause systemic vasculopathy, including MMD and PH, via caveolin-1 expression changes [19,20]. With interest to the prior reports on caveolin-1 and vascular changes, we examined caveolin-1 expression using the immunohistochemical analysis. In the present study, immunohistochemical analysis indicated that caveolin-1 expression correlates with the pathological changes observed in the intima and media, as well as with the distribution of RNF213 protein expression. The results also suggested that the proliferative cells responsible for this pathological change are smooth muscle cells. These findings suggest a pathological link between the morphological changes of the arteries and altered RNF213 and caveolin-1 expression. The specific functions of RNF213 and caveolin-1, and their association with vascular development, will require future studies.
5. Conclusion
In conclusion, we have reported the autopsy results of a case with PPAS and MMD with a homozygous mutation in RNF213. To the best of our knowledge, this report is the first to histologically describe the vasculopathy observed in a case with combined PPAS and MMD. Future basic and clinical studies are warranted to further elucidate the impact of this genetic abnormality in the pathogenesis of PPAS and MMD.
Declaration of competing interest
Authors have no conflicts of interest to disclose.
Contributor Information
Kei Takahashi, Email: kay_lei_may@yahoo.co.jp.
Ichizo Tsujino, Email: itsujino@med.hokudai.ac.jp.
References
- 1.McLaughlin V.V., Archer S.L., Badesch D.B., Barst R.J., Farber H.W., Lindner J.R., Mathier M.A., McGoon M.D., Park M.H., Rosenson R.S., Rubin L.J., Tapson V.F., Varga J., Harrington R.A., Anderson J.L., Bates E.R., Bridges C.R., Eisenberg M.J., Ferrari V.A., Grines C.L., Hlatky M.A., Jacobs A.K., Kaul S., Lichtenberg R.C., Lindner J.R., Moliterno D.J., Mukherjee D., Pohost G.M., Rosenson R.S., Schofield R.S., Shubrooks S.J., Stein J.H., Tracy C.M., Weitz H.H., Wesley D.J. ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American college of cardiology foundation task force on expert consensus documents and the American heart association: developed in collaboration with the American college of chest physicians, American thoracic society, inc., and the pulmonary hypertension association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Inglessis I., Landzberg M. Interventional catheterization in adult congenital heart disease. Circulation. 2007;115:1622–1633. doi: 10.1161/CIRCULATIONAHA.105.592428. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzer J., Landzberg M.J., Preminger T.J., Mandell V.S., Treves S.T., Reid L.M., Lock J.E. Isolated peripheral pulmonary artery stenoses in the adult. Circulation. 1996;93:1417–1423. doi: 10.1161/01.cir.93.7.1417. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki J., Takuku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 5.Kamada F., Aoki Y., Narisawa A., Abe Y., Komatsuzaki S., Kikuchi A., Kanno J., Niihori T., Ono M., Ishii N., Owada Y., Fujimura M., Mashimo Y., Suzuki Y., Hata A., Tsuchiya S., Tominaga T., Matsubara Y., Kure S. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011;56:34–40. doi: 10.1038/jhg.2010.132. [DOI] [PubMed] [Google Scholar]
- 6.Liu W., Morito D., Takashima S., Mineharu Y., Kobayashi H., Hitomi T., Hashikata H., Matsuura N., Yamazaki S., Toyoda A., Kikuta K., Takagi Y., Harada K.H., Fujiyama A., Herzig R., Krischek B., Zou L., Kim J.E., Kitakaze M., Miyamoto S., Nagata K., Hashimoto N., Koizumi A. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukushima H., Takenouchi T., Kosaki K. Homozygosity for moyamoya disease risk allele leads to moyamoya disease with extracranial systemic and pulmonary vasculopathy. Am. J. Med. Genet. A. 2016;170:2453–2456. doi: 10.1002/ajmg.a.37829. [DOI] [PubMed] [Google Scholar]
- 8.Sawabe M., Saito M., Naka M., Kasahara I., Saito Y., Arai T., Hamamatsu A., Shirasawa T. Standard organ weights among elderly Japanese who died in the hospital, including 50 centenarians. Pathol. Int. 2006;56(6):315–323. doi: 10.1111/j.1440-1827.2006.01966.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim E.H., Yum M.S., Ra Y.S., Park J.B., Ahn J.S., Kim G.H., Goo H.W., Ko T.S., Yoo H.W. Importance of RNF213 polymorphism on clinical features and long-term outcome in moyamoya disease. J. Neurosurg. 2016;124:1221–1227. doi: 10.3171/2015.4.JNS142900. [DOI] [PubMed] [Google Scholar]
- 10.Miyatake S., Miyake N., Touho H., Nishimura-Tadaki A., Kondo Y., Okada I., Tsurusaki Y., Doi H., Sakai H., Saitsu H., Shimojima K., Yamamoto T., Higurashi M., Kawahara N., Kawauchi H., Nagasaka K., Okamoto N., Mori T., Koyano S., Kuroiwa Y., Taguri M., Morita S., Matsubara Y., Kure S., Matsumoto N. Homozygous c.14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012;78:803–810. doi: 10.1212/WNL.0b013e318249f71f. [DOI] [PubMed] [Google Scholar]
- 11.Ou P., Dupont P., Bonnet D. Fibromuscular dysplasia as the substrate for systemic and pulmonary hypertension in the setting of Moya-Moya disease. Cardiol. Young. 2006;16:495–497. doi: 10.1017/S104795110600045X. D. [DOI] [PubMed] [Google Scholar]
- 12.Tokunaga K., Hishikawa T., Sugiu K., Date I. Fatal outcomes of pediatric patients with moyamoya disease associated with pulmonary arterial hypertension. Report of two cases. Clin. Neurol. Neurosurg. 2013;115:335–338. doi: 10.1016/j.clineuro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Chang S.A., Song J.S., Park T.K., Yang J.H., Kwon W.C., Kim S.R., Kim S.M., Cha J., Jang S.Y., Cho Y.S., Kim T.J., Bang O.Y., Song J.Y., Ki C.S., Kim D.K. Nonsyndromic peripheral pulmonary artery stenosis is associated with homozygosity of RNF213 p.Arg4810Lys regardless of Co-occurrence of moyamoya disease. Chest. 2018;153:404–413. doi: 10.1016/j.chest.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli A.R., Ahmed M., Hamed F., Prieto L.R. Peripheral pulmonary artery stenosis as a cause of pulmonary hypertension in adults. Pulm. Circ. 2015;5:204–210. doi: 10.1086/679727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takagi Y., Kikuta K., Nozaki K., Hashimoto N. Histopathological features of middle cerebral arteries from patients treated for moyamoya disease. Neurol. Med. Chir. (Tokyo) 2007;47:1–4. doi: 10.2176/nmc.47.1. [DOI] [PubMed] [Google Scholar]
- 16.Bang O.Y., Fujimura M., Kim S.K. The pathophysiology of moyamoya disease: an update. J. Stroke. 2016;18:12–20. doi: 10.5853/jos.2015.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moteki Y., Onda H., Kasuya H., Yoneyama T., Okada Y., Hirota K., Mukawa M., Nariai T., Mitani S., Akagawa H. Systematic validation of RNF213 coding variants in Japanese patients with moyamoya disease. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung J.W., Kim D.H., Oh M.J., Cho Y.H., Kim E.H., Moon G.J., Ki C.S., Cha J., Kim K.H., Jeon P., Yeon J.Y., Kim G.M., Kim J.S., Hong S.C., Bang O.Y. Cav-1 (Caveolin-1) and arterial remodeling in adult moyamoya disease. Stroke. 2018 Nov;49(11):2597–2604. doi: 10.1161/STROKEAHA.118.021888. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H., Kabata R., Kinoshita H., Morimoto T., Ono K., Takeda M., Choi J., Okuda H., Liu W., Harada K.H., Kimura T., Youssefian S., Koizumi A. Rare variants in RNF213, a susceptibility gene for moyamoya disease, are found in patients with pulmonary hypertension and aggravate hypoxia-induced pulmonary hypertension in mice. Pulm. Circ. 2018 Jul-Sep;8(3) doi: 10.1177/2045894018778155. 2045894018778155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang O.Y., Chung J.W., Kim S.J., Oh M.J., Kim S.Y., Cho Y.H., Cha J., Yeon J.Y., Kim K.H., Kim G.M., Chung C.S., Lee K.H., Ki C.S., Jeon P., Kim J.S., Hong S.C., Moon G.J. Caveolin-1, Ring finger protein 213, and endothelial function in Moyamoya disease. Int. J. Stroke. 2016 Dec;11(9):999–1008. doi: 10.1177/1747493016662039. [DOI] [PubMed] [Google Scholar]
- 21.Frank P.G., Woodman S.E., Park D.S., Lisanti M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]