Abstract
There is an increasing demand for carotenoids due to their applications in the food, flavor, pharmaceutical and feed industries, however, the extraction and synthesis of these compounds can be expensive and technically challenging. Microbial production of carotenoids provides an attractive alternative to the negative environmental impacts and cost of chemical synthesis or direct extraction from plants. Metabolic engineering and synthetic biology approaches have been widely utilized to reconstruct and optimize pathways for carotenoid overproduction in microorganisms. This review summarizes the current advances in microbial engineering for carotenoid production and divides the carotenoid biosynthesis building blocks into four distinct metabolic modules: 1) central carbon metabolism, 2) cofactor metabolism, 3) isoprene supplement metabolism and 4) carotenoid biosynthesis. These four modules focus on redirecting carbon flux and optimizing cofactor supplements for isoprene precursors needed for carotenoid synthesis. Future perspectives are also discussed to provide insights into microbial engineering principles for overproduction of carotenoids.
Keywords: Carotenoids, Synthetic biology, Metabolic engineering, Mevalonate, Isopentenol utilization pathway, Modular engineering
Graphical abstract
Highlights
-
•
Recent advances in microbial engineering for carotenoid production.
-
•
Dividing the building blocks for carotenoid biosynthesis into four distinct modules.
-
•
Systematic engineering of these modules to increase carotenoid production.
-
•
Challenges and directions in microbial engineering for carotenoid production.
1. Introduction
To improve the overall quality of life, natural products have been utilized for many different purposes, including as industrial supplements, flavors or fragrances. Among natural products, carotenoids are one of the most common. Carotenoids represent a subfamily of terpenoids that usually contain 40 carbon atoms with 8 isoprene molecules and have yellow, orange, and red hues attributable to an extensively conjugated polyene chain. This characteristic chemical structure is responsible for their physiological function as an antioxidant and provitamin A nutrient, as well as its ability to protect from UV radiation. In fungi, for example, carotenoids play a key role as potent photoprotective and stress-protecting agents (Avalos and Limón, 2015). Moreover, carotenoids are reported to play a vital role in gene regulation and apoptosis in mammalian cells (Tapiero et al., 2004; Walsh and Tang, 2018), which are metabolic processes essential for maintaining the health of animals.
Owing to these biological activities, carotenoids have been widely used as additives in food and feed, nutraceuticals, and cosmetics (Saini and Keum, 2019). The global market for carotenoids is reported to reached $1.5 billion in 2018, and will increase to an estimated value of $2.0 billion in 2022 (McWilliams, 2018). Although the demand for carotenoids is rapidly increasing, their supply is limited due to inefficient production methods (Ambati et al., 2014; Saini and Keum, 2018). Many of the structurally more complex carotenoids cannot be chemically synthesized via economically viable methods (Zhang et al., 2008). Most carotenoids are extracted from natural sources with low yields due to complicated multi-step processes and low concentrations in the raw materials, which can also be further affected by unfavorable environmental conditions (Adadi et al., 2018; Luo et al., 2015). Therefore, microbial production of carotenoids provides an attractive alternative, as it does not require direct land use or the time invested to grow crops for harvest and extraction. Recent advances in genetic and metabolic engineering of microorganisms make it increasingly possible to modify, design and optimize host microorganisms to be advanced microbial cell factories (Jia and Schwille, 2019; Ma et al., 2019b; Simon et al., 2019). Combining different strategies and advanced metabolic engineering tools will enable the microbial production of carotenoids to achieve high yields and meet the increasing carotenoid demand. Recently, a systematic approach was developed to engineer secondary metabolism described as ‘multivariate modular metabolic engineering’ (MMME) (Ajikumar et al., 2010). Reduced regulatory complexities are achieved by grouping multiple genes into modules as a method to assess and eliminate regulatory and pathway bottlenecks (Yadav et al., 2012; Zhang et al., 2018b). Using a MMME approach, the microbial biosynthesis of carotenoids can be divided into four distinct modules (Fig. 1). Increasing flux into these modules and balancing the metabolic flux among these modules become critical in order to obtain higher carotenoid yields. The main scope of this review is to describe key strategies and current advances in engineering these modules for maximizing carotenoid production.
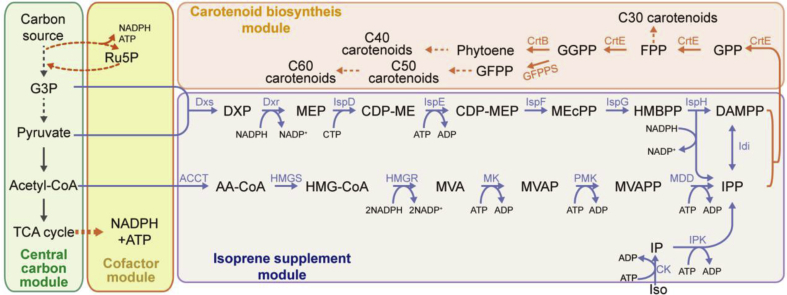
Fig. 1.
Scheme of carotenoid biosynthesis. The whole metabolic pathway divides into four modules, striating from a carbon source and leading to carotenoids. Enzymes are as follows. Isoprene supplement module: (1) MVA pathway: ACCT, Acetoacetyl-CoA thiolase; HMGS, HMG-CoA synthase; HMGR, HMG-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; MDD, mevalonate diphosphate decarboxylase; Idi: isopentenyl pyrophosphate isomerase. (2) MEP pathway: Dxs, 1-deoxy-D-xylulose 5-phosphate synthase; Dxr, 1-deoxy-D-xylulose 5-phosphate reductoisomerase; IspD, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; IspE, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; IspF, 4-diphosphocytidyl-2-Cmethyl-D-erythritol kinase; IspG, 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase; IspH, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase. (3) IUP: CK, choline kinase; IPK, isopentenyl monophosphate kinase. Carotenoid biosynthesis module: CrtE, geranylgeranyl pyrophosphate synthase; CrtB, 15-cis-phytoene synthase; GFPPS, geranylfarnesyl diphosphate synthase. Abbreviations can be found in the appendix. The dashed arrows indicate multiple steps.
2. Biosynthesis of carotenoids
To date, more than one thousand natural carotenoids have been found in plants, microalgae, fungi and bacteria (Yabuzaki, 2017). Carotenoids are derived from the universal isoprene (C5) precursors isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP). These can be synthesized through two different natural pathways, the mevalonate (MVA) pathway and the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway, as well as an artificial pathway, the isopentenol utilization pathway (IUP) (Fig. 1) (Chatzivasileiou et al., 2019). The MVA pathway is mainly present in eukaryotes and archaea, while the MEP pathway is typically found in most bacteria and plant plastids (Boronat and Rodríguez-Concepción, 2014). The substrates of the MVA and MEP pathways are acetyl-CoA, glyceraldehyde-3-phosphate (G3P) and pyruvate, all provided by the central carbon pathway (Wang et al., 2016; Zhao et al., 2013). In addition, microbial hosts typically use the central carbon pathway to supply critical cofactors (ATP and NADPH) for carotenoid biosynthesis (Alper et al., 2005a). To produce one molecule of IPP, 1.5 molecules of glucose and three molecules of ATP are consumed and four molecules of NADPH are produced using the MVA pathway, while one molecule of glucose, three molecules of ATP and two molecules of NADPH are required using the MEP pathway (Li et al., 2019).
In the MVA pathway, two molecules of acetyl-CoA are condensed to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), followed by a reduction step that generates mevalonate and three phosphorylation steps and one decarboxylation step to produce IPP (Fig. 1) (Moise et al., 2014). The biosynthesis of IPP in the MEP pathway begins with the condensation of G3P and pyruvate, followed by a reductive isomerization reaction, a subsequent coupling with cytidine triphosphate (CTP), and finally a phosphorylation and cyclization reaction (Fig. 1) (Moise et al., 2014).
In the IUP, the substrate is the isopentenol isomer isoprenol or/and prenol, which can be directly supplemented in production media (Chatzivasileiou et al., 2019; Lund et al., 2019) or introduced via intracellular biosynthesis (Clomburg et al., 2019). One molecule of isoprenol/prenol and two molecules of ATP are consumed to produce one molecule of IPP/DMAPP (Chatzivasileiou et al., 2019). The IUP only contains two steps through sequential phosphorylation of isoprenol/prenol, compared with six steps in the MVA pathway and seven steps in the MEP pathway (Fig. 1).
Following the generation of the isoprene precursors, one molecule of DMAPP and three molecules of IPP are condensed into one geranylgeranyl diphosphate (GGPP) in three prenyl-transferase reactions (Fig. 1). Two molecules of GGPP can be condensed to form phytoene (C40). Four double bonds are introduced into phytoene to form lycopene, which then becomes a precursor to a variety of carotenoids, e.g., lycopene, β-carotene, zeaxanthin and astaxanthin (Fig. 2). During the condensation of DMAPP and IPPs, variation of the prenyltransferases of the condensation reactions can form farnesyl diphosphate (FPP, C15) and geranylfarnesyl diphosphate (GFPP, C25), which serve as the precursors of minor C30 and C50 carotenoids (Wang et al., 2019). The C50 carotenoids can be elongated with isoprene units by lycopene C5-elongases and C50-cyclases to generate unique C60 carotenoids (Li et al., 2019a).
Fig. 2.
Derivatives of lycopene. Enzymes are as follows: LcbB, lycopene bifunctional β-cyclase; Bhy, β-carotene hydroxylase; CrtW, β-carotene 4-ketolase; CCD1, carotenoid cleavage dioxygenase 1; Blh, 15, 15 cleavage dioxygenase; Rdh, retinol dehydrogenase; LcmE, lycopene monofunctional ε-cyclase; Cehy, α-hydroxylase; LcbE, lycopene bifunctional ε-cyclase. The dashed arrows indicate multiple steps.
3. Modular engineering for carotenoids production
Potential commercial interests in sustainable industrial production of carotenoids have led to research breakthroughs that increase carotenoid titer via metabolic engineering. A summary of these strategies can be found in Table 1. The basic principle of metabolic engineering is to maximize carbon flux from substrates to the target compound while minimizing flux to unnecessary byproducts. The carotenoid biosynthesis pathways, in particular, can be divided into four metabolism modules: 1) central carbon metabolism, 2) cofactor metabolism, 3) isoprene supplement metabolism, and 4) carotenoid biosynthesis (Fig. 1). Increasing and balancing carbon flux into and among these modules is important for carotenoid overproduction.
Table 1.
Carotenoid production in microorganisms using various metabolic engineering strategies.
| Major carotenoid | Host microorganism | Fermentation conditions | Yield (titer or/and content) | Metabolic engineering approach | Reference |
|---|---|---|---|---|---|
| Lycopene | Escherichia coli | Shake Flask | 448 mg/g | Regulation of lycopene synthesis pathway | Coussement et al. (2016) |
| Lycopene | Escherichia coli | Batch | 220 mg/L | Introduction of IUP | Chatzivasileiou et al. (2019) |
| Lycopene | Saccharomyces cerevisiae | Fed-batch | 2300 mg/L | Modular enzyme assembly | Kang et al. (2019) |
| β-carotene | Escherichia coli | Shake Flask | 44.2 mg/g | Increasing membrane synthesis | Wu et al. (2017) |
| β-carotene | Escherichia coli | Fed-batch | 2100 mg/L | Engineering central metabolic modules | Zhao et al. (2013) |
| β-carotene | Saccharomyces cerevisiae | Fed-batch | 2370 mg/L (73.3 mg/g) | Combined modular engineering | Ma et al. (2019a) |
| β-carotene | Yarrowia lipolytica | Fed-batch | 4000 mg/L | Iterative integration of multiple-copy pathway genes | Gao et al. (2017) |
| β-carotene | Yarrowia lipolytica | Fed-batch | 6500 mg/L (90 mg/g) | Optimization of promoter-gene pairs of crt | Larroude et al. (2018) |
| Astaxanthin | Corynebacterium glutamicum | Shake Flask | 1.7 mg/g | Screening different β-carotene hydroxylases | Henke et al. (2016) |
| Astaxanthin | Escherichia coli | Fed-batch | 385.0 mg/L (7.0 mg/g) | Membrane fused expression of β-carotene hydroxylase | Park et al. (2018a) |
| Astaxanthin | Escherichia coli | Fed-batch | 320 mg/L | Modular engineering | Zhang et al. (2018b) |
3.1. Engineering the carotenoid biosynthesis module
The carotenoids biosynthesis module is directly related to carotenoid yield (Das et al., 2007; Wang et al., 2019). Inefficient carotenoid biosynthesis leads to a cellular accumulation of IPP/DMAPP that can be toxic to cells (Sung et al., 2007) and a lower yield of carotenoids. To date, many strategies have been employed to engineer the carotenoid biosynthesis module, including selection of novel enzymes, engineering key enzymes, localized expression of key enzymes, optimization of gene expression and increasing carotenoid storage in producing cells (Fig. 3).
Fig. 3.
Strategies for engineering the carotenoid biosynthesis module. Astaxanthin biosynthesis route is used as an example. Engineering enzymes to have higher activity, regulating their expression level, increasing carotenoid storage and localizing expression of enzymes to membranes can all increase carotenoid production. Enzymes are as follows: CrtE, geranylgeranyl pyrophosphate synthase; CrtB: 15-cis-phytoene synthase; CrtI: phytoene desaturase; LcbB: lycopene bifunctional β-cyclase; Bhy: β-carotene hydroxylase; CrtW: β-carotene 4-ketolase. Abbreviations can be found in the appendix. The dashed arrows indicate multiple steps.
3.1.1. Screening and engineering enzymes with high activity
Common cell factory hosts, e.g., Escherichia coli and Saccharomyces cerevisiae, do not harbor all the genes for carotenoid biosynthesis. Therefore, heterologous genes are required to construct a carotenoid biosynthesis pathway. Heterologous proteins have different expression levels in different hosts (Demain and Vaishnav, 2009) so gene sources for carotenoid biosynthesis need to be well chosen. In E. coli, for example, changing the crtE/B/I genes from Pantoea ananatis to Pantoea agglomerans nearly doubles lycopene production (Yoon et al., 2007). In S. cerevisiae, the crtE/B/I genes from diverse species were screened and crtE from Taxus x media, crtB from P. agglomerans, and crtI from Blakeslea trispora resulted in the highest amounts of lycopene (Chen et al., 2016). Besides plants, marine bacteria are another natural source for both common and uncommon valuable carotenoids (Torregrosacrespo et al., 2018). β-carotene hydroxylase from the marine bacterium Fulvimarina pelagi allows a better astaxanthin production than that from P. ananatis in Corynebacterium glutamicum (Henke et al., 2016). In addition, some novel bifunctional enzymes have been screened for carotenoid biosynthesis. Single genes encoding for a protein with both phytoene synthase and lycopene cyclase activities from several species (Guo et al., 2014; Lodato et al., 2003; Velayos et al., 2000; Wang et al., 2017) have been functionally analyzed and applied to carotenoid biosynthesis. These fusion enzymes can remove competition with precursors (Camagna et al., 2019) and aid in the elimination of unwanted side reactions (Albertsen et al., 2011), leading towards high yields of target products. Artificial fused proteins have similar benefits. An engineered carotenoid cleavage dioxygenase 1 (CCD1) from Osmanthus fragans (OfCCD1) was fused with lycopene ε-cyclase, facilitating substrate channeling and increasing product production by fifty percent (Chen et al., 2019).
Identified and screened wild-type enzymes usually have low activity, low affinity, and insufficient expression levels in heterologous hosts. Multiple approaches employing protein engineering and expression level regulation have been developed to improve carotenoid production. Due to the inherent color of carotenoids, simple, color-based high-throughput screening methods have been developed for isolating positive mutants. The following directed evolution studies all utilized color-based screening methods to improve enzyme function and activity. At the beginning of carotenoid biosynthesis, DMAPP to GGPP conversion by GGPP synthase was found to be a rate-limiting step (Kang et al., 2005). In order to increase flux through this pathway, directed evolution of GGPP synthase (Jakočiūnas et al., 2018; Leonard et al., 2010; Wang et al., 2000) has been applied to increase carotenoid production. Similarly, directed evolution of a bifunctional enzyme containing phytoene synthase and lycopene cyclase (Xie et al., 2015a), as well as the single enzyme β-carotene ketolase (Zhou et al., 2017b, 2019), also increased carotenoid production. In addition, a mutant FPP synthase that altered its chain-length specificity (Lee et al., 2005) can also increase GGPP synthesis and supply needs for downstream products. Besides random mutations, rational design has been developed to generate mutants of CCD1 from Petunia hybrid (PhCCD1) (Werner et al., 2019) and the aforementioned OfCCD1 (Chen et al., 2019), which increased production of derivatives from β-carotene and ε-carotene by 300% and 200%, respectively.
In addition to single/multiple point mutations of key enzymes, several researchers have explored engineering the N/C-terminal for enhancing carotenoid biosynthesis. Since enzyme activity and solubility are correlated for a variety of proteins (Zhou et al., 2012a), N/C-terminal fusion tags that significantly affect protein solubility were tested for improved overall activity (Esposito and Chatterjee, 2006). As an example, the solubility of enzymes involved in the synthesis of the terpene taxadiene was optimized through a Cas9-based toolkit (Reider Apel et al., 2016), including 10 fusion tags, which increased taxadiene production by 15-fold. This toolkit could be further explored for improving carotenoid production. In another example, a truncation of 50-residues in the N-terminal of lycopene ε-cyclase from Lactuca sativa, which is thought to enhance its solubility and activity, increased ε-lycopene production by 122% (Zhang et al., 2018a). In addition to protein engineering for high solubility, a machine learning-based regression predictive model has been developed to predict lycopene β-cyclase solubility based on the known solubility of proteins in the solubility dataset (Han et al., 2019). Using this method, enzymes with higher solubility can be selected from the protein database (PDB) and analyzed, which can then be applied in carotenoid overproduction. Fusion tags can also affect protein stabilization and improve production (Kazufumi et al., 2011). A fusion tag from thioredoxin (trxA) was attached to both the N- and C- terminals of a β-carotene ketolase from Chlamydomonas reinhardtii to allow stable expression, which increased astaxanthin production by 60% (Park et al., 2018a).
After carotenoids are synthesized, they are expected to bind to lipid membranes due to their hydrophobicity (Gruszecki, 1999). Localizing enzymes to the membrane can offer a greater chance for enzymes to interact with the hydrophobic substrate, improving the overall conversion efficiency. Co-localizing β-carotene ketolase to the E. coli membrane using the signal peptide of outer membrane protein (OmpF) resulted in nearly 60% higher astaxanthin production (Park et al., 2018a). Similarly, localizing PhCCD1 to the membrane through the signal peptide of rat lymphocyte cell-specific protein tyrosine kinase (Lck) increased ionone production by 170% (Werner et al., 2019). In another example, β-carotene ketolase and hydroxylase fused to glycerol channel protein (GlpF) were localized to the membrane, increasing astaxanthin production by 215% (Ye et al., 2018).
3.1.2. Regulating gene expression level
Other than protein engineering and localized expression, increasing efficiency in a given pathway is related to enzyme expression levels, which is considered to increase the metabolic flux towards target production. Overexpression of heterologous or endogenous proteins is related to a variety of aspects, including promoter engineering, ribosome binding site (RBS) engineering, gene copy number and gene regulation (Huo et al., 2019; Li et al., 2015a; Peti and Page, 2007). Selecting and mutating promoters from various heterologous sources has been applied to improve gene expression in carotenoid biosynthesis modules in both bacteria (Qiang et al., 2019) and yeast (Gao et al., 2017; Hara et al., 2014; Larroude et al., 2018) for carotenoid overproduction. In addition, engineering the RBS of key enzymes in carotenoid synthesis can also increase carotenoid production in E. coli (Jin et al., 2015; Wang et al., 2009a; Ye et al., 2010).
Another strategy to improve carotenoid production is to increase gene copy numbers of key enzymes (Gao et al., 2017; Henke et al., 2016; Larroude et al., 2018; Shi et al., 2019) since gene copy number can be directly related to its protein expression level (Huo et al., 2019). Due to the genetic instability of plasmids, an approach named “chemically induced chromosomal evolution” was applied to control gene copy integration into the chromosome by triclosan induction (Chen et al., 2013a; Tyo et al., 2009) for carotenoid overproduction. This method uses RecA to mediate DNA crossover between the leading and trailing homologous regions for high copy genome integration. In addition, the order of genes in the operon affected the yield of carotenoids since genes farther away from a promoter in an operon have lower levels of expression (Colloms et al., 2013; Xu et al., 2016b). Splitting up this large operon into smaller operons consisting of two or three genes controlled by a strong promoter can eliminate this issue and improve overall flux (Ye et al., 2016).
The promoter controlling the carotenoid biosynthesis module can be repressed by various transcription factors and repressors, decreasing overall carotenoids production. Knocking out the crtR repressor, for example, deregulates the crt operon, leading to a several-fold increase of lycopene and β-carotene in C. glutamicum (Henke et al., 2017). Increasing cofactor expression can also improve enzyme activity. Oxygen is a cofactor of β-carotene ketolase and hydroxylase, whose activities can be increased by introducing the hemoglobin gene from Methylomonas sp. 16a, improving astaxanthin conversion by 60% in E. coli (Tao et al., 2006).
3.1.3. Engineering carotenoid storage
Carotenoid yield can be limited by the intracellular capacity of the host organism to store these molecules (Gruszecki, 1999) and the fact that high intracellular concentrations of carotenoids can be toxic to the host (Sung et al., 2007). Increasing the ability of a host organism to store carotenoids while limiting toxic effects is therefore a major challenge. The yeast Yarrowia lipolytica, which can produce lipid bodies and store larger amounts of carotenoids (Mlíčková et al., 2004), is an excellent host for carotenoid overproduction because of its increased storage capacity and increasing the size of its lipid bodies can further enhance lycopene production (Matthäus et al., 2014). Triacylglycerol lipid droplets were also introduced into a lycopene production strain of S. cerevisiae and increased lycopene production by 25% (Ma et al., 2019a).
Natural carotenoids are considered to be mainly stored in the membranes of plants and bacteria (Gruszecki, 1999). Increasing the amount of membrane structures and expanding the membrane surface area can increase carotenoid production in E. coli (Wu et al., 2017, 2018). Overexpression of a glycerol-3-phosphate O-acyltransferase and a 1-acyl-sn-glycerol-3-phosphate acyltransferase that can increase membrane synthesis improved β-carotene production by 43%, while a monoglucosyldiacylglycerol synthase that can increase the total membrane area improved lycopene production by 37.5% (Wu et al., 2017). Though increasing membrane synthesis can increase the intracellular storage of carotenoids in bacteria (Gruszecki, 1999), accumulation of carotenoids can disturb the membrane structure, which could have a toxic effect on cells. To address this problem, a novel artificial membrane vesicle transport system was constructed in E. coli to efficiently secrete hydrophobic molecules, leading to a 61% increase of β-carotene (Wu et al., 2019a). In this system, knockouts of tolA and nlpI, which encodes lipoproteins, was thought to enhance the outer membrane vehicles formation and thus increased OMV-mediated β-carotene excretion. This strategy could be applied to other hosts to improve total membrane storage of carotenoids. To relieve carotenoid toxicity, membrane flexibility was increased by overexpressing the gene encoding stearoyl-CoA 9-desaturase, leading to an increase of 30% lycopene production in S. cerevisiae (Hong et al., 2019). In addition, various membrane components were modified (e.g. ergosterol) by overexpressing the gene ino2, which encodes for a transcription factor involved in stress response and biosynthesis of phospholipids and sterol, enhancing lycopene production in S. cerevisiae by nearly 10% (Chen et al., 2016).
3.2. Engineering the isoprene supplement module
To obtain a high yield of microbial carotenoids, IPP is considered to be a key precursor (Park et al., 2018b; Vachali et al., 2012). As introduced earlier, natural IPP is synthesized from the MVA and MEP pathways. Overexpression of the whole MEP or/and MVA pathway does not appear to increase the IPP precursor supply due to the accumulation of toxic intermediates and feedback inhibition (Li et al., 2019). Several methods, including optimized expression of key enzymes and regulation of the pathway has been applied to improve engineering the MVA and MEP pathways (Fig. 4).
Fig. 4.
Strategies for engineering central carbon, cofactor and isoprene supplement modules. Enzymes are as follows. (1) Central carbon module: GapA, glyceraldehyde 3-phosphate dehydrogenase; Pps, phosphoenolpyruvate synthase; Por, pyruvate ferredoxin oxidoreductase; Ald6p, aldehyde dehydrogenase; Adh2, alcohol dehydrogenase; GltA, citrate synthase; AcnAB, aconitate hydratase; Icd, aconitate hydratase; SucAB, α-ketoglutarate dehydrogenase; SucCD, succinyl-CoA synthetase; SdhABCD, succinate dehydrogenase; FumAC, fumarate hydratase; Mdh, malate dehydrogenase. (2) Cofactor module: Zwf1, glucose 6-phosphate dehydrogenase; Pgl, 6-phosphogluconolactonase; Gnd, 6-phosphogluconate dehydrogenase; Rpe, ribulose 5-phosphate 3-epimerase; Rpi, ribose-5-phosphate isomerase; TktA, transketolase subunit A; TktB, transketolase subunit B; TalB, transaldolase; Nuo, NADH-quinone oxidoreductase; CYD, cytochrome bd-I oxidase; Cyo, cytochrome bo oxidase; Atp, ATP synthase. (3) Isoprene supplement module: MVA pathway: ACCT, Acetoacetyl-CoA thiolase; HMGS, HMG-CoA synthase; HMGR, HMG-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; MDD, mevalonate diphosphate decarboxylase; Idi: isopentenyl pyrophosphate isomerase. MEP pathway: Dxs, 1-deoxy-D-xylulose 5-phosphate synthase; Dxr, 1-deoxy-D-xylulose 5-phosphate reductoisomerase; IspD, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; IspE, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase; IspF, 4-diphosphocytidyl-2-Cmethyl-D-erythritol kinase; IspG, 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase; IspH, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase. IUP: CK, choline kinase; IPK, isopentenyl monophosphate kinase. RibB, 3,4-dihydroxy 2-butanone 4-phosphate synthase. Abbreviations can be found in the appendix. The dashed circles around the proteins indicate that these enzymes are the rate-limiting step in carotenoid biosynthesis. The proteins in green color indicate that the genes encoding these proteins are overexpressing. The dashed arrows indicate multiple steps. The red forks indicate derepression. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2.1. Engineering the MVA pathway to maximize IPP precursor supply
In the MVA pathway, the intermediate hydroxymethylglutaryl-CoA (HMG-CoA) can be toxic to cells (Pitera et al., 2007) and thus the low expression level of HMG-CoA reductase is one of the rate-limiting steps in the MVA pathway. This can be solved by HMG-CoA overexpression (Wang and Keasling, 2002; Woo et al., 2013) or replacement with a more active one from Staphylococcus aureus (Tsuruta et al., 2009). The expression of mevalonate kinase (MK) can be strongly feedback inhibited by GPP and FPP (Dorsey and Porter, 1968; Hinson et al., 1997). Directed evolution (Anthony et al., 2009) and overexpression (Chen et al., 2018b) of MK has been applied to increase yields of IPP downstream products. In addition, several feedback-resistant MKs have been identified and characterized (Kazieva et al., 2017; Primak et al., 2011), which can be expressed in other microorganisms for carotenoid overproduction. Similarly, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR1) can be feedback inhibited by FPP, which limits the carotenoid yield. This problem can be alleviated by overexpressing an N-terminal truncated HMGR1 (tHMGR1) (Donald et al., 1997; Polakowski et al., 1998). Overexpression of key enzymes is not always an ideal solution for supplementing IPP, however, as a balanced expression system can improve carotenoid production and eliminate bottlenecks within the pathway. Overexpression of the first three genes involved in the upper MVA pathway can lead to accumulation of HMG-CoA and result in poor cell growth and low MVA production, but modulating expression of these genes by selecting promoters and controlling copy number increased MVA production by 700% (Pitera et al., 2007). Moreover, optimized expression of the upper MVA pathway by tunable intergenic regions (TIGRs) (Pfleger et al., 2006), which uses oligonucleotides to control the stability of mRNA fragments, or scaffold assembly (Dueber et al., 2009), which assembles enzymes in a more appropriate ratio, can also increase the IPP precursor supply. Tunable expression of the lower MVA pathway led to an increase of zeaxanthin production (Shen et al., 2016). For tunable expression of the whole MVA, galactose regulated promoters were attached to the genes of the MVA pathway, which can decrease generation of toxic intermediates and increase IPP supply (Westfall et al., 2012). In addition, deletion of ROX1, a transcriptional regulator that inhibits genes involved in the MVA pathway (Özaydın et al., 2013), can also enhance the IPP supply (Bröker et al., 2018). All the above modifications focus on cytoplasm engineering, however, mitochondria are another compartment that produces large amounts of acetyl-CoA, the precursor for the MVA pathway. Localized expression of the entire MVA pathway in mitochondria increases the supply of acetyl-CoA and significantly improved the yield of the IPP downstream product isoprene in S. cerevisiae (Lv et al., 2016b). These strategies to improve flux through the MVA pathway can be applied for enhancement of carotenoid production.
3.2.2. Engineering the MEP pathway to maximize IPP precursor supply
In the MEP pathway, 1-deoxyxylulose-5-phosphate synthase (Dxs) and 1-deoxyxylulose-5-phosphate reductoisomerase (Dxr), which catalyze the first two steps, are generally considered as the key rate-limiting enzymes. Overexpression of dxs has been widely applied to increase carotenoid production in bacteria (Heider et al., 2014; Vadali et al., 2005; Zhou et al., 2013b). The product catalyzed by Dxs, DXP, is also the substrate for thiamine and pyridoxal synthesis in E. coli (Julliard and Douce, 1991), which can reduce the flux into the MEP pathway. Besides down-regulation of these pathways, DXP can also be synthesized from ribulose 5-phosphate by introducing a shunt pathway (Kirby et al., 2015), which can be applied to increase the total DXP concentration. During overexpression of genes in the MEP pathway, methylerythritol cyclodiphosphate (MEcPP) was found to secret from the cells, which can be reduced by overexpressing 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase (IspG) that used MEcPP as a substrate (Zhou et al., 2012b). Moreover, overexpression of the iron-sulfur cluster operon, which supplies the cofactor for IspG and 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (IspH) (Zou et al., 2013), can increase the IPP precursor supply.
An additional challenge to increase flux through this pathway is to eliminate feedback inhibition. IPP and DMAPP inhibit Dxs (Aparajita et al., 2013) and FPP inhibits IspF (Chang et al., 2013), which can limit downstream bacterial carotenoid production. Introducing the heterologous MVA pathway into bacteria, which can evade endogenous feedback regulation, has been developed to offset feedback inhibition in the MEP pathway and improve the overall concentration of IPP (Heider et al., 2014; Yang and Guo, 2014; Zurbriggen et al., 2012). In addition, introducing both pathways simultaneously provides a synergistic effect. The MVA pathway provides a net gain of NADPH, which provides reducing power for ATP formation, a highly required cofactor in the MEP pathway. This synergistic effect contributed to improving overall lycopene production in E. coli (Yang et al., 2016). To functionally introduce the MEP pathway into eukaryotes, the last two steps catalyzed by IspG and IspH were bottlenecks that needed to be alleviated (Carlsen et al., 2013; Partow et al., 2012). A number of orthologous genes encoding IspG and IspH, as well as the redox partners flavodoxin (Fld) and flavodoxin/ferredoxin NADP+-reductase (FNR) from different species were screened and characterized in S. cerevisiae (Kirby et al., 2016). ispG from Bacillus thuringiensis IBL 200, ispH from E. coli and fld from Bacillus subtilis subsp. subtilis str. 168 resulted in the highest amounts of flux through the MEP pathway for carotenoid biosynthesis in S. cerevisiae. More attempts are needed to explore the regulation of the MEP pathway and the relationship between the MEP and MVA pathways in eukaryotes.
3.2.3. Introducing the novel isopentenol utilization pathway to provide a new pathway for IPP precursor supply
As shown above, a lot of attempts have been applied to improve the efficiency of the MEP and MVA pathways, although these attempts still rely on the initial substrate for regulation. A novel IUP was constructed and produced nearly 100-fold more IPP than the MEP pathway within 5 mins (Chatzivasileiou et al., 2019). More than 95% of carbon from the target product taxadiene came from IUP compared to less than 5% from the MEP pathway, suggesting that IUP can be an efficient pathway for IPP supply in carotenoid biosynthesis. Moreover, IUP is thought to be non-regulated by any pathway intermediates (Ward et al., 2019), which can significantly increase flux into IPP synthesis. One major limitation to using isoprenoid alcohol as a feedstock is the fact that it is derived from chemical synthesis, which means that any downstream product must be marketed as non-natural. In order to circumvent this problem, an isoprenoid alcohol (IPA) pathway was constructed with novel enzymes that use glycerol as the carbon source and IPA as a natural intermediate (Clomburg et al., 2019). More attempts are needed, however, to optimize the natural supplement of isoprenoid alcohol due to the low efficiency of the IPA pathway (Clomburg et al., 2019).
3.2.4. Engineering the isomerization of IPP
After IPP generation, isopentenyl diphosphate isomerase (Idi), which catalyzes the isomerization of IPP to DMAPP, is a key rate-limiting enzyme for carotenoid biosynthesis (Berthelot et al., 2012). The function of Idi is limited by its low expression level, enzyme activity, short half-life, and weak substrate affinity (Ramos-Valdivia et al., 1997). Overexpression of idi from several species has been applied to increase carotenoid production (de Ruyck et al., 2011; Zhou et al., 2013a). Type 2 Idi (mainly exists in plants, some gram-positive eubacteria and archaea) tends to perform better than the type 1 (has a conserved cysteine in the NxxCxHP consensus sequence and a conserved glutamate in the ExE consensus sequence that encode for the active site and mainly exists in plants, mammals, some Gram-positive eubacteria and most Gram-negative eubacteria) for isoprenoid production in E. coli (Rad et al., 2012). In addition, directed evolution of idi from S. cerevisiae increased its activity and affinity, leading to a 1.8-fold increase of lycopene production in E. coli (Chen et al., 2018a).
3.3. Engineering the central carbon module
Acetyl-CoA, G3P, and pyruvate, the precursors of the MVA and MEP pathways, are also the substrates for many other pathways. The ‘push-pull’ strategies that limit their bypass are the most common approaches for improving the supply of these precursors for carotenoid synthesis (Fig. 4). In addition, increasing the flux from carbon sources and balancing the precursors are also important for carotenoid overproduction.
In the MVA pathway, three acetyl-CoAs are required for each isoprene unit. Acetyl-CoA is also the precursor to lactate, acetate, and ethanol so elimination of these byproducts can lead to an improvement of carotenoid production (Kim et al., 2016; Lian et al., 2014). In addition, reducing the acetyl-CoA consumption in the TCA cycle by a knock-out of the gene encoding for the subunit of 2-oxoglutarate dehydrogenase (SucA) increased more flux into the MVA pathway (Wang et al., 2016). In S. cerevisiae, the only cytosolic route to produce acetyl-CoA is from activation of acetate but the glycolytic flux trends toward ethanol formation during glucose cultivation (Pronk et al., 1996). Overexpression of cytosolic acetaldehyde dehydrogenase (Ald6p) and acetyl-CoA synthetase (Acs) from Salmonella enterica can pull acetyl-CoA from acetate (Ma et al., 2019a; Shiba et al., 2007) and overexpression of alcohol dehydrogenase (Adh2) can prevent ethanol oxidation by acetaldehyde (Chen et al., 2013b; Ma et al., 2019a). These strategies have been applied to redirect the carbon flux from byproducts to acetyl-CoA. In addition, to increase acetyl-CoA concentrations, redirecting the pentose phosphate (PP) pathway to supply acetyl-CoA (Meadows et al., 2016) and directly converting pyruvate to acetyl-CoA (Lian et al., 2014) has been used to enhance carotenoid production.
In the MEP pathway, G3P and pyruvate are both precursors and utilized in equal amounts. Limitations on either of these precursors are sufficient to reduce carotenoid biosynthesis. G3P is considered a major bottleneck, as G3P is mainly converted to pyruvate and the flux is biased to pyruvate. Redirecting the flux from pyruvate to G3P by downregulation of glyceraldehyde 3-phosphate dehydrogenase (GapA) (Jung et al., 2016) or overexpressing phosphoenolpyruvate (PEP) synthase (Pps) (Farmer and Liao, 2001) increased lycopene production in E. coli by 45% and 250%, respectively. In addition, regulated expression of pps that is controlled by acetyl-phosphate-inducible artificial Ntr regulons increased pyruvate utilization, leading to an increase of lycopene production (Farmer and Liao, 2000). The Entner-Doudoroff (ED) pathway, which produces equal amounts of G3P and pyruvate, can also be utilized and optimized for carotenoid overproduction (Li et al., 2015b; Liu et al., 2013, 2014).
Acetyl-CoA, G3P, and pyruvate are mainly derived from an initial carbon source, which is glucose in most cases (Borowitzka, 2010). Enhancing glucose uptake by replacing the PEP-dependent phosphotransferase system with the ATP-dependent galactose permease and glucose kinase system can increase flux into the MEP pathway (Zhang et al., 2015) and thus can be applied for carotenoid overproduction. Besides glucose, utilizing xylose as a carbon source by pathway engineering can increase flux from ethanol to acetyl-CoA and provide an additional substrate for carotenoid production (Henke et al., 2018; Kwak et al., 2017; Sun et al., 2019).
3.4. Engineering the cofactor module
NADPH and ATP, the cofactors of the MVA and MEP pathways, are critical to carotenoid biosynthesis. Sixteen molecules of NADPH, eight molecules of ATP and eight molecules of CTP are required for one molecule of β-carotene derived through the MEP pathway (Alper et al., 2005a). Two NADPH are required for HMGR in the MVA pathway (Fig. 4). NADPH mainly comes from the PP pathway and TCA cycle (Sauer et al., 2004). In order to regenerate and increase the amount of NADPH needed for carotenoid overproduction, multiple strategies have been used to engineer the PP pathway, including overexpressing glucose-6-phosphate dehydrogenase (Zwf1) (Zhao et al., 2015) or overexpressing transketolase I (TktA) and TransaldolaseB (talB) (Zhao et al., 2013). Engineering the TCA cycle by overexpression of malate dehydrogenase (Mdh) (Choi et al., 2010) or citrate synthase (GltA), α-ketoglutarate dehydrogenase (SucAB), and succinate dehydrogenase (SdhABCD) (Zhao et al., 2013) can also improve NAD(P)H supply and carotenoid production. In addition, activating NADPH production by optimized expression of a transcription factor Stb5 that can regulate genes in the pentose phosphate pathway increased lycopene production by 14% (Hong et al., 2019). Likewise, overexpression of a mitochondrial NADH kinase (Pos5) that converts NADH to NADPH increased both lycopene and β-carotene production (Zhao et al., 2015). To balance NADPH, limiting off-target consumption, such as knocking out the gene encoding glutamate dehydrogenase (GdhA) (Alper et al., 2005a; Choi et al., 2010) or the non-essential NADPH-dependent aldehyde reductase YjgB (Zhou et al., 2017a) to increase NAPDH accumulation can also be applied to increase carotenoid production.
ATP is synthesized through ATP synthase and the electron transfer chain in E. coli. Increasing the ATP supplement by modulating expression of NADH-quinone oxidoreductase, cytochrome bd-I oxidase, cytochrome bo oxidase and ATP synthase enhanced β-carotene production by 120% (Zhao et al., 2013). Furthermore, several novel synthetic cofactor systems, such as a rewired ED pathway that can increase NADPH and/or ATP supplements (Korman et al., 2017; Ng et al., 2015) are potential powerful tools for carotenoid overproduction.
3.5. Multi-module engineering for flux balance
Focusing on engineering one module can increase accumulation of the precursors required for subsequent modules, leading to potential toxicity (e.g. IPP and DMAPP (George et al., 2018)) and suboptimal production, resulting in unwanted bottlenecks. Multi-module engineering can solve the bottlenecks between adjacent modules and drive the flux into carotenoid biosynthesis. Combining the engineering of the MEP pathway, ATP and NADPH supply, and β-carotene biosynthesis pathway in E. coli led to 2100 mg/L β-carotene in a bioreactor (Zhao et al., 2013). Step-by-step engineering of the MVA pathway, β-carotene biosynthesis pathway, NAPDH supply, and carotenoid storage in S. cerevisiae led to an accumulation of 2370 mg/L β-carotene in a bioreactor (Ma et al., 2019a). In addition, different modules can be spatially segregated in two different microbes, which allows for taking advantage of different cellular machinery that is optimized for a specific module and avoiding overburdening one host cell. For example, co-culture of an engineered E. coli, which harbors the taxadiene biosynthesis module and has a faster growth rate than yeast, with an engineered S. cerevisiae that harbors the taxane biosynthesis module and has an efficient protein expression system, leads to complex taxane overproduction (Zhou et al., 2015). These experiments can provide novel insights into complex carotenoid biosynthesis.
Though these combined modules can easily obtain high yields of carotenoids, the flux balance between different modules is still difficult to probe. Optimized expression level of modules, known as MMME, is a powerful strategy to balance different modules. The expression level of the MEP pathway and taxane biosynthesis pathway were modulated by promoter engineering and optimizing gene copy number, leading to taxane overproduction (Ajikumar et al., 2010). To better optimize module expression, experimental design-aided systematic pathway optimization (EDASPO) was developed, which can decrease the time cost and the overall workload (Zhang et al., 2015). Using this method, experimental data were analyzed using a linear regression model and allowed researchers to screen promoters that would optimize module efficiency. Though promoter screening can enhance the overall module expression level, individual gene expression levels within the module need to be adjusted as well to reduce accumulation of intermediates. Therefore, a multidimensional heuristic process was developed to coordinate intra-module activities by RBS screening and enzyme variants (Zhang et al., 2018b). This method regulated transcription as well as translation and enzyme activity of fifteen essential genes that were partitioned into four modules, leading to 320 mg/L astaxanthin production in E. coli. To improve modular pathway engineering, statistical-based modeling to predict optimal gene expression levels is another important tool (Xu et al., 2013, 2016a). In addition, regulation of the key enzymes can also balance the flux between different modules. Directed co-evolution of idi, dxs and dxr, which balanced the expression levels between the isoprene supplement and carotenoid biosynthesis modules (Fig. 1), increased lycopene production by 80% in E. coli (Lv et al., 2016a). Linking Idi and CrtE, which are the key enzymes in the isoprene supplement and carotenoid biosynthesis modules (Fig. 1), by short peptide tag assembly leads to a 58% increase of lycopene production in S. cerevisiae (Kang et al., 2019). By linking these enzymes from different modules together, it is hypothesized to improve conversion and reduce the accumulation of intermediates that cause pathway bottlenecks.
Overproduction of carotenoids, which are secondary metabolites, can increase the metabolic burden of the host and thus decrease cell growth, which can be alleviated by dynamic control of modules to separate cell processes into a growth phase that achieve a high cell density and a production phase that focuses on carotenoid overproduction. An inducer/repressor-free sequential control strategy that repressed carotenoid biosynthesis during the growth phase through the use of a glucose-repression promoter was used to obtain a high cell density. After consumption of the glucose during the growth phase, carotenoid synthesis was activated and carotenoid overproduction could be achieved without sacrificing cell density, leading to 1156 mg/L β-carotene in a bioreactor (Xie et al., 2015b). Moreover, a quorum-sensing system were developed in B. subtilis that can control transcription of target genes based on cell density (Cui et al., 2019). This system was utilized to maximize production of menaquinone-7 by controlling flux through the central carbon module, IPP supplement module and essential byproduct pathway during different phases of fermentation. This system could be used to regulate similar modules in other organisms for carotenoid overproduction. In addition, rewiring gene expression with feedback genetic circuits and microbial interactions to maintain metabolic homeostasis and balance the cell growth fitness and productivity (Lv et al., 2019; Xu, 2018) can aid in improving carotenoid production by limiting carotenoid synthesis until after the growth phase is complete.
In addition, omics analysis is a useful tool to better understand the entire cell performance and can further improve carotenoid production. Comparative proteomic analysis of a zeaxanthin overproduction strain (Shen et al., 2016) and transcriptomic analysis of a lycopene strain using fructose as carbon source (Du et al., 2016) identified regulated pathways and genes that can be further enhanced for carotenoid production. From the comparative proteomic analysis (Shen et al., 2016), a correlation of the precursor pool, cofactor availability, oxidative stress response, membrane storage capacity, and cell morphology with increased zeaxanthin production was observed. From the transcriptomic analysis (Du et al., 2016), 384 differentially expressed genes involved in pyruvate catabolism, tricarboxylic acid cycle, and oxidative phosphorylation were identified and are thought to provide more precursors, cofactors and energy needed for growth and lycopene production. For pathway regulation, transcription factors not only regulate expression of specific genes, but also entire pathways, making them important targets for intramodular engineering. For example, overexpressing sigma factors sigA (Taniguchi et al., 2017), an important cofactor in the MEP pathway, and rpoD (Jin and Stephanopoulos, 2007), a cofactor involved in altering cellular oxidative status, increases carotenoid production in C. glutamicum and E. coli, respectively.
In addition to modular engineering, computational or random systemic engineering have been applied to identify gene targets that would lead to a global phenotype optimum. A genome-wide stoichiometric flux balance analysis, which calculates metabolic fluxes by flux balance analysis and identifies potential knockout genes by minimization of metabolic adjustment (MOMA), was developed for discovering putative genes impacting network properties and cellular phenotype (Alper et al., 2005a; Asadollahi et al., 2009). Using this method, knockouts of three simulated genes increased lycopene production by 40% (Alper et al., 2005a). To identify the potential amplification genes, genomic library screening by systematic and combinatorial approaches (Jin and Stephanopoulos, 2007) and flux scanning based on enforced objective flux (FSEOF) (Choi et al., 2010) were developed. Using FSEOF, potential genes are selected based on metabolic flux scanning, which selects fluxes toward product formation. Eight genes were identified to amplify for lycopene overproduction by FSEOF, leading to an increase of 523% (Choi et al., 2010).
Due to the many unknown kinetic and regulatory factors that are unaccounted for in stoichiometric models and cannot be considered by computational simulation, random targeting and screening is also applied for carotenoid overproduction. Targets were identified by colorimetric screening of a shot-gun library (Kang et al., 2005), a global transposon library search (Alper et al., 2005b), and modeling and transposon mutagenesis (Alper and Stephanopoulos, 2008) for improving lycopene production. In addition, mutagenesis by atmospheric and room temperature plasma (ARTP) has been employed to generate a mutagenesis library in S. cerevisiae, one of which had a 83% increase in astaxanthin yield (Jin et al., 2018). To generate a mutagenesis library of programming multiple target genes, multiplex automated genome engineering (MAGE) based on the RecT recombinase was developed in E. coli, one of which had a nearly 450% increase in lycopene yield (Wang et al., 2009b).
4. Conclusion and outlook
Metabolic engineering techniques have greatly improved carotenoid overproduction in microorganisms. Novel enzyme and target gene screening, protein engineering, and regulation tools have been successfully applied in the central carbon metabolism module, cofactor metabolism module, isoprene supplement metabolism module and carotenoid biosynthesis module for improved carotenoid production. Few engineered microbial carotenoid strains, however, are widely applied in industry and more efforts are needed for microbial carotenoid overproduction. First, the genetic stability of microbial strains limits their application in industry. Only a few bioprocesses focus on chromosomal genes and most still use plasmids for gene regulation, which are less stable, requires antibiotics and bear a higher metabolic burden (Furubayashi et al., 2015). Several strategies have been employed to solve these problems, such as novel marker-free genetic integration tools based on the CRISPR-Cas system (Klompe et al., 2019; Liu et al., 2019), stable gene expression systems based on an incoherent feedforward loop (iFFL) (Segall-Shapiro et al., 2018) and antibiotic-free plasmids based on Stable and TunAble PLasmid (STAPL) system that encode for translation initiation factors required by a corresponding auxotroph (Kang et al., 2018). Second, most enzymes of carotenoid biosynthesis are from plants and have a low catalytic efficiency in microorganisms. Novel enzyme screening (Han et al., 2019) and protein evolution (Wu et al., 2019b), assisted with machine learning, can be applied to obtain efficient enzymes. Moreover, unnatural base pairs (Zhang et al., 2017) and unnatural amino acids (Ravikumar et al., 2015) can make protein evolution more diverse. Third, though several omics analyses have been done in carotenoid overproduction strains, the relationship between pathway regulation and metabolite consumption must be better understood. Coupling multi-omics analysis, including transcriptomics, proteomics and metabolomics can lead to a deeper understanding of carotenoid biosynthesis, which is beneficial for bottleneck analysis and carotenoid overproduction. Fourth, carotenoids are secondary metabolites, that when overproduced, can be a burden to the overall cell metabolism. The IUP that used isoprenol as a secondary substrate to supply IPP, which does not have to compete with the rest of the cell for carbon flux (Chatzivasileiou et al., 2019), can relieve this metabolic burden. Due to the toxicity of isoprenol, improvement of the IUP is needed to increase carotenoid yield. Nevertheless, further efforts, including but not limited to these approaches, will be required to better understand carotenoid biosynthesis and improve microbial engineering for carotenoid overproduction.
Authors’ contribution
CL, CAS and AJS designed the study. CL collected the literature. CL, CAS and AJS wrote the paper.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgements
This work was financially supported by the Singapore-MIT Alliance for Research and Technology (SMART) program entitled Disruptive & Sustainable Technologies for Agricultural Precision (DiSTAP).
Glossary
- (G6P)
Glucose 6-phosphate
- (G3P)
Glyceraldehyde-3-phosphate
- (Cit)
Citrate
- (Isocit)
Isocitrate
- (AKG)
α-Ketoglutarate
- (Suc-CoA)
Succinyl-CoA
- (Suc)
Succinate
- (Fum)
Fumarate
- (Mal)
Malate
- (OAA)
Oxaloacetate
- (6-P-G-I)
6-Phosphoglucono-δ-lactone
- (Glu6P)
6-Phosphogluconate
- (Ru5P)
Ribulose 5-phosphate
- (X5P)
Xylulose 5-phosphate
- (R5P)
Ribose 5-phosphate
- (S7P)
Sedoheptulose 7-phosphate
- (G3P)
Glyceraldehyde 3-phosphate
- (E4P)
Erythrose 4-phosphate
- (F6P)
Fructose 6-phosphate
- (DXP)
1-Deoxy-D-xylulose-5-phosphate
- (MEP)
Methylerythritol phosphate
- (CDP-ME)
4-Diphosphocytidyl-2C-methyl-D-erythritol
- (CDP-MEP)
4-Diphosphocytidyl-2C-methyl-D-erythritol-2- phosphate
- (MEcPP)
2C-Methyl-D-erythritol-2,4-cyclo-diphosphate
- (HMBPP)
4-Hydroxy-3-methyl-2-(E)-butenyl-4-diphosphate
- (IPP)
Isopentenyl diphosphate
- (DMAPP)
Dimethylallyl diphosphate
- (AA-CoA)
Acetoacetyl-CoA
- (HMG-CoA)
3-Hydroxy-3-methylglutaryl-CoA
- (MVA)
Mevalonate
- (MVAP)
Mevalonate-5-phosphate
- (MVAPP)
Mevalonate-5-pyrophosphate
- (Iso)
Isopentenol
- (IP)
Isopentenyl phosphate
- (GPP)
Geranyl diphosphate
- (FPP)
Farnesyl diphosphate
- (GFPP)
Geranylfarnesyl diphosphate
- (GGPP)
Geranylgeranyl diphosphate
- (ED pathway)
Entner–Doudoroff pathway
- (TCA cycle)
Tricarboxylic acid cycle
Contributor Information
Cheng Li, Email: chengl@mit.edu.
Charles A. Swofford, Email: cswoff@mit.edu.
Anthony J. Sinskey, Email: asinskey@mit.edu.
References
- Adadi P., Barakova N.V., Krivoshapkina E.F. Selected methods of extracting carotenoids, characterization, and health concerns: a review. J. Agric. Food Chem. 2018;66:5925–5947. doi: 10.1021/acs.jafc.8b01407. [DOI] [PubMed] [Google Scholar]
- Ajikumar P.K., Xiao W.-H., Tyo K.E., Wang Y., Simeon F., Leonard E., Mucha O., Phon T.H., Pfeifer B., Stephanopoulos G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen L., Chen Y., Bach L.S., Rattleff S., Maury J., Brix S., Nielsen J., Mortensen U.H. Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl. Environ. Microbiol. 2011;77:1033–1040. doi: 10.1128/AEM.01361-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper H., Jin Y.-S., Moxley J.F., Stephanopoulos G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab. Eng. 2005;7:155–164. doi: 10.1016/j.ymben.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Alper H., Miyaoku K., Stephanopoulos G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat. Biotechnol. 2005;23:612–616. doi: 10.1038/nbt1083. [DOI] [PubMed] [Google Scholar]
- Alper H., Stephanopoulos G. Uncovering the gene knockout landscape for improved lycopene production in E. coli. Appl. Microbiol. Biotechnol. 2008;78:801–810. doi: 10.1007/s00253-008-1373-x. [DOI] [PubMed] [Google Scholar]
- Ambati R., Phang S.-M., Ravi S., Aswathanarayana R. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J.R., Anthony L.C., Nowroozi F., Kwon G., Newman J.D., Keasling J.D. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug precursor amorpha-4, 11-diene. Metab. Eng. 2009;11:13–19. doi: 10.1016/j.ymben.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Aparajita B., Yan W., Rahul B., Yue L., Honggao Y., Sharkey T.D. Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J. Biol. Chem. 2013;288:16926–16936. doi: 10.1074/jbc.M113.464636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadollahi M.A., Maury J., Patil K.R., Schalk M., Clark A., Nielsen J. Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab. Eng. 2009;11:328–334. doi: 10.1016/j.ymben.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Avalos J., Limón M.C. Biological roles of fungal carotenoids. Curr. Genet. 2015;61:309–324. doi: 10.1007/s00294-014-0454-x. [DOI] [PubMed] [Google Scholar]
- Berthelot K., Estevez Y., Deffieux A., Peruch F. Isopentenyl diphosphate isomerase: a checkpoint to isoprenoid biosynthesis. Biochimie. 2012;94:1621–1634. doi: 10.1016/j.biochi.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Boronat A., Rodríguez-Concepción M. Biotechnology of Isoprenoids. Springer; 2014. Terpenoid biosynthesis in prokaryotes; pp. 3–18. [Google Scholar]
- Borowitzka M.A. Elsevier; 2010. Carotenoid Production Using Microorganisms. Single Cell Oils; pp. 225–240. [Google Scholar]
- Bröker J.N., Müller B., van Deenen N., Prüfer D., Gronover C.S. Upregulating the mevalonate pathway and repressing sterol synthesis in Saccharomyces cerevisiae enhances the production of triterpenes. Appl. Microbiol. Biotechnol. 2018;102:6923–6934. doi: 10.1007/s00253-018-9154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camagna M., Grundmann A., Bär C., Koschmieder J., Beyer P., Welsch R. Enzyme fusion removes competition for geranylgeranyl diphosphate in carotenogenesis. Plant Physiol. 2019;179:1013–1027. doi: 10.1104/pp.18.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen S., Ajikumar P.K., Formenti L.R., Zhou K., Phon T.H., Nielsen M.L., Lantz A.E., Kielland-Brandt M.C., Stephanopoulos G.J. A.m., biotechnology Heterologous expression and characterization of bacterial 2-C-methyl-D-erythritol-4-phosphate pathway in Saccharomyces cerevisiae. 2013;97:5753–5769. doi: 10.1007/s00253-013-4877-y. [DOI] [PubMed] [Google Scholar]
- Chang W.-c., Song H., Liu H.-w., Liu P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013;17:571–579. doi: 10.1016/j.cbpa.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzivasileiou A.O., Ward V., Edgar S.M., Stephanopoulos G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. 2019;116:506–511. doi: 10.1073/pnas.1812935116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li M., Liu C., Zhang H., Xian M., Liu H. Enhancement of the catalytic activity of Isopentenyl diphosphate isomerase (IDI) from Saccharomyces cerevisiae through random and site-directed mutagenesis. Microb. Cell Factories. 2018;17:65. doi: 10.1186/s12934-018-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Liu C., Li M., Zhang H., Xian M., Liu H. Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene. RSC Adv. 2018;8:15021–15028. doi: 10.1039/c8ra01783b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Shukal S., Zhang C. Integrating enzyme and metabolic engineering tools for enhanced α-ionone production. J. Agric. Food Chem. 2019;67:13451–13459. doi: 10.1021/acs.jafc.9b00860. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Y., Shen H.-J., Cui Y.-Y., Chen S.-G., Weng Z.-M., Zhao M., Liu J.-Z. Chromosomal evolution of Escherichia coli for the efficient production of lycopene. BMC Biotechnol. 2013;13:6. doi: 10.1186/1472-6750-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Daviet L., Schalk M., Siewers V., Nielsen J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab. Eng. 2013;15:48–54. doi: 10.1016/j.ymben.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Chen Y., Xiao W., Wang Y., Liu H., Li X., Yuan Y. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb. Cell Factories. 2016;15:113. doi: 10.1186/s12934-016-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.S., Lee S.Y., Kim T.Y., Woo H.M. In silico identification of gene amplification targets for improvement of lycopene production. Appl. Environ. Microbiol. 2010;76:3097–3105. doi: 10.1128/AEM.00115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clomburg J.M., Qian S., Tan Z., Cheong S., Gonzalez R. The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis. Proc. Natl. Acad. Sci. 2019;116:12810–12815. doi: 10.1073/pnas.1821004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloms S.D., Merrick C.A., Olorunniji F.J., Stark W.M., Smith M.C., Osbourn A., Keasling J.D., Rosser S.J. Rapid metabolic pathway assembly and modification using serine integrase site-specific recombination. Nucleic Acids Res. 2013;42 doi: 10.1093/nar/gkt1101. e23-e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussement P., Bauwens D., Maertens J., De Mey M.J. A.s. b. vol. 6. 2016. pp. 224–232. (Direct Combinatorial Pathway Optimization). [DOI] [PubMed] [Google Scholar]
- Cui S., Lv X., Wu Y., Li J., Du G., Ledesma-Amaro R., Liu L.J.A.S.B. 2019. Engineering a Bifunctional Phr60-Rap60-Spo0A Quorum-Sensing Molecular Switch for Dynamic Fine-Tuning of Menaquinone-7 Synthesis in Bacillus Subtilis. [DOI] [PubMed] [Google Scholar]
- Das A., Yoon S.-H., Lee S.-H., Kim J.-Y., Oh D.-K., Kim S.-W. An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007;77:505. doi: 10.1007/s00253-007-1206-3. [DOI] [PubMed] [Google Scholar]
- de Ruyck J., Wouters J., Poulter C.D. Inhibition studies on enzymes involved in isoprenoid biosynthesis. Focus on two potential drug targets: dxr and idi-2 enzymes. Curr. Enzym. Inhib. 2011;7:79–95. doi: 10.2174/157340811796575317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A.L., Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009;27:297–306. doi: 10.1016/j.biotechadv.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Donald K., Hampton R.Y., Fritz I.B. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1997;63:3341–3344. doi: 10.1128/aem.63.9.3341-3344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey J.K., Porter J.W. The inhibition of mevalonic kinase by geranyl and farnesyl pyrophosphates. J. Biol. Chem. 1968;243:4667–4670. [PubMed] [Google Scholar]
- Du W., Song Y., Liu M., Yang H., Zhang Y., Fan Y., Luo X., Li Z., Wang N., He H., Zhou H., Ma W., Zhang T. Gene expression pattern analysis of a recombinant Escherichia coli strain possessing high growth and lycopene production capability when using fructose as carbon source. Biotechnol. Lett. 2016;38:1571–1577. doi: 10.1007/s10529-016-2133-0. [DOI] [PubMed] [Google Scholar]
- Dueber J.E., Wu G.C., Malmirchegini G.R., Moon T.S., Petzold C.J., Ullal A.V., Prather K.L., Keasling J.D. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 2009;27:753. doi: 10.1038/nbt.1557. [DOI] [PubMed] [Google Scholar]
- Esposito D., Chatterjee D.K. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006;17:353–358. doi: 10.1016/j.copbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Farmer W.R., Liao J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol. 2000;18:533. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- Farmer W.R., Liao J.C. Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol. Prog. 2001;17:57–61. doi: 10.1021/bp000137t. [DOI] [PubMed] [Google Scholar]
- Furubayashi M., Ikezumi M., Takaichi S., Maoka T., Hemmi H., Ogawa T., Saito K., Tobias A.V., Umeno D. A highly selective biosynthetic pathway to non-natural C 50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 2015;6:7534. doi: 10.1038/ncomms8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Tong Y., Zhu L., Ge M., Zhang Y., Chen D., Jiang Y., Yang S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017;41:192–201. doi: 10.1016/j.ymben.2017.04.004. [DOI] [PubMed] [Google Scholar]
- George K.W., Thompson M.G., Kim J., Baidoo E.E.K., Wang G., Benites V.T., Petzold C.J., Chan L.J.G., Yilmaz S., Turhanen P., Adams P.D., Keasling J.D., Lee T.S. Integrated analysis of isopentenyl pyrophosphate (IPP) toxicity in isoprenoid-producing Escherichia coli. Metab. Eng. 2018;47:60–72. doi: 10.1016/j.ymben.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Gruszecki W.I. 1999. Carotenoids in Membranes. [Google Scholar]
- Guo W., Tang H., Zhang L. Lycopene cyclase and phytoene synthase activities in the marine yeast Rhodosporidium diobovatum are encoded by a single gene crtYB. J. Basic Microbiol. 2014;54:1053–1061. doi: 10.1002/jobm.201300920. [DOI] [PubMed] [Google Scholar]
- Han X., Wang X., Zhou K. Develop machine learning-based regression predictive models for engineering protein solubility. Bioinformatics. 2019;35:4640–4646. doi: 10.1093/bioinformatics/btz294. [DOI] [PubMed] [Google Scholar]
- Hara K.Y., Morita T., Endo Y., Mochizuki M., Araki M., Kondo A. Evaluation and screening of efficient promoters to improve astaxanthin production in Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2014;98:6787–6793. doi: 10.1007/s00253-014-5727-2. [DOI] [PubMed] [Google Scholar]
- Heider S.A.E., Wolf N., Hofemeier A., Peters-Wendisch P., Wendisch V.F. Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2014;2 doi: 10.3389/fbioe.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke N.A., Heider S.A.E., Hannibal S., Wendisch V.F., Peters-Wendisch P. Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke N.A., Heider S.A.E., Peters-Wendisch P., Wendisch V.F. Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar. Drugs. 2016;14:124. doi: 10.3390/md14070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke N.A., Wiebe D., Pérez-García F., Peters-Wendisch P., Wendisch V.F. Coproduction of cell-bound and secreted value-added compounds: simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour. Technol. 2018;247:744–752. doi: 10.1016/j.biortech.2017.09.167. [DOI] [PubMed] [Google Scholar]
- Hinson D., Chambliss K., Toth M., Tanaka R., Gibson K. Post-translational regulation of mevalonate kinase by intermediates of the cholesterol and nonsterol isoprene biosynthetic pathways. J. Lipid Res. 1997;38:2216–2223. [PubMed] [Google Scholar]
- Hong J., Park S.-H., Kim S., Kim S.-W., Hahn J.-S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl. Microbiol. Biotechnol. 2019;103:211–223. doi: 10.1007/s00253-018-9449-8. [DOI] [PubMed] [Google Scholar]
- Huo L., Hug J.J., Fu C., Bian X., Zhang Y., Müller R. Heterologous expression of bacterial natural product biosynthetic pathways. Nat. Prod. Rep. 2019;36:1412–1436. doi: 10.1039/c8np00091c. [DOI] [PubMed] [Google Scholar]
- Jakočiūnas T., Pedersen L.E., Lis A.V., Jensen M.K., Keasling J.D. CasPER, a method for directed evolution in genomic contexts using mutagenesis and CRISPR/Cas9. Metab. Eng. 2018;48:288–296. doi: 10.1016/j.ymben.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Jia H., Schwille P. Bottom-up synthetic biology: reconstitution in space and time. Curr. Opin. Biotechnol. 2019;60:179–187. doi: 10.1016/j.copbio.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Jin J., Wang Y., Yao M., Gu X., Li B., Liu H., Ding M., Xiao W., Yuan Y.J. B.f. b. vol. 11. 2018. p. 230. (Astaxanthin Overproduction in Yeast by Strain Engineering and New Gene Target Uncovering). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Xu X., Jiang L., Zhang Z., Li S., Huang H. Putative carotenoid genes expressed under the regulation of Shine–Dalgarno regions in Escherichia coli for efficient lycopene production. Biotechnol. Lett. 2015;37:2303–2310. doi: 10.1007/s10529-015-1922-1. [DOI] [PubMed] [Google Scholar]
- Jin Y.-S., Stephanopoulos G. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab. Eng. 2007;9:337–347. doi: 10.1016/j.ymben.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Julliard J.H., Douce R. Biosynthesis of the thiazole moiety of thiamin (vitamin B1) in higher plant chloroplasts. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2042–2045. doi: 10.1073/pnas.88.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Lim J.H., Kim S.Y., Im D.-K., Seok J.Y., Lee S.-J.V., Oh M.-K., Jung G.Y. Precise precursor rebalancing for isoprenoids production by fine control of gapA expression in Escherichia coli. Metab. Eng. 2016;38:401–408. doi: 10.1016/j.ymben.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Kang C.W., Lim H.G., Yang J., Noh M.H., Seo S.W., Jung G.Y. J.M.e. vol. 48. 2018. pp. 121–128. (Synthetic Auxotrophs for Stable and Tunable Maintenance of Plasmid Copy Number). [DOI] [PubMed] [Google Scholar]
- Kang M.J., Lee Y.M., Yoon S.H., Kim J.H., Ock S.W., Jung K.H., Shin Y.C., Keasling J.D., Kim S.W. Identification of genes affecting lycopene accumulation in Escherichia coli using a shot-gun method. Biotechnol. Bioeng. 2005;91:636–642. doi: 10.1002/bit.20539. [DOI] [PubMed] [Google Scholar]
- Kang W., Ma T., Liu M., Qu J., Liu Z., Zhang H., Shi B., Fu S., Ma J., Lai L.T.F. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nat. Commun. 2019;vol. 10:1–11. doi: 10.1038/s41467-019-12247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazieva E., Yamamoto Y., Tajima Y., Yokoyama K., Katashkina J., Nishio Y. Characterization of feedback-resistant mevalonate kinases from the methanogenic archaeons Methanosaeta concilii and Methanocella paludicola. Microbiology. 2017;163:1283. doi: 10.1099/mic.0.000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazufumi T., Tomohiro O., Jun O., Shun-Ichi T., Clement A., Yuichi K., Shigenori K. Stabilization by fusion to the C-terminus of hyperthermophile Sulfolobus tokodaii RNase HI: a possibility of protein stabilization tag. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-H., Wang C., Jang H.-J., Cha M.-S., Park J.-E., Jo S.-Y., Choi E.-S., Kim S.-W. Isoprene production by Escherichia coli through the exogenous mevalonate pathway with reduced formation of fermentation byproducts. Microb. Cell Factories. 2016;15:214. doi: 10.1186/s12934-016-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J., Dietzel K.L., Wichmann G., Chan R., Antipov E., Moss N., Baidoo E.E., Jackson P., Gaucher S.P., Gottlieb S.J. M.e. vol. 38. 2016. pp. 494–503. (Engineering a Functional 1-Deoxy-D-Xylulose 5-phosphate (DXP) Pathway in Saccharomyces cerevisiae). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J., Nishimoto M., Chow R.W.N., Baidoo E.E.K., Wang G., Martin J., Schackwitz W., Chan R., Fortman J.L., Keasling J.D. Enhancing terpene yield from sugars via novel routes to 1-deoxy-D-xylulose 5-phosphate. Appl. Environ. Microbiol. 2015;81:130–138. doi: 10.1128/AEM.02920-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompe S.E., Vo P.L., Halpin-Healy T.S., Sternberg S.H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature. 2019;1 doi: 10.1038/s41586-019-1323-z. [DOI] [PubMed] [Google Scholar]
- Korman T.P., Opgenorth P.H., Bowie J.U. J.N.c. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. 2017;8:15526. doi: 10.1038/ncomms15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak S., Kim S.R., Xu H., Zhang G.-C., Lane S., Kim H., Jin Y.-S. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017;114:2581–2591. doi: 10.1002/bit.26369. [DOI] [PubMed] [Google Scholar]
- Larroude M., Celinska E., Back A., Thomas S., Nicaud J.M., Ledesma-Amaro R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018;115:464–472. doi: 10.1002/bit.26473. [DOI] [PubMed] [Google Scholar]
- Lee P.C., Petri R., Mijts B.N., Watts K.T., Schmidt-Dannert C. Directed evolution of Escherichia coli farnesyl diphosphate synthase (IspA) reveals novel structural determinants of chain length specificity. Metab. Eng. 2005;7:18–26. doi: 10.1016/j.ymben.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Leonard E., Ajikumar P.K., Thayer K., Xiao W.-H., Mo J.D., Tidor B., Stephanopoulos G., Prather K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. 2010;107:13654–13659. doi: 10.1073/pnas.1006138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Hou F., Wu T., Jiang X., Li F., Liu H., Xian M., Zhang H. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories. Nat. Prod. Rep. 2019 doi: 10.1039/c9np00016j. [DOI] [PubMed] [Google Scholar]
- Li C., Lin Y., Zheng X., Pang N., Liao X., Liu X., Huang Y., Liang S. Combined strategies for improving expression of Citrobacter amalonaticus phytase in Pichia pastoris. BMC Biotechnol. 2015;15:88. doi: 10.1186/s12896-015-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ying L.-Q., Zhang S.-S., Chen N., Liu W.-F., Tao Y. Modification of targets related to the Entner–Doudoroff/pentose phosphate pathway route for methyl-d-erythritol 4-phosphate-dependent carotenoid biosynthesis in Escherichia coli. Microb. Cell Factories. 2015;14:117. doi: 10.1186/s12934-015-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Furubayashi M., Wang S., Maoka T., Kawai-Noma S., Saito K., Umeno D. Genetically engineered biosynthetic pathways for nonnatural C60 carotenoids using C5-elongases and C50-cyclases in Escherichia coli. Sci. Rep. 2019;9:2982. doi: 10.1038/s41598-019-39289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Si T., Nair N.U., Zhao H. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab. Eng. 2014;24:139–149. doi: 10.1016/j.ymben.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Liu H., Sun Y., Ramos K.R.M., Nisola G.M., Valdehuesa K.N.G., Lee W.K., Park S.J., Chung W.-J. Combination of Entner-Doudoroff pathway with MEP increases isoprene production in engineered Escherichia coli. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang Y., Tang Q., Kong W., Chung W.-J., Lu T. MEP pathway-mediated isopentenol production in metabolically engineered Escherichia coli. Microb. Cell Factories. 2014;13:135. doi: 10.1186/s12934-014-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Shi X., Song L., Liu H., Zhou X., Wang Q., Zhang Y., Cai M. CRISPR–Cas9-mediated genomic multiloci integration in Pichia pastoris. Microb. Cell Factories. 2019;18:1–11. doi: 10.1186/s12934-019-1194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato P., Alcaino J., Barahona S., Retamales P., Cifuentes V. Alternative splicing of transcripts from crtI and crtYB genes of Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2003;69:4676–4682. doi: 10.1128/AEM.69.8.4676-4682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S., Hall R., Williams G.J. An artificial pathway for isoprenoid biosynthesis decoupled from native hemiterpene metabolism. ACS Synth. Biol. 2019;8:232–238. doi: 10.1021/acssynbio.8b00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Li B.-Z., Liu D., Zhang L., Chen Y., Jia B., Zeng B.-X., Zhao H., Yuan Y.-J. Engineered biosynthesis of natural products in heterologous hosts. Chem. Soc. Rev. 2015;44:5265–5290. doi: 10.1039/c5cs00025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X., Gu J., Wang F., Xie W., Liu M., Ye L., Yu H. Combinatorial pathway optimization in Escherichia coli by directed co-evolution of rate-limiting enzymes and modular pathway engineering. Biotechnol. Bioeng. 2016;113:2661–2669. doi: 10.1002/bit.26034. [DOI] [PubMed] [Google Scholar]
- Lv X., Wang F., Zhou P., Ye L., Xie W., Xu H., Yu H. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat. Commun. 2016;7:12851. doi: 10.1038/ncomms12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Qian S., Du G., Chen J., Zhou J., Xu P.J. M.e. 2019. Coupling Feedback Genetic Circuits with Growth Phenotype for Dynamic Population Control in Metabolic Engineering. [DOI] [PubMed] [Google Scholar]
- Ma T., Shi B., Ye Z., Li X., Liu M., Chen Y., Xia J., Nielsen J., Deng Z., Liu T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019;52:134–142. doi: 10.1016/j.ymben.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Ma X., Liang H., Cui X., Liu Y., Lu H., Ning W., Poon N.Y., Ho B., Zhou K. A standard for near-scarless plasmid construction using reusable DNA parts. Nat. Commun. 2019;10:3294. doi: 10.1038/s41467-019-11263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthäus F., Ketelhot M., Gatter M., Barth G. Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2014;80:1660–1669. doi: 10.1128/AEM.03167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams A. The global market for carotenoids. 2018. https://www.bccresearch.com
- Meadows A.L., Hawkins K.M., Tsegaye Y., Antipov E., Kim Y., Raetz L., Dahl R.H., Tai A., Mahatdejkul-Meadows T., Xu L. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 2016;537:694. doi: 10.1038/nature19769. [DOI] [PubMed] [Google Scholar]
- Mlíčková K., Roux E., Athenstaedt K., d’Andrea S., Daum G., Chardot T., Nicaud J.-M. Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2004;70:3918–3924. doi: 10.1128/AEM.70.7.3918-3924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise A.R., Al-Babili S., Wurtzel E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014;114:164–193. doi: 10.1021/cr400106y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.Y., Farasat I., Maranas C.D., Salis H.M. J.M.e. vol. 29. 2015. pp. 86–96. (Rational Design of a Synthetic Entner–Doudoroff Pathway for Improved and Controllable NADPH Regeneration). [DOI] [PubMed] [Google Scholar]
- Özaydın B., Burd H., Lee T.S., Keasling J.D. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng. 2013;15:174–183. doi: 10.1016/j.ymben.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Binkley R.M., Kim W.J., Lee M.H., Lee S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018;49:105–115. doi: 10.1016/j.ymben.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Yang D., Ha S.H., Lee S.Y. Metabolic engineering of microorganisms for the production of natural compounds. Adv. Biosyst. 2018;2:1700190. [Google Scholar]
- Partow S., Siewers V., Daviet L., Schalk M., Nielsen J.J.P.O. vol. 7. 2012. (Reconstruction and Evaluation of the Synthetic Bacterial MEP Pathway in Saccharomyces cerevisiae). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti W., Page R. Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr. Purif. 2007;51:1–10. doi: 10.1016/j.pep.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Pfleger B.F., Pitera D.J., Smolke C.D., Keasling J.D. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat. Biotechnol. 2006;24:1027. doi: 10.1038/nbt1226. [DOI] [PubMed] [Google Scholar]
- Pitera D.J., Paddon C.J., Newman J.D., Keasling J.D. Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab. Eng. 2007;9:193–207. doi: 10.1016/j.ymben.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Polakowski T., Stahl U., Lang C. Overexpression of a cytosolic hydroxymethylglutaryl-CoA reductase leads to squalene accumulation in yeast. Appl. Microbiol. Biotechnol. 1998;49:66–71. doi: 10.1007/s002530051138. [DOI] [PubMed] [Google Scholar]
- Primak Y.A., Du M., Miller M.C., Wells D.H., Nielsen A.T., Weyler W., Beck Z.Q. Characterization of a feedback-resistant mevalonate kinase from the archaeon Methanosarcina mazei. Appl. Environ. Microbiol. 2011;77:7772–7778. doi: 10.1128/AEM.05761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk J.T., Yde Steensma H., van Dijken J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi: 10.1002/(sici)1097-0061(199612)12:16<1607::aid-yea70>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Qiang S., Su A.P., Li Y., Chen Z., Hu C.Y., Meng Y.H. Elevated β-carotene synthesis by the engineered Rhodobacter sphaeroides with enhanced CrtY expression. J. Agric. Food Chem. 2019;67:9560–9568. doi: 10.1021/acs.jafc.9b02597. [DOI] [PubMed] [Google Scholar]
- Rad S.A., Zahiri H.S., Noghabi K.A., Rajaei S., Heidari R., Mojallali L. Type 2 IDI performs better than type 1 for improving lycopene production in metabolically engineered E. coli strains. World J. Microbiol. Biotechnol. 2012;28:313–321. doi: 10.1007/s11274-011-0821-4. [DOI] [PubMed] [Google Scholar]
- Ramos-Valdivia A.C., van der Heijden R., Verpoorte R. Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function. Nat. Prod. Rep. 1997;14:591–603. doi: 10.1039/np9971400591. [DOI] [PubMed] [Google Scholar]
- Ravikumar Y., Nadarajan S.P., Yoo T.H., Lee C.-s., Yun H. Unnatural amino acid mutagenesis-based enzyme engineering. Trends Biotechnol. 2015;33:462–470. doi: 10.1016/j.tibtech.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Reider Apel A., d’Espaux L., Wehrs M., Sachs D., Li R.A., Tong G.J., Garber M., Nnadi O., Zhuang W., Hillson N.J., Keasling J.D., Mukhopadhyay A. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2016;45:496–508. doi: 10.1093/nar/gkw1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R.K., Keum Y.-S. Carotenoid extraction methods: a review of recent developments. Food Chem. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- Saini R.K., Keum Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: an updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019;46:657–674. doi: 10.1007/s10295-018-2104-7. [DOI] [PubMed] [Google Scholar]
- Sauer U., Canonaco F., Heri S., Perrenoud A., Fischer E. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 2004;279:6613–6619. doi: 10.1074/jbc.M311657200. [DOI] [PubMed] [Google Scholar]
- Segall-Shapiro T.H., Sontag E.D., Voigt C.A. J.N.b. Engineered promoters enable constant gene expression at any copy number in bacteria. 2018;36:352. doi: 10.1038/nbt.4111. [DOI] [PubMed] [Google Scholar]
- Shen H.-J., Cheng B.-Y., Zhang Y.-M., Tang L., Li Z., Bu Y.-F., Li X.-R., Tian G.-Q., Liu J.-Z. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng. 2016;38:180–190. doi: 10.1016/j.ymben.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Shi B., Ma T., Ye Z., Li X., Huang Y., Zhou Z., Ding Y., Deng Z., Liu T. Systematic metabolic engineering of Saccharomyces cerevisiae for lycopene overproduction. J. Agric. Food Chem. 2019;67:11148–11157. doi: 10.1021/acs.jafc.9b04519. [DOI] [PubMed] [Google Scholar]
- Shiba Y., Paradise E.M., Kirby J., Ro D.-K., Keasling J.D. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids. Metab. Eng. 2007;9:160–168. doi: 10.1016/j.ymben.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Simon A.J., d’Oelsnitz S., Ellington A.D. Synthetic evolution. Nat. Biotechnol. 2019;1 doi: 10.1038/s41587-019-0157-4. [DOI] [PubMed] [Google Scholar]
- Sun L., Kwak S., Jin Y.-S. Vitamin A production by engineered Saccharomyces cerevisiae from xylose via two-phase in situ extraction. ACS Synth. Biol. 2019;8:2131–2140. doi: 10.1021/acssynbio.9b00217. [DOI] [PubMed] [Google Scholar]
- Sung W.S., Lee I.-S., Lee D.G. Damage to the cytoplasmic membrane and cell death caused by lycopene in Candida albicans. J. Microbiol. Biotechnol. 2007;17:1797–1804. [PubMed] [Google Scholar]
- Taniguchi H., Henke N.A., Heider S.A.E., Wendisch V.F. Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: application to production of β-carotene and the non-native linear C50 carotenoid bisanhydrobacterioruberin. Metab. Eng. Commun. 2017;4:1–11. doi: 10.1016/j.meteno.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Sedkova N., Yao H., Ye R.W., Sharpe P.L., Cheng Q. Expression of bacterial hemoglobin genes to improve astaxanthin production in a methanotrophic bacterium Methylomonas sp. Appl. Microbiol. Biotechnol. 2006;74:625. doi: 10.1007/s00253-006-0708-8. [DOI] [PubMed] [Google Scholar]
- Tapiero H., Townsend D., Tew K.J.B., Pharmacotherapy The role of carotenoids in the prevention of human pathologies. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosacrespo J., Montero Z., Fuentes J.L., Reig M.G., Garbayo I., Vílchez C., Martínezespinosa R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs. 2018;16:203. doi: 10.3390/md16060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta H., Paddon C.J., Eng D., Lenihan J.R., Horning T., Anthony L.C., Regentin R., Keasling J.D., Renninger N.S., Newman J.D. High-level production of amorpha-4, 11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS One. 2009;4:e4489. doi: 10.1371/journal.pone.0004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyo K.E., Ajikumar P.K., Stephanopoulos G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat. Biotechnol. 2009;27:760. doi: 10.1038/nbt.1555. [DOI] [PubMed] [Google Scholar]
- Vachali P., Bhosale P., Bernstein P.S. Springer; 2012. Microbial Carotenoids. Microbial Carotenoids from Fungi; pp. 41–59. [DOI] [PubMed] [Google Scholar]
- Vadali R.V., Fu Y., Bennett G.N., San K.-Y. Enhanced lycopene productivity by manipulation of carbon flow to isopentenyl diphosphate in Escherichia coli. Biotechnol. Prog. 2005;21:1558–1561. doi: 10.1021/bp050124l. [DOI] [PubMed] [Google Scholar]
- Velayos A., Eslava A.P., Iturriaga E.A. A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur. J. Biochem. 2000;267:5509–5519. doi: 10.1046/j.1432-1327.2000.01612.x. [DOI] [PubMed] [Google Scholar]
- Walsh C., Tang Y. The chemical biology of human vitamins. R. Soc. Chem. 2018;1 [Google Scholar]
- Wang C.-w., Oh M.-K., Liao J.C. Directed evolution of metabolically engineered Escherichia coli for carotenoid production. Biotechnol. Prog. 2000;16:922–926. doi: 10.1021/bp000124f. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhao S., Shao X., Park J.-B., Jeong S.-H., Park H.-J., Kwak W.-J., Wei G., Kim S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Factories. 2019;18:55. doi: 10.1186/s12934-019-1105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-Y., Keasling J.D. Amplification of HMG-CoA reductase production enhances carotenoid accumulation in Neurospora crassa. Metab. Eng. 2002;4:193–201. doi: 10.1006/mben.2002.0225. [DOI] [PubMed] [Google Scholar]
- Wang H.H., Isaacs F.J., Carr P.A., Sun Z.Z., Xu G., Forest C.R., Church G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.H., Isaacs F.J., Carr P.A., Sun Z.Z., Xu G., Forest C.R., Church G.M.J.N. vol. 460. 2009. p. 894. (Programming Cells by Multiplex Genome Engineering and Accelerated Evolution). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Niyompanich S., Tai Y.-S., Wang J., Bai W., Mahida P., Gao T., Zhang K. Engineering of a highly efficient Escherichia coli strain for mevalonate fermentation through chromosomal integration. Appl. Environ. Microbiol. 2016;82:7176–7184. doi: 10.1128/AEM.02178-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jing P., Zheng Y., Jiang P., Gong W., Chen X., Chen D. Genetic manipulation of the bifunctional gene, carRA, to enhance lycopene content in Blakeslea trispora. Biochem. Eng. J. 2017;119:27–33. [Google Scholar]
- Ward V.C., Chatzivasileiou A.O., Stephanopoulos G. Cell free biosynthesis of isoprenoids from isopentenol. Biotechnol. Bioeng. 2019;116:3269–3281. doi: 10.1002/bit.27146. [DOI] [PubMed] [Google Scholar]
- Werner N., Ramirez-Sarmiento C.A., Agosin E. Protein engineering of carotenoid cleavage dioxygenases to optimize β-ionone biosynthesis in yeast cell factories. Food Chem. 2019;299:125089. doi: 10.1016/j.foodchem.2019.125089. [DOI] [PubMed] [Google Scholar]
- Westfall P.J., Pitera D.J., Lenihan J.R., Eng D., Woolard F.X., Regentin R., Horning T., Tsuruta H., Melis D.J., Owens A. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. 2012;109:E111–E118. doi: 10.1073/pnas.1110740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H.M., Murray G.W., Batth T.S., Prasad N., Adams P.D., Keasling J.D., Petzold C.J., Lee T.S. Application of targeted proteomics and biological parts assembly in E. coli to optimize the biosynthesis of an anti-malarial drug precursor, amorpha-4, 11-diene. Chem. Eng. Sci. 2013;103:21–28. [Google Scholar]
- Wu T., Li S., Ye L., Zhao D., Fan F., Li Q., Zhang B., Bi C., Zhang X. Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of β-carotene in Escherichia coli. ACS Synth. Biol. 2019;8:1037–1046. doi: 10.1021/acssynbio.8b00472. [DOI] [PubMed] [Google Scholar]
- Wu T., Ye L., Zhao D., Li S., Li Q., Zhang B., Bi C. Engineering membrane morphology and manipulating synthesis for increased lycopene accumulation in Escherichia coli cell factories. Biotech. 2018;8:269. doi: 10.1007/s13205-018-1298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ye L., Zhao D., Li S., Li Q., Zhang B., Bi C., Zhang X. Membrane engineering - a novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab. Eng. 2017;43:85–91. doi: 10.1016/j.ymben.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Wu Z., Kan S.J., Lewis R.D., Wittmann B.J., Arnold F.H. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc. Natl. Acad. Sci. 2019;116:8852–8858. doi: 10.1073/pnas.1901979116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Lv X., Ye L., Zhou P., Yu H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab. Eng. 2015;30:69–78. doi: 10.1016/j.ymben.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Xie W., Ye L., Lv X., Xu H., Yu H. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng. 2015;28:8–18. doi: 10.1016/j.ymben.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Xu P., Gu Q., Wang W., Wong L., Bower A.G., Collins C.H., Koffas M.A. J.N.c. Modular optimization of multi-gene pathways for fatty acids production in. E. Coli. 2013;4:1409. doi: 10.1038/ncomms2425. [DOI] [PubMed] [Google Scholar]
- Xu P., Rizzoni E.A., Sul S.-Y., Stephanopoulos G.J. A.s. b. vol. 6. 2016. pp. 148–158. (Improving Metabolic Pathway Efficiency by Statistical Model-Based Multivariate Regulatory Metabolic Engineering). [DOI] [PubMed] [Google Scholar]
- Xu P. Production of chemicals using dynamic control of metabolic fluxes. Curr. Opin. Biotechnol. 2018;vol. 53:12–19. doi: 10.1016/j.copbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Xu X., Jin W., Jiang L., Xu Q., Li S., Zhang Z., Huang H. A high-throughput screening method for identifying lycopene-overproducing E. coli strain based on an antioxidant capacity assay. Biochem. Eng. J. 2016;112:277–284. [Google Scholar]
- Yabuzaki J. Carotenoids Database: structures, chemical fingerprints and distribution among organisms. Database. 2017 doi: 10.1093/database/bax004. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V.G., De Mey M., Lim C.G., Ajikumar P.K., Stephanopoulos G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab. Eng. 2012;14:233–241. doi: 10.1016/j.ymben.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Gao X., Jiang Y., Sun B., Gao F., Yang S. Synergy between methylerythritol phosphate pathway and mevalonate pathway for isoprene production in Escherichia coli. Metab. Eng. 2016;37:79–91. doi: 10.1016/j.ymben.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Yang J., Guo L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Factories. 2014;13:160. doi: 10.1186/s12934-014-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Zhang C., Bi C., Li Q., Zhang X.J. M.c. f. vol. 15. 2016. p. 202. (Combinatory Optimization of Chromosomal Integrated Mevalonate Pathway for β-carotene Production in Escherichia coli). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Zhu X., Wu T., Wang W., Zhao D., Bi C., Zhang X. Optimizing the localization of astaxanthin enzymes for improved productivity. Biotechnol. Biofuels. 2018;11:278. doi: 10.1186/s13068-018-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Cao H., Yan M., Cao F., Zhang Y., Li X., Xu L., Chen Y., Xiong J., Ouyang P. Construction and co-expression of a polycistronic plasmid encoding carbonyl reductase and glucose dehydrogenase for production of ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour. Technol. 2010;101:6761–6767. doi: 10.1016/j.biortech.2010.03.099. [DOI] [PubMed] [Google Scholar]
- Yoon S.-H., Kim J.-E., Lee S.-H., Park H.-M., Choi M.-S., Kim J.-Y., Lee S.-H., Shin Y.-C., Keasling J.D., Kim S.-W. Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl. Microbiol. Biotechnol. 2007;74:131–139. doi: 10.1007/s00253-006-0623-z. [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen X., Lindley N.D., Too H.P. A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol. Bioeng. 2018;115:174–183. doi: 10.1002/bit.26462. [DOI] [PubMed] [Google Scholar]
- Zhang C., Seow V.Y., Chen X., Too H.-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018;9:1858. doi: 10.1038/s41467-018-04211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zou R., Chen X., Stephanopoulos G., Too H.-P. Experimental design-aided systematic pathway optimization of glucose uptake and deoxyxylulose phosphate pathway for improved amorphadiene production. Appl. Microbiol. Biotechnol. 2015;99:3825–3837. doi: 10.1007/s00253-015-6463-y. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Y., Pfeifer B.A. Bacterial hosts for natural product production. Mol. Pharm. 2008;5:212–225. doi: 10.1021/mp7001329. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ptacin J.L., Fischer E.C., Aerni H.R., Caffaro C.E., San Jose K., Feldman A.W., Turner C.R., Romesberg F.E. A semi-synthetic organism that stores and retrieves increased genetic information. Nature. 2017;551:644. doi: 10.1038/nature24659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Li Q., Sun T., Zhu X., Xu H., Tang J., Zhang X., Ma Y. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab. Eng. 2013;17:42–50. doi: 10.1016/j.ymben.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Zhao X., Shi F., Zhan W. Overexpression of ZWF1 and POS5 improves carotenoid biosynthesis in recombinant Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2015;61:354–360. doi: 10.1111/lam.12463. [DOI] [PubMed] [Google Scholar]
- Zhou C., Li Z., Wiberley-Bradford A.E., Weise S.E., Sharkey T.D. Isopentenyl diphosphate and dimethylallyl diphosphate/isopentenyl diphosphate ratio measured with recombinant isopentenyl diphosphate isomerase and isoprene synthase. Anal. Biochem. 2013;440:130–136. doi: 10.1016/j.ab.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yang L., Wang C., Choi E.-S., Kim S.-W. Enhanced performance of the methylerythritol phosphate pathway by manipulation of redox reactions relevant to IspC, IspG, and IspH. J. Biotechnol. 2017;248:1–8. doi: 10.1016/j.jbiotec.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Zhou K., Qiao K., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015;33:377. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Zou R., Stephanopoulos G., Too H.-P. Enhancing solubility of deoxyxylulose phosphate pathway enzymes for microbial isoprenoid production. Microb. Cell Factories. 2012;11:148. doi: 10.1186/1475-2859-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Zou R., Stephanopoulos G., Too H.-P. Metabolite profiling identified methylerythritol cyclodiphosphate efflux as a limiting step in microbial isoprenoid production. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Zou R., Zhang C., Stephanopoulos G., Too H.-P. Optimization of amorphadiene synthesis in bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol. Bioeng. 2013;110:2556–2561. doi: 10.1002/bit.24900. [DOI] [PubMed] [Google Scholar]
- Zhou P., Li M., Shen B., Yao Z., Bian Q., Ye L., Yu H. Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin. J. Agric. Food Chem. 2019;67:1072–1080. doi: 10.1021/acs.jafc.8b05003. [DOI] [PubMed] [Google Scholar]
- Zhou P., Xie W., Li A., Wang F., Yao Z., Bian Q., Zhu Y., Yu H., Ye L. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2017;100:28–36. doi: 10.1016/j.enzmictec.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Zou R., Zhou K., Stephanopoulos G., Too H.P. Combinatorial engineering of 1-deoxy-D-xylulose 5-phosphate pathway using cross-lapping in vitro assembly (CLIVA) method. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Kirst H., Melis A. Isoprene production via the mevalonic acid pathway in Escherichia coli (bacteria) BioEnergy Res. 2012;5:814–828. [Google Scholar]