Abstract
Studies in human immunodeficiency virus (HIV)–infected individuals suggest excess weight gain with integrase inhibitor–based antiretroviral therapy. The HIV Prevention Trials Network Study 077 evaluated changes in weight and fasting metabolic parameters in HIV-uninfected individuals randomized to cabotegravir or a placebo. No differences between arms were found for change in weight or fasting metabolic parameters overall or for subgroups.
Keywords: cabotegravir, CAB, weight gain, HIV uninfected
Antiretroviral therapy (ART) that contains an integrase inhibitor has been associated with weight gain and increased waist circumference, with changes of greater magnitude seen among women, blacks, and those with lower CD4 and higher human immunodeficiency virus (HIV) RNA prior to starting ART [1–7]. Some studies have observed a mitigating effect of tenofovir disoproxil fumarate (TDF) in those regimens [1], and these changes have been associated with increases in body mass index (BMI) category [8]—a change that has been associated in other studies with morbidity and mortality [9].
Cabotegravir (CAB) is a novel integrase inhibitor in development for HIV prevention and as part of combination ART for treatment. CAB is available both as an oral tablet for daily administration (being developed only for lead-in to the injectable product) and as a long-acting suspension for monthly or every-other-month intramuscular injection [10]. Weight changes for HIV-infected participants in trials of CAB as part of combination ART have not yet been published.
The HIV Prevention Trials Network Study 077 (HPTN 077) was a phase 2a safety, tolerability, and pharmacokinetic study that enrolled 199 HIV-uninfected low-risk participants at 8 sites globally [11]. The study provides a unique opportunity to evaluate changes in weight and metabolic parameters among participants exposed to long-acting injectable CAB (CAB LA) or a placebo (PBO), absent HIV infection or additional antiretroviral agents. We performed a post hoc analysis to explore the hypothesis that changes in weight and metabolic parameters would not be different between participants in the CAB and PBO study arms.
METHODS
Study Design
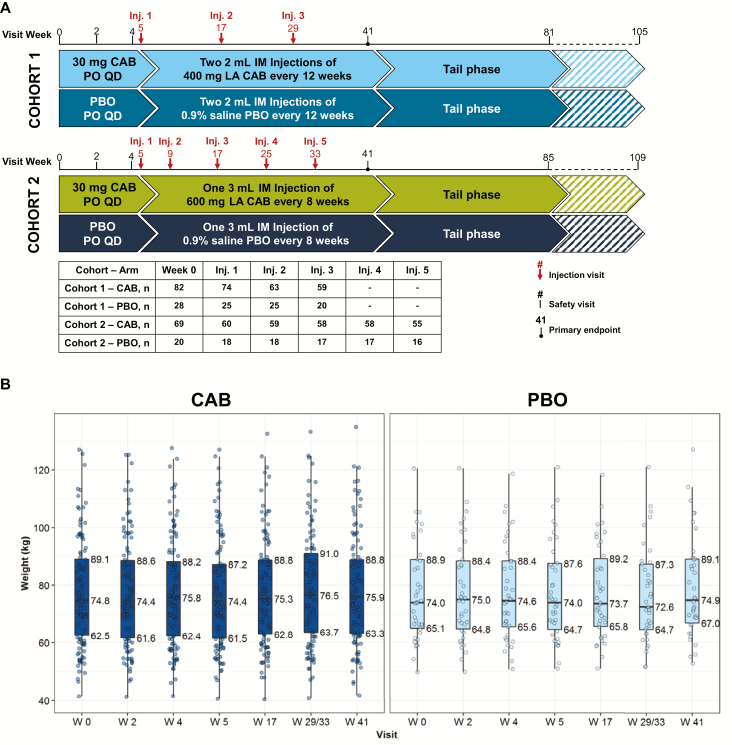
In HPTN 077, participants were randomized 3:1 to CAB or a PBO course, during which they received a daily oral tablet for 4 weeks, a 1-week hiatus, and then a series of injections with a primary safety and tolerability endpoint 41 weeks after study entry. Two dose cohorts were enrolled sequentially, the first with an injection phase consisting of 3 quarterly injections of 800 mg of CAB (or a 0.9% saline placebo) and the second characterized by 5 total injections of 600 mg of CAB (or saline placebo) at 8-week intervals after a 4-week initial separation. Participants were then followed for 48–72 weeks after their final injection.
Assessments
Weight was measured at study entry, during oral product administration at weeks 2 and 4, and during injectable product administration at weeks 5, 17, 19, 29 or 33, and 41 [1]. Demographics were collected at baseline, and BMI was calculated from baseline height measurement and week 0 (W0) and week 41 (W41) weight measurements. Fasting glucose and fasting lipids were collected at W0 and W41.
Analysis
Wilcoxon rank sum tests were used to compare distributions of intraparticipant changes in weight and metabolic parameters; only participants with paired W0 and W41 data available for a given parameter were included. Generalized estimating equations (GEE) were used to model longitudinal weight data over time. Mean modeled intraparticipant weight changes were compared between CAB and PBO groups as an additional sensitivity analysis to the primary comparison. The GEE model included all 177 participants and available weight data at all available timepoints of interest (above) for a given participant.
We estimated that the sample size of 177 participants provided 90% power with a 5% significance alpha to rule out a 2.4 kg or larger mean difference in overall weight change between the CAB and PBO arms.
RESULTS
Study and Analysis Population
The numbers of participants who entered the study in each arm of each dose cohort and who received each sequential injection are shown in Figure 1A. For the current analysis, we included 177 participants who received at least 1 injection (134 CAB and 43 PBO).
Figure 1.
A, HIV Prevention Trials Network study 077 study design. B, Median and interquartile range participant weight by study arm over time. Abbreviations: CAB, cabotegravir; IM, intramuscular; Inj, injection; LA, long-acting; PBO, placebo; PO, by mouth (orally); QD, everyday.
The overall study population has been previously described [11]; the analysis population (n = 177) had a median age of 31.5 (interquartile range [IQR], 24–39) years, was 66% female, 40% black, and 26% Latino. Slightly more than half (55%) were from US sites, 23% from sub-Saharan Africa, and 21% from Brazil. Ten percent of the population reported smoking at study entry. Characteristics were balanced between the CAB and PBO arms.
The median baseline weight of study participants was 74.7 (IQR, 62.4–91.2) kg and median BMI was 26.6 (IQR, 23.4–32.7), with no significant difference between CAB and PBO groups (Figure 1B and Supplementary Figure 1A). At baseline, median fasting glucose was 85 (IQR, 80–90) mg/dL. Median total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides were 170.5 (IQR, 151–192), 99 (IQR, 80–121), 51 (IQR, 41–63), and 80 (IQR, 56–121). Baseline fasting metabolic parameters were also not different between CAB and PBO treated participants (Supplementary Figure 1C). No participant met the fasting glucose criterion for diabetes.
Outcomes
Among the 146 participants with paired weights, between W0 and W41 the median increase in weight for CAB-treated participants was 1.1 (IQR, –0.9, +3.0) kg; median 1.0 (IQR, –.2, +3.2) kg was gained by PBO-treated participants (∆ = +0.1 kg, P = .66). The distribution of weight changes across the 41-week treatment period did not differ between CAB- and PBO-treated participants nor when divided into the W0–W4 oral phase (∆ = +0.3 kg, P = .6) and the W5–W41 injection phase (∆ = +0.2 kg, P = .65). A 5% or greater increase in weight from W0 to W41 was seen in 24 (22%) CAB participants and in 7 (18%) PBO participants (P = .62).
Distributions of changes in weight from W0 to W41 were also not different among racial and ethnic subgroups nor by sex at birth, injectable product dose cohort, BMI category (greater than or less than or equal to the overall study population median), or baseline smoking status (Supplementary Figure 2). Distributions of changes in weight were also not different across geographic region (United States, Brazil, sub-Saharan Africa).
Frequency of transitions between BMI categories (using standard definitions) were not significantly different between arms (Supplementary Figure 1B). GEE models of all available weight data over time were consistent with the sparse analysis using only W0 and W41 data (Supplementary Tables 1–3).
Distributions of changes in fasting glucose and lipid parameters also were not different between CAB- and PBO-treated participants (Supplementary Figure 3).
DISCUSSION
Clinical experience, observational cohort data, and randomized trials strongly suggest, but do not confirm, an association between integrase inhibitor–based ART and weight gain. Observational data from treatment studies in persons living with HIV have described transitions in BMI category among integrase inhibitor–treated participants [8] that have the potential to increase risk for metabolic complications and increase risk for cardio- and cerebrovascular disease and altered glucose homeostasis. These data are potentially confounded by the presence of HIV infection and its inflammatory sequalae, a return to health phenomenon, as well as potential mitigating or exacerbating effects of nucleoside reverse transcriptase inhibitors, which are used as “backbones” of ART regimens.
We explored changes in weight and fasting metabolic parameters assessed as part of the phase 2 development program of CAB LA for HIV prevention. Absent HIV infection and potentially confounding additional antiretroviral drugs, we found no significant differences in changes in weight or fasting metabolic parameters between participants randomized to CAB or PBO. The observed weight changes were modest (approximately 1.0 kg in each arm over approximately 9.5 months).
Important limitations to this analysis are its post hoc exploratory design, nonstandardization of weight measurement across study sites, and the modest sample size of the overall study and the subgroups analyzed. Two ongoing double-blind, double-dummy phase 3 HIV prevention trials, HPTN 083 (NCT02720094) and HPTN 084 (NCT03164564), with a planned total enrollment of 7700 participants will provide more definitive data to address this question.
The modest sample size limited our ability to rule out weight change differences smaller than approximately 2.4 kg between arms. However, if these negative results are confirmed in the larger ongoing phase 3 prevention studies of CAB, it is possible that the weight increases seen in treatment studies of HIV-infected individuals are attributable to differential effects of integrase inhibitors on the HIV-affected immunologic milieu, a molecule-specific (rather than class) effect, and/or bystander activity of other antiretrovirals. It is also possible that participants who did not receive the full complement of injections in each cohort (Figure 1A) and therefore were exposed to waning CAB levels over the observation period further diluted any metabolic effects. TDF/emtricitabine was associated with a 5% or greater unintentional weight loss in the iPrEX study [12]; absence of weight gain, if confirmed, could increase the acceptability of integrase inhibitor–based preexposure prophylaxis. While we did not observe a difference in fasting glucose between arms, we did not collect fasting insulin/homeostatic model assessment for insulin resistance, a more sensitive measure for detecting changes in glucose tolerance.
In a moderately sized randomized study of CAB vs PBO in HIV-uninfected participants, no differences in changes in weight or fasting metabolic parameters were apparent between study arms. Ongoing phase 3 efficacy studies of CAB for HIV prevention will provide an opportunity to further examine these potential relationships.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The study team thanks the study participants and their families; the clinical research staff at the 8 sites conducting the study; and Carlee Moser, PhD, for her helpful discussions and review of the manuscript.
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH; grants UM1AI068619, UM1AI068613, and UM1AI068617). ViiV Healthcare provided study products for the conduct of HPTN 077.
Potential conflicts of interest. R. J. L. received personal fees from Gilead Sciences, Merck Inc, and Roche. J. J. E. received grants from the NIH and grants and personal fees from ViiV Healthcare, Gilead Sciences, Merck, and Janssen. H. D. received personal fees from Adcock Ingram South Africa and MSD–South Africa and support from MSD–South Africa and Pfizer. M. M. received grants from NIH/Division of AIDS (DAIDS)/HPTN. A. Y. L. received support from Gilead Sciences. D. A. M. received support from ViiV Healthcare. A. R. received support from ViiV Healthcare. M. S. C. received support from Merck and Gilead. J. S. C. received grants from Theratechnologies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5:41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gatell JM, Assoumou L, Moyle G, et al. ; NEAT022 Study Group . Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31:2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis 2012; 12:111–8. [DOI] [PubMed] [Google Scholar]
- 4. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390:2073–82. [DOI] [PubMed] [Google Scholar]
- 5. Sculier D, Doco-Lecompte T, Yerly S, Metzner KJ, Decosterd LA, Calmy A. Stable HIV-1 reservoirs on dolutegravir maintenance monotherapy: the MONODO study. HIV Med 2018; 19:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourgi KJC, Rebeiro PF, Lake JE, et al. Greater weight gain among treatment-naive persons starting integrase inhibitors [abstract 670]. In: the Conference on Retroviruses and Opportunistic Infections Seattle, WA, 2019. [Google Scholar]
- 7. Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018; 73:2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design . Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388: 776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spreen WR, Margolis DA, Pottage JC Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.