Plasma levels of fungal antigen (1→3)-β-D-Glucan are elevated during early and chronic human immunodeficiency virus (HIV) infection and do not normalize despite long-term antiretroviral therapy. Such elevation is associated with immune activation in a well-defined cohort of untreated and treated people living with HIV.
Keywords: HIV, immune activation, microbial translocation, (1→3)-β-D-glucan, antiretroviral therapy
Abstract
Background
Microbial translocation from the gut to systemic circulation contributes to immune activation during human immunodeficiency virus (HIV) infection and is usually assessed by measuring plasma levels of bacterial lipopolysaccharide (LPS). Fungal colonization in the gut increases during HIV-infection and people living with HIV (PLWH) have increased plasma levels of fungal polysaccharide (1→3)-β-D-Glucan (βDG). We assessed the contribution of circulating DG to systemic immune activation in PLWH.
Methods
Cross-sectional and longitudinal assessments of plasma βDG levels were conducted along with markers of HIV disease progression, epithelial gut damage, bacterial translocation, proinflammatory cytokines, and βDG-specific receptor expression on monocytes and natural killer (NK) cells.
Results
Plasma βDG levels were elevated during early and chronic HIV infection and persisted despite long-term antiretroviral therapy (ART). βDG increased over 24 months without ART but remained unchanged after 24 months of treatment. βDG correlated negatively with CD4 T-cell count and positively with time to ART initiation, viral load, intestinal fatty acid–binding protein, LPS, and soluble LPS receptor soluble CD14 (sCD14). Elevated βDG correlated positively with indoleamine-2,3-dioxygenase-1 enzyme activity, regulatory T-cell frequency, activated CD38+Human Leukocyte Antigen - DR isotype (HLA-DR)+ CD4 and CD8 T cells and negatively with Dectin-1 and NKp30 expression on monocytes and NK cells, respectively.
Conclusions
PLWH have elevated plasma βDG in correlation with markers of disease progression, gut damage, bacterial translocation, and inflammation. Early ART initiation prevents further βDG increase. This fungal antigen contributes to immune activation and represents a potential therapeutic target to prevent non–acquired immunodeficiency syndrome events.
(See the Editorial Commentary by Hoenigl on pages 242–4.)
Human immunodeficiency virus (HIV) infection is characterized by a rapid decline in CD4 T-cell count, early gut mucosal damage, and subsequent translocation of microbial products from the leaky gut [1, 2]. Microbial translocation encompasses the translocation of microbes and/or their products into systemic circulation and contributes to systemic immune activation as well as the development of acquired immunodeficiency syndrome (AIDS) and non-AIDS events [2–5]. Microbial translocation is usually determined by measuring plasma levels of markers of bacterial translocation such as lipopolysaccharide (LPS), LPS-binding protein (LBP), and soluble LPS receptor CD14 (sCD14). While the gut microbiome consists of bacteria, fungi, archaea, viruses, and eukaryotic microbes, there has been limited investigation into the consequences of translocation of nonbacterial products in health and disease [6, 7]. There is emerging evidence regarding elevated circulation of fungal products in people living with HIV (PLWH) [8–10].
Fungal cell walls are composed of mannoproteins, chitins, and α- and β-linked glucans [11]. (1→3)-β-D-Glucan (βDG) represents one of the most abundant components of fungal cell walls and serves as a potent pathogen-associated molecular pattern in triggering antifungal immunity [12]. Detection of circulating βDG is currently used for the presumptive clinical diagnosis of Candida, Aspergillus, and Pneumocystis jiroveci infections [13]. Reports have shown elevated plasma levels of βDG in PLWH without invasive fungal infection (IFI). Elevated plasma levels of βDG together with increased plasma levels of sCD14 and reduced abundance of lactobacilli in the distal gut have been associated with cardiopulmonary and neurocognitive dysfunction [8–10]. While previous findings support a potentially important role for circulating βDG during HIV infection, they were conducted in small cohorts. Furthermore, these studies did not assess whether elevated circulation of βDG plays an active role in inducing systemic immune activation.
Inflammation in PLWH is characterized by increased plasma levels of proinflammatory cytokines, increased monocyte activation, increased frequency of activated T cells, increased indoleamine-2,3-dioxygenase-1 (IDO-1) activity, and increased frequency of regulatory T cells (Tregs) [1, 14–18]. βDG is known to induce systemic immune activation during fungal infections by interacting with βDG-specific receptors to trigger the production of proinflammatory cytokines [19, 20]. βDG may contribute to systemic immune activation in PLWH by interacting with Dectin-1 and NKp30 on monocytes and natural killer (NK) cells, respectively, to induce the production of proinflammatory cytokines. Such inflammation will activate myeloid and T cells. βDG has been previously shown to induce IDO-1 activity in vitro, which could promote Treg differentiation in PLWH [15, 21].
In this report, we cross-sectionally and longitudinally quantified βDG in PLWH without IFI during both early and chronic stages of infection, including those who are antiretroviral therapy (ART) naive and those receiving ART. We determined the significance of elevated βDG in PLWH by assessing its correlation with markers of disease progression, gut damage, bacterial translocation, and immune activation.
METHODS
Study Design and Population
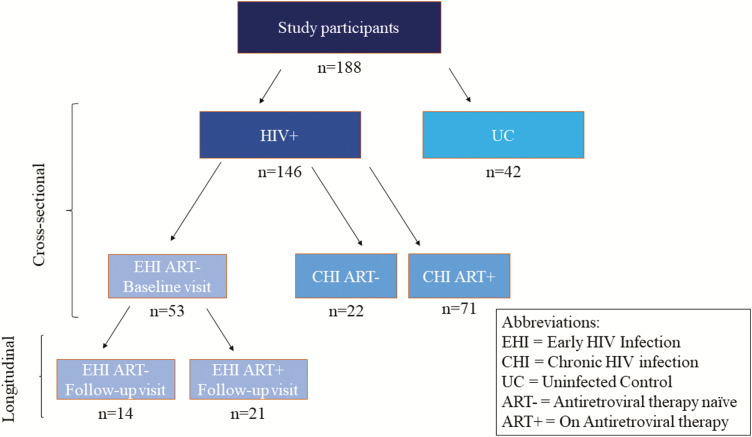
In a cross-sectional analysis, a total of 146 adult PLWH were enrolled from the Montreal Primary HIV Infection Study, Chronic Viral Illness Service, at the McGill University Health Centre, and Canadian HIV and Aging Cohort Study [22]. PLWH were categorized into those with early HIV infection (EHI) (n = 53), defined as being within 6 months of the estimated date of infection using the criteria by the National Institutes of Health Acute HIV Infection and Early Diagnosis Research Program [22], and those with chronic HIV infection (CHI) who were either untreated (n = 22) or ART treated (n = 71) [23]. PLWH were compared with 42 HIV-uninfected controls (UCs) who were also enrolled from the Montreal Primary HIV Infection Study at the Chronic Viral Illness Service as well as the Canadian HIV and Aging Cohort Study. We prospectively followed 35 PLWH for 2 years, 21 of whom were followed before and after 2 year on ART while 14 participants were remained ART naive (Figure 1). All participants were fasting at the time of blood collection. Participants were excluded if they presented with P. jiroveci pneumonia (PJP), oral and/or esophageal candidiasis, Aspergillus infection, chronic colitis, or any other acute conditions [22]. To account for potential confounders, we recorded the usage of antibiotics, renal and liver function, serum lipid levels, and co-infections and adjusted their influence on βDG.
Figure 1.
Study design and classification of study participants. The study population included 146 PLWH and 42 UCs. PLWH were then further classified into various subgroups depending on the duration of infection and whether they had initiated treatment. Abbreviations: ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; PLWH, people living with HIV; UC, uninfected control.
Laboratory Measurements
HIV infection was diagnosed by measuring plasma HIV-1 p24 antigen/antibody and confirmed by Western blot as previously reported [13, 22]. Plasma viral load was measured by the Abbott RealTime HIV-1 assay (Abbott Laboratories). CD4 and CD8 T-cell counts were measured using 4-color flow cytometry [22, 24]. Plasma and peripheral blood mononuclear cell samples of study participants were stored at −80°C and in liquid nitrogen, respectively.
Measurement of Plasma βDG and Galactomannan Levels
Plasma βDG was measured by the Fungitell Limulus Amebocyte Lysate (LAL) assay (Associates of Cape Cod, Inc) in duplicate as per the manufacturer’s instructions. Plasma galactomannan levels were quantified using the Platelia assay (Bio-Rad Laboratories).
Measurement of Markers of Epithelial Gut Damage and Bacterial Translocation
Intestinal fatty acid–binding protein (I-FABP) and LPS were measured as previously described [15]. sCD14 was measured in duplicate by immunoassay (Quantikine; R&D Systems).
Multiplex Measurement of Soluble Inflammatory Markers
Plasma levels of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-8 were measured in duplicate using the Meso Scale Discovery (MSD) U-Plex Pro-Inflammatory Combo 4 kit.
Measurement of IDO-1 Enzyme Activity
Tryptophan and kynurenine were measured in plasma using an automated on-line solid-phase extraction–liquid chromatographic–tandem mass spectrometric method [25].
Flow Cytometry Analyses
Frozen peripheral blood mononuclear cells were rapidly thawed and stained for 20 minutes at 4°C using 2 fluorochrome-conjugated antibody panels (Supplemental Figure 1). Cells were then washed and fixed in 2% paraformaldehyde for acquisition. Tregs were characterized as previously described [15]. Fluorescence minus one color controls were used to discriminate autofluorescence from positive signals. Cells were acquired using a BD Fortessa X20 (BD Biosciences) flow cytometer. Data were analyzed using FlowJo 10.0.7 (FlowJo, LLC).
Statistical Analyses
Descriptive analyses were performed with means and standard deviations calculated for the variables with normal distribution and the median with interquartile range calculated for variables with a nonnormal distribution. Unpaired comparisons were conducted using t tests or Mann-Whitney U tests, as appropriate. Paired comparisons were conducted using Wilcoxon matched-pairs test. Spearman rank correlation test identified associations between 2 quantitative measures. The Kruskal-Wallis test was used to compare more than 2 study groups. P values < .05 were considered significant. Multivariable linear regression analysis was conducted to determine the independent association of βDG with HIV infection adjusting for confounding factors. SPSS 24.0 (IBM SPSS) and GraphPad Prism 7.0 (GraphPad Software) were used to perform statistical analyses.
Ethical Considerations
This study was approved by the McGill University Health Centre research ethics board and by each participating medical center. It was conducted in accordance with the principals of the Declaration of Helsinki, and all study participants provided written informed consent for study enrollment.
RESULTS
Study Participant Characteristics
The median (interquartile range) age of participants was 48 (36–56) years and 87.1% were male. Untreated PLWH had a significantly lower CD4 T-cell count, 480 (321–658 cells/μL), which improved among those receiving ART to 552 (410–691). Conversely, untreated PLWH had a higher CD8 T-cell count (772 [611–1073) cells/μL] than those receiving ART (727 [552–953]). Median log10 viral loads per milliliter of plasma in ART-naive EHI and CHI groups were 4.5 (4.2–5.1) and 5.1 (4.4–5.5), respectively. PLWH receiving ART all had suppressed viremia of less than 50 copies/mL (Table 1).
Table 1.
Characteristics of Study Participants
| Characteristics | EHI ART− (n = 42) | EHI ART+ (n = 11) | CHI ART− (n = 22) | CHI ART+ (n = 71) | UCs (n = 42) |
|---|---|---|---|---|---|
| Age, y | |||||
| Median (IQR) | 34 (31–42) | 40 (29–45) | 38 (33–50) | 55 (50–61) | 52 (46–59) |
| Range | 21–53 | 22–58 | 22–56 | 26–75 | 23–76 |
| Sex, no. (%) | |||||
| Male | 55 (98.2) | 11 (100) | 17 (77.3) | 64 (90.1) | 31 (73.8) |
| Female | 1 (1.8) | 0 (0) | 5 (22.7) | 7 (9.9) | 11 (26.2) |
| CD4 T cells/µL | |||||
| Median (IQR) | 480 (260–670) | 530 (430–660) | 220 (35–345) | 546 (408–700) | 821 (519–1022) |
| Range | 210–910 | 369–912 | 3–489 | 161–1083 | 281–1173 |
| CD8 T cells/µL | |||||
| Median (IQR) | 750 (640–1120) | 590 (392–820) | 770 (406–1147) | 734 (557–997) | 373 (273–535) |
| Range | 300–1460 | 268–1325 | 54–1425 | 300–1213 | 188–843 |
| CD4:CD8 | |||||
| Median (IQR) | 0.6 (0.4–0.8) | 0.8 (0.6–1.4) | 0.2 (0.1–0.4) | 0.7 (0.5–1.0) | 2.1 (1.2–3.0) |
| Range | 0.2–1.2 | 0.5–1.9 | 0.1–1.0 | 0.4–1.2 | 0.4–4.0 |
| VL, log10 copies/mL | |||||
| Median (IQR) | 4.5 (4.2–5.1) | <1.7 | 5.1 (4.4–5.5) | <1.7 | NA |
| Range | 2.8–5.5 | NA | 3.9–5.5 | NA | NA |
N = 146 people living with HIV and 42 UCs.
Abbreviations: ART, antiretroviral therapy; ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; UC, uninfected control; VL, viral load.
βDG Levels Were Elevated in HIV-infected Participants and Did Not Change With ART
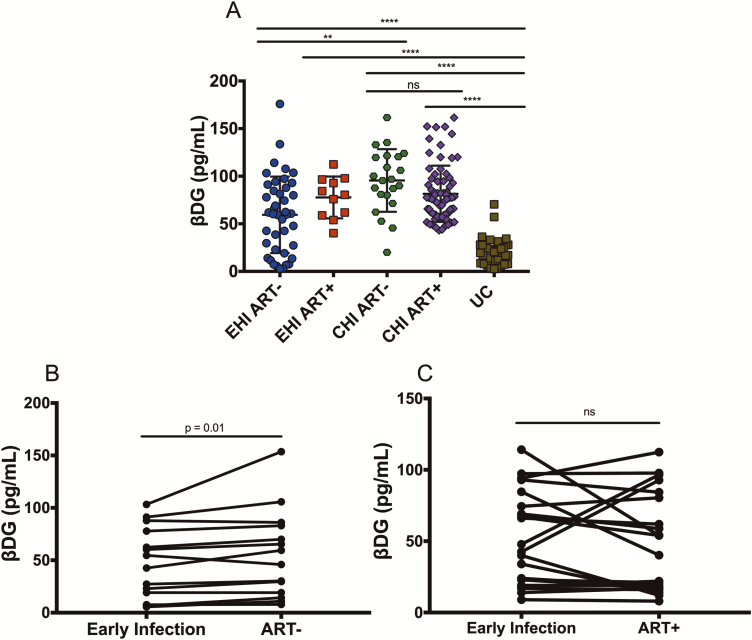
Cross-sectional analysis showed higher plasma βDG levels in EHI (67.9 ± 40.7 pg/mL) and CHI (88.5 ± 30.9 pg/mL) participants compared with UCs (20.4 ± 13.3 pg/mL) (P < .001 for both, Kruskal-Wallis test with Dunn’s post test). Elevated βDG levels among PLWH were independent of age, sex, CD4 and CD8 T-cell count, I-FABP, and LPS (data not shown). βDG did not correlate with plasma levels of glucose, cholesterol, creatine, albumin, low-density lipoprotein, high-density lipoprotein, or triglycerides. PLWH with or without syphilis, gonorrhea, and/or mononucleosis co-infections had no difference in their βDG levels (data not shown). There was no difference in plasma levels of βDG in males compared with females among either PLWH or HIV-uninfected controls (data not shown). βDG was higher in CHI than in EHI individuals (P < .001) and did not differ on ART (Figure 2A). Longitudinal assessment of 14 PLWH showed that βDG increased from 47.8 ± 33.8 pg/mL to 55.8 ± 41.9 pg/mL over a 24-month interval in the EHI group not receiving ART (Figure 2B; P = .01). Conversely, 21 EHI participants who initiated ART during follow-up had stable βDG levels after 24 months (Figure 2C; P > .99). Nonparametric analyses showed that duration of ART had no influence on βDG in CHI participants (r = −0.123, P = .328) while duration of infection before initiation of ART was correlated with βDG (r = 0.254, P = .04; data not shown). This suggests that early initiation of ART limits βDG plasma levels in PLWH. Plasma levels of galactomannan are used for the clinical diagnosis of Aspergillus diseases and were confirmed to be negative in our participants (data not shown) [26]. As expected, higher plasma levels of LPS were observed in EHI (68.72 ± 36.81 pg/mL) and CHI (91.86 ± 25.7 pg/mL) participants compared with UCs (28.31 ± 16.82 pg/mL) (P < .001; data not shown). In contrast to plasma βDG, lower levels of plasma LPS were detected in CHI participants receiving ART as compared with those not receiving treatment (P < .001) (data not shown).
Figure 2.
Cross-sectional and longitudinal plasma levels of βDG during different stages of HIV infection. A, βDG concentrations increased from EHI to CHI and remained unchanged with ART (EHI ART−, n = 42; EHI ART+, n = 11; CHI ART−, n = 22; CHI ART+, n = 71; UCs, n = 42). B, Longitudinal analysis showed an increase in βDG levels over 24 months in the absence of ART (n = 14). C, Longitudinal analysis showed that 24 months of ART, initiated during EHI, prevented an increase in βDG plasma levels (n = 21). P values show Kruskal-Wallis tests with Dunn’s post hoc test between different groups. Abbreviations: ART, antiretroviral therapy; ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; UC, uninfected controls; βDG, (1→3)-β-D-glucan. *P < .05; **P < .01; ***P < .001; ****P < .0001.
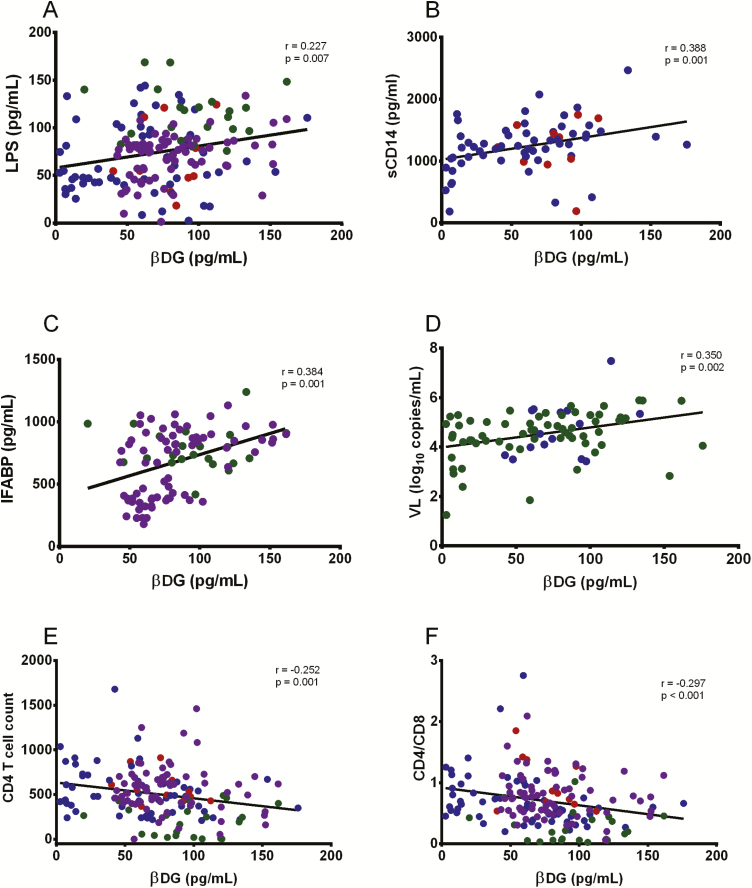
βDG Levels Were Associated With Markers of Bacterial Translocation, Gut Mucosal Damage, and HIV Disease Progression
Plasma βDG levels were positively correlated with LPS (r = 0.227, P = .007) (Figure 3A) and sCD14 (r = 0.388, P = .001) (Figure 3B). Plasma βDG levels were also positively correlated with increased plasma levels of I-FABP in CHI participants (r = 0.384, P = .001) but not in EHI participants (data not shown) (Figure 3C). βDG levels correlated positively with viral load (r = 0.350; P = .002) (Figure 3D) in untreated PLWH and inversely with CD4 T-cell count (r = −0.252; P = .001) (Figure 3E) and CD4:CD8 ratio (r = −0.297; P < .001) in untreated PLWH (Figure 3F) suggesting βDG as a marker of disease progression [5]. PLWH with CD4 T-cell count below 200 cells/μL were receiving trimethoprim and sulfamethoxazole as PJP prophylaxis, these individuals did not present with higher plasma βDG levels compared to PLWH with CD4 T-cell count >200 cells/μL (Figure 3E).
Figure 3.
Comparison of βDG with markers of bacterial translocation, gut damage, and HIV disease progression. A, βDG levels correlated with plasma LPS in EHI and CHI participants (ART+ and ART−) (n = 146). B, βDG concentrations correlated with plasma sCD14 in EHI participants (ART+ and ART−) (n = 67). C, βDG concentrations in plasma were associated with plasma I-FABP in CHI participants (n = 93). D, βDG concentrations were correlated with plasma viral load in untreated participants (n = 78). E, βDG concentrations were correlated with CD4 T-cell count in EHI and CHI participants (ART+ and ART−) (n = 146). F, βDG concentrations were correlated with CD4-to-CD8 ratio in EHI and CHI participants (ART+ and ART−) (n = 146). P values show nonparametric Spearman correlations. EHI ART− = blue; EHI ART+ = red; CHI ART− = green; CHI ART+ = purple. Abbreviations: ART, antiretroviral therapy; ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; I-FABP, intestinal fatty acid–binding protein; LPS, lipopolysaccharide; sCD14, soluble LPS receptor; VL, viral load; βDG, (1→3)-β-D-glucan.
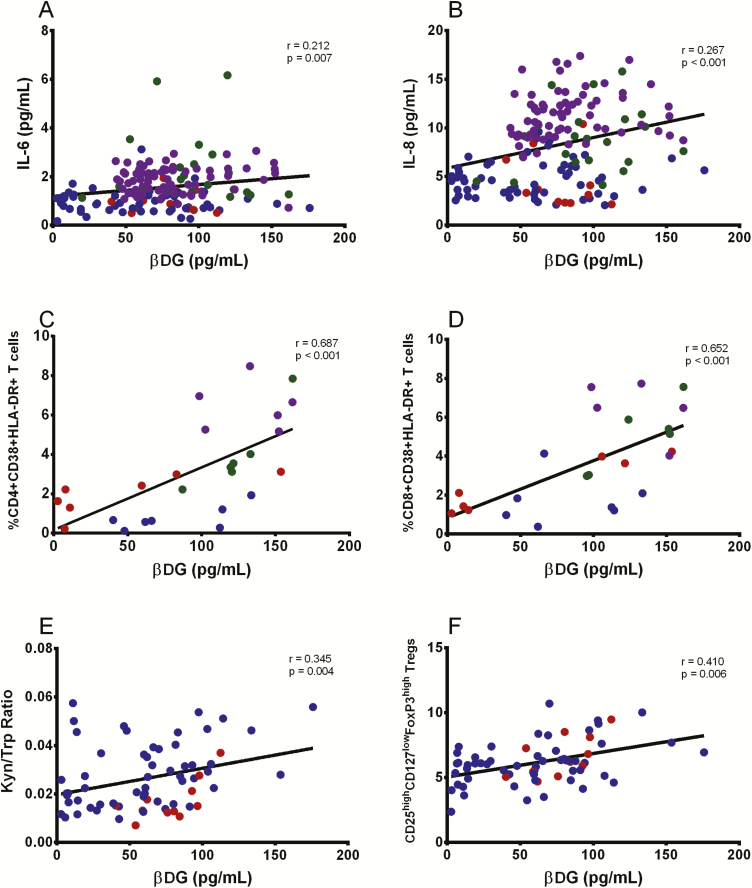
βDG Levels Correlated With Markers of Systemic Immune Activation
Plasma levels of IL-6 and IL-8, which have been linked to the development of non-AIDS events, correlated with plasma βDG levels (r = 0.212, P = .007, and r = 0.267, P < .001, respectively) (Figure 4A and 4B) [27, 28]. Conversely, plasma levels of IL-1β and TNF-α were not associated with plasma βDG levels (Supplemental Table 1). The frequency of activated CD4 and CD8 T cells, measured by co-expression of CD38 and HLA-DR, positively correlated with βDG (r = 0.687, P < .001, and r = 0.652, P < .001, respectively) (Figure 4C and 4D).
Figure 4.
βDG correlated with markers of immune activation ex vivo. βDG levels correlated with plasma IL-6 (n = 146) (A) and IL-8 (n = 146) (B) in PLWH. βDG levels correlated with frequency of CD4+CD38+HLA-DR+ T cells (n = 26) (C) and CD8+CD38+HLA-DR+ T cells (n = 26) (D) in PLWH. E, βDG levels correlated with kynurenine (Kyn)-to-tryptophan (Trp) ratio (n = 67). F, βDG levels correlated with frequency of CD25highCD127lowFoxP3high Tregs (n = 67). P values show nonparametric Spearman correlations. EHI ART− = blue; EHI ART+ = red; CHI ART− = green; CHI ART+ = purple. Abbreviations: ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; IL, interleukin; PLWH, people living with HIV; Treg, regulatory T cell; βDG, (1→3)-β-D-glucan.
βDG Levels Were Linked to IDO-1 Activity and Frequency of Regulatory T Cells
βDG levels positively correlated with the kynurenine to tryptophan ratio (r = 0.345, P = .004) (Figure 4E) as a marker of IDO-1 activity, which has been linked to gut damage and microbial translocation during HIV infection [15, 25, 28]. A positive correlation of βDG was also observed with the frequency of Tregs (r = 0.410, P = .006) (Figure 4F). Conversely, LPS did not correlate with IDO-1 activity and Treg frequency (Supplemental Table 1).
βDG Levels Correlated With a Decrease in Dectin-1 Expression on Monocytes and NKp30 Expression on Mature CD56dim NK Cells
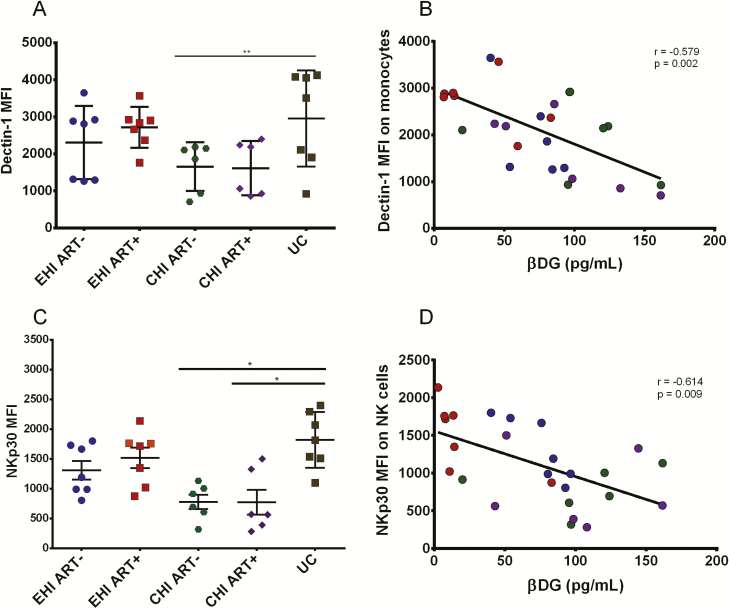
Because βDG is known to trigger Dectin-1 and NKp30, we measured expression, as the mean fluorescence intensity, of these receptors on monocytes and NK cells, respectively. Dectin-1 expression on monocytes in different groups of PLWH (Figure 5A) was inversely correlated with βDG levels (r = −0.579, P = .002) (Figure 5B) (gating strategy in Supplemental Figure 2).
Figure 5.
Analysis of βDG receptors on monocytes and NK cells ex vivo. A, Dectin-1 MFI on monocytes of participants in different stages of HIV infection (EHI ART−, n = 7; EHI ART+, n = 7; CHI ART−, n = 6; CHI ART+, n = 6; UCs, n = 7). B, βDG levels were inversely correlated with Dectin-1 MFI on monocytes during HIV infection (n = 26). C, NKp30 MFI on CD56dim NK cells of participants in different stages of HIV infection (EHI ART−, n = 7; EHI ART+, n = 7; CHI ART−, n = 6; CHI ART+, n = 6; UCs, n = 7). D, βDG levels inversely correlated with NKp30 MFI on CD56dim NK cells in HIV-infected participants (n = 26). P values show nonparametric Kruskal-Wallis test between different groups. EHI ART− = blue; EHI ART+ = red; CHI ART− = green; CHI ART+ = purple; UC = Brown. *P < 0.05, **P < 0.01. Abbreviations: ART−, antiretroviral therapy naive; ART+, on antiretroviral therapy; CHI, chronic HIV infection; EHI, early HIV infection; HIV, human immunodeficiency virus; MFI, mean fluorescence intensity; NK, natural killer; UC, uninfected controls; βDG, (1→3)-β-D-glucan.
Lower NKp30 expression levels were observed in CD56dim NK cells in PLWH compared with UCs (Figure 5C), which inversely correlated with βDG (r = −0.614, P = .009) (Figure 5D). Furthermore, plasma levels of LPS did not correlate with neither Dectin-1 (r = −0.106, P = .487) nor NKp30 (r = −0.113, P = .689) expression (Supplemental Table 1). Overall, these findings indicate a potential role of elevated βDG in activating monocytes and NK cells in an LPS-independent manner.
DISCUSSION
We confirmed in a large group of participants that plasma βDG levels were elevated in PLWH compared with UCs and further demonstrated that βDG levels increased from EHI to CHI. Using longitudinal measurements, we showed that early initiation of ART, but not duration of ART, reduced plasma βDG levels in PLWH. Furthermore, we showed associations between plasma βDG levels and inflammatory markers, implying that βDG likely contributes to systemic immune activation in PLWH.
Multivariate analysis confirmed that elevation of plasma βDG during HIV infection was independent of age, sex, CD4 and CD8 T-cell count, I-FABP, and LPS. Of note, LPS is decreasingly used as the principal marker of microbial translocation due to methodological challenges in its usual measurement by LAL assays, its low precision, and association with serum lipid levels. Furthermore, plasma levels of LPS measured by the LAL assay have been shown to have potential cross-reactivity with βDG [29]. To avoid this effect, we used an enzyme-linked immunosorbent assay to measure plasma levels of LPS; however, we found levels of circulating LPS in PLWH similar to those in previous studies using the LAL assay [1]. Apart from the LPS receptor sCD14, βDG was shown to be a better marker of disease progression and immune activation. We also report the absence of elevated levels of another fungal antigen, galactomannan, indicating that these participants did not have Aspergillus diseases. Diagnosis of PJP is partially based on plasma βDG levels above 80 pg/mL [30]. Our findings suggest that 43% of PLWH will have a false-positive result using this test, suggesting a need to re-evaluate the threshold for PLWH developing PJP or other IFI. Along these lines, elevated plasma βDG levels could be measured in PLWH without IFI because its strong association with immune activation may be a predictor for the risk of developing non-AIDS events in the ART era.
We also showed that IDO-1 activity and Treg frequency were strongly correlated with plasma βDG. We, as well as others, have shown that increased IDO-1 activity is linked to an increased frequency of Tregs, epithelial gut damage, degree of microbial translocation, immune activation, HIV disease progression, and HIV reservoir size [15, 25, 28, 31–33]. Because IDO-1 is known to be expressed in myeloid cells, which also express βDG receptors, these findings suggest that elevated βDG may participate in the induction of IDO-1 activity.
We showed significant associations between plasma βDG levels and markers of systemic immune activation. Plasma βDG levels positively correlated with the frequency of activated CD4 and CD8 T cells and plasma sCD14 and negatively correlated with Dectin-1 and NKp30 expression on monocytes and NK cells, respectively. Such relationships are supported by previous findings of βDG stimulation leading to monocyte and NK cell activation as well as IL-6 and IL-8 production via Dectin-1 and NKp30, respectively [34–37]. Our study showed there was an inverse correlation between βDG levels with Dectin-1 and NKp30 expression in PLWH, demonstrating that βDG contributes to activation of monocytes and NK cells in vivo. These findings warrant investigation into therapeutic strategies against Dectin-1 and NKp30 signaling to reduce systemic immune activation during HIV infection.
Previous studies have shown that elevated plasma levels of microbial products, such as LPS, in PLWH are a result of epithelial gut damage and subsequent microbial translocation [1]. It has also been shown that such translocation leads to mucosal dysbiosis in PLWH [15]. We do not demonstrate direct evidence that elevated βDG originates from the gut; however, we showed correlations between βDG with plasma LPS and a validated marker of epithelial gut damage, I-FABP. Furthermore, it is known that more than 60% of healthy individuals have Candida and more than 90% have Saccharomyces colonization in the gastrointestinal tract and that mucosal dysbiosis makes PLWH highly susceptible to increased proportions of fungal colonization [6]. Hence, persistence of plasma βDG despite long-term ART may be explained by the high frequency of Candida and Saccharomyces colonization, prolonged mucosal T-helper (Th) 17/Th22 dysfunction, and related translocation of fungal products [38, 39].
We acknowledge certain limitations to this study. Due to constraints in sample availability, certain measurements were conducted on a subset of participants. While clinical measurement of βDG using the Fungitell kit is validated in serum samples, its use in research [5, 8, 9, 40] has frequently relied on plasma samples. A meta-analysis by Karageorgopoulos et al [41] has shown similar average sensitivity and specificity of measuring βDG levels for the diagnosis of IFIs in serum or plasma samples. Therefore, it is unlikely that our selection of plasma samples has biased the measurement of βDG levels. We demonstrated correlations of βDG with systemic immune activation, and we did not identify the origin of elevated βDG in PLWH. This opens avenues to analyze gut epithelium to directly characterize mucosal damage as well as fecal and blood samples to show evidence of fungal colonization and translocation. Data were not available for factors such as smoking, alcohol consumption, and drug abuse, all of which are more prevalent among PLWH [22]. Future studies should consider these confounding factors to further determine the independent contribution of βDG to immune activation and its use as a prognostic marker. In addition, we did not record information regarding dietary habits of study participants. While elevated βDG can be due to a high seaweed/mushroom or health-food diet, it is unlikely that diet would differ among our Canadian study participants of PLWH and UCs.
In the ART era, despite viral suppression, PLWH still suffer from residual immune activation leading to increased risk of non-AIDS events [27]. Along these lines, elevated βDG has been associated with cardiopulmonary and neurocognitive dysfunction [8, 10]. Furthermore, Hoenigl et al [5] recently showed that plasma levels of βDG are an independent predictor of non-AIDS events in ART-treated PLWH. Overall, our study findings suggest that elevated levels of plasma βDG during HIV infection contribute to systemic immune activation via monocytes and NK cells, increased IDO-1 activity, and production of proinflammatory cytokines in an LPS-independent manner. Further, we demonstrate that early ART, rather than duration of ART, prevents an increase in βDG plasma levels in PLWH. Understanding the implications of elevated βDG levels may facilitate the development of therapeutic strategies against chronic systemic immune activation and the development of non-AIDS events despite long-term ART.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. V. M. and R. R. performed the experiments, analyzed the data, wrote the first draft, and revised the final draft of the manuscript. S. I., F. P. D., R. P., J. C., I. K., and M.-A. J. contributed to the experiments, data analysis, and critical review of the first and final drafts of the manuscript. J.-P. R. designed the study, contributed to data analysis, and critically reviewed the first and final drafts of the manuscript. N. F. B. and D. C. S. contributed to the study design and critical review of the manuscript. C. C., B. L., R. T., P. C., R. L., J.-G. B., M. D., C. C-L., C. T., and P. A. all contributed to recruitment and follow-up of study participants and critically reviewed the manuscript. All authors have read and approved the contents of this manuscript.
Acknowledgments. The authors are highly grateful to the study participants for their contribution. They thank Angie Massicotte, Josée Girouard, and Danica Albert for study coordination and assistance as well as Mario Legault, Stéphanie Matte, Olfa Debbeche, and Sylla Mohamed for coordinating the Montreal Primary HIV Infection Study and Canadian HIV and Aging Cohort Study. They also acknowledge Professor Jean-Marc Cavaillon, Institut Pasteur, Paris, France, for his scientific discussion on lipopolysaccharide and inflammation. They are thankful to the following physicians for their contribution: C. Milne, S. Lavoie, J. Friedman, M. Duchastel, F. Villielm, F. Asselin, M. Boissonnault, P. J. Maziade, S. Lavoie, M. Milne, N. Z. Miaki., M. E. Thériault., at Clinique médicale l’Actuel. B. Lessard, M. A. Charron, S. Dufresne, M. E. Turgeon, S. Vézina, E. Huchet, J. P. Kerba, M. Poliquin, S. Poulin, P. Rochette, P. Junod, D. Longpré, R. Pilarski, E. Sasseville, L. Charest, A. Hamel, A. Cloutier-Blais, S. Massoud, F. Chano, B. Trottier at Clinique médicale urbaine du Quartier Latin; L. Labrecque, C. Fortin, V. Hal-Gagne, M. Munoz, B. Deligne, V. Martel-Laferrière, B. Trottier, M. E. Goyer at Unité Hospitalière de Recherche, d’Enseignement et de Soins sur le Sida Centre Hospitalier de l’Université de Montréal Hôtel-Dieu and Notre-Dame; and M. Teltscher, A de Pokomandy, J. Cox, E. Beauchamp, L. P. Haraoui at Mcgill University Health Centre Chronic Viral Illness Service.
Financial support. This work was supported by the Fonds de la Recherche Québec-Santé (FRQ-S), Réseau SIDA/Maladies Infectieuses and Thérapie Cellulaire; the Canadian Institutes of Health Research (CIHR; grant numbers MOP 103230 and 154051); the Vaccines & Immunotherapies Core of the CIHR Canadian HIV Trials Network (grant number CTN 257); the Canadian Foundation for AIDS Research (CANFAR; grant number 02-512); and the Canadian HIV Cure Enterprise Team (grant number HIG-133050) awarded by the CIHR in partnership with CANFAR. V. M. was supported by an FRQ-S Postdoctoral Fellowship Award. R. R. is an undergraduate student supported by the H. Grenville Smith Studentship. S. I. is a postdoctoral fellow supported by the William Turner research fellowship. C. T. C. is an FRQ-S Junior 1 chercheur-boursier-clinicien. M.-A. J. holds the CIHR Canada Research Chair tier 2 in Immunovirology. J.-P. R. is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University, and the William Turner award holder from the McGill University Health Centre.
Potential conflicts of interest. R. T. reports personal fees from Gilead, Merck, and ViiV, outside the submitted work. D. C. S. reports grants and personal fees from Merck, outside the submitted work. C. T. reports grants and personal fees from Merck, Gilead, and ViiV, as well as personal fees from Theratechnologie, outside the submitted work. J-G. B. reports grants and personal fees from ViiV Healthcare, Merck Canada, and Gilead, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 22nd International AIDS Conference (AIDS-2018), Amsterdam, The Netherlands, and Conference on Retroviruses and Opportunistic Infections (CROI-2018), Boston, Massachusetts.
Contributor Information
Montreal Primary HIV Infection Study and Canadian HIV and Aging Cohort Study Groups:
C Milne, S Lavoie, J Friedman, M Duchastel, F Villielm, F Asselin, M Boissonnault, P J Maziade, S Lavoie, M Milne, N Z Miaki, M E Thériault, B Lessard, M A Charron, S Dufresne, M E Turgeon, S Vézina, E Huchet, J P Kerba, M Poliquin, S Poulin, P Rochette, P Junod, D Longpré, R Pilarski, E Sasseville, L Charest, A Hamel, A Cloutier-Blais, S Massoud, F Chano, B Trottier, L Labrecque, C Fortin, V Hal-Gagne, M Munoz, B Deligne, V Martel-Laferrière, B Trottier, M E Goyer, M Teltscher, A de Pokomandy, J Cox, E Beauchamp, and L P Haraoui
References
- 1. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 2. Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther 2016; 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009; 338:a3172. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team . Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoenigl M, Moser C, Funderburg N, et al. Soluble urokinase plasminogen activator receptor (suPAR) is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nash AK, Auchtung TA, Wong MC, et al. The gut mycobiome of the human microbiome project healthy cohort. Microbiome 2017; 5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waldman AJ, Balskus EP. The human microbiota, infectious disease, and global health: challenges and opportunities. ACS Infect Dis 2018; 4:14–26. [DOI] [PubMed] [Google Scholar]
- 8. Hoenigl M, de Oliveira MF, Pérez-Santiago J, et al. (1→3)-β-D-Glucan levels correlate with neurocognitive functioning in HIV-infected persons on suppressive antiretroviral therapy: a cohort study. Medicine (Baltimore) 2016; 95:e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoenigl M, Pérez-Santiago J, Nakazawa M, et al. (1→3)-β-d-Glucan: a biomarker for microbial translocation in individuals with acute or early HIV infection? Front Immunol 2016; 7:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris A, Hillenbrand M, Finkelman M, et al. Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr 2012; 61:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang X, Kirui A, Muszyński A, et al. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat Commun 2018; 9:2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farhour Z, Mehraj V, Chen J, Ramendra R, Lu H, Routy JP. Use of (1→3)-β-d-glucan for diagnosis and management of invasive mycoses in HIV-infected patients. Mycoses 2018; 61:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013; 21:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenabian MA, El-Far M, Vyboh K, et al. ; Montreal Primary Infection and Slow Progressor Study Groups . Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 16. Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahalingam M, Peakman M, Davies ET, Pozniak A, McManus TJ, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol 1993; 93:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noss I, Doekes G, Thorne PS, Heederik DJ, Wouters IM. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun 2013; 19:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elder MJ, Webster SJ, Chee R, Williams DL, Hill Gaston JS, Goodall JC. β-Glucan size controls dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Front Immunol 2017; 8:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karumuthil-Melethil S, Gudi R, Johnson BM, Perez N, Vasu C. Fungal β-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J Immunol 2014; 193:3308–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehraj V, Cox J, Lebouche B, et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc 2018; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durand M, Chartrand-Lefebvre C, Baril JG, et al. ; Investigators of the Canadian HIV and Aging Cohort Study . The Canadian HIV and Aging Cohort Study—determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: rationale and study protocol. BMC Infect Dis 2017; 17:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boulassel MR, Mercier F, Gilmore N, Routy JP. Immunophenotypic patterns of CD8+ T cell subsets expressing CD8alphaalpha and IL-7Ralpha in viremic, aviremic and slow progressor HIV-1-infected subjects. Clin Immunol 2007; 124:149–57. [DOI] [PubMed] [Google Scholar]
- 25. Mehraj V, Jenabian MA, Ponte R, et al. ; Montreal Primary HIV Infection and the Canadian Long-Term Non-Progressors Study Groups . The plasma levels of soluble ST2 as a marker of gut mucosal damage in early HIV infection. AIDS 2016; 30:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24(Suppl 1):e1–38. [DOI] [PubMed] [Google Scholar]
- 27. Borges AH, O’Connor JL, Phillips AN, et al. Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis 2016; 214:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong J, Zhang Y, Patidar A, Vilar E, Finkelman M, Farrington K. Is endotoxemia in stable hemodialysis patients an artefact? Limitations of the limulus amebocyte lysate assay and role of (1→3)-β-D glucan. PLoS One 2016; 11:e0164978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sax PE, Komarow L, Finkelman MA, et al. ; AIDS Clinical Trials Group Study A5164 Team . Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis 2011; 53:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehraj V, Routy JP. Tryptophan catabolism in chronic viral infections: handling uninvited guests. Int J Tryptophan Res 2015; 8:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen J, Xun J, Yang J, et al. Plasma indoleamine 2,3-dioxygenase activity is associated with the size of HIV reservoir in patients receiving antiretroviral therapy. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jenabian MA, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One 2013; 77:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heldt S, Prattes J, Eigl S, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect 2018; 77:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonfim CV, Mamoni RL, Blotta MH. TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med Mycol 2009; 47:722–33. [DOI] [PubMed] [Google Scholar]
- 36. Hefter M, Lother J, Weiß E, et al. Human primary myeloid dendritic cells interact with the opportunistic fungal pathogen Aspergillus fumigatus via the C-type lectin receptor Dectin-1. Med Mycol 2017; 55:573–8. [DOI] [PubMed] [Google Scholar]
- 37. Spear GT, Zariffard MR, Cohen MH, Sha BE. Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J Reprod Immunol 2008; 78:76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gosselin A, Monteiro P, Chomont N, et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol 2010; 184:1604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panpetch W, Somboonna N, Bulan DE, et al. Gastrointestinal colonization of Candida albicans increases serum (1→3)-β-D-glucan, without candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock 2018; 49:62–70. [DOI] [PubMed] [Google Scholar]
- 40. Hoenigl M, de Oliveira MF, Perez-Santiago J, et al. Correlation of (1–>3)-beta-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 2011; 52:750–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.