Abstract
Objective
The aim of this study was to evaluate whether the establishment of a preoperative screening and decolonization protocol for Staphylococcus aureus carriers undergoing total knee arthroplasty (TKA) could decrease the incidence of periprosthetic joint infection (PJI) caused by this microorganism.
Methods
We conducted a retrospective study comparing a control group comprising 400 patients (134 men, and 266 women; mean age: 72.2 ± 6.8 years) who went through surgery between January 2009 and December 2013, with a second intervention group of 403 patients (125 men, and 278 women; mean age: 72.4 ± 6.9 years) in which the protocol of screening and decolonization of S. aureus nasal carriers was applied between January 2014 and December 2016. During this latter period patients were preoperatively screened and, if positive, treated with mupirocin nasal ointment and chlorhexidine soap, for 5 days prior to surgery.
Results
In the control group, 17 of 400 patients (4.2%) had a SSI, 8 (2%) of them caused by S. aureus and 9 (2.2%) by other microorganisms. In the intervention group 20.6% of patients had a positive S. aureus nasal swab and were treated according to the protocol. 5 of 403 patients (1.2%) in this group had a SSI, 1 (0.2%) due to S. aureus and 4 (1%) to other microorganisms. When comparing surgical-site infection (SSI) rates between the two groups, we found a statistically significant reduction in both global SSI (p = 0.009) and specifically S. aureus SSI (p = 0.02), in the intervention group. No decolonized S. aureus nasal carrier presented a SSI.
Discussion
In patients undergoing TKA a preoperative screening and decolonization protocol for S. aureus nasal carriers, using mupirocin nasal ointment and chlorhexidine soap, is an effective measure to reduce the rate of SSI caused by this microorganism.
Level of Evidence
Level III; Therapeutic Study.
Keywords: S. aureus, TKA infection, Screening protocol, Decolonization protocol, TKR infection
Introduction
Periprosthetic joint infection is one of the most challenging complications after total knee arthroplasty (TKA).1, 2 S. aureus is considered to be a major pathogen implicated in surgical site infection (SSI) generally and in prosthetic joint infection specifically.3
It is well known that up to one third of the population is colonized with S. aureus, the anterior nares being the most common location for isolation of this organism, but which also resides on skin surfaces as extranasal colonization.4, 5 The incidence of SSI by S. aureus after TKA has been estimated to be 63% of all cases and this is the most commonly identified organism in such infections.5 It has been shown that being a nasal carrier of S. aureus is a significant risk factor for developing a SSI. The association between S. aureus nasal colonization and infection was first reported in 1931.5 Since then, it has been well established that development of SSI involving S. aureus is associated with preoperative nasal colonization with the microorganism.5 Furthermore, some studies have proven an endogenous origin of these infections in more than 80% of cases.3, 4, 5 A molecular DNA analysis of S. aureus isolates causing SSI reveals that a majority of the infecting strains are part of the patient's resident normal nasal flora.4, 5, 6
Major morbidity and the enormous healthcare costs of total knee arthroplasty infections make preventive measures to reduce infection rates of huge importance.7, 8, 9 Thus, a reasonable approach to decrease postoperative S. aureus SSI could be elimination of S. aureus nasal carriage from patients prior to surgery. Currently, there is good bibliographic evidence showing that preoperative decolonization of S. aureus carriers decreases post-operative risk of infection, although there is little consensus between screening and decolonization protocols among studies.3, 5, 10, 11, 12, 13, 14, 15, 16, 17, 18 Thus, when evaluating the literature, we can find different detection and decolonization protocols, most of them using topical nasal mupirocin, but not all of which include decolonization of extranasal sites. The possibility of universal decolonization for patients undergoing TKA is also reported, although this is still a subject of much debate due to the potential development of mupirocin resistance.18, 19, 20, 21 Furthermore, not all of these screening and decolonization protocols are universally applied, frequently because of logistical difficulties in their implementation. Even though the beneficial effects of eradicating S. aureus carriage before surgery are well established, survey results show that only between 37% and 60% of hospitals in the United States have implemented decolonization strategies.22, 23
The purpose of our study was to evaluate whether in our hospital, the establishment of a screening and decolonization protocol of intranasal carriers of S. aureus among patients who underwent total knee replacement, using mupirocin nasal ointment and chlorhexidine soap, could reduce the incidence of post-surgical infection by this microorganism.
Materials and methods
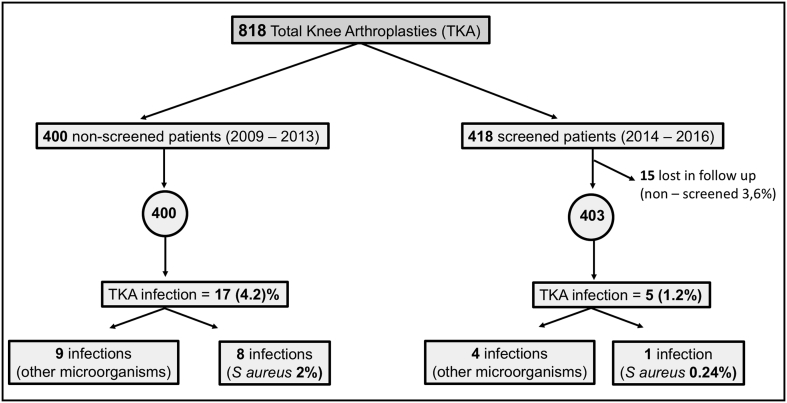
A retrospective study was conducted including a total of 818 consecutive patients who had undergone primary TKA in our hospital during the reviewed period. One group of patients (n = 400) operated on between January 1, 2009 and December 31, 2013 served as historical controls. The second group of 418 patients comprised the intervention group, whose surgery was performed between January 1, 2014 and December 31, 2016. In this group a protocol of nasal S. aureus carrier detection and decolonization was applied. However, 15 patients in this cohort did not undergo screening for a variety of reasons, and were therefore excluded from our analysis. The intervention group therefore comprised n = 403 patients.
To assess comparability of the groups, data recorded for every patient was: age, gender, co-morbidities such as diabetes mellitus or rheumatoid arthritis, National Nosocomial Infection Surveillance System (NISS) risk score, which includes the scale of the American Society of Anesthesiologists (ASA), status and surgical time, and if there was a SSI or not (by S. aureus or any other microorganism).
Medical records of all study patients were examined for 1 year after surgery to determine if any developed an infection. Periprosthetic joint infection (PJI) was defined in accordance with the criteria of the Musculoskeletal Infection Society.24
The type of infection was recorded as superficial incisional, deep incisional, or organ space/joint, according to definitions established by the Centers for Disease Control and Prevention.25 The superficial infections were confirmed by a positive culture of the drainage of the surgical incision together with the local symptoms and a negative intra-articular culture.
The control group did not undergo preoperative screening before the implantation of the TKA. In the intervention group, patients were screened for nasal S. aureus 3–4 weeks before surgery. Nasal swab samples were collected by a trained nurse during the pre-anaesthesia visit or during scheduled preoperative tests or educational programs. Each swab was cultured on an enriched broth medium (thioglycollate, bioMerieux) which was incubated for 24 h at 37 °C. Afterwards each broth was subcultured aerobically on selective mannitol salt agar medium. Biochemical identification and sensitivity analysis was performed on MicroScan (Pos Combo Panel 38, Beckman). The result of the culture was reviewed by the nosocomial infection nurse and, in all positive cases, she arranged a new appointment with the patient before surgery to provide and familiarise him/her with the decolonization kit consisting of 2% mupirocin nasal ointment (Bactroban Nasal) and 4% chlorhexidine-gluconate soap. Intranasal mupirocin had to be applied three times a day, with the last dose taken the night before surgery, and chlorhexidine soap used as a daily shower, both for 5 days prior to surgery.
Preoperative antibiotic prophylaxis protocol was the same in both groups: 2 g of cefazolin or 1 g of vancomycin in patients allergic to betalactamic antibiotics or known to be methicillin-resistant S. aureus (MRSA) carriers. In the intervention group, we implemented other additional measures such as a modification of the surgical site skin antiseptic from povidone-iodine to alcoholic chlorhexidine.
Statistical analysis
Statistical analysis to estimate the cumulative incidence of infection and its confidence interval was performed using the asymptotic approximation of the Binomial distribution to Normal distribution.
Statistical significance was defined as p < 0.05. All statistical analysis was performed using the statistical package R 3.3.3 version for Windows.
Results
In total, 803 patients were analysed, 400 included in the historical control group and 403 in the intervention (screening and decolonization) group (Fig. 1). There was a loss of 15 patients (3.6%) in the intervention group due to some administrative problems. In the intervention group 18.7% were positive for MSSA and 1.9% were positive for MRSA. Both groups were comparable regarding gender, age, NNIS score and co-morbidities. Only the ASA score in the intervention group was changed, but the difference was only marginally significant (Table 1).
Fig. 1.
Participants flow chart.
Table 1.
Baseline Demographics of TKA patients in control (2009/13) and intervention (2014/16) groups.
| Variables | 2009/13 n = 400 | 2014/16 n = 403 | p value | |
|---|---|---|---|---|
| Age | Mean (SD) | 72.2 (6.8) | 72.4 (6.9) | p = 0.48 |
| Median (Q1–Q3) | 73.0 (68.0–77.0) | 73.0 (68.8–77.2) | ||
| Sex | Man | 134 (33.5%) | 125 (31%) | p = 0.32 |
| Female | 266 (66.5%) | 278 (69%) | ||
| NNIS | 0 | 257 (64.2%) | 251 (62.3%) | p = 0.69 |
| 1 | 118 (29.5%) | 128 (31.7%) | ||
| 2 | 24 (6.0%) | 24 (6%) | ||
| Missing | 1 (0.2%) | 0 (0.0%) | ||
| ASA | I | 32 (8.0%) | 19 (4.7%) | p = 0.05 |
| II | 305 (76.2%) | 265 (65.8%) | ||
| III | 62 (15.5%) | 117 (29%) | ||
| Missing | 1 (0.2%) | 2 (0.5%) | ||
| R. Arthritis | No | 387 (96.8%) | 390 (96.8%) | p = 1.00 |
| Yes | 13 (3.2%) | 13 (3.2%) | ||
| D. Mellitus | No | 306 (76.5%) | 334 (77.7%) | p = 0.6 |
| Yes | 94 (23.5%) | 90 (22.3%) | ||
| Surgery time | Mean (SD) | 91.2 (38.5) | 92.9 (29.6) | p = 0.44 |
| Median (Q1–Q3) | 81.0 (58.0–119.0) | 84.0 (74.0–105.0) |
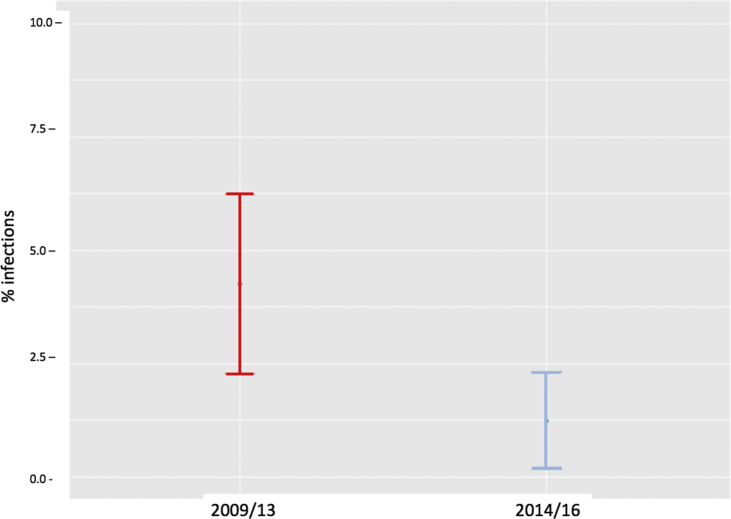
The number of patients in the control group with a surgical site infection was 17, representing a cumulative incidence of infection of 4.25% (95% confidence interval [CI] 2.27%–6.23%) (Fig. 2). Of these infections, 7 were classified as superficial infection and 10 as organ space/joint, according to definitions established by the Centers for Disease Control and Prevention.25
Fig. 2.
Cumulative incidence of all infections in control patients and in patients who underwent preoperative screening and decolonization. Comparison of proportions test: Chi2 = 6.82, df = 1, p-value = 0.009.
The number of infections decreased to 5 in the intervention group, corresponding to a significant reduction in the cumulative incidence of infection to 1.24% (95% CI 0.16%–2.32%) (Fig. 2). Of these, 2 infections were superficial and 3 were classified as organ space/joint.
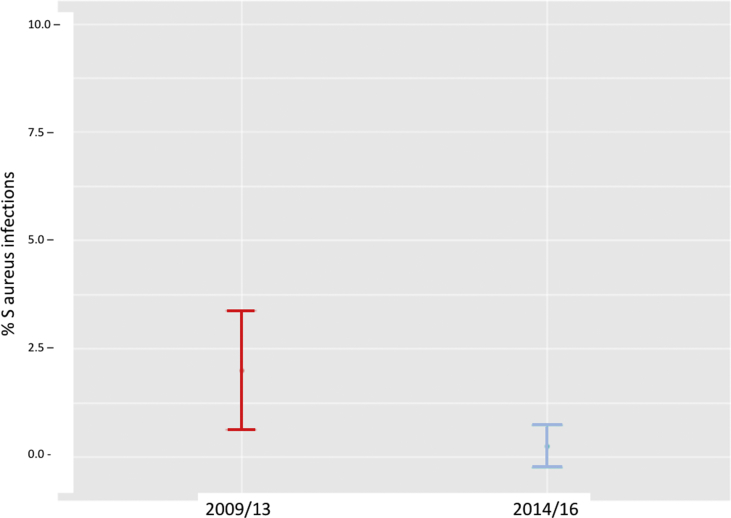
The cumulative incidence of infection by S. aureus specifically (all of them MSSA) was also significantly lower in the intervention group (with only 1 infection by S. aureus in a non-carrier patient) compared to 8 in the control group. These figures represent a rate of 2% in the control group (95%CI 0.63%–3.37%) compared to 0.24% in the intervention group (95%CI 0.00%–0.73%) (Fig. 3).
Fig. 3.
Cumulative incidence of S. aureus infection in control patients and in patients who underwent preoperative screening and decolonization. Comparison of proportions test: Chi2 = 5.56, df = 1, p-value = 0.01838/Fisher test p-valor = 0.02043.
These values represented a reduction in global SSIs of 71% and a reduction in specific S. aureus SSIs of 88%. Furthermore, no decolonized nasal carrier of S. aureus presented a surgical site infection by this microorganism.
Discussion
Surgical site infections involving S. aureus are a major adverse event in TKA. Any program to reduce its incidence would be important. Implementing a screening and targeted decolonization strategy in daily practice is complicated. But the logistical challenges of applying such protocols can be overcome within a population undergoing elective surgery in a medium size hospital like ours, with a low daily number of surgeries.
Microbiological identification of S. aureus carriers susceptible to decolonization can be achieved by different laboratory methods. In our study, we used conventional culture of nasal swabs, which is a commonly used and accepted method to identify nasal carriers of S. aureus and valid for our study population of patients undergoing elective surgery.3, 4, 6, 11, 15, 16, 26, 27 Nevertheless, other authors have used the real-time PCR diagnostic test to identify nasal carriers because of its rapidity, which can be an advantage when rapid detection is a priority.28
In our study we detected an incidence of 20.6% of S. aureus nasal carriers, with an incidence of only 1.9% for MRSA. Surveillance studies suggest similar colonization rates in the general population, with variations worldwide, and with a rate of 20% for persistent nasal carriage and 30% for intermittent carriage.29 Chen et al in a literature review found a prevalence for MSSA nasal carriage of between 21% and 30% and between 1% and 19% for MRSA in different studies, including patients undergoing a TKA or Total Hip Arthroplasty (THA).14
In our work, we evaluated the reduction in SSI rates after applying a protocol to detect S. aureus nasal carriers before surgery and subsequent preoperative decolonization using a combination of nasal mupirocin ointment and chlorhexidine body wash for 5 days prior to surgery. Using this protocol, no nasal carrier of S. aureus who was decolonized subsequently presented a surgical site infection by this microorganism, with an overall incidence of S. aureus SSI of 0.2% (1 case in a patient who was a not a nasal carrier). Hacek et al found a fourfold decrease of S. aureus SSI in a group of 223 patients identified as colonized with S. aureus and treated preoperatively with mupirocin alone.27 In the current literature, the reduction in incidence of S. aureus SSI when using mupirocin alone was only 29%–57%, compared to 13%–81% using both mupirocin and chlorhexidine, or up to 200% with mupirocin and triclosan.6, 28, 29, 30, 31, 32, 33 When analysing the effectiveness of decolonization protocols, some studies have reported a persistent incidence of nasal colonization after decolonization using mupirocin alone of as high as 20%.10, 34 Moroski et al also found a significant decrease in S. aureus colonization using only nasal decolonization in a population of Total Arthroplasties, although 5.2% of the patients remained MSSA positive and 0.35% MRSA positive postoperatively.16 Treatment failure risks when using mupirocin alone have been associated with colonization at multiple anatomical sites, longer hospitals stays and bacterial resistance to the antibiotic.35 Therefore, it seems reasonable to avoid using mupirocin alone for these protocols. Chlorhexidine body wash is recommended by the CDC for preoperative skin preparation and it is frequently used as an adjunct to mupirocin ointment in decolonization protocols to reduce bacterial density at extra-anatomical sites. Alternatives for mupirocin ointment include neomycin and fusidic acid, but clinical trials comparing the effectiveness of these agents to mupirocin are lacking. Anderson et al, in a study of MSSA and MRSA decolonization in total joint arthroplasties, also recommended the use of effective iodine or chlorhexidine-based agents for nasal application, which would avoid the potential for the emergence of antibiotic resistance.18 This method was also recommended in a recent study by Parvizi et al.21 In fact, nasal povidone-iodine was shown to be equally effective when compared to mupirocin in preventing deep S. aureus SSI.35 Nevertheless, the World Health Organization (WHO) strongly recommends using mupirocin 2% for treating known nasal carriers, while they give no recommendation for or against the use of a chlorhexidine body wash for the purpose of decolonization.36 For our study, we finally decided to follow the recommendations of the WHO.
In our study the global SSI incidence was decreased by 71% after applying the decolonization protocol. Regarding specific S. aureus SSI, almost half of the SSIs in the control group were caused by S. aureus while only 20% were caused by this microorganism after implementing the screening and decolonizing protocol. Thus, the reduction in S. aureus infection rate was 88% and was statistically significant. On this issue, our findings were similar to previous studies. For example, Stambough et al in a large series including more than 4000 patients (THA and TKA), observed a significant decrease in incidence of S. aureus SSI from 0.5% to 0.09% after applying the protocol.8 Chen et al in a systematic review analysed 19 studies which examined the ability of the decolonization protocol to reduce SSIs in elective orthopaedic (total joints, spine, and sports) and trauma patients.13 Furthermore, they observed a reduction in total SSIs of between 13% and 200% and a decrease in specific S. aureus SSI of between 40% and 200% for MSSA and 29%–149% for MRSA.13 However, their review found that there was a wide range of study designs from retrospective to prospective, and while all of the studies included in the meta-analysis suggested a decrease in the rates of SSI with decolonization, five of them were underpowered and the changes found did not reach statistical significance. Furthermore, in these studies the protocol also saved costs when comparing the costs of screening and decolonization with the reduction of SSI.13 However, other authors using the same screening and decolonization protocol have observed a decrease in S. aureus SSI which was not statistically significant, and they concluded that no clear benefit could be demonstrated using the protocol, and recommended performing a mega-trial investigating the efficiency and cost-effectiveness of a decolonization strategy in nasal S. aureus carriers.3, 11, 35, 36
However, our study has certain limitations. First of all it is a retrospective non-aleatorized study, although both groups had similar characteristics regarding risk factors for infection. The SSI rate in the control group was 4.2%, which is above the universally accepted prevalence for SSI in TKR of less than 2%.7, 37, 38 In the intervention group, in order to improve this abnormal average, we implemented other additional measures such as a modification of the surgical site skin antiseptic from povidone-iodine to alcoholic chlorhexidine. Furthermore, we started a fast-track recovery protocol in our centre. Using this protocol, we achieved among other advantages, a shorter hospital stay and a preoperative optimization of our patients for different medical conditions such as anemia. These measures may have had an impact on the reduction in the global rate of prosthetic infection in the intervention group. Nevertheless, the incidence of specific S. aureus SSI was decreased in to a greater degree compared to global SSI after implementing the screening and decolonization protocol. In our opinion, this reduction in the specific S. aureus SSI was fundamentally related to the implemented protocol.
Another limitation of our study is that we did not determine preoperatively the proportion of patients successfully decolonized. This would have added implementation difficulties and costs. The effectiveness of mupirocin in the decolonization of S. aureus nasal carriage was demonstrated in a meta-analysis which found a success rate of up to 90% after one week of follow-up, which decreased to 60% after at least two weeks.39 Even higher rates (98%) of decolonization have been achieved, when using mupirocin together with chlorhexidine soap in a joint elective and urgent arthroplasty population.40 Moreover, in our protocol decolonization was carried out only in the 5 days before surgery, minimizing possibilities of recolonization. In fact, we found no SSI in any decolonized nasal carrier of S. aureus.
In conclusion, our findings show that a screening and nasal and extranasal decolonization program for S. aureus prior to TKA resulted in a significant decrease in S. aureus surgical site infections. Our results provide further evidence to support the use of such protocols as a routine in patients undergoing TKA.
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Xavier Pelfort, Email: xavierpelfortlopez@gmail.com.
Alba Romero, Email: alba.romero.c@gmail.com.
Montserrat Brugués, Email: mbrugues@csa.cat.
Amparo García, Email: agarciag@cli.cat.
Sergi Gil, Email: sgilgo@csa.cat.
Anna Marrón, Email: annamarronpuigdueta@gmail.com.
References
- 1.Kurtz S., Ong K., Lau E. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–781. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S., Lau E., Watson H. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61–62. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Sousa R.J., Barreira P.M., Leite P.T., Santos A.M., Ramos M.H., Oliveira A.F. Preoperative Staphylococcus aureus screening/decolonization protocol before total joint arthroplasty - results of a small prospective randomized trial. J Arthroplasty. 2016;31:234–239. doi: 10.1016/j.arth.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Bode L.G., Kluytmans J.A., Wertheim H.F. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. New Engl J Med. 2010;362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 5.Weiser M.C., Moucha C.S. The current state of screening and decolonization for the prevention of Staphylococcus aureus surgical site infection after total hip and knee arthroplasty. J Bone Joint Surg Am. 2015;97:1449–1458. doi: 10.2106/JBJS.N.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen A.F., Heyl A.E., Xu P.Z., Rao N., Klatt B.A. Preoperative decolonization effective at reducing Staphylococcal colonization in total joint arthroplasty patients. J Arthroplasty. 2013;28:18–20. doi: 10.1016/j.arth.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Slover J., Hass J.P., Quirno M., Phillips M.S., Bosco J.A. Cost-effectiveness of a Staphylococcus aureus screening and decolonization program for high-risk orthopaedic patients. J Arthroplasty. 2011;26:360–365. doi: 10.1016/j.arth.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Stambough J.B., Nam D., Warren D.K. Decreased hospital costs and surgical site infection incidence with a universal decolonization protocol in primary total joint arthroplasty. J Arthroplasty. 2017;32:728–734. doi: 10.1016/j.arth.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Courville X.F., Tomek I.M., Kirkland K.B., Birhle M., Kantor S.R., Finlayson S.R. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2012;33:152–159. doi: 10.1086/663704. [DOI] [PubMed] [Google Scholar]
- 10.Kalmeijer M.D., Coertjens H., van Nieuwland-Bollen P.M. Surgical site infections in orthopaedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo controlled study. Clin Infect Dis. 2002;35:353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 11.Hadley S., Immerman I., Hutzler L., Slover J., Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;ID924518:1–4. doi: 10.1155/2010/924518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer M., Perencevich E., McDanel J. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopaedic surgery: systematic review and meta-analysis. BMJ. 2013;346:1–13. doi: 10.1136/bmj.f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A.F., Wessel C.B., Rao N. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res. 2013;471:2383–2399. doi: 10.1007/s11999-013-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A.F., Wessel C.B., Rao N. Reply to the letter to the editor: Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Clin Orthop Relat Res. 2013;471:3712–3713. doi: 10.1007/s11999-013-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao N., Cannella B.A., Crossett L.S., Yates A.J., McGough R., Hamilton C.W. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection. Prospective cohort study with 2-year follow-up. J Arthroplasty. 2011;26:1501–1507. doi: 10.1016/j.arth.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Moroski N.M., Woolwine S., Scharzkopf R. Is preoperative staphylococcal decolonization efficient in total joint arthroplasty. J Arthroplasty. 2015;30:444–446. doi: 10.1016/j.arth.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Sporer S.M., Rogers T., Abella L. Methicillin-resistant and methicillin-sensitive Staphylococcus aureus screening and decolonization to reduce surgical site infection in elective total joint arthroplasty. J Arthroplasty. 2016;31:144–147. doi: 10.1016/j.arth.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Parvizi J., Shohat N., Gehrke T. Prevention of periprosthetic joint infection. Bone Joint J. 2017;99:3–10. doi: 10.1302/0301-620X.99B4.BJJ-2016-1212.R1. [DOI] [PubMed] [Google Scholar]
- 19.Jain R., Kralovic S.M., Evans M.E. Veterans Affairs initiative to prevent methicillin-resistant Staphyloccocus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 20.Dancer S.J., Christison F., Eslami A. Is it worth screening elective orthopaedic patients for carriage of Staphyloccocus aureus? A part-retrospective case-control study in a Scottish hospital. BMJ Open. 2016;6:011642. doi: 10.1136/bmjopen-2016-011642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson M.J., David M.L., Scholz M. Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares. Antimicrob Agents Chemother. 2015;59:2765–2773. doi: 10.1128/AAC.04624-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kline S., Highness M., Herwaldt L.A., Perl T.M. Variable screening and decolonization protocols for Staphylococcus aureus carriage prior to surgical procedures. Infect Control Hosp Epidemiol. 2014;35:880–882. doi: 10.1086/676866. [DOI] [PubMed] [Google Scholar]
- 23.Diekema D., Johansson B., Herwaldt L. Current practice in Staphylococcus aureus screening and decolonization. Infect Control Hosp Epidemiol. 2011;32:1042–1044. doi: 10.1086/661917. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J., Tan T., Goswami K. The 2018 Definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309–1314. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 25.Horan T.C., Gaynes R.P. Surveillance of nosocomial infections. In: Mayhall G.C., editor. Hospital Epidemiology and Infection Control. 3rd ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2004. pp. 1659–1702. [Google Scholar]
- 26.Economedes D.M., Deirmengian G.K., Deirmengian C.A. Staphylococcus aureus colonization among arthroplasty patients previously treated by a decolonization protocol: a pilot study. Clin Orthop Relat Res. 2013;471:3128–3132. doi: 10.1007/s11999-013-2856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hacek D.M., Robb W.J., Paule S.M., Kudrna J.C., Stamos V.P., Peterson L.R. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466:1349–1355. doi: 10.1007/s11999-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sankar B., Hopgood P., Bell K.M. The role of MRSA screening in joint-replacement surgery. Int Orthop. 2005;29:160–163. doi: 10.1007/s00264-005-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paule S.M., Pasquariello A.C., Hacek D.M. Direct detection of Staphylococcus aureus from adult and neonate nasal swab specimens using real-time polymerase chain reaction. J Mol Diag. 2004;6:191–196. doi: 10.1016/S1525-1578(10)60509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wertheim H.F., Melles D.C., Vos M.C. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 31.Nixon M., Jackson B., Varghese P., Taylor G. Methicillin-resistant Staphylococcus aureus on orthopaedic wards: incidence, spread, mortality, cost and control. J Bone Joint Surg Br. 2006;88:812–817. doi: 10.1302/0301-620X.88B6.17544. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox M.H., Hall J., Pike H. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54:196–201. doi: 10.1016/s0195-6701(03)00147-6. [DOI] [PubMed] [Google Scholar]
- 33.Baratz M.D., Hallmark R., Odum S.M., Springer B.D. Twenty percent of patients remain colonized with methicilin-resistant Staphylococcus aureus despite a decolonization protocol in patients undergoing elective total joint arthroplasty. Clin Orthop Relat Res. 2015;473:2283–2290. doi: 10.1007/s11999-015-4191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perl Trish M., Cullen Joseph J., Wenzel Richard P. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 35.Phillips M., Rosenberg A., Shopsin B. Preventing surgical site infections: a randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infect Control Hosp Epidemiol. 2014;35:826–832. doi: 10.1086/676872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhoeven P.O., Berthelot P., Chapelle C. Staphylococcus aureus screening and decolonization in orthopaedic surgery and reduction of surgical site infections. Letter to the editor. Clin Orthop Relat Res. 2013;471:3709–3711. doi: 10.1007/s11999-013-3254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO Global guidelines on the prevention of surgical site infection. http://www.who.int/gpsc/ssi-prevention-guidelines/en.
- 38.Barnes S. An enhanced benchmark for prosthetic joint replacement infection rates. Am J Infect Control. 2006;34:669–670. doi: 10.1016/j.ajic.2006.04.207. [DOI] [PubMed] [Google Scholar]
- 39.Ammerlaan H.S., Kluytmans J.A., Wertheim H.F., Nouwen J.L., Bonten M.J. Eradication of methicillin-resistant S. aureus carriage: a systematic review. Clin Infect Dis. 2009;48:922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- 40.Barbero Allende J.M., Romanyk Cabrera J., Montero Ruiz E. Eradication of Staphylococcus aureus in carrier patients undergoing joint arthroplasty. Enferm Infecc Microbiol Clin. 2015;33:95–100. doi: 10.1016/j.eimc.2014.03.004. [DOI] [PubMed] [Google Scholar]