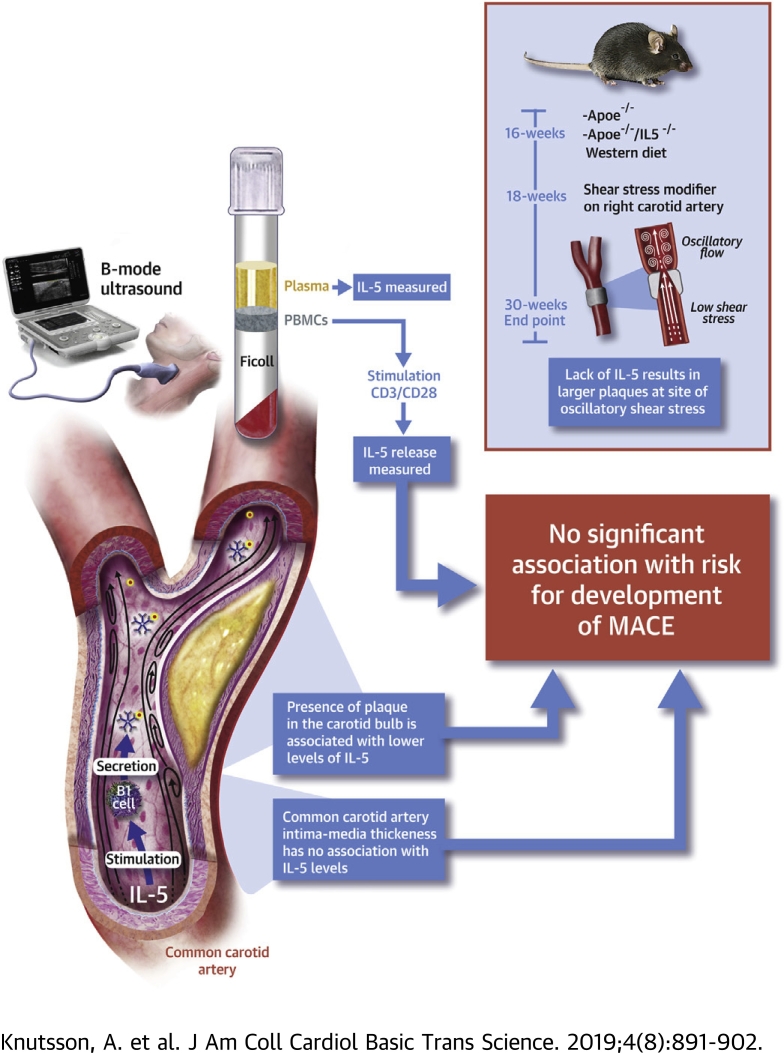
Visual Abstract
Key Words: interleukin 5, atherosclerosis, myocardial infarction, stroke
Abbreviations and Acronyms: ApoE, apolipoprotein E; CVD, cardiovascular disease; HR, hazard ratio; IL-5, interleukin-5; ILC2, type 2 innate lymphoid cells; MACE, major adverse cardiac events; OR, odds ratio
Highlights
-
•
There is strong experimental evidence that IL-5 has a protective role in atherosclerosis but the clinical importance of this remains poorly studied.
-
•
In a prospective study involving 696 subjects with a follow-up of close to 17 years we show that baseline plasma levels of IL-5 do not predict risk for coronary events and stroke.
-
•
However, subjects with high levels of IL-5 were less likely to have a carotid plaque at the baseline investigation.
-
•
Experimental studies using a shear stress-modifying cast to the carotid artery of Apoe−/− mice deficient for IL-5 showed that lack of IL-5 was associated with increased plaque formation at sites of oscillatory blood flow.
-
•
The findings are in line with previous experimental observations of an atheroprotective role of IL-5 but do not support the use of IL-5 measurement in cardiovascular risk prediction.
Summary
Experimental studies have suggested an atheroprotective role of interleukin (IL)-5 through the stimulation of natural immunoglobulin M antibody expression. In the present study we show that there are no associations between baseline levels of IL-5 and risk for development of coronary events or stroke during a 15.7 ± 6.3 years follow-up of 696 subjects randomly sampled from the Malmö Diet and Cancer study. However, presence of a plaque at the carotid bifurcation was associated with lower IL-5 and IL-5 deficiency resulted in increased plaque development at sites of oscillatory blood flow in Apoe−/− mice suggesting a protective role for IL-5 in plaque development.
Aggregation and oxidation of low-density lipoprotein (LDL) particles in the arterial intima initiates a complex array of innate and adaptive immune responses that play important roles in the development of atherosclerosis 1, 2, 3. Many of these responses are pro-inflammatory and contribute to plaque growth and de-stabilization, but a number of atheroprotective immune responses have also been identified. Antibodies reacting with danger-associated molecular patterns represent one of the best-characterized atheroprotective immune responses 4, 5. These are typically immunoglobulin M (IgM) antibodies that react with oxidation-specific epitopes on damaged cells and lipoproteins and are referred to as natural antibodies because they are germline encoded. Natural antibodies play important housekeeping functions by facilitating the removal of potentially toxic structures thus limiting inflammation and injury to the tissue (6). They are produced by a relatively small subset of B cells called B1 cells that primarily resides in the spleen and the peritoneum (7). The notion that B1 cells and natural antibodies have atheroprotective functions has gained support from experimental studies showing that transfer of B1 cells in particular reverses the proatherogenic effect of splenectomy and that this effect is dependent on the ability of B1 cells to secrete IgM antibodies 8, 9, 10. The mechanisms regulating the expression of danger-associated molecular pattern–specific IgM antibodies by B1 cells remains to be fully characterized (11), but interleukin (IL)-5 released from type 2 innate lymphoid cells (ILC2) has been identified as one important stimuli (11). The observation that deletion of ILC2 in hypercholesterolemic mice accelerates atherosclerosis adds further support to the notion of an atheroprotective role of natural antibodies produced through activation of the ILC2/B1 immune pathway (12). There is also evidence from experimental studies that IL-5 plays a critical role in this atheroprotective immune pathway. Selective genetic ablation of ILC2 in LDL receptor–deficient mice accelerates the development of atherosclerosis, which is prevented by reconstitution with wild-type but not IL5−/− ILC2 (12). Similar observations were made following reconstitution of irradiated LDL receptor–deficient mice with wild-type, but not IL-5 deficient, bone marrow (13). Moreover, Zhao et al. (14) found that a macrophage overexpression of IL-5 was associated with increased plasma levels of natural antibodies and attenuation of atherosclerosis. Despite the strong experimental evidence of an atheroprotective role of IL-5, little is known about its possible importance for development of cardiovascular disease (CVD) in man. In a case-control study comparing 931 subjects with prevalent coronary heart disease and 974 controls, Clarke et al. (15) found that those in the highest tertile of plasma IL-5 had a 50% higher risk for coronary heart disease. This observation is in apparent contrast to the atheroprotective role of IL-5 suggested by experimental studies but could possibly represent activation of protective responses in subjects with prevalent CVD. To resolve this issue, we studied the association between baseline levels of IL-5 (both in plasma and the release from activated leukocytes) and the risk for development of cardiovascular events during a follow-up period of more than 15 years. We also studied the association between IL-5 and carotid atherosclerosis in the study cohort as well as the development of carotid plaques at sites of artificially modified shear stress in Apoe−/−mice with or without IL-5 deficiency.
Methods
Study population
The study population consisted of a random sample (n = 700) recruited from the cardiovascular arm of the Malmö Diet and Cancer study (16) as previously described (17). Data regarding the incidence of major coronary events (fatal and nonfatal myocardial infarction, coronary artery by-pass grafting, and percutaneous coronary intervention) and stroke between the baseline investigation from 1991 to 1994 and December 31, 2014, were obtained from the Swedish Discharge Registry and the Cause of Death Registry of Sweden. Based on coronary event rate of 14% in the original cohort (n = 6,102), it was calculated that a subsample of 700 subjects would allow identification of a 5% difference in any given biomarker between incident coronary cases and controls with an alpha value of 0.01 and a power of 0.80. The random subsampling of 700 subjects was performed by an investigator not otherwise involved in the present study. Blood pressure, body mass index, cholesterol, smoking, and lipid levels were determined as previously described (18). Four subjects were excluded due to incomplete data. The study was approved by the Ethics Committee of Lund University and was conducted in accordance with the Helsinki Declaration. All subjects gave written informed consent.
B-mode ultrasound
Analysis of common carotid intima-media thickness (IMT) and carotid bulb plaque thickness were performed at the baseline investigation using an Acuson 128 CT system (Siemens AG, Erlangen, Germany) with a 7-MHz transducer as described previously (18). Images of IMT and plaque thickness were obtained in the longitudinal projection showing the thickest intima-media complex. Plaque was defined as a focal thickening of the IMT exceeding 1.2 mm and a plaque area >10 mm2 (19).
Isolation of mononuclear leukocytes
Blood was collected in heparin-containing BD Vacutainer tubes, (Becton Dickinson, Franklin Lakes, New Jersey) and mononuclear leukocytes isolated with FicollPaque Plus (GE Healthcare, Waukesha, Wisconsin) density gradient centrifugation according to the instructions of the manufacturer. The isolated cells were then suspended in 500 μl autologous serum with 500 μl 20% cold dimethyl sulfoxide in Roswell Park Memorial Institute (RPMI) 1640 medium, transferred into cryofreezing containers, and then frozen at −80°C for at least 1 h or overnight. The tubes were then transferred to −140°C and stored until analysis. At the time of analysis, cells were thawed, washed with phosphate-buffered saline supplemented with 1% human serum, and centrifuged at 330g for 10 min at room temperature. The cells were then resuspended in RPMI 1640 media containing 10% human serum (Gibco, Life Technologies, Bleiswijk, the Netherlands) and different T cell subsets (CD4+ T cells, CD4+/interferon-γ+ Th1 cells, CD4+/IL-4+ Th2 cells, CD4+/FoxP3+ regulatory T cells, and CD8+ T cells) were analyzed by flow cytometry as previously described 17, 20, 21.
IL-5 analysis
To determine the release of activated mononuclear leukocytes, 4 × 105 cells were cultured in complete RPMI and stimulated with CD3/CD28 beads (MiltenyiBiotec, Bergisch Gladbach, Germany) for 72 h at 37°C in a cell incubator (5% CO2). Thereafter, the cell supernatants were stored at −80°C until analysis. The concentration of IL-5 in plasma and conditioned leukocyte cell culture medium were determined by multiplex technology (MesoScale Discovery, Gaithersburg, Maryland).
Experimental animal study
Apolipoprotein E–deficient (Apoe−/−) mice (B6.129P2-Apoetm1Unc/J, The Jackson Laboratory) and IL-5–deficient mice (C57BL/6-Il5tm1Kopf/J, The Jackson Laboratory) were crossed. IL5−/−Apoe−/− mice were used in experiments with Apoe−/− mice as controls. Starting at the age of 16-weeks, the mice were fed an atherogenic 0.15% cholesterol-containing Western diet (WD; R638, Lantmännen, Sweden). At 18-weeks, a perivascular shear stress modifier (referred to as a cast) was placed around the right common carotid artery to generate atherosclerotic plaques by altering the pattern of hemodynamic flow, as described by Cheng et al. (22). In short, the surgery was performed under anesthesia with oxygen-carried isoflurane. Buprenorphine was administered subcutaneously at 0.1 mg/kg before and after surgery. The mice were euthanized at 30 weeks of age. Blood was collected by cardiac puncture and placed into ethylenediaminetetraacetic acid–coated tubes. Plasma was retrieved by centrifugation at 3,000 rpm for 15 min at 4 °C. The colorimetric assay Infinity Total Cholesterol (Thermo Scientific, Liverpool, United Kingdom) was used to quantify total plasma cholesterol and triglycerides and the Bio-Plex Pro Mouse Cytokine Assay (BIO-RAD) was used to quantify plasma cytokine concentrations of with IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A, and tumor necrosis factor-α. Both analyses were performed according to instructions from the manufacturer. Sera were diluted between 1:100 and 1:500 and IgM antibodies to copper-oxidized LDL, malondialdehyde modified (MDA)–LDL, and phosphoryl choline-bovine serum albumin (PC-BSA) were measured by chemiluminescent enzyme-linked immunosorbent assay as previously described (23). The animal studies were approved by the Malmoe/Lund regional ethical committee (Sweden).
Sample preparation and histologic analyses
The carotid arteries were fixed in Histochoice (Amresco), embedded in paraffin, and sectioned at 5 μm. Carotid artery sections were stained with Accustain trichrome (Masson) (Sigma-Aldrich) according to the manufacturer’s instructions to determine collagen content. Carotid artery sections were immunohistochemically stained using antibodies against Mac-2 (Cedarlane; Burlington, Ontario, Canada) and IgM (Vector Laboratories, Cat. No: BA-2020). Sections were deparaffinized and rehydrated in xylene and a graded series of alcohols before heat-induced antigen epitope retrieval was performed (pH 6.0, 20 min). The ImmPRESS HRP anti-rat (mouse absorbed) polymer detection kit (Vector Laboratories, MP-7444) was used for the MAC-2 staining procedure according to the manufacturer’s instructions. The Vectastain ABC-kit (Vector Laboratories, PK-6100) was used for the IgM staining procedure according to the manufacturer’s instructions. To detect apoptosis in the plaques, the terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labelling (TUNEL) assay kit—HRP-DAB (Abcam, ab206386)—was used according to the manufacturer’s instructions, with the exception of interchanging the counterstain methyl green with Meyer's hematoxylin. Flat preparations of descending aortas were stained with 0.3% Oil Red O for 50 min and mounted with Mountquick (Daido Sangyo Co. LTD, Tokyo, Japan). Immunohistochemically stained sections and flat preparations were scanned and digitalized using an Aperio ScanScope digital slide scanner (Scanscope Console v8.2.0.1263, Aperio Technologies, Vista, California). Image analysis was performed using BioPix iQ software (BioPixAB, Gothenburg, Sweden). The atherosclerotic carotid lesion size is expressed as absolute area.
Statistics
Values are given as mean ± SD for variables with normal distribution and as median and interquartile 25th and 75th percentiles for skewed variables. Statistical comparisons of baseline variables between cases and controls were performed using univariable Cox proportional hazards regression models. In the experimental studies Student’s t-test or the Mann-Whitney U test were used for statistical comparison between groups as appropriate. The Spearman rank test was used to calculate correlation coefficients. Kaplan-Meier plots and log rank test were used to analyze associations of IL-5 tertiles and IL-5 categories with coronary events and stroke. Cox proportional hazards regression models adjusting for the pre-specified covariates age, sex, current smoking, body mass index (BMI), diabetes, LDL, and high-density lipoprotein (HDL) cholesterol, triglycerides, systolic blood pressure, and high-sensitivity C-reactive protein (hsCRP) was used to investigate the possible influence of other risk factors on the associations of IL-5 tertiles and IL-5 categories with coronary events and stroke. The proportional hazards assumption was confirmed by visual inspection of the survival plots. In a sensitivity analysis we excluded 24 prevalent cases of major adverse cardiac events (MACE) to only study incident cases. Competing risk regression according to Fine and Gray (with deaths from causes unrelated to MACE or stroke as competing events) was used to examine the association between plasma IL-5/leukocyte IL-5 and incidence of MACE/stroke. Sub-hazard ratios (HRs) were obtained with the adjustment for potential confounding factors. Logistic regression models for used to adjust for the influence of conventional risk factors on the association between IL-5 and presence of a carotid plaque and the results presented as odds ratios (ORs) with 95% confidence interval (CI). IBM SPSS statistics version 22 was used for analyses of the clinical data and GraphPad Prism version 7 for the experimental data. A p value < 0.05 was considered statistically significant.
Results
Associations of IL-5 with risk for development of MACE and stroke
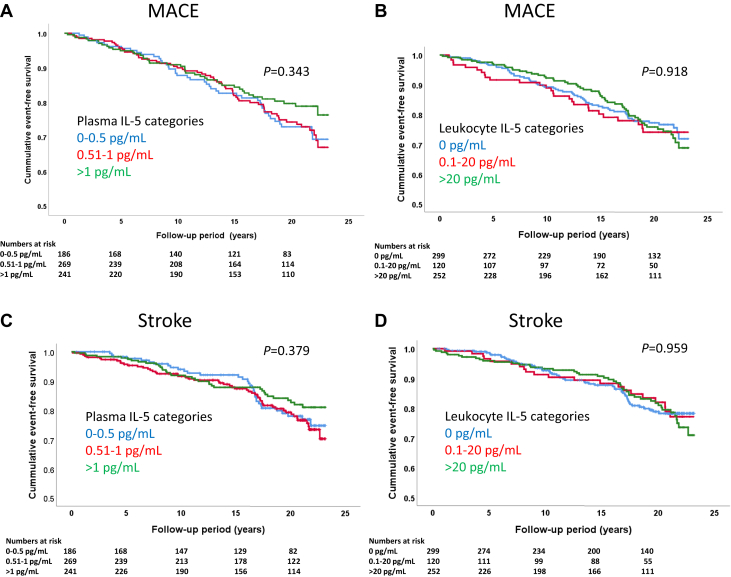
Among the 696 study subjects, a total of 142 MACE (fatal and nonfatal myocardial infarction, coronary artery by-pass grafting, and percutaneous coronary interventions) were identified in national registers during a mean follow-up time of 15.7 ± 6.3 years. Subjects with MACE were more often males and suffered from diabetes (Table 1). They also had higher baseline fasting glucose, hsCRP, and systolic blood pressure as well as lower HDL cholesterol. There was no difference in plasma levels of IL-5 between those with and without MACE (Table 1). To further investigate a possible association between IL-5 and risk of MACE, we analyzed the release of IL-5 from activated peripheral blood mononuclear cells (PBMCs). These PBMCs were isolated at the baseline investigation and stored at –140°C with more than 95% of the cells remaining viable when thawed 20 years later (24). The cells were activated by exposure to CD3/CD28 beads for 72 h followed by analysis of IL-5 in the cell culture medium. Again, we found no difference between those with or without MACE (Table 1). Because IL-5 levels both in plasma and cell medium were skewed, we categorized IL-5 levels into low, medium, and high using the intervals 0 to 0.50, 0.51 to 1.00, and >1.00 pg/ml, respectively, for plasma and 0, 0.1 to 20.0, and >20.0 pg/ml, respectively, for cell-conditioned medium; these IL-5 categories lacked association with risk of MACE (Figures 1A and 1B). Controlling for age, sex, current smoking, BMI, diabetes, LDL and HDL cholesterol, triglycerides, systolic blood pressure, and hsCRP in Cox proportional hazards regression models, there were still no significant associations between IL-5 categories and risk of MACE (HR: 0.74; 95% CI: 0.46 to 1.17; and HR: 1.30; 95% CI: 0.87 to 2.10) for the highest versus the lowest category for plasma and leukocyte release of IL-5, respectively. There were 24 cases of prevalent MACE at baseline. Excluding those did not change the lack of association between IL-5 categories and risk of MACE (HR: 0.69; 95% CI: 0.42 to 1.12 and HR: 1.39; 95% CI: 0.92 to 2.12) for the highest versus the lowest category for plasma and leukocyte release of IL-5, respectively, using the same covariates in Cox regression models as above. Adjusting plasma IL-5 levels for total circulating numbers of CD4+ T cells (106 cells/ml of blood) did not affect the lack of association between IL-5 and MACE (data not shown). The adjusted sub-HR, taking into account deaths from other causes unrelated to MACE, remained nonsignificant.
Table 1.
Baseline Clinical Characteristics and Interleukin 5 Levels in Subjects With or Without a Major Adverse Cardiac Event or Stroke During Follow-Up
| MACE |
Stroke |
|||||||
|---|---|---|---|---|---|---|---|---|
| No (n = 553) | Yes (n = 143) | HR (95% CI) | p Value | No (n = 574) | Yes (n = 122) | HR (95% CI) | p Value | |
| Age, yrs | 65.6 ± 1.1 | 65.7 ± 1.2 | 1.13 (0.97-1.31) | ns | 65.6 ± 1.1 | 65.6 ± 1.2 | 1.00 (0.85-1.18) | ns |
| Males, % | 36.7 | 52.4 | 1.78 (1.29-2.45) | <0.001 | 40.1 | 48.4 | 1.54 (1.08-2.20) | ns |
| Current smokers, % | 17.4 | 20.1 | 1.46 (0.97-2.21) | ns | 17.8 | 18.6 | 1.54 (0.97-2.46) | ns |
| Diabetes, % | 11.5 | 23.7 | 2.35 (1.59-3.47) | <0.001 | 13.3 | 17.2 | 1.53 (0.94-2.48) | ns |
| BMI, kg/m2 | 26.3 ± 4.0 | 26.7 ± 3.9 | 1.03 (0.99-1.07) | ns | 26.3 ± 4.0 | 26.3 ± 3.8 | 1.00 (0.96-1.05) | ns |
| f-glucose, mmol/l | 5.3 ± 1.4 | 5.6 ± 2.0 | 1.13 (0.97-1.31) | <0.001 | 5.3 ± 1.4 | 5.6 ± 1.8 | 1.15 (1.04-1.28) | 0.009 |
| LDL, mmol/l | 4.4 ± 1.0 | 4.4 ± 1.0 | 0.99 (0.84-1.17) | ns | 4.5 ± 1.0 | 4.2 ± 1.1 | 0.80 (0.66-0.98) | 0.028 |
| HDL, mmol/l | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.41 (0.24-0.69) | 0.001 | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.67 (0.39-1.16) | ns |
| Triglycerides, mmol/l | 1.3 (0.9-1.8) | 1.3 (1.0-1.8) | 1.16 (0.95-1.41) | ns | 1.3 (0.9-1.8) | 1.3 (1.0-1.8) | 1.17 (0.95-1.45) | ns |
| Systolic BP, mm Hg | 150 ± 19 | 155 ± 20 | 1.02 (1.01-1.02) | <0.001 | 151 ± 20 | 152 ± 19 | 1.01 (1.00-1.02) | ns |
| hsCRP, mg/l | 1.6 (0.8-3.10) | 1.7 (0.7-1.1) | 1.04 (1.01-1.07) | 0.005 | 1.6 (0.8-3.6) | 1.5 (0.6-3.1) | 1.03 (1.00-1.07) | ns |
| Plasma IL-5, pg/ml | 0.73 (0.50-1.36) | 0.70 (0.47-1.14) | 1.00 (1.00-1.01) | ns | 0.75 (0.50-1.34) | 0.65 (0.47-1.26) | 1.00 (1.00-1.01) | ns |
| Leukocyte IL-5 release, pg/ml | 3.8 (0-58.1) | 4.6 (0-84.1) | 1.00 (1.00-1.00) | ns | 4.40 (0-59.0) | 3.1 (0-62.9) | 1.00 (1.00-1.00) | ns |
Values are mean ± SD or median (interquartile range). Statistical comparisons between cases and controls were done using univariable Cox proportional hazards regression models.
BMI = body mass index; BP = blood pressure; CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio; hsCRP = high-sensitivity C-reactive protein; IL = interleukin; LDL = low-density lipoprotein; MACE = major adverse cardiac event.
Figure 1.
Kaplan-Meier Plots
Kaplan-Meier plots showing the associations of intervals of (A) plasma interleukin (IL)-5 and (B) IL-5 released from activated leukocytes and major adverse cardiac events (MACE) as well as the associations of intervals of (C) plasma IL-5 and (D) IL-5 released from activated leukocytes and stroke. No significant associations were identified using the log rank test.
There were 122 cases of incident stroke during the follow-up period. There was no difference in plasma levels of IL-5, or the release of IL-5 from activated PBMCs, between those with and without incident stroke (Table 1). There were also no associations between IL-5 categories and risk for development of stroke (Figures 1C and 1D), and this remained the same when controlling for the same covariates as above in Cox proportional hazards regression models. The adjusted sub-HR, taking into account deaths from other causes unrelated to stroke, also remained nonsignificant.
Low plasma levels of IL-5 is associated with presence of carotid plaques
To study the possible association of IL-5 with atherosclerosis severity, we determined how the presence of a plaque in the right carotid bifurcation at baseline related to plasma levels and PBMC release of IL-5. A plaque was defined as a focal thickening of the intima-media layer >1.2 mm and a plaque area >10 mm2. Subjects with carotid plaques (n = 324) had higher plasma levels of IL-5, whereas there was no significant difference for the release of IL-5 from activated PBMC between those with and without a carotid plaque (Table 2). Similar findings were made when adjusting plasma IL-5 levels for total circulating numbers of CD4+ T cells (Table 2). When adjusting for age and sex in a logistic regression model, those in the highest plasma IL-5 category (>1.0 pg/ml) had a decreased OR for the presence of carotid plaque of 0.63 (95% CI: 0.42 to 0.94; p = 0.023). However, this association became of borderline significance when also including current smoking, diabetes, LDL cholesterol, HDL cholesterol, and systolic blood pressure in the model (OR: 0.65; 95% CI: 0.42 to 1.00; p = 0.05). There was no significant association between plasma IL-5 and the IMT of the common carotid artery (r = -0.06, p = 0.124). The plasma levels of IL-5 decreased with age and correlated with hsCRP, but otherwise the IL-5 levels did not correlate significantly with cardiovascular risk factors (Table 3).
Table 2.
Baseline Clinical Characteristics and Interleukin 5 Levels in Subjects With or Without a Plaque in the Carotid Bifurcation at Baseline
| Carotid Plaque |
|||
|---|---|---|---|
| No (n = 339) | Yes (n = 324) | p Value | |
| Age, yrs | 65.7 ± 1.1 | 65.5 ± 1.2 | ns |
| Males, % | 38.9 | 43.5 | ns |
| Current smokers, % | 12.0 | 24.3 | <0.001 |
| Diabetes, % | 12.4 | 15.6 | ns |
| BMI, kg/m2 | 26.5 ± 3.8 | 25.8 ± 3.7 | <0.018 |
| f-glucose, mmol/l | 5.28 ± 1.28 | 5.46 ± 1.72 | ns |
| LDL, mmol/l | 4.34 ± 0.98 | 4.47 ± 1.05 | ns |
| HDL, mmol/l | 1.37 ± 0.36 | 1.37 ± 0.38 | ns |
| Triglycerides, mmol/l | 1.21 (0.90-1.75) | 1.31 (0.95-1.81) | ns |
| Systolic BP, mm Hg | 149 ± 20 | 153 ± 20 | <0.009 |
| hsCRP, mg/l | 1.60 (0.80-3.10) | 1.60 (0.70-3.10) | ns |
| Plasma IL-5, pg/ml | 0.81 (0.50-1.43) | 0.67 (0.49-1.13) | <0.037 |
| Plasma IL-5/106 CD4+ T cells | 1.65 (0.96-3.19) | 1.35 (0.77-2.80) | <0.046 |
| Leukocyte IL-5 Release, pg/ml |
3.47 (0-42.2) | 4.58 (0-103.1) | ns |
Values are mean ± SD or median (interquartile range). Student’s t-test or the Mann-Whitney U test was used statistical comparison as appropriate.
Abbreviations as in Table 1.
Table 3.
Associations of Interleukin 5 With Cardiovascular Risk Factors and T Cell Subsets
| Plasma IL-5 | p Value | PBMC IL-5 Release |
p Value | |
|---|---|---|---|---|
| Risk factors | ||||
| Age | −0.07 | ns | −0.11 | 0.006 |
| BMI | 0.03 | ns | 0.01 | ns |
| f-glucose | 0.00 | ns | 0.05 | ns |
| LDL | 0.00 | ns | 0.04 | ns |
| HDL | −0.05 | ns | −0.07 | ns |
| Triglycerides | −0.05 | ns | 0.04 | ns |
| hsCRP | 0.10 | 0.015 | 0.04 | ns |
| T cell subtypes | ||||
| CD4+ T cells | −0.07 | ns | 0.13 | 0.001 |
| Th1 T cells | −0.02 | ns | 0.13 | 0.001 |
| Th2 T cells | 0.04 | ns | 0.05 | ns |
| Regulatory T cells | −0.03 | ns | −0.03 | ns |
| CD8+ T cells | −0.13 | 0.001 | 0.13 | 0.001 |
| Oxidized LDL IgM | ||||
| MDA-p45 | 0.06 | ns | 0.09 | 0.036 |
| MDA-p210 | 0.01 | ns | −0.04 | ns |
Correlations are shown as Spearman rank correlation coefficients. The values for T cell subsets used in the analyses are the total numbers of each subset per milliliter of blood.
PBMC = peripheral blood mononuclear cell; other abbreviations as in Table 1.
Plasma levels of IL-5 were inversely related to the number of CD8+ T cells in the blood, but there were no significant associations with other T cell subsets including the number of Th2 (IL-4+/CD4+) cells (Table 3). When comparing the release of IL-5 from activated leukocytes, we instead found significant positive associations with both the number of CD4+ and CD8+ T cells in the blood (Table 2). Finally, we determined the association between IL-5 and 2 different oxidized LDL-specific IgM antibodies and found a weak significant association between IL-5 released from activated leukocytes and IgM against the MDA modified p45 sequence (amino acids 661-680) of apolipoprotein B-100 (Table 2). There was no significant association between the release of IL-5 from activated leukocytes and IL-5 in plasma (r = 0.01; p = 0.845).
IL-5 deficiency is associated with increased formation of plaques at sites of oscillatory stress in Apoe−/− mice
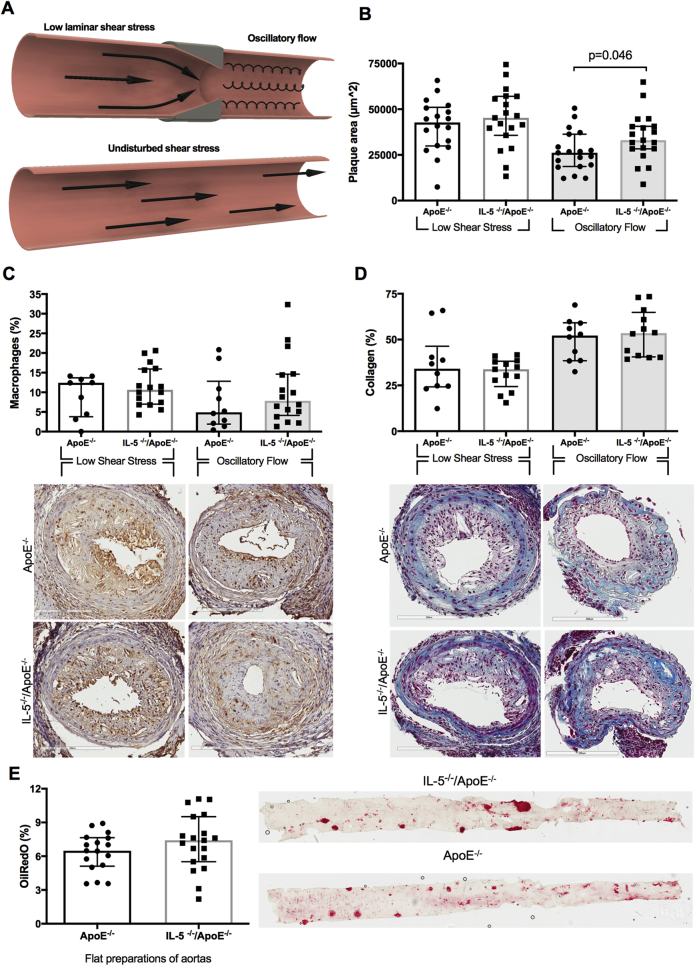
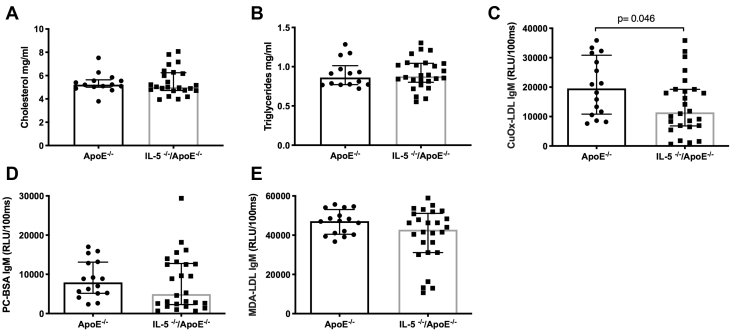
The clinical observations described above imply an association between IL-5 and atherosclerotic plaque formation at sites of oscillatory blood flow (carotid bifurcation), but not at sites with laminar flow (common carotid artery). To investigate the possible interaction between blood flow patterns, IL-5, and plaque formation, we generated IL5−/−Apoe−/− mice and applied a cast to the right carotid artery that generates a laminar, low shear stress proximal to the cast, and an oscillatory flow distal to the cast. This model generates lipid-rich, inflammatory plaques at the proximal site, whereas plaques at the distal site become more fibrous (Figure 2A). IL5−/−Apoe−/− mice developed larger plaques at the site with oscillatory flow than Apoe−/− mice with intact IL-5 expression. There were no differences in plaque size at the site with low shear, laminar flow (Figure 2B). IL-5 deficiency did not affect the macrophage and collagen content in plaques at either site (Figures 2C and 2D). Atherosclerosis in the descending aorta, a site where blood flow also is predominantly laminar with high shear stress, was assessed by en face staining with Oil Red O. Again, we found no difference between IL5−/−Apoe−/− and Apoe−/− mice (Figure 2E). IL-5 deficiency did not affect plasma levels of IL-6, monocyte chemotactic protein-1, tumor necrosis factor-α, IL-10, IL-13, and IL-17, suggesting that the IL-5-deficiency has no effect on systemic inflammation (data not shown). It also was without effect on plasma cholesterol and triglyceride levels (Figures 3A and 3B). IL-5 is known to stimulate the synthesis of the natural, germline-encoded IgM antibodies that facilitates the removal of certain micro-organisms, apoptotic cells, and oxidized LDL. In accordance, IL-5–deficient mice had lower levels of IgM against oxidized LDL, whereas there were no differences in IgM against phosphoryl choline or MDA (Figures 3C to 3E). The lower levels of IgM against oxidized LDL in IL-5–deficient mice were not associated with reduced accumulation of IgM or an increased presence of apoptotic cells in carotid plaques (Figures 4A and 4B).
Figure 2.
Effect of Interleukin 5 Deficiency on Atherosclerotic Plaque Formation in Apoe−/− Mice
(A) To induce plaque formation in Apoe−/− and IL5−/−Apoe−/− mice a cast with a fixed geometry was applied to the right carotid artery generating a laminar, low shear stress proximal to the cast and an oscillatory flow distal to the cast. (B)IL5−/−Apoe−/− mice developed larger plaques at the site with oscillatory flow than Apoe−/− mice with intact interleukin 5 (IL-5) expression. There were no differences in plaque size at the site with low shear, laminar flow. (C, D) IL-5 deficiency did not affect the macrophage or collagen content in plaques at either site. (E) Atherosclerosis in the descending aorta, a site where blood flow also is predominantly laminar, was assessed by en face staining with Oil Red O. No difference was found between IL5−/−Apoe−/−and Apoe−/− mice. Graphs are plotted as median with interquartile range. The p values were calculated using the Mann-Whitney U test.
Figure 3.
Effect of Interleukin 5 Deficiency on Plasma Lipids and Low-Density Lipoprotein–Associated Autoantibodies
Effect of interleukin 5 (IL-5) deficiency on plasma lipids and low-density lipoprotein (LDL)–associated autoantibodies in Apoe-/- mice (A,B).Apoe-/- and IL5-/-/Apoe-/- mice had similar levels of cholesterol and triglycerides. (C to E) IL-5 is known to stimulate in the expression of the natural immunoglobulin M (IgM) antibodies. In accordance, IL-5–deficient mice had lower levels of IgM against copper oxidized LDL, whereas there were no differences in IgM against phosphoryl choline and malondialdehyde. Graphs are plotted as median with interquartile range. The p values were calculated using the Mann-Whitney U test. BSA = bovine serum albumin; PC = phosphoryl choline; RLU = relative luminescence intensities.
Figure 4.
Effect of Interleukin 5 Deficiency on Immunoglobulin M Accumulation and Apoptosis
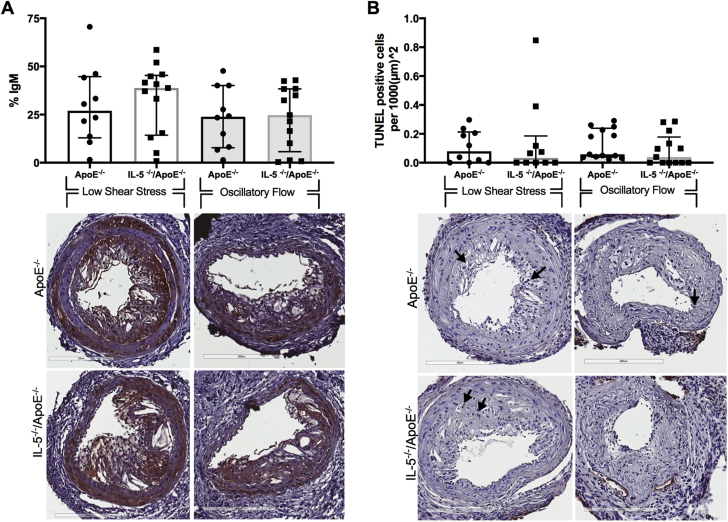
Effect of interleukin 5 (IL-5) deficiency on immunoglobulin M (IgM) accumulation and apoptosis in plaques of Apoe−/− mice IL-5 deficiency did not affect (A) the IgM content or (B) amount of terminal deoxynucleotidyl transferase deoxyuridine triphosphatase nick end labeling (TUNEL)–positive cells in plaques from low shear stress sites or in the oscillatory flow sites. Representative images of the IgM stain and TUNEL stain are shown under the respective graphs. IgM is shown in brown and TUNEL positive cells are shown with black arrows. Graphs are plotted as median with interquartile range. p values were calculated using the Mann-Whitney U test.
Discussion
In the present study, we found no association between plasma levels of IL-5 at baseline and the risk for development of coronary events during a follow-up period of more than 15 years. There was also no association between the release of IL-5 from activated leukocytes and the risk of coronary events. Accordingly, IL-5 is likely not a clinically useful marker of cardiovascular risk. Although the present observational studies do not allow any conclusions regarding causal relationships to be made, our findings do not support an important protective role of IL-5 in CVD. They are also not in line with the observations from the PROCARDIS (Precocious Coronary Artery Disease) study in which subjects with prevalent CVD had higher IL-5 levels indicating a potential harmful role of IL-5 (15). Possible explanations to the finding of higher plasma levels of IL-5 in subjects with prevalent coronary heart disease could be that protective IL-5 responses are activated as a result of a more severe atherosclerosis in these subjects or that they have a general activation of immunity. However, the present observation that subjects with a plaque in the carotid bulb have lower plasma levels of IL-5 argue against this possibility. In a study, involving 1,011 middle-aged Finnish subjects Sämpi et al. (25) also found that high plasma levels of IL-5 were associated with a smaller IMT in carotid bulb but not in the common carotid artery. Accordingly, both this and our study found that high levels of IL-5 is associated with less atherosclerosis in the carotid bulb, observations which are more in accordance with the atheroprotective effects of IL-5 found in experimental studies 12, 13, 14. In both studies, the inverse association between IL-5 and atherosclerosis was only observed in the carotid bulb, but not in the common carotid artery, suggesting that it may be dependent on the pattern of blood flow. Blood flow in the common carotid artery is laminar, producing a high shear stress that is anti-inflammatory and has an anti-atherogenic effect. In contrast, the arterial bifurcation in the carotid bulb results in an oscillatory flow and reduced shear stress that is known to activate inflammatory responses and promote atherogenesis 22, 26, 27. To further investigate how blood flow patterns and shear stress influence the effect of IL-5 on plaque development, we generated IL5−/−Apoe−/− mice and induced different shear stress patterns by implanting a shear stress modifier cast around 1 of the carotid arteries. At the distal site of the cast, where an oscillatory blood flow results in formation of predominantly fibrous lesions, there was increased plaque development in IL5−/−Apoe−/− mice as compared to IL-5 competent Apoe−/− mice. Proximal to the cast, where a laminar but low shear stress flow accelerates the formation of lesions rich in lipid and inflammatory cells, IL-5 deficiency did not affect plaque development. This observation suggests that the atheroprotective effect of IL-5 primarily is effective at sites with oscillatory blood flow. This is also well in line with the clinical observations of an inverse association between IL-5 and atherosclerosis in carotid bulb but not in the common carotid artery. It should be kept in mind that the low shear stress created by the carotid cast in the mouse model primarily is representative of the flow pattern proximal to a large plaque significantly reducing the blood flow in humans and did thus not correspond to the carotid blood flow patterns in our clinical study. The site-specificity of the atheroprotective effect of IL-5 is intriguing. Because there is no evidence that IL-5 in itself has direct influence on plaque development, it can be assumed that IL-5 is atheroprotective through stimulation of the expression of natural antibodies. Arterial bifurcations generate oscillatory blood flow resulting in impaired endothelial ability to exclude lipoprotein infiltration (28). As a result, there is commonly subendothelial aggregation and oxidation lipoproteins at such sites. It is possible that natural antibodies are particularly important for the clearance of such lipoproteins. We did not find evidence for reduced accumulation of total IgM in the plaque of IL5−/−Apoe−/− mice, but this does not exclude reduced accumulation of certain subtypes of IgM such as natural antibodies.
Several experimental studies have shown a functional role for IL-5 in the expression of natural antibodies binding to oxidized LDL 5, 29. The present observation of reduced levels of IgM recognizing oxidized LDL in IL5−/−Apoe−/− mice add further support to this notion. The relative importance of this pathway for expression of natural antibodies in humans is less well characterized. Sämpi et al. (25) found a correlation between plasma IL-5 and IgM against both oxidized and MDA-modified LDL in middle-aged Finnish cohort. In the present study, we observed an association between IgM reacting with the MDA-modified p45 amino acid sequence of apolipoprotein B-100. We have previously shown that low levels of this autoantibody is associated with an increased risk for myocardial infarction (30). This could represent a possible mechanism through which IL-5 could be atheroprotective in man, but this notion remains speculative.
There are some limitations of the present study that should be considered. In the clinical study, we only had access to IL-5 values from a single time point (e.g., the baseline investigation). There is no published data on how the plasma level of IL-5 varies over time in the same individual. A study where blood samples were repeatedly drawn from healthy volunteers during a 2-month period found correlations coefficients ranging from 0.6 to 0.9 for IL-1β, IL-6, and IL-8 suggesting that plasma cytokine levels in healthy individuals are relatively stable (31). Furthermore, it cannot be excluded that the long storage period affected the possibility to correctly analyze plasma IL-5. Finally, the cohort used in the present study may have been too small for identification of associations between IL-5 and cardiovascular risk.
In conclusion, we applied a translational approach to study the role of IL-5 in CVD. We found evidence for a protective role of IL-5 in the development of atherosclerotic plaques at sites of oscillatory blood flow but found no evidence that this was associated with a reduced risk of cardiovascular events.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Despite experimental data of an atheroprotective role of IL-5, plasma analysis of this cytokine does not help to identify subjects at risk of cardiovascular events.
TRANSLATIONAL OUTLOOK: Low levels of IL-5 are associated in presence of plaques in the carotid bifurcation, an observation that can be replicated in experimental studies.
Acknowledgments
The authors thank Maria Ozsvar Kozma for excellent technical assistance, and Yan Borné for statistical advice.
Footnotes
This study was supported by grants from the Swedish Medical Research Council, the Swedish Heart-Lung foundation, Swedish Foundation for Strategic Research, and the European Union's Seventh Framework Program (FP7/2007-2013) under grant agreement VIA n°603131. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and US Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman A.H., Binder C.J., Tsimikas S., Witztum J.L. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Lichtman A.H., Hansson G.K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder C.J., Shaw P.X., Chang M.K. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Tsiantoulas D., Diehl C.J., Witztum J.L., Binder C.J. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw P.X., Horkko S., Chang M.K. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri G., Nicoletti A., Poirier B., Hansson G.K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw T., Tay C., Krishnamurthi S. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 10.Ait-Oufella H., Herbin O., Bouaziz J.D. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgarth N. B-1 Cell Heterogeneity and the regulation of natural and antigen-induced IgM production. Front Immunol. 2016;7:324. doi: 10.3389/fimmu.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newland S.A., Mohanta S., Clement M. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat Commun. 2017;8:15781. doi: 10.1038/ncomms15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder C.J., Hartvigsen K., Chang M.K. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W., Lei T., Li H. Macrophage-specific overexpression of interleukin-5 attenuates atherosclerosis in LDL receptor-deficient mice. Gene Ther. 2015;22:645–652. doi: 10.1038/gt.2015.33. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R., Valdes-Marquez E., Hill M. Plasma cytokines and risk of coronary heart disease in the PROCARDIS study. Open Heart. 2018;5:e000807. doi: 10.1136/openhrt-2018-000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglund G., Elmstahl S., Janzon L., Larsson S.A. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 17.Wigren M., Bjorkbacka H., Andersson L. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32:2000–2004. doi: 10.1161/ATVBAHA.112.251579. [DOI] [PubMed] [Google Scholar]
- 18.Hedblad B., Nilsson P., Janzon L., Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17:299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Li C., Engstrom G., Berglund G., Janzon L., Hedblad B. Incidence of ischemic stroke in relation to asymptomatic carotid artery atherosclerosis in subjects with normal blood pressure. A prospective cohort study. Cerebrovasc Dis. 2008;26:297–303. doi: 10.1159/000149577. [DOI] [PubMed] [Google Scholar]
- 20.Engelbertsen D., Andersson L., Ljungcrantz I. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol. 2013;33:637–644. doi: 10.1161/ATVBAHA.112.300871. [DOI] [PubMed] [Google Scholar]
- 21.Kolbus D., Ljungcrantz I., Andersson L. Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med. 2013;274:41–51. doi: 10.1111/joim.12038. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C., Tempel D., van Haperen R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 23.Binder C.J., Horkko S., Dewan A. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 24.Berg K.E., Ljungcrantz I., Andersson L. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 25.Sampi M., Ukkola O., Paivansalo M., Kesaniemi Y.A., Binder C.J., Horkko S. Plasma interleukin-5 levels are related to antibodies binding to oxidized low-density lipoprotein and to decreased subclinical atherosclerosis. J Am Coll Cardiol. 2008;52:1370–1378. doi: 10.1016/j.jacc.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Gimbrone M.A., Jr., Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown A.J., Teng Z., Evans P.C., Gillard J.H., Samady H., Bennett M.R. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol. 2016;13:210–220. doi: 10.1038/nrcardio.2015.203. [DOI] [PubMed] [Google Scholar]
- 28.Tabas I., Williams K.J., Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 29.Mantani P.T., Duner P., Bengtsson E. IL-25 inhibits atherosclerosis development in apolipoprotein E deficient mice. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorkbacka H., Alm R., Persson M., Hedblad B., Nilsson J., Fredrikson G.N. Low Levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler Thromb Vasc Biol. 2016;36:765–771. doi: 10.1161/ATVBAHA.115.306938. [DOI] [PubMed] [Google Scholar]
- 31.Alves Dias J., Hellstrand S., Ericson U. Plasma variation and reproducibility of oxidized LDL-cholesterol and low-grade inflammation biomarkers among participants of the Malmo Diet and Cancer cohort. Biomarkers. 2016:1–10. doi: 10.3109/1354750X.2016.1160431. [DOI] [PubMed] [Google Scholar]