Abstract
Objective
The aim of this biomechanical experimental study was to evaluate the resistance of each posterior ligamentous complex structure of the thoracic and lumbar spine to compression forces and to measure the shifting load to the intervertebral disc when each PLC structure was interrupted.
Method
The study was conducted on 4 groups for thoracic and lumbar region as intact, supraspinous ligament interrupted, interspinous ligament/ligamentum flavum combination interrupted and facet joint capsule interrupted. Pre and post anterior vertebral body height, the highest compression force and pressure changes in the intervertebral disc during 40 N loading were measured.
Results
A significantly different degree of resistance to compression force was determined in each posterior ligamentous complex structure in the thoracic and lumbar spine samples. The combination of interspinous ligament and ligamentum flavum was found to be the most effective structure to resist compression forces (p = 0.001 in both groups). The effect of the supraspinous ligament in thoracic and lumbar segments was found to be similar to that of the interspinous ligament and ligamentum flavum combination (p = 0.008 and p = 0.006, respectively). The least effective structure was observed to be the facet joint capsule. Compression forces were significantly increased in the intervertebral disc as a result of the disruption of supraspinous ligament (p = 0.0032 and p = 0.0029, respectively in thoracic and lumbar segments) and combination of interspinous ligament/ligamentum flavum (p = 0.0019 and p = 0.0021, respectively in thoracic and lumbar segments).
Conclusion
The interspinous ligament/ligamentum flavum combination and supraspinous ligament are the largest contributor to resisting applied compression moments in the sheep thoracic and lumbar spine. As a result of the loss of resistance to compression forces, there will be a shift of a great proportion of this force onto the intervertebral disc.
Level of evidence
Level V.
Keywords: Posterior ligamentous complex, Intradiscal pressure, Traumatic disc disease, Resistance to compression forces of the spine, Interspinous ligament, Supraspinous ligament, Facet joint capsule
Introduction
Thoracolumbar spine fractures are not only an osseous pathology, the importance of ligamentous structures has been included in current classifications and treatments.1, 2, 3 Although vertebral fractures are seen as a result of high-energy trauma with the occurrence of fragmentation of the vertebra corpus, fragment diastasis and traumatic kyphosis, damage to the PLC plays an important role both in making the decision for surgical treatment and in the form of surgical treatment.
As an important factor in defining fracture stability, PLC has been included in several studies and classifications.4, 5, 6, 7 Historically, Frank Holdsworth emphasized the importance of PLC in spinal instability in 1963, classifying post-traumatic spinal instability according to fracture classification used in the current study, instability is defined as fragmentation of the bone structures, soft tissue damage and especially PLC damage.1 If PLC damage is not noticed and insufficient treatment is applied, it is clear that in the future, deformity will develop in the affected segments.5 In 2009, Vaccaro et al introduced the “thoracolumbar injury severity scale (TLISS)” and “thoracolumbar injury classification and severity score (TLICS)” as new scoring systems for spine fractures. Accordingly, PLC damage comes under one of three main headings and if the PLC is damaged, three points are scored in this system, and with 4 points in total, the fracture is considered unstable.8
In a study made on a porcine model by Callaghan et al, it was reported that after the application of repeated flexion and extension forces, disc herniation developed and that disc herniation was a cumulative process, with more severe disc herniation resulting from increased force applied to the disc.9 We have hypothesized that when the PLC is damaged, the ability of the spinal column to resist compression forces decreases as a result of the loss of posterior tension-band characteristics of the spine, which results as anterior load increase and consequently higher intervertebral disc loadings.
The resistance of components of the PLC to compression force has been well defined in literature but the effect of the injury of each PLC component under compression forces on the intervertebral disc has not been described in detail. In addition, all of other studies have been made on the lumbar spine while the thoracic spine has not been evaluated. The aim of this biomechanical experimental study was to evaluate separately the resistance of each element forming the posterior ligamentous complex of the thoracic and lumbar spine to compression forces and to measure the shifting load to the intervertebral disc when each PLC structure was interrupted.
Method
Specimen preparation
The “sheep spine” model was selected for the experiments. All specimens were obtained from a fresh meat production company. All the samples were of the same sex and similar weight, with an average age of 12 months, and no additional disease confirmed by veterinary examination. The specimens were dissected and stripped of muscle and other soft tissue but preserving the ligaments, within 2 h post-mortem. The samples were transported in appropriate cold conditions (+4 °C) to the research laboratory within an average of 2 h.
All the experiments were performed at room temperature (average 24 °C). During the preparation of the samples, care was taken not to lose water from the soft tissues, so during preparation and the experiments, the specimens were protected by wetting with 0.9% NaCl solution at regular intervals. Samples were weighed before and after the experiment. Those that did not display any irregularities were included in the experiment.
Cervical and sacral areas were excluded from the experiment. A total of 15 sheep spines were dissected into lumbar and thoracic regions with two vertebrate in each sample (dual segment model). Thoracic vertebrae were dissected as T6-T7, T8-T9, T10-T11, T12-T13 and the lumbar vertebrae were dissected as L1-L2, L3-L4, L5-L6 without creating posterior ligamentous complex or disc damage.
Experimental protocol
The samples were divided into 4 groups; Group 1(I): Intact, Group 2(SS): Supraspinous ligament interrupted, Group 3(IS + LF): Interspinous ligament and ligamentum flavum interrupted, Group 4(FJC): Facet joint capsule interrupted.
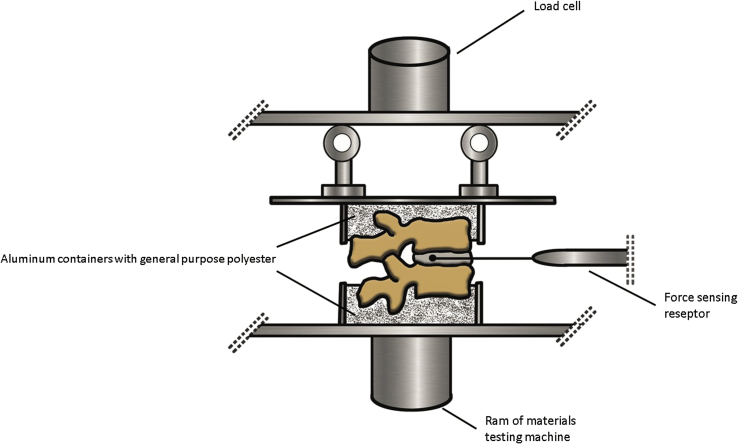
Separate experiments were applied to the lumbar and thoracic regions of the spines. There were 15 dual segment samples in each group of thoracic region and 11 in each group of lumbar region. After the segments had been divided into the above-mentioned groups, they were placed in aluminum containers with general purpose polyester which gave a low exothermic reaction and were frozen. The fracture strengths of the vertebrae were observed with the loading speed of 20 N/sec in a compression machine (ELE International, PO BOX 389, Loveland, CO 80539–0389, U.S.A.). A force sensor (Interlink Electronics, Inc.546 Flynn Road, Camarillo, CA 93012, USA) was placed in the intervertebral disc and the force transmitted on the disc was measured with a load of 40 N which was determined with preliminary experiments, and the force changes were noted. The resulting force was converted to the pressure value (N/cm2) by calculating each vertebral corpus area in centimeter-square (Fig. 1, Fig. 2).
Fig. 1.
Image of design the experiment.
Fig. 2.
Image during the experiment.
Data evaluation
The obtained data were processed and transferred to the SPSS statistics program (SPSS for Windows 15.0 SPSS Inc. 2006). As a result of the power analysis, it was concluded that 60 dual segment samples for thoracic region and 44 for lumbar region of sheep spines would suffice. The data obtained were found not to have normal distribution when evaluated with the Kolmogorov–Smirnov and Shapiro Wilk tests. Therefore, non-parametric tests were preferred to statistical tests. The Mann–Whitney U test was used for binary comparison, and the Kruskal–Wallis test was used for multiple comparisons.
Results
A total of 30 preliminary experiments were performed on both the thoracic and lumbar dual segments to determine the value of the force at the time of fracture. After initial experiments, it was observed that the lowest compression force to create vertebrae fracture was 40 N in the lumbar spine and 43.0 N in the thoracic spine. The mean corpus radius was 9.41 mm (ranged, 9.3–9,5) and 11.37 m (ranged, 10,5–12,1), the mean vertebra weight was 63.904gr (ranged, 61,22–65,12) and 97.2gr (ranged, 95,2–99,5), the mean interspinous distance was 12 mm (ranged, 11,5–12,2) and 13.53 mm (ranged, 13-13,8), respectively, for thoracic spine and lumbar spine. No statistically significant differences were determined between the specimens in each group (p > 0.05). The measurements of the vertebral anterior height taken before and after the experiment in the thoracic region were 52.28 mm (ranged, 49,98–53,35) and 23.52 mm (ranged, 21,25-24,52), respectively, and 67.26 mm (ranged, 65,21–69,25) and 30.26 mm (ranged, 28,65-31,12) in the lumbar region, respectively. A mean 45% loss of height was determined in the anterior vertebra corpus heights in both groups and this was found to be statistically significant (p = 0.004, p = 0.007, respectively, for thoracic and lumbar region).
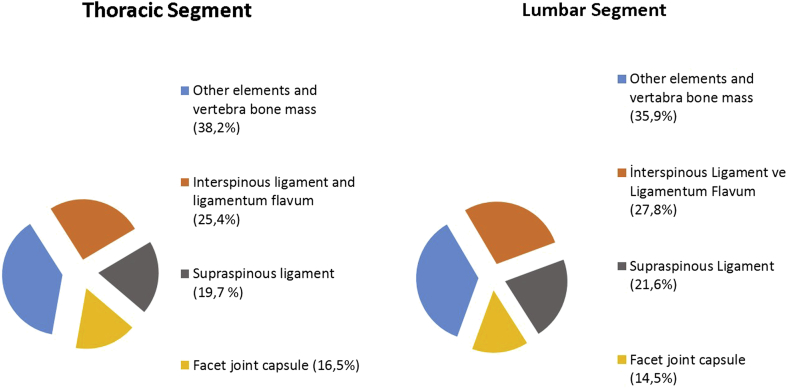
In terms of compression force necessary to form a fracture measured in the thoracic and lumbar segments, the highest values were obtained in Group 1(I) as average 72,3N (ranged, 70,5–73,7) and 77,1N (ranged, 75,5–78,8), respectively. These values were determined to be statistically significantly higher than those of the other groups. Other groups were listed as follows: Group 4(FJC) as average 64,2N (ranged, 62,1–65,1) and 59,3N (ranged, 58,2–60), Group 2(SS) as average 58,5N (ranged, 58–61,2) and 55,8N (ranged, 54,5–57,5), Group 3(IS + LF) as average 47,2N (ranged, 46,1–48,7) and 40,1N (ranged, 39–41,2), respectively, for thoracic and lumbar segments. Statistical results comparing the groups with each other are presented in Table 1. According to these results Group 3(IS + LF) showed the least resistance to compression forces in both segments. This was followed by Group 2(SS), Group 4(FJC) and Group 1(I), respectively. The contributions of each PLC structure in both segments are shown in Fig. 3.
Table 1.
Compression force values necessary to form a fracture when PLC elements are sequential resected and intact. Group 1(I): Intact, Group 2(SS): Supraspinous ligament interrupted, Group 3(IS + LF): Interspinous ligament and ligamentum flavum interrupted, Group 4(FJC): Facet joint capsule interrupted.
| Groups | Thoracic segments |
Lumbar segments |
||||
|---|---|---|---|---|---|---|
| Mean Compression force(N)a | Statistical Difference (p value) | Mean Compression force(N)a | Statistical Difference (p value) | |||
| Group 1(I) – Group 2(SS) | 72,3 | 58,5 | p = 0.008b | 77,1 | 55,8 | p = 0.006b |
| Group 1(I) – Group 3(IS + LF) | 72,3 | 47,2 | p = 0.001b | 77,1 | 40,1 | p = 0.001b |
| Group 1(I) – Group 4(FJC) | 72,3 | 64,2 | P = 0.054 | 77,1 | 59,3 | p = 0.030b |
| Group 2(SS) – Group 3(IS + LF) | 58,5 | 47,2 | p = 0.182 | 55,8 | 40,1 | p = 0.061 |
| Group 2(SS) – Group 4(FJC) | 58,5 | 64,2 | p = 1.000 | 55,8 | 59,3 | p = 1.000 |
| Group 3(IS + LF) – Group 4(FJC)b | 47,2 | 64,2 | p = 0.031b | 40,1 | 59,3 | p = 0.04b |
Mean compression forces to form a compression fracture with average 45% loss of height.
Statistically significant.
Fig. 3.
The contribution percentage of each PLC structure to resist compression forces.
Intradiscal pressure (N/cm2) measured from the intervertebral disc during the 40N compression load was as follows: Group 3(IS + LF) as mean value 10,79 N/cm2 (ranged, 9,95–12,01) and 7,65 N/cm2 (ranged, 6,68-8,85), Group 2(SS) as mean value 9,89 N/cm2 (ranged, 8,75–11,05) and 6,98 N/cm2 (ranged, 5,95–8,02), Group 4(FJC) as mean value 6.60 N/cm2 (ranged, 6,45–6,92) and 4,96 N/cm2 (ranged, 3,82–6,12), Group 1(I) as mean value 6,29 N/cm2 (ranged, 5,41–7,12) and 4,74 N/cm2 (ranged, 4,05–5,55), respectively, for thoracic and lumbar segment. The load shifting to the intervertebral disc increased at most in Group 3(IS + LF) in both segments. This was followed by Group 2(SS), Group 4(FJC) and Group 1(I), respectively. Statistical results are presented in Table 2.
Table 2.
Intradiscal pressure values measured during 40 N load and comparison between groups. Group 1(I): Intact, Group 2: Supraspinous ligament interrupted, Group 3: Interspinous ligament and ligamentum flavum interrupted, Group 4: Facet joint capsule interrupted.
| Groups | Thoracic segments |
Lumbar segments |
||||
|---|---|---|---|---|---|---|
| Mean Intradiscal Pressure (N/cm2) | Statistical Difference (p value) | Mean Intradiscal Pressure (N/cm2) | Statistical Difference (p value) | |||
| Group 1(I) – Group 2(SS)a | 6,29 | 9,89 | p = 0.032a | 4,74 | 6,98 | p = 0.029a |
| Group 1(I) – Group 3(IS + LF)a | 6,29 | 10,79 | p = 0.019a | 4,74 | 7,65 | p = 0.021a |
| Group 1(I) – Group 4(FJC) | 6,29 | 6,60 | p = 0.90 | 4,74 | 4,96 | p = 1.000 |
| Group 2(SS) – Group 3(IS + LF) | 9,89 | 10,79 | p = 1.000 | 6,98 | 7,65 | p = 0.95 |
| Group 2(SS) – Group 4(FJC) | 9,89 | 6,60 | p = 0.065 | 6,98 | 4,96 | p = 0,081 |
| Group 3(IS + LF) – Group 4(FJC)a | 10,79 | 6,60 | p = 0.031a | 7,65 | 4,96 | p = 0.04a |
Statistically significant.
Discussion
The stability of the ligaments of the spine is not only associated with intrinsic robustness. Izzo et al reported that the distance between the attachment site to the bone and the instant axis of rotation (IAR), in other words, the moment arm length, plays an important role in ligament stability. A strong ligament with a short moment arm contributes less to stability than a weaker ligament with a long moment arm.10 As the supraspinous and interspinous ligaments are located further from the IAR compared to the ligamentum flavum, they have been reported to have greater resistance to spinal flexion.11
In a biomechanical cadaveric study Yao et al hypothesized that the injury in PLC appeared in a row, starting initially from the facet joint capsule rupture to be followed by interspinous ligament, supraspinous ligament and finally ligamentum flavum. As a result, they found that the flexion and extension intervals significantly increased in those whose supraspinous and ligamentum flavum were ruptured.12 We think that injury of PLC may not always happen in a row, the row can be change due to features of trauma, both structural and age-related changes in the bone and connective tissue of the patient, so it is necessary to evaluate each ligament separately. In the biomechanical study performed by Gillespie and Dickey on a pig model, they found contributions to counteracting flexion forces as supraspinous/interspinous complex by 35.9%, intervertebral disc by 25.2% and ligamentum flavum by 24.7%.13 In this study, we hypothesized that supraspinous and interspinous ligaments should be evaluated separately. Because, the resistance to tensile forces and the flexing capacities of these structure are different from each other.21 In the literature there is no study showing that these structures are torn together. However, Pizones and colleagues have shown that ligamentum flavum always tears together with the (100%) interspinous ligament.20 Therefore we evaluated ligamentum flavum and interspinous ligament together in same group. In our study, the contributions to counteracting compression forces of the combination of interspinous ligament and ligamentum flavum was 25.4% and 27.8%, supraspinous ligament 19.7% and 21.6%, facet joint capsule 16.5% and 14.5%, other elements and the bone were 38.2% and 35.9%, respectively for thoracic and lumbar spine(Fig. 3). In a study by Heurer et al, they reported that range of motion (ROM) and lordosis angle increased after sequential removal of each posterior spinal structure. According to this, when supraspinous and interspinous ligaments were removed, an increase was determined in lumbar extension and when ligamentum flavum and facet joint capsule were removed, an increase incurred in the lumbar flexion.14 For us, the greatest limitation of this study is that it may occur intrinsic injury in the ligament when the previous step of the structure to be evaluated. We believe that in this study, the ligaments we want to evaluate is giving more accurate results because it has not been subjected to any processing before. However, they stated that the spine becomes more mobile, resulting in more stress and load transfer on the disk when PLC structures are removed. There are only a few studies in literature that examine the relationship between posterior ligamentous complex and disc. In one of these, in a biomechanical study, Heuer et al reported a 22.6% increase in axial stress on the intervertebral disc when PLC and posterior vertebral arch were removed.15 In another study made by Heuer et al, PLC structures were cut in from outside stepwise, and when each structure was cut, a significant pressure change was detected on the disc. According to this, most pressure change was the result of facet joint removal.16 As mentioned above the intrinsic injury of the ligament that may occurred in the previous phase was not taken into account in this study. In addition, only the capsule of facet joint is one of the PLC structures, not the bone structure of the facet joint. In our study, the effect of each structure was separately calculated since PLC structures were removed separately. According to this, in 40N load, approximately three-quarters of this force was measured on the intervertebral disc when supraspinous ligament and interspinous ligament/ligamentum flavum combination separately resected. Thus, compared to the PLC intact segment, there was a significant increase in the pressure on the disc when supraspinous ligament was resected (37,5% and 32,1% increase, respectively for thoracic and lumbar segments) and interspinous ligament/ligamentum flavum combination was resected (42,7% and 38,1% increase, respectively for thoracic and lumbar segments. In addition, Alanay et al, in their MRI study, did not detect any significant additional disc pathology in the conservative treatment of burst fractures with intact PLC integrity.17 These studies support the opinion that in the future after vertebral fractures with supraspinous or interspinous ligament damage, chronic post-traumatic disc diseases would develop.
Similar results with previous studies was found in our study. As Izzo et al stated that the resistance of the spine, supraspinous and interspinous ligaments to compression forces, was greater than that of the facet joint capsule because the distance between IAR and PLC increase.10 Unlike other biomechanical studies about PLC, we found no significant effect of the facet joint capsule on compression forces at the thoracic level. According to our knowledge, there is no biomechanical study about PLC on the thoracic spine, however it can be attributed to the assumption that the facet joints in the thoracic region are morphologically horizontal.
However, some limitations exist in this study. Firstly; it would have been possible to obtain results closer to reality if the study had been made on human cadavers. However, the reason for using in vitro animal models in this study was the availability of animal cadavers, they are inexpensive and show little variation. The sheep was selected as the animal model because the sheep spine is resembles the human spine in terms of biomechanics and it holds its head upright. Previous experimental studies have shown that the sheep spine is a suitable model for humans in terms of anatomy and biomechanics.18, 19 Secondly; there are many injury patterns on the spine but we chose the compression type injury model because it is the most common one. Thirdly, the absence of muscle attachments, costas and tissue fatigue. Fourthly, we have resected the interspinous ligament and ligamentum flavum together in this study. Because in a study conducted by Pizones et al, they determined that ligamentum flavum ruptures always (100%) occur together with interspinous ligament injuries by evaluating intra-operative findings and MRIs.20
Conclusion
In conclusion, supraspinous or interspinous ligaments are the most resistant structures of the PLC to compression forces. Facet joint capsule contributes less to resistance to compression forces. However, when there are injuries to the PLC elements, as a result of the loss of resistance to compression forces a major proportion of this force shifts onto the intervertebral disc.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Magerl F., Aebi M., Gertzbein S., Harms J., Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 2.Vaccaro A.R., Lehman R.A., Hurlbert R.J. A new classification of thoracolumbar injuries the importance of injury morphology , the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976) 2005;30(20):2325–2333. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 3.Lee J., Vaccaro A., Lim M., Oner F., Hulbert R. Thoracolumbar Injury Classification and Severity Score : A New Paradigm for the Treatment of Thoracolumbar Spine Trauma. J Orthop Sci. 2005;10(6):671–675. doi: 10.1007/s00776-005-0956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James K., Wenger K., Schlegel J., Dunn H. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine (Phila Pa 1976) 1994;19(15):1731–1740. doi: 10.1097/00007632-199408000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Oner F., Van Gils A., Faber J., Dhert W., Verbout A. Some complications of common treatment schemes of thoracolumbar spine fractures can be predicted with magnetic resonance imaging: prospective study of 53 patients with 71 fractures. Spine (Phila Pa 1976) 2002;27(6):629–636. doi: 10.1097/00007632-200203150-00012. [DOI] [PubMed] [Google Scholar]
- 6.Rihn J a, Yang N., Fisher C. Using magnetic resonance imaging to accurately assess injury to the posterior ligamentous complex of the spine: a prospective comparison of the surgeon and radiologist. J Neurosurg Spine. 2010;12(4):391–396. doi: 10.3171/2009.10.SPINE08742. [DOI] [PubMed] [Google Scholar]
- 7.Whang P., Vaccaro A., Poelstra K., Patel A., Anderson D., Albert T. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine (Phila Pa 1976) 2007;32(7):791–795. doi: 10.1097/01.brs.0000258882.96011.47. [DOI] [PubMed] [Google Scholar]
- 8.Vaccaro A.R., Rihn J.A., Saravanja D. Injury of the posterior ligamentous complex of the thoracolumbar spine: a prospective evaluation of the diagnostic accuracy of magnetic resonance imaging. Spine (Phila Pa 1976) 2009;34(23):E841–E847. doi: 10.1097/BRS.0b013e3181bd11be. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan J.P., McGill S.M. Intervertebral disc herniation: studies on a porcine model exposed to highly repetitive flexion/extension motion with compressive force. Clin Biomech (Bristol, Avon) 2001;16(1):28–37. doi: 10.1016/s0268-0033(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 10.Izzo R., Guarnieri G., Guglielmi G., Muto M. Biomechanics of the spine. Part I: spinal stability. Eur J Radiol. 2013;82(1):118–126. doi: 10.1016/j.ejrad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Chazal J., Tanguy A., Bourges M. Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J Biomech. 1985;18(3):167–176. doi: 10.1016/0021-9290(85)90202-7. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Shen Z., Huang M., Wang X. Stepwise resection of the posterior ligamentous complex for stability of a thoracolumbar compression fracture. Med (United States) 2017;96(35):1–6. doi: 10.1097/MD.0000000000007873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie K.A., Dickey J.P. Biomechanical role of lumbar spine ligaments in flexion and Extension : determination using a parallel linkage robot and a porcine model complex. Spine (Phila Pa 1976) 2004;29(11):1208–1216. doi: 10.1097/00007632-200406010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Heuer F., Schmidt H., Klezl Z., Claes L., Wilke H.-J. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J Biomech. 2007;40(2):271–280. doi: 10.1016/j.jbiomech.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Heuer F., Schmidt H., Wilke H.J. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J Biomech. 2008;41(9):1953–1960. doi: 10.1016/j.jbiomech.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Heuer F., Schmidt H., Claes L., Wilke H.-J. Stepwise reduction of functional spinal structures increase vertebral translation and intradiscal pressure. J Biomech. 2007;40(4):795–803. doi: 10.1016/j.jbiomech.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Alanay A., Yazici M., Acaroglu E., Turhan E. Course of nonsurgical management of burst fractures with intact posterior ligamentous complex : an MRI study. Spine (Phila Pa 1976) 2004;29(21):2425–2431. doi: 10.1097/01.brs.0000143169.80182.ac. [DOI] [PubMed] [Google Scholar]
- 18.Cain C., Fraser R. Bony and vascular anatomy of the normal cervical spine in the sheep. Spine (Phila Pa 1976) 1995;20(7):759–765. doi: 10.1097/00007632-199504000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wilke H., Claes L., Kettler A. Are the sheep spines a valid biomechanical model for human spines? Spine (Phila Pa 1976) 1997;22(20):2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Pizones J., Zúñiga L., Sánchez-Mariscal F., Alvarez P., Gómez-Rice A., Izquierdo E. MRI study of post-traumatic incompetence of posterior ligamentous complex: importance of the supraspinous ligament. Prospective study of 74 traumatic fractures. Eur Spine J. 2012;21(11):2222–2231. doi: 10.1007/s00586-012-2403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hukins D.W., Kirby M.C., Skioryn T.A. Comprasion of structure, mechanical properties, and functions of lumbar ligaments. Spine (Phila Pa 1976) 1990;15(8):787–795. [PubMed] [Google Scholar]