Abstract
Background
Tuberculosis (TB) remains an urgent global public health priority, causing 1.5 million deaths worldwide in 2018. There is evidence that psychosocial factors modulate immune function; however, how this may influence TB risk or BCG vaccine response, and whether this pathway can be modified through social protection, has not been investigated. This paper aims to: a) systematically review evidence of how psychosocial factors influence the expression of biomarkers of immunity, and b) apply this general evidence to propose plausible TB-specific pathways for future study.
Methods
Papers reporting on the impact of psychosocial stressors on immune biomarkers in relation to infectious disease risk were identified through a search of the databases MEDLINE, PsycINFO, Global Health and PsycEXTRA alongside reference list and citation searching of key papers. Data extraction and critical appraisal were carried out using a standardised form. The findings were tabulated and synthesised narratively by infectious disease category, and used to propose plausible mechanisms for how psychosocial exposures might influence immune outcomes relevant to TB and BCG response.
Results
27,026 citations were identified, of which 51 met the inclusion criteria. The literature provides evidence of a relationship between psychosocial factors and immune biomarkers. While the direction and strength of associations is heterogenous, some overarching patterns emerged: adverse psychosocial factors (e.g. stress) were generally associated with compromised vaccine response and higher antibody titres to herpesviruses, and vice versa for positive psychosocial factors (e.g. social support).
Conclusions
The evidence identifies pathways linking psychosocial factors and immune response: co-viral infection and immune suppression, both of which are potentially relevant to TB and BCG response. However, the heterogeneity in the strength and nature of the impact of psychosocial factors on immune function, and lack of research on the implications of this relationship for TB, underscore the need for TB-specific research.
Keywords: Tuberculosis, Immunity, BCG vaccine, Psychosocial, Stress, Social protection
Highlights
-
•
It is unknown whether and how psychosocial factors could modulate immunity to TB.
-
•
We systematically review studies of psychosocial influences on immune function.
-
•
There is evidence for an association between psychosocial factors and immunity.
-
•
The nature and strength of associations are heterogeneous.
-
•
Further research is required to establish TB-specific pathways.
1. Introduction
1.1. A bio-social approach to TB research
With an estimated 1.5 million deaths worldwide in 2018, tuberculosis (TB) remains the leading cause of death from a single infectious agent (World Health Organization, 2019).
Biomedical advances, including diagnostics, antibiotic treatment and the Bacille Calmette-Guérin (BCG) vaccine, are important contributors to the reduction in TB mortality and morbidity. However, it is widely acknowledged that TB is closely associated with social determinants, and is considered a sensitive marker of impoverished and unequal societies (Oxlade & Murray, 2012). As such, human development remains the biggest driver of TB incidence decline worldwide (Hargreaves et al., 2011).
Despite the pervasive role of social determinants in the epidemiology and control of TB, research developments that explore biomedical and social lines of enquiry have proceeded largely independently, and examples of unified conceptual models linking biological and social determinants of TB are rare. These theoretical models can inform a more holistic approach to tackling TB based on better integration of social development strategies and biomedical interventions (Dowd, Fletcher, & Boccia, 2018).
1.2. VALIDATE project
The VALIDATE project ‘Enhancing BCG Efficacy: the Social Technology Lab Initiative’ has attempted to bridge biomedical and social models by advocating for an environ-vaccinology approach to the research and development of vaccines against diseases of poverty like TB (Dowd et al., 2018). According to this framework, social determinants can influence immunity and contribute to the differential efficacy of vaccines and susceptibility to disease often observed across populations of different socioeconomic status (Dowd et al., 2018). From this it follows that the response to existing or new immunisation tools can be enhanced by combining their administration with poverty-reduction strategies in order to boost the immunity of populations living in severely deprived conditions.
This can be achieved through social protection interventions, a set of risk management measures that aim to prevent, manage, and overcome situations that adversely affect people's wellbeing, which are widely implemented in low- and middle-income countries (LMICs) (Schmitt & De, 2013). Social protection encompasses social security and safety net measures with the aim of raising people out of extreme poverty and protecting them from the risks and consequences of livelihood shocks (Adato, 2008). This includes social protection programmes implemented to improve resilience to natural disasters, which – like other social protection programmes – can lead to poverty-reduction and associated improvements in mental and physical health and wellbeing (Pelham, Clay, & Braunholz, 2011). Such social protection interventions may reduce the burden of infectious diseases like TB through a number of mechanisms, including improving socio-economic factors contributing to transmission of disease (e.g. living conditions, hygiene and sanitation, access to healthcare), reducing psychosocial stress, and supporting immune function (see Box 1).
Box 1. Background literature supporting the postulated conceptual framework.
The biological and epidemiological plausibility of the postulated conceptual framework shown in Fig. 1 is supported by a variable quantity and quality of literature. The framework should therefore be interpreted as a visual representation of potential mechanisms, which will require future testing to confirm which putative causal pathways are supported. Below we discuss the evidence that is available for some of the main pathways in the conceptual framework.
Social protection, poverty and TB.
There is strong and consistent evidence that social protection interventions such as cash transfers and microfinance can reduce poverty and inequalities (Goldberg, 2005; Hagen-Zanker et al., 2016). There is also more limited evidence that they can influence risk factors relevant for TB, and very little evidence that they can directly impact TB outcomes, due to a paucity of studies of social protection interventions that specifically address TB (Boccia et al., 2011). However, a country's social protection level has been shown to be inversely associated with TB prevalence, incidence and mortality, adjusting for various confounders (Siroka, Ponce, & Lonnroth, 2016).
Social protection and psychosocial factors.
Social protection interventions may affect psychological stress by acting on underlying drivers of stress such as poverty (i.e. inability to meet basic needs or needs that are deemed important to satisfy individual wellbeing or the wellbeing of family members) and severe socioeconomic inequalities. The effect of social protection on these two social determinants is well documented (Bastagli et al., 2016; World Bank, 2015). Additionally, by acting on poverty and inequalities, social protection not only improves material living conditions, but indirectly mitigates the sense of exclusion and injustice often reported by people living in poverty or exposed to inequalities, with associated improvements in psychosocial stressors and mental health (Kilburn, Handa, Angeles, Tsoka, & Mvula, 2018; Natali, Handa, Peterman, Seidenfeld, & Tembo, 2018; Ozer, Fernald, Weber, Flynn, & VanderWeele, 2011; Powell-Jackson et al., 2016).
Impacts of poverty on TB: material, psychosocial and behavioural.
Poverty may influence health by multiple pathways, including material living conditions, psychosocial factors and health-related behaviours (Bartley, 2017). In the context of TB, the impact of material (e.g. overcrowding, malnutrition) and behavioural (e.g. smoking, diabetes) factors have been discussed extensively (Bothamley, 2005; Cegielski & McMurray, 2004; Dubos & Dubos, 1996; Harries et al., 2016). However, evidence on the influence of psychosocial factors on TB outcomes is lacking.
Psychosocial factors/mental health and TB.
Given the association between TB and poverty (Lönnroth, Jaramillo, & Williams, 2009), and between poverty and chronic psychosocial stressors (Jani, 2013) and mental disorders (Lund et al., 2010), many TB patients are likely to have been exposed to various psychosocial factors prior to TB infection or the development of active disease. Indeed, TB patients are known to have a high prevalence of psychosocial factors such as stress and anxiety; for example, a study in South Africa found high levels of distress among TB patients, associated with underlying stressors such as poverty (Peltzer et al., 2012).
In the 1950s Thomas Holmes, a physician at a Seattle sanatorium, found that individuals who had experienced stressors such as divorce, being widowed, or unemployment were more likely to contract TB and less likely to recover (Lerner, 1996). While the relationships between TB and psychosocial factors/mental health are complex and bidirectional (Sweetland et al., 2017), several cohort studies indicate that depression often precedes and is therefore possibly a risk factor for TB (Cheng, Liao, Lin, & Lai, 2017; Oh, Choi, Kim, Kim, & Cho, 2017). Further prospective studies are required to fully disentangle the complex causal pathways involved.
Psychosocial factors/mental health and immunity.
Psychosocial stressors are known to modulate the immune response. For example, those experiencing chronic stress have been shown to have reductions in the numbers of B-cells (Futterman, Wellisch, Zighelboim, Luna-Raines, & Weiner, 1996; Kiecolt-Glaser et al., 1987a, 1987b), T-cells (McKinnon, Weisse, Reynolds, Bowles, & Baum, 1989), and salivary IgA secretion (Deinzer, Kleineidam, Stiller-Winkler, Idel, & Bachg, 2000; Jemmott et al., 1983; Jemmott & Magloire, 1988) compared with controls. Such associations occur via interactions between the central nervous system (CNS), endocrine and immune systems. Stressors can activate the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medullary (SAM) axes, provoking the release of hormones including catecholamines (e.g. adrenaline) and glucocorticoids (e.g. cortisol), which can in turn modulate immune function. This may occur directly, by the hormone binding to immune cell-surface receptors, or indirectly, for example by inducing the dysregulation of cytokines such as IFNγ and interleukins (Glaser & Kiecolt-Glaser, 2005; Padgett & Glaser, 2003; Webster, Tonelli, & Sternberg, 2002).
It is possible that mental health may mediate the relationship between psychosocial factors and immune response. While there is no existing evidence that directly tests this pathway in entirety, we postulate this as a plausible mechanism given evidence of the links between psychosocial factors and mental health and between mental health and immunity. For example, stressful life events can precipitate depressive episodes in vulnerable persons (Kendler, Karkowski, & Prescott, 1999), and childhood abuse or neglect increases risk of depression in adulthood (Pechtel & Pizzagalli, 2011). Additionally, evidence links mental health to immune function. A meta-analysis found that depression is associated with various immunological assays, including impairments in T-cell mediated activities and reduced NK cell counts and cytotoxicity (Zorrilla et al., 2001).
Psychosocial factors, immunity and TB outcomes.
There are several biological pathways by which psychosocial factors could influence immune response to TB:
-
a)
There is evidence that psychosocial stressors can partially suppress certain aspects of immune function (Glaser & Kiecolt-Glaser, 2005). Given that protective immunity against M.tb depends on various innate and adaptive immune mechanisms, there are various pathways that could be disrupted by immune suppression. For example, the T-cell mediated immune response is vital in host control of M.tb infection. In particular, the capacity of CD4+ T-cells to produce IFNγ, which stimulates phagocytes to contain intracellular M.tb, is crucial. This is supported by studies of host genetic susceptibility showing that individuals with deficiencies in signalling pathways for the cytokines IFNγ and interleukin-12 (IL-12) have increased susceptibility to mycobacterial infection (Altare et al., 1998). Additionally, CD4+ T-cell decline in HIV-infected individuals is associated with increased susceptibility to TB disease (Lawn, Myer, Edwards, Bekker, & Wood, 2009). Given this, if psychosocial stress could suppress CD4+ T-cell proliferation and/or IFNγ secretion, this would likely reduce host ability to contain M.tb infection. A potential association between psychosocial stress and impaired T-cell immunity is supported by evidence in animal models. For example, rats exposed to repeated stress show significant decreases in mononuclear cell count, especially CD8+ T cells, and reduced IL-2 production (Batuman, Sajewski, Ottenweller, Pitman, & Natelson, 1990), and mice exposed to experimental stressors show supressed production of IFNγ (Curtin, Boyle, Mills, & Connor, 2009; Zhang, Okutsu, Kanemi, Gametchu, & Nagatomi, 2005). It is also supported by evidence in humans. In medical students, stress due to examinations is associated with reduced overall numbers of T cells, as well as a reduction in helper T cell and suppressor T cell counts compared with 6 weeks earlier (Glaser et al., 1985; Kiecolt-Glaser et al., 1984). In addition, caregivers of dementia patients show poor HSV-1 specific memory T cell proliferation compared with controls (Glaser & Kiecolt-Glaser, 1997). It is also possible that immune suppression could interfere with BCG response, perhaps through limiting the BCG-associated expansion of CD4+ and CD8+ T-cells (Godfrey, Uldrich, McCluskey, Rossjohn, & Moody, 2015) and release of IFNγ (Fletcher et al., 2016).
-
b)
There is evidence of an association between psychosocial stressors and herpesvirus infection and reactivation (Jenkins & Baum, 1995), and also between certain herpesviruses and TB (Cobelens, Nagelkerke, & Fletcher, 2018), suggesting a further plausible pathway. TB patients are more likely to be cytomegalovirus (CMV) seropositive (Olaleye, Omilabu, & Baba, 1990) and have higher titres of anti-CMV antibodies (Amran et al., 2016) (reflecting increased CMV exposure) compared with controls. This was demonstrated recently in a Ugandan cohort, in which increased CMV-specific immunoglobulin G levels were associated with active pulmonary TB (Stockdale et al., 2018). Similarly, the CMV-specific IFNγ response has been associated with a greater than twofold increased TB disease risk in infants (Muller et al., 2019). Indeed, active CMV infection could be involved in progression from latent TB infection to active disease, a proposal supported by the similarity in the two infections' age-sex distributions (Cobelens et al., 2018). It is likely that the relationship between CMV and TB is bi-directional, and some of the observed association may be accounted for by their shared epidemiology and risk factors, especially poverty. However, in a prospective study in rural Uganda, CMV IgG was associated with increased risk of active TB disease up to 10 years before TB diagnosis (p=0.006), suggesting that CMV may indeed be a risk factor for TB disease (Stockdale et al., 2019). Beyond CMV, fewer studies have investigated the link between other herpesviruses and TB. However, a study in Finland showed that EBV antibody levels were higher in patients with pulmonary TB than in controls (Nikoskelainen, Hannuksela, & Palva, 1974), and a study in Taiwan showed that TB patients have a higher rate of seropositivity (p=0.01) and higher antibody titres (p=0.006) for HHV-8 than matched controls (Su et al., 2015). In a study in Georgia, prevalence of HHV-2 prevalence was 92% in TB patients and 29% in controls; for CMV prevalence was 68.5% in TB patients 5.8% in healthy controls (Karalian, 2008).
Alt-text: Box 1
The currently available vaccine against TB, Mycobacterium bovis BCG, represents a key proof-of-concept for the environ-vaccinology model. BCG is notoriously only partially effective; while it reliably protects against disseminated forms of TB including meningitis and miliary disease in infants, efficacy in protecting against pulmonary TB is highly variable across geographic populations, especially in adults (World Health Organization, 2018a). While there are several new TB candidate vaccines in development, some of which are in advanced stages of clinical trials, none of these are likely to become widely available in the near future (World Health Organization, 2018a). In the meantime, mathematical modelling studies suggest that combining existing biomedical tools against TB with social protection interventions would accelerate the global decline in TB incidence; for example, one model demonstrates that increasing social protection coverage – defined by the proportion of people covered by labour market protections, social assistance, and social insurance – to 100% (as set for one of the Sustainable Development Goal 1 targets) could lead to a 76% reduction of TB incidence by 2035 (Carter et al., 2018).
However, the mechanisms by which social protection might influence BCG response and TB risk are not understood. In particular, there has been little research on the potential role for social factors in the immune response to TB and BCG. This is despite evidence that improved BCG efficacy is associated with higher latitudes, an observation that may be partially accounted for by the variation in socio-economic conditions seen across latitudes (Wilson, Fineberg, & Colditz, 1995). For example, BCG response as measured by increase in interferon-γ (IFN-γ) production to Mycobacterium tuberculosis purified protein derivative (M.tb PPD) is much larger in adolescents in the UK than in Malawian adolescents (Lalor et al., 2009).
1.3. Conceptual framework
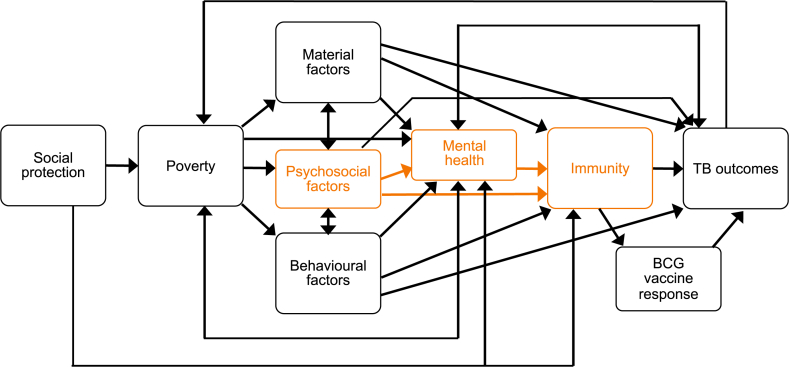
The conceptual model in Fig. 1 illustrates several pathways by which social protection interventions could alter immune function, via material, psychosocial or behavioural pathways. In turn, modulated immune function may lead to better resilience against TB infection and disease or improved BCG response.
Fig. 1.
Conceptual framework. This conceptual framework depicts several pathways through which social protection interventions may affect TB outcomes. ‘TB outcomes’ here includes TB exposure, infection, disease, and adverse outcomes. In this paper we focus on the pathway linking psychosocial factors with biomarkers of immunity (orange boxes in the figure) and apply the findings to the context of TB. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The biological and epidemiological plausibility of this conceptual framework is supported by a variable quantity and quality of literature (Box 1). In particular, evidence on the effect of psychosocial factors on immunity remains inconclusive. Psychosocial theory postulates that vulnerability to disease is linked to psychological stress, in particular arising from perceptions and experiences of adverse living conditions and social inequalities compounded by a perceived lack of agency and control (Marmot & Wilkinson, 2001).
A growing body of research in the field of psychoneuroimmunology explores how psychosocial stressors modulate the immune system, influencing infectious disease risk/outcomes (Kemeny & Schedlowski, 2007) and vaccination response (Burns, Carroll, Ring, & Drayson, 2003b). For example, in healthy volunteers inoculated with a respiratory virus, severity of infection and symptoms increased in a dose-response relationship with psychosocial stress index score (Cohen, Tyrrell, & Smith, 1991). However, while psychosocial influences on immune biomarkers have been investigated (see Box 1), and applied for example to human immunodeficiency virus (HIV) (Kemeny, 2003), the application of this knowledge to TB is lacking.
1.4. Aims and objectives
This paper seeks to address this research gap, reviewing existing literature on how psychosocial factors influence immunity, and applying this evidence to the context of TB. We focus on the relationship between psychosocial factors and immunity in relation to TB with the aim of drawing attention to this specific and understudied pathway vis a vis material and behavioural pathways between poverty and TB/immune function, which have been studied more extensively. This research will increase understandings of potential mechanisms underlying TB risk, and through its application within the VALIDATE project will contribute to the development of a more creative, interdisciplinary response to tackling TB, combining social protection interventions with existing biomedical tools.
Firstly, to understand how psychosocial factors influence biomarkers of immunity, a systematic literature review will be undertaken. This literature has not been systematically examined since 1993 (Van Rood, Bogaards, Goulmy, & Van Houwelingen, 1993). It is anticipated that no or very little existing literature will explore psychosocial influences on the immune response to TB specifically. Therefore, the scope of this review encompasses knowledge relating to any infectious disease. Secondly, these general findings will be used to make inferences about plausible mechanisms by which psychosocial factors could influence the expression of biomarkers of immunity in ways relevant to TB.
While these proposed mechanisms are putative and will require further research to confirm or refute, the extrapolation of general findings to TB is a valid exercise given that many markers of immune response are overlapping and not pathogen-specific. Various immune correlates have been shown to be significantly associated with increased risk of TB disease in case-control studies which control for non-specific immune activation in the host environment (Fletcher et al., 2016; Zak et al., 2016). While not diagnostic of TB disease, such markers are significantly associated with increased risk of developing TB disease and are therefore relevant to our conceptual framework.
This paper has two aims, which are reflected in the structure of the paper:
-
1.
To systematically review research into psychosocial influences on immune function (reported in 3. Results)
-
2.
To use the existing general evidence to propose plausible TB-specific pathways for future study (reported in 4.2. Implications for TB and BCG response)
2. Methods
2.1. Inclusion criteria
This systematic review sought to identify primary epidemiological research investigating the impact of psychosocial factors on the expression of biomarkers of immunity relevant to infectious diseases including TB (see Table 1). Included papers therefore assessed psychosocial factors as exposures and immune biomarkers as outcomes. Studies that examine the impact of psychosocial stress on epidemiological or clinical evidence of infection, without assessment of immunological correlates, were excluded, given the review's aims of understanding the biological pathways between psychosocial factors and infectious disease and applying the findings to TB.
Table 1.
Inclusion criteria.
| Population |
|
| Exposure |
|
| Control |
|
| Outcome |
|
| Study design |
|
Following existing literature (Brunner, 2017), psychosocial factors of interest include stress, anxiety, stigma, isolation/exclusion, discrimination, control, demands, autonomy, lack of self-esteem and social support. However, most of the literature focuses on stress/anxiety and social support/isolation. Despite significant evidence that such factors are associated with various health outcomes (Martikainen, Bartley, & Lahelma, 2002), their definition and measurement varies widely and is often unclear (Epel et al., 2018). The term ‘stress’ is used to refer to stressful exposures/life events (both acute (short-term) and chronic (long-term)), the perception of such exposures, and associated physiological responses. Here, we included papers that defined psychosocial factors by the factor itself (e.g. stressful life events) or perception thereof (e.g. anxiety), rather than by the physiological response (e.g. cortisol). These are usually measured using questionnaire tools, such as the Perceived Stress Scale (PSS), which assesses how often individuals have felt stressed during the past month based on several questions on a scale of 0 (never) to 4 (very often) (Cohen, Kamarck, & Mermelstein, 1983). We included papers with mental health as a mediator, but those looking solely at the impact of mental health on immunity without reference to underlying psychosocial causes were excluded. Since the findings had to be relevant to infections including TB, we only included papers presenting any changes in immune biomarkers in relation to infectious disease risk.
The review aimed to understand pathways relating commonly-experienced psychosocial stresses that are potentially amenable to modification by social protection to immune functioning in the general population. Therefore, studies of ‘extreme’ stressors, defined as situations involving intensely stressful experiences (either acute or chronic) not experienced by the general population, either because of their abrupt onset or catastrophic consequences (e.g. natural disasters, military stressors, caregiving), are excluded, as these are distinct from the widespread poverty-related stressors commonly experienced in TB-affected communities. Moreover, studies in HIV-infected populations are excluded, as the immunosuppression acquired immunodeficiency syndrome (AIDS) entails is considered a strong effect modifier which could obscure the pattern of how psychosocial factors influence immune functioning in the general population. Similarly, laboratory and animal studies are excluded, as this review focuses on pathways between psychosocial factors and immunity that operate in situ.
2.2. Search strategy
MEDLINE (1946–2018), PsycINFO (1806–2018) and Global Health (1910–2018) were searched from inception to 11/05/2018, combining free text terms and subject headings for psychosocial factors, immunity, and infectious disease including TB (shown in Appendix A). The search was restricted to papers in English and relating to humans. For grey literature, PsycEXTRA (1908–2018) was searched from inception to 15/07/2018 using the same search strategy. Key reviews (Burns et al., 2003b; Glaser & Kiecolt-Glaser, 2005; Kemeny & Schedlowski, 2007) had their reference lists examined and citation searching carried out using the Web of Science citation search tool. All references were imported into EndNote, and duplicates deleted, followed by title, abstract, and full-text screening to assess eligibility. Finally, bibliographic screening of the included papers was undertaken.
2.3. Data extraction
Data were extracted using the form in Appendix B. This is adapted from the Cochrane EPOC group's data collection form (Cochrane Effective Practice and Organisation of Care (EPOC), 2017). The extracted data were tabulated, detailing participants, methods and results of included studies.
2.4. Critical appraisal
The quality of all included studies was assessed by commenting on the methods’ suitability for answering the review question at each relevant stage of the data extraction form. Comments were made following questions on the CASP checklists for cohort (Critical Appraisal Skills Programme, 2018b) and case-control (Critical Appraisal Skills Programme, 2018a) studies, and the AXIS tool for cross-sectional studies (EpiNet). This critical appraisal process informed a judgement on overall study quality: high, moderate or low.
2.5. Synthesis
The eligible studies identified through the systematic review of the literature were categorised into three thematic areas:
-
1.
Impact of psychosocial factors on immune response to vaccination. This was considered especially pertinent given our interest in understanding how social protection interventions could influence BCG vaccine response.
-
2.
Impact of psychosocial factors on immune response to herpesviruses. This was considered particularly relevant given the increasing evidence indicating an association between herpesviruses and TB, discussed in Box 1.
-
3.
Impact of psychosocial factors on immunity to other infectious diseases. This was considered relevant given the communicable nature of TB.
Studies within each category often measured the same type of biomarkers of immunity, although there remained significant heterogeneity even within categories. Nevertheless, the categorisation facilitated direct comparisons among studies where appropriate and allowed a more coherent synthesis of the study findings.
Given the variety of study designs, psychosocial factors and immune biomarkers measured, and metrics for reporting findings, meta-analysis was deemed inappropriate. A narrative synthesis was therefore completed, separately by thematic area, using the tabulated data to draw out relevant findings and salient points regarding study design and methods. Within each study group, the findings were reviewed to identify whether direction and strength of association varied according to the psychosocial factors and immune biomarkers measured, or by study design and quality. Additionally, any mediation was reported. The collective evidence was assessed as to whether and how psychosocial factors influence biomarkers of immunity, and the extent to which the findings could be extrapolated to the case of TB.
3. Results
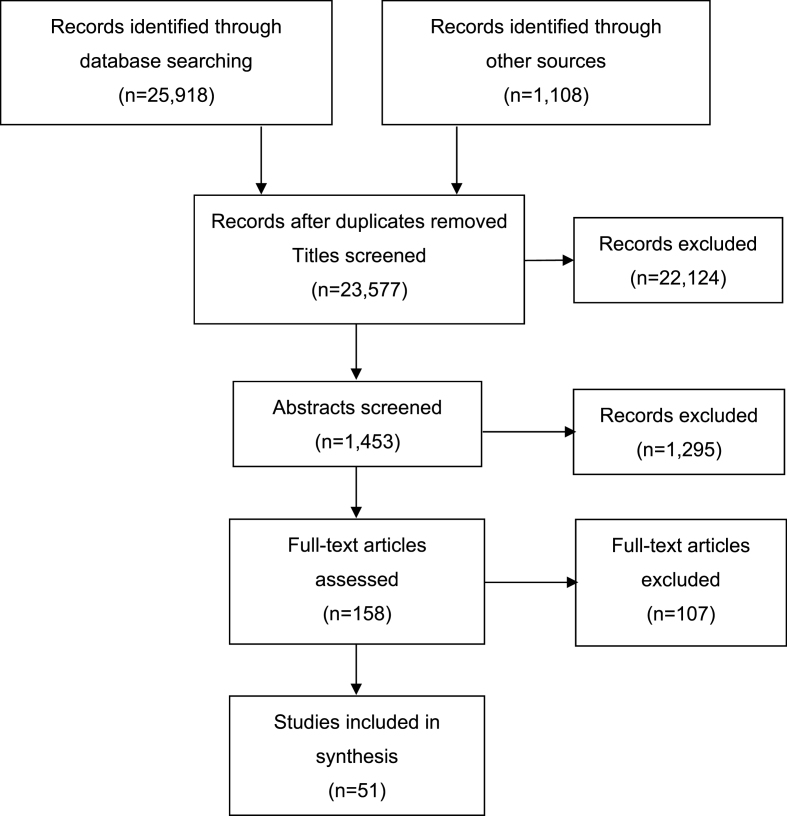
51 articles met the inclusion criteria (Fig. 2). Included studies fall into three broad thematic areas regarding their infectious disease application: vaccine response (18 studies), herpesviruses (19 studies) and other infections (14 studies). The vaccine response studies are primarily longitudinal, whereas the herpesvirus and other infection studies are mostly cross-sectional. The median number of participants is 95 overall: 78.5 for the vaccine response studies, 121 for the herpesvirus studies, and 109.5 for other infection studies. Study quality is highest for the vaccine response studies and lowest for the studies of other infections.
Fig. 2.
Flow chart of literature search. Adapted from PRISMA 2009 Flow Diagram (Moher, Liberati, Tetzlaff, Altman, & The Prisma Group, 2009). 107 articles were excluded following full-text screening because they did not meet the inclusion criteria. The most common reasons for exclusion at this stage were: HIV-infected population, ‘extreme’ stressor, onset of infection precedes psychosocial stressor, and not relating to infectious disease.
3.1. Vaccine response studies
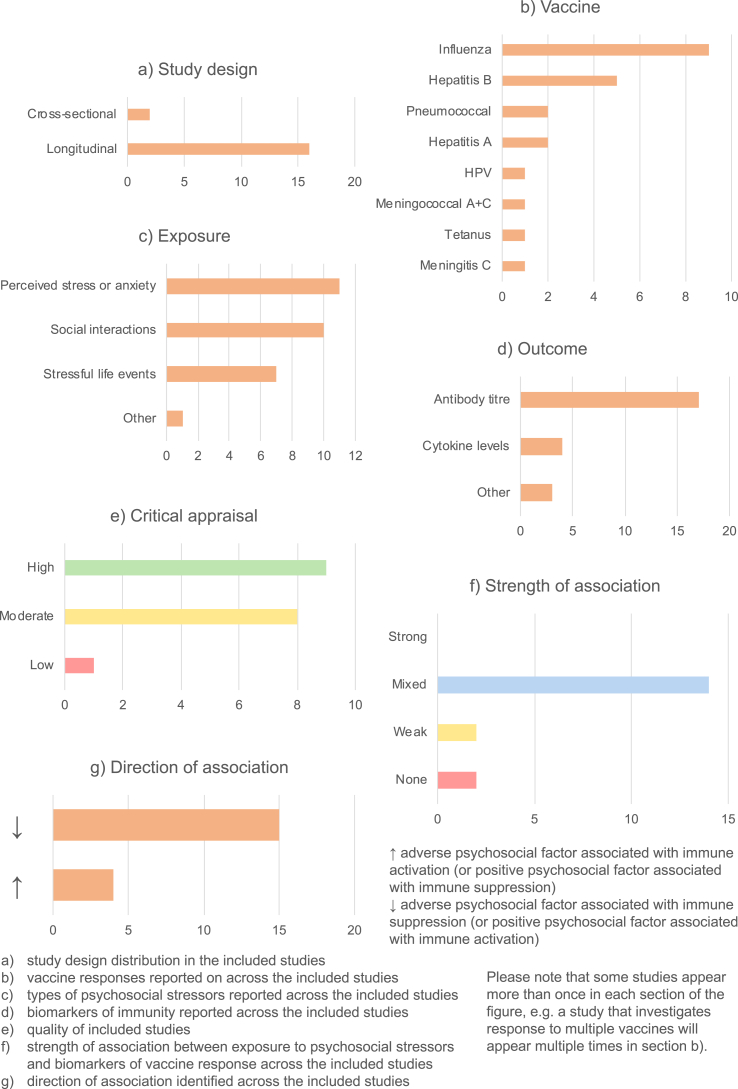
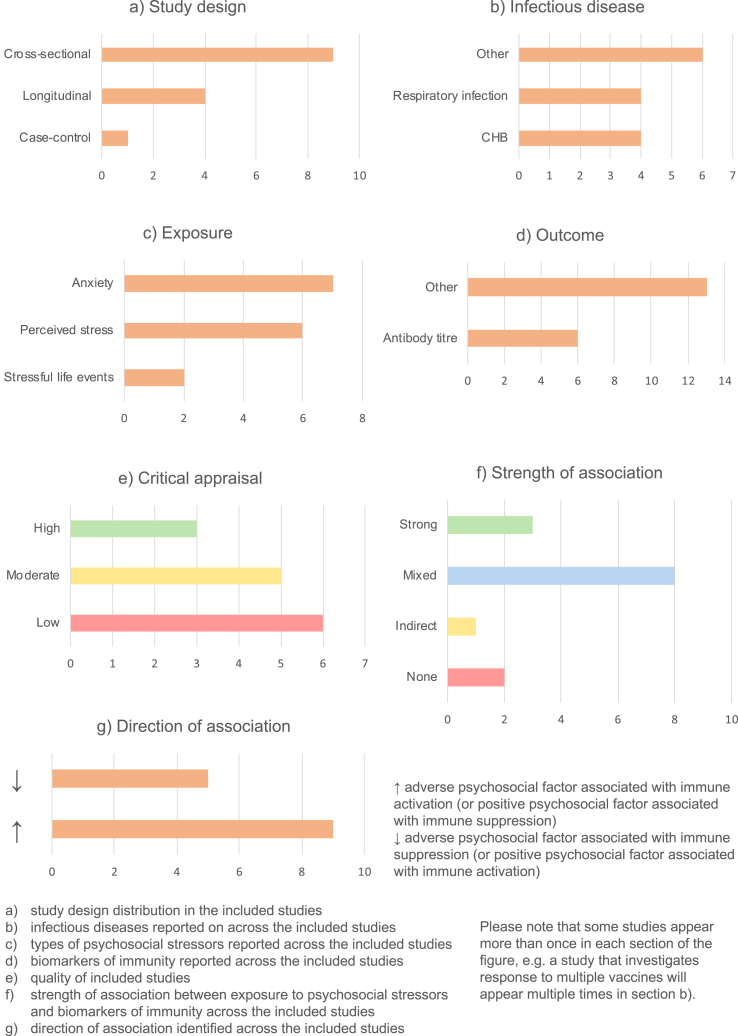
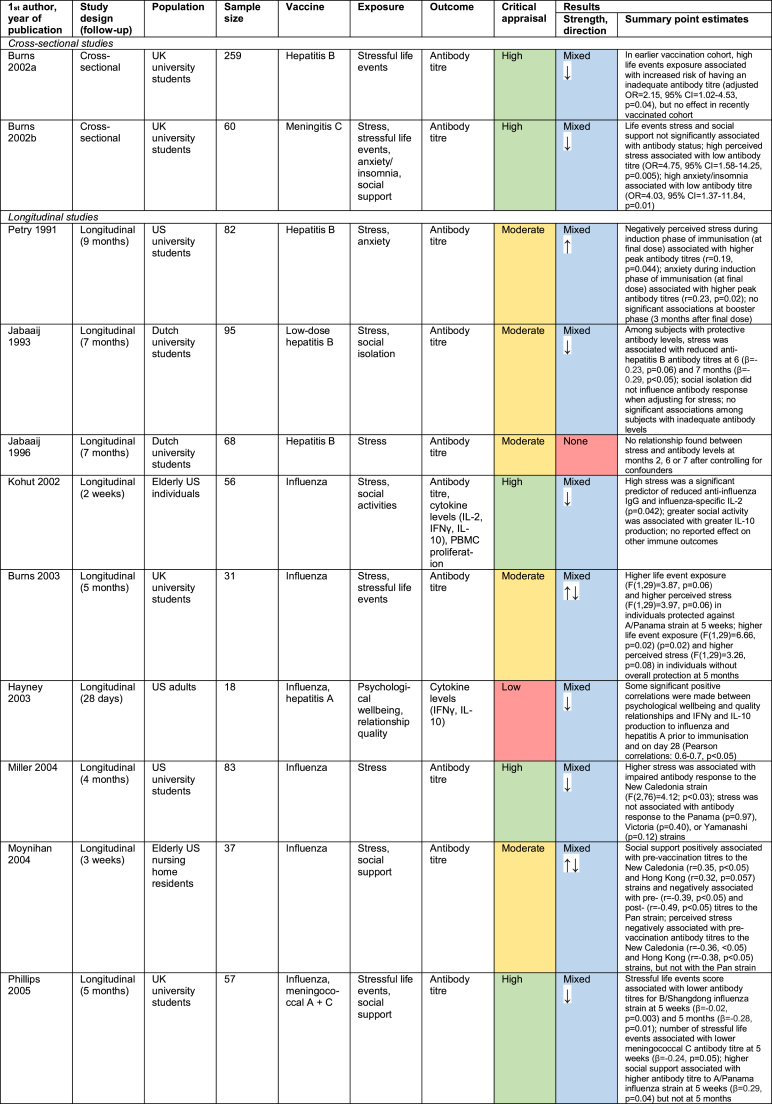
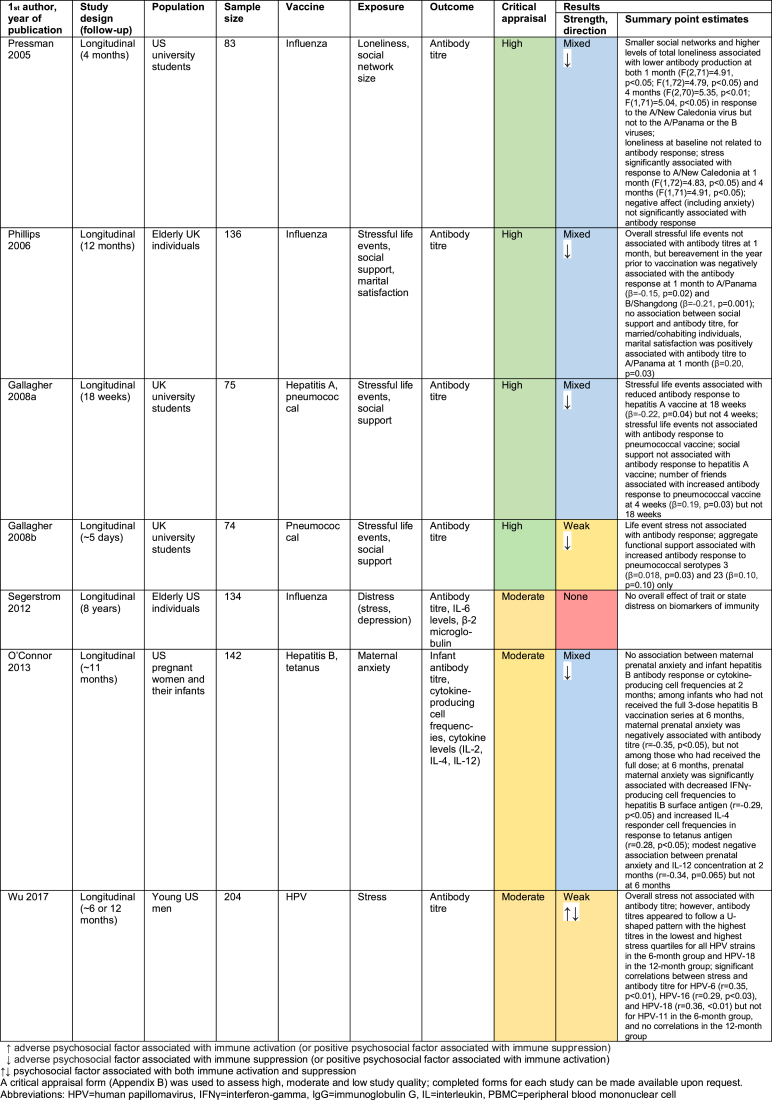
18 papers out of 51 investigated the relationship between psychosocial factors and vaccination response (Burns, Carroll, Drayson, Whitham, & Ring, 2003a; Burns, Carroll, Ring, Harrison, & Drayson, 2002a; Burns, Drayson, Ring, & Carroll, 2002b; Gallagher, Phillips, Ferraro, Drayson, & Carroll, 2008a, 2008b; Hayney et al., 2003; Jabaaij et al., 1993; Jabaaij et al., 1996; Kohut, Cooper, Nickolaus, Russell, & Cunnick, 2002; Miller et al., 2004; Moynihan et al., 2004; O'Connor et al., 2013; Petry, Weems, & Livingstone, 1991; Phillips, Burns, Carroll, Ring, & Drayson, 2005; Phillips et al., 2006; Pressman et al., 2005; Segerstrom, Hardy, Evans, & Greenberg, 2012; Wu, Zimmerman, & Lin, 2017). Findings are summarised in Fig. 3 and detailed in Appendix C.
Fig. 3.
Summary of vaccine response studies.
Most (16/18) vaccination studies were longitudinal (Fig. 3a), assessing psychosocial factors and administering vaccines at baseline, and following up participants to assess their response. The two cross-sectional studies (Burns et al., 2002a, 2002b) assessed psychosocial factors and vaccine response in previously-vaccinated participants. The most common infection vaccinated against was influenza (8 studies), with others including hepatitis A and B, meningitis A and C, pneumococcal infections and human papillomavirus (HPV) (Fig. 3b). Various psychosocial factors were assessed, broadly relating to stress and social interactions (Fig. 3c). The most common biomarker of immunity measured was antibody titre, in 17 studies, although four additionally or instead measure cytokine levels in response to vaccination (Fig. 3d). Most studies are moderate- or high-quality, with only one low-quality study (Hayney et al., 2003) (Fig. 3e).
The findings were varied. 14/18 studies gave mixed results, with significant associations only uncovered for some of the psychosocial factors and immune biomarkers measured. A further two studies showed a weak association, and two others no association (Fig. 3f).
In general, where associations were observed, adverse psychosocial exposures (e.g. stress) were associated with compromised vaccine response, whereas positive psychosocial factors (e.g. social support) were associated with improved response (Fig. 3g). There were, however, some exceptions. For example, one study found that higher life event exposure and perceived stress were associated with protective antibody titres at five weeks, with the reverse true at five months (Burns et al., 2003a).
Four papers investigated mediation. In one, coping strategy mediated the association between stressful life events and antibody titre: acceptance coping was protective whereas coping by substance use increased risk of having an inadequate antibody titre (Burns et al., 2002a). In other studies, sleep quantity mediated the relationship between stress and antibody response (Miller et al., 2004), and stress mediated the association between loneliness and antibody titre (Pressman et al., 2005). No other significant mediation effects were found.
There was no apparent overall trend between study quality and association strength. However, the amount of time between psychosocial assessment/vaccination and assessment of immune biomarkers seems to influence the findings, with studies with the longest follow-up showing the weakest associations (Jabaaij et al., 1996; Segerstrom et al., 2012; Wu et al., 2017). This variation is also seen within longitudinal studies, with some finding significant associations at early timepoints but not later during follow-up (Phillips et al., 2005, 2006; Wu et al., 2017). However, the relationship is not entirely straightforward, and varies according to the psychosocial factor and vaccine response assessed (Gallagher, Phillips, Ferraro, Drayson, & Carroll, 2008a).
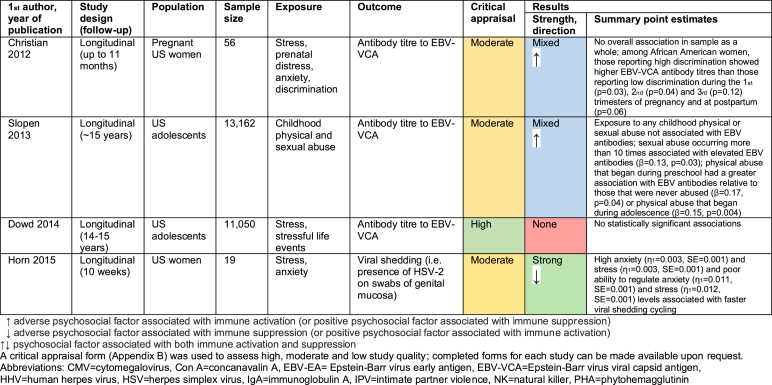
3.2. Herpesvirus studies
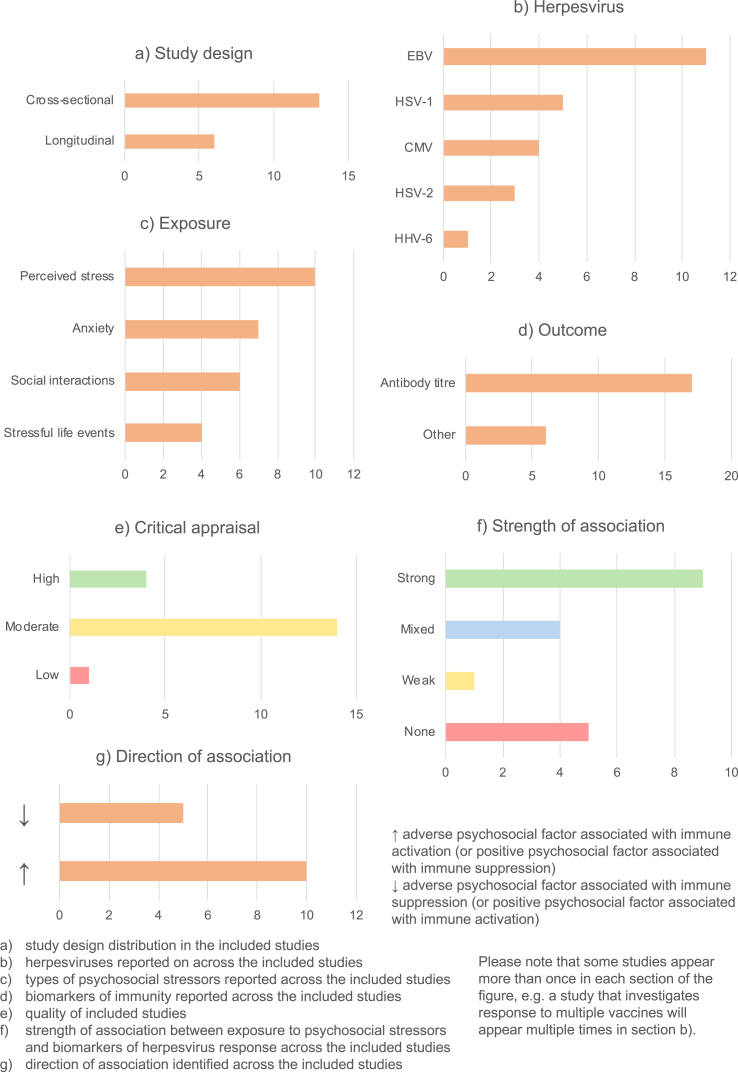
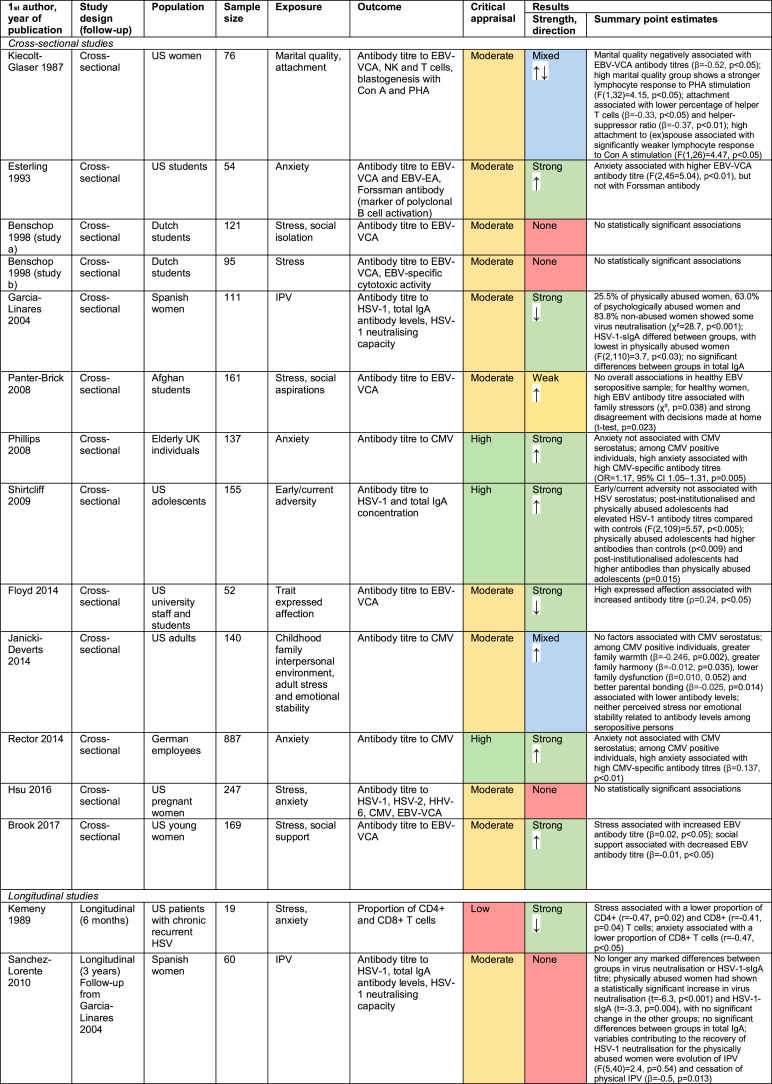
19 studies explored the relationship between psychosocial factors and immune response to herpesviruses (Benschop, Jabaaij, Oostveen, Vingerhoets, & Ballieux, 1998; Brook, Christian, Hade, & Ruffin, 2017; Christian, Iams, Porter, & Glaser, 2012; Dowd, Palermo, Chyu, Adam, & McDade, 2014; Esterling, Antoni, Kumar, & Schneiderman, 1993; Floyd, Hesse, Boren, & Veksler, 2014; Garcia-Linares, Sanchez-Lorente, Coe, & Martinez, 2004; Horn, Turkheimer, & Strachan, 2015; Hsu et al., 2016; Janicki-Deverts, Cohen, Doyle, Marsland, & Bosch, 2014; Kemeny, Cohen, Zegans, & Conant, 1989; Kiecolt-Glaser et al., 1987a; Panter-Brick, Eggerman, Mojadidi, & McDade, 2008; Phillips, Carroll, Khan, & Moss, 2008; Rector et al., 2014; Sanchez-Lorente, Blasco-Ros, Coe, & Martinez, 2010; Shirtcliff, Coe, & Pollak, 2009; Slopen, McLaughlin, Dunn, & Koenen, 2013). Findings are summarised in Fig. 4 and detailed in Appendix D.
Fig. 4.
Summary of herpesviruses studies.
13/19 studies were cross-sectional (Fig. 4a), assessing psychosocial exposures and immune outcomes at a single timepoint. The remaining six were longitudinal, either measuring psychosocial factors at baseline and immune response at follow-up, or involving repeated measures of both psychosocial and immunological factors. The herpesvirus most-commonly investigated was Epstein-Barr virus (EBV, in 11 studies), although herpes simplex viruses (HSV-1 and HSV-2) and cytomegalovirus (CMV) were also common (Fig. 4b). Various psychosocial factors were investigated, mostly relating to stress and social support (Fig. 4c). In most studies, the biomarker of immunity measured was antibody titre to a herpesvirus (Fig. 4d). Study quality was moderate in 14 studies, high in 4, and low in 1 (Fig. 4e).
Results were extremely diverse, with nine studies showing strong results, five no evidence of associations between psychosocial factors and immunity, and the remaining five mixed or weak results (Fig. 4f). Notably, only one study was carried out in a low-income country (LIC) (Panter-Brick et al., 2008). Herpesviruses are much more common in LICs than high-income countries (HICs); in LICs where data is available, over 90% of the population are seropositive for CMV, compared with ~40–70% in HICs (Manicklal, Emery, Lazzarotto, Boppana, & Gupta, 2013). It is therefore difficult to extrapolate the findings from HICs to LICs, where TB is more common. However, given that antibody response studies are typically undertaken among the seropositive, the immune responses observed in this group in HICs can be used to infer likely responses among the seropositive in LICs.
With only one exception, where associations were observed, adverse psychosocial exposures were associated with higher antibody titres to herpesviruses, whereas positive psychosocial factors were associated with lower antibody titres (Fig. 4g). High herpesvirus-specific antibody titres reflect greater herpesvirus exposure, and greater risk of immune exhaustion resulting in impaired immunity to other infections. There was no consistent relationship between the type of psychosocial factor or herpesvirus studied and the direction or strength of associations. However, given relatively few papers considered herpesviruses other than EBV (only five studies investigate HSV-1, and fewer for other herpesviruses), this cannot be concluded with certainty. Finally, there were no clear correlations between type of immune biomarker and strength of findings, because all studies measured antibody titre. In the three studies that measured total antibody concentrations, no associations were found between psychosocial factors and this biomarker, indicating that any significant associations were due to herpesvirus-specific antibodies rather than overall immune system changes (Garcia-Linares et al., 2004; Sanchez-Lorente et al., 2010; Shirtcliff et al., 2009). Moreover, in studies that measured any biomarker of immunity other than antibody titre or herpesvirus neutralising capacity, adverse psychosocial factors were associated with immune suppression rather than activation (Horn et al., 2015; Kemeny et al., 1989; Kiecolt-Glaser et al., 1987a).
Six papers undertook mediation analysis. Potential mediators investigated included lifestyle and behavioural factors, mental health, inflammation and cortisol. However, only one study found evidence of mediation: the follow-up intimate partner violence (IPV) study found that evolution and cessation of IPV mediated the recovery of HSV-1 neutralisation (defined as the ability of saliva to inhibit the formation of virus-induced cytopathologic effects in cell cultures incubated with HSV-1) between baseline and follow-up in physically-abused women (Sanchez-Lorente et al., 2010).
No overall pattern appeared between study quality and strength of findings. Cross-sectional studies more often gave strong findings, with 53.8% of cross-sectional studies uncovering strong associations compared with 33.3% of longitudinal studies. This suggests that associations found in cross-sectional studies could be partially attributable to relationships in the other direction, with immune response to herpesviruses somehow influencing psychosocial factors (e.g. if poor immune response increases risk of herpesvirus infection or reactivation, or worsens symptoms, this may be a cause of stress or stigmatisation) or, perhaps more likely, to common causes of both.
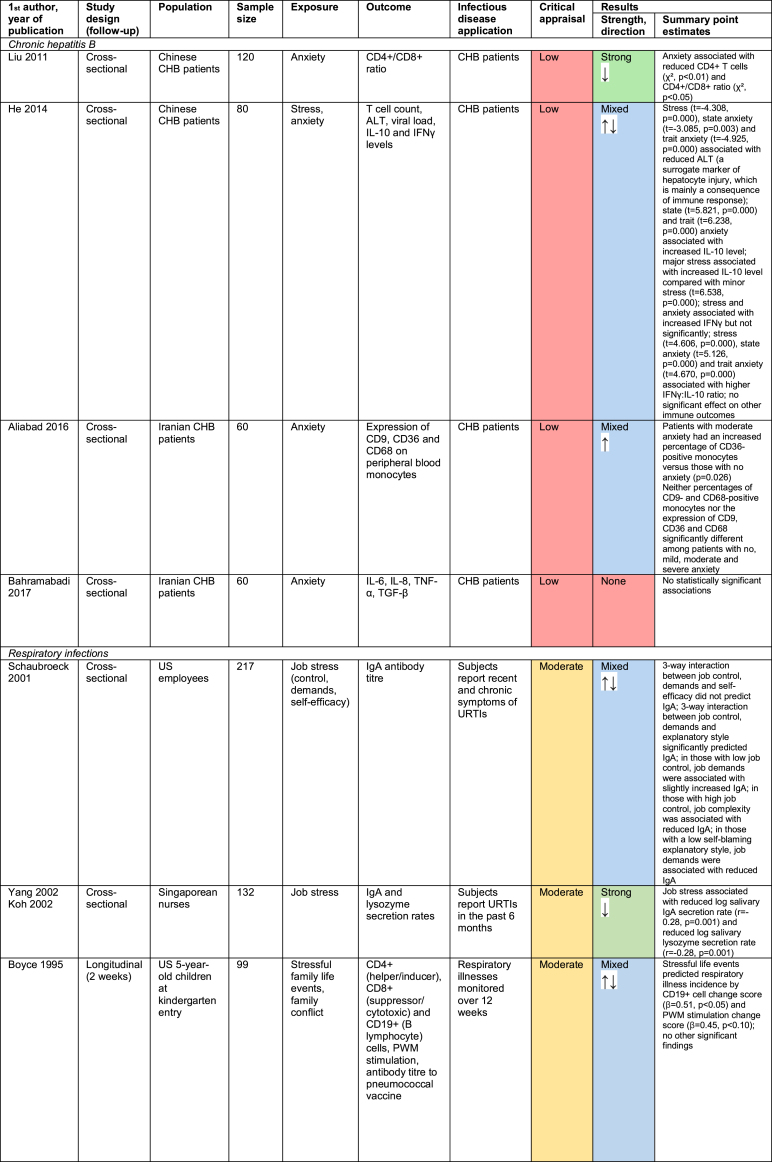
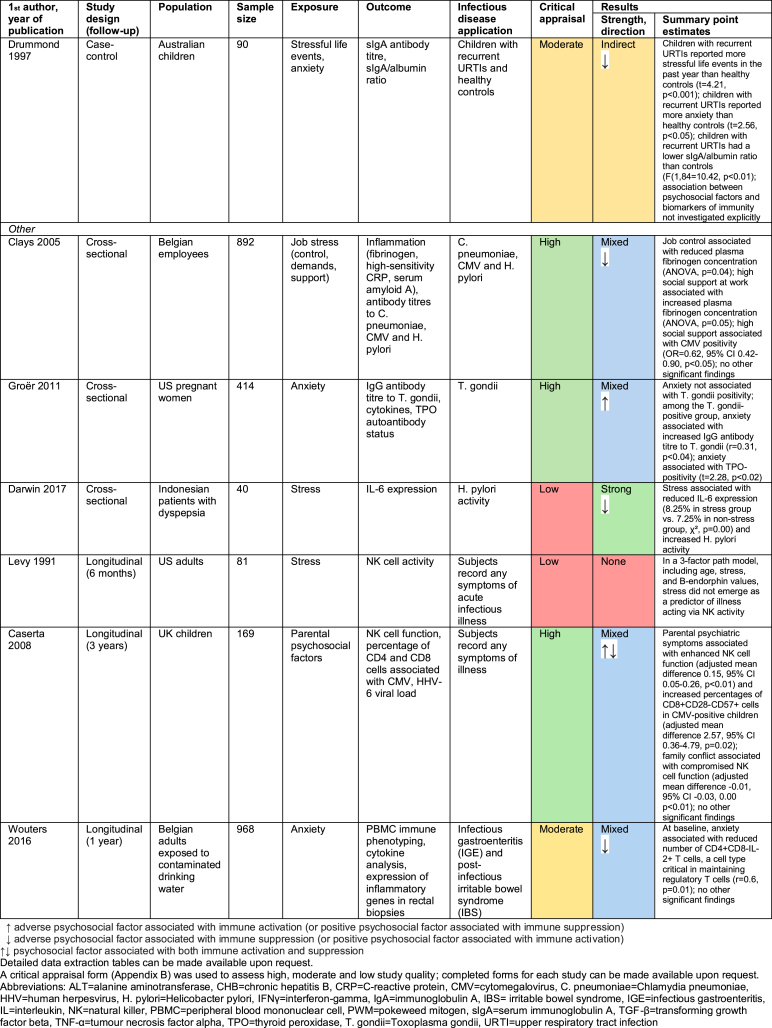
3.3. Studies of other infectious diseases
14 studies investigated the relationship between psychosocial factors and immunity in the context of infectious diseases other than vaccine response or herpesviruses. This includes four studies in chronic hepatitis B (CHB) patients (Aliabad et al., 2016; Bahramabadi et al., 2017; He, Gao, Li, & Zhao, 2014; Liu, Zhang, & Jiang, 2011), four relating to respiratory infections (Boyce et al., 1995; Drummond & Hewson-Bower, 1997; Koh, Yong, Ng, & Chia, 2002; Schaubroeck, Jones, & Xie, 2001; Yang et al., 2002), and six relating to other infectious diseases (Caserta et al., 2008; Clays et al., 2005; Darwin, Murni, & Nurdin, 2017; Groër et al., 2011; Levy et al., 1991; Wouters et al., 2016). Findings are summarised in Fig. 5 and detailed in Appendix E.
Fig. 5.
Summary of other infectious disease studies.
Study design and quality were highly variable, something unsurprising given the variety of infectious diseases and biomarkers of immunity investigated. The results were also extremely heterogenous. More studies found that adverse psychosocial factors have a suppressive effect on immune function (nine studies) than have an enhancing effect (five studies). However, the relatively small number of studies and their diverse nature makes identifying any overarching trend difficult.
Among CHB patients, anxiety was associated with reduced CD4+ T-cells and CD4+/CD8+ ratio (Liu et al., 2011), and an increased percentage of CD36-positive monocytes (Aliabad et al., 2016), and both stress and anxiety were correlated with increased interleukin 10 (IL-10) level and IFNγ:IL-10 ratio (He et al., 2014). Psychosocial factors therefore appear to suppress some aspects of immunity while enhancing others in CHB patients. The respiratory infection and other infectious disease studies similarly indicate that psychosocial stressors can both suppress and enhance different aspects of immune function.
The strength of the findings was also variable: three studies showed strong associations, eight were mixed, one was indirect, and two showed no association. Neither direction nor strength of findings seemed to vary by infectious disease investigated or immune biomarker measured. However, it is hard to detect patterns given the paucity of studies relating to each disease/biomarker. Similarly, no clear overall pattern exists between study quality and strength of findings.
4. Discussion
4.1. Summary of findings
Overall, most studies (42/51) found some relationship between psychosocial factors and biomarkers of immunity. However, associations were generally only uncovered for some of the psychosocial factors and biomarkers investigated. Moreover, the direction and strength of associations were mixed and not dependent on the type of biomarker or psychosocial factor investigated. Some overarching patterns can be cautiously identified: adverse psychosocial factors were often associated with compromised vaccine response and higher antibody titres to herpesviruses, with the reverse true for positive psychosocial factors. However, there were exceptions to both trends.
Such inconsistency is perhaps unsurprising, given the breadth of psychosocial factors, immune biomarkers, and infectious diseases investigated. A possible explanation may also lie in the nature of the psychosocial stressors and their measurement, in particular whether they were acute or chronic stressors. Both evolutionary theory (McEwen, 1998) and studies in mice exposed to brief stressors (Glaser & Kiecolt-Glaser, 2005) suggest that acute stressors might enhance aspects of immune function, whereas chronic stressors are usually immunosuppressive. However, this cannot be demonstrated in our review since most of the studies included (whether cross-sectional or longitudinal) only measured psychosocial factors at a single timepoint, rendering it impossible to differentiate between chronic and acute stressors.
A further interesting finding of the vaccine response studies is that the time between measurement of psychosocial factors and immune outcomes seems to matter for the strength and nature of findings, but in a complex manner. This is an important limitation of all the cross-sectional studies included: since immune system functioning is constantly fluctuating, immune activation measured at a given timepoint following a stressor may be followed by a period of immune suppression, and vice versa. More longitudinal studies are therefore required, measuring psychosocial exposures and immune outcomes at multiple timepoints.
4.2. Implications for TB and BCG response
Although the relationship between psychosocial factors and biomarkers of immunity was investigated for various infectious diseases, no study examined this in relation to TB risk, progression from latent to active disease, or BCG response. This makes it impossible to draw direct conclusions for TB, meaning that future TB-specific research in this area is essential. However, inferences for TB can be made based on the general evidence. In particular, this review has found evidence supporting two mechanisms through which psychosocial factors affect immune response, both of which are potentially relevant to the immunological processes involved in TB and BCG response:
4.2.1. Co-viral infection
The studies in this review found that adverse psychosocial circumstances are generally associated with an increase in antibody titres to herpesviruses, suggesting that psychosocial factors could precipitate herpesvirus infection, reinfection or reactivation. This is consistent with evidence that individuals of low socio-economic position (something commonly associated with adverse psychosocial factors (Jani, 2013)) are more likely to be seropositive for CMV (Dowd, Aiello, & Alley, 2009) and have higher CMV-specific circulating antibody levels across the life-course (Dowd & Aiello, 2009). An increase in the CMV-specific IFNγ response (which, like increased antibody titre, indicates CMV exposure) has been associated with increased TB disease risk among infants (Muller et al., 2017) and a lower BCG antigen-specific T-cell response following vaccination (Fletcher et al., 2016). A plausible mechanism underlying such associations could be that herpesvirus-associated chronic immune activation of the T-cell response (Bekker et al., 2005; Elwenspoek et al., 2017; Fülöp, Larbi, & Pawelec, 2013; Slyker et al., 2012) ultimately leads to immune exhaustion and senescence, reducing the host's ability to respond to TB infection. For example, primary CMV infection is characterised by expansion of CMV-specific CD4+ and CD8+ T-cells and natural killer (NK) cells, both of which are important for defence against TB (Fletcher et al., 2016; Muller et al., 2019). This herpesvirus-specific expansion can ultimately result in immune exhaustion, with CMV-specific NK cells showing reduced IFNγ secretion in response to other pathogens such as TB (Cobelens et al., 2018). Moreover, expansion of CMV-specific T-cells, a phenomenon known as memory inflation (something associated with immune senescence), may displace naïve T-cells that could otherwise respond to M.tb antigens (White, Beard, & Barton, 2012). While CMV is used as an example here, it is not the only chronic viral infection known to be associated with TB (see Box 1), meaning that the proposed mechanisms are potentially more widely applicable.
4.2.2. Immune suppression
The studies in this review found that adverse psychosocial factors are generally associated with compromised immune response to vaccination, including influenza, hepatitis A and B, meningitis C and pneumococcal vaccines. BCG is absent from this list, meaning that firm conclusions cannot be drawn about whether a similar effect would be seen of psychosocial factors on BCG response. However, it is a plausible future research avenue. Adverse psychosocial factors were generally associated with compromised vaccine response evidenced by reduced antibody titres, something relevant to TB given increasing evidence for the antibody response's role in host defence against M.tb (Lu et al., 2016). However, a few studies also investigated vaccine response by cytokine levels; for example psychosocial factors were associated with both antigen-specific IFNγ levels (Hayney et al., 2003) and antigen-specific IFNγ-producing cell frequencies (O'Connor et al., 2013). This is particularly relevant to TB, given that IFNγ production by CD4+ T-cells is central to the immune response to BCG vaccination (Fletcher et al., 2016). An additional tentative pathway may be suggested based on two studies in which stress and anxiety correlate with a reduced proportion of CD4+ and CD8+ T-cells among patients with chronic recurrent HSV (Kemeny et al., 1989) and CHB (Liu et al., 2011). These findings suggest a direct pathway by which adverse psychosocial factors could provoke immune suppression manifested as a lower proportion of CD4+ and CD8+ T-cells. More indirectly, they could be a result of the pathway outlined above in which chronic immune activation due to persistent viral infection leads to T-cell exhaustion, precipitating reduced T-cell proliferation and cytokine production. A lower number and compromised cytokine-producing ability of CD4+ and CD8+ T-cells would likely lead to reduced IFNγ secretion and thus increased risk of TB disease. Evidence supports this; in infants, BCG-specific T-cells secreting IFNγ associate with reduced risk of TB (Fletcher et al., 2016).
4.3. Strengths and limitations
This study represents the first attempt to systematically assess the association between a wide range of psychosocial factors and biomarkers of immunity and apply this knowledge to strengthen our understanding of the impact of psychosocial factors on BCG response. However, the lack of TB-specific evidence means that our review can only produce provisional hypotheses in this respect. Other limitations impose caution in drawing conclusions: while synthesis across disparate studies has allowed the evidence to be brought together and analysed holistically, this diversity makes drawing direct comparisons between studies difficult, and meta-analysis inappropriate. Moreover, there remain gaps in the evidence base. Most studies are based in HICs, making it difficult to infer whether the findings hold for populations in LICs.
An important limitation to take into consideration is that it is beyond the scope of the included studies to fully account for the upstream social environment that underlies the stressors measured. While some studies adjust for education, income and/or occupation as underlying causes, this is not the case in all studies. Upward bias in the estimates of stressors is thus possible, since psychosocial stress is associated with socio-economic deprivation (Jani, 2013). For example, poverty may influence immunity via material or behavioural pathways in addition to psychosocial mechanisms (see Fig. 1), thus lack of adjustment for socioeconomic factors or other pathways may overestimate the effect of stress. Given the complexity of the causal pathways involved, it is difficult to draw conclusions with certainty. However, that an effect of psychosocial stress on immunity is seen in longitudinal studies and across different population groups, and that the findings are consistent with experimental evidence in animals (Batuman et al., 1990; Curtin et al., 2009; Zhang et al., 2005), suggests that the possibility of a real effect of psychosocial factors on immunity should not be discounted.
In all systematic reviews, publication bias is a risk, since studies with no or weak findings are less likely to be published. However, a robust approach was used to systematically identify relevant published literature. The large number of titles screened – 23,577 after de-duplication – the use of additional strategies beyond database searching including bibliographic screening and citation searching, and critical appraisal of included papers ensured a comprehensive examination of existing evidence in this field. Whilst the review did not include non-English language papers, ‘extreme’ stressors, HIV-infected populations, or laboratory studies, future reviews could focus on these categories.
5. Conclusions
This is the first study to our knowledge to systematically examine psychosocial influences on immunity since 1993 (Van Rood et al., 1993), and the first to apply the findings to TB and BCG response. Firstly, the reviewed evidence suggests that psychosocial factors can influence immune response to infectious diseases and immunisation, but in a highly heterogeneous manner. This renders the current lack of TB-specific studies an important gap in the evidence base. Secondly, this review provides evidence to support the plausibility of the hypothesised conceptual framework linking social protection interventions and BCG response, underscoring the need for further research to confirm or refute whether a psychosocial framework can describe the nature of the hypothesised relationship between social determinants and BCG vaccine response. Specifically, this review can postulate two pathways linking psychosocial factors to immune biomarkers relevant to TB: co-viral infection and suppressed immune response. Future research must confirm whether these mechanisms indeed hold true in the case of TB and BCG response.
From a broader perspective, we postulate that better integration between social protection and BCG immunisation may improve vaccine response among severely deprived populations. To our knowledge, there is no evidence as yet about the influence of social protection and social policies in general on biomarkers of immunity. In particular, there is a significant paucity of data about the influence of social adversities on the immune response to TB. In addition, very little is known on how and the extent to which any alteration to these processes could be modified or recovered through interventions, like social protection, able to tackle adverse living conditions. Understanding the pathways between social determinants, psychosocial exposures, and immune response to TB and BCG, and whether these can be modulated by social protection policies, is therefore an important agenda for further research.
Funding
This work was supported by the GCRF Networks in Vaccines Research and Development VALIDATE Network (www.validate-network.org), which was co-funded by the MRC and BBSRC. This UK funded award is part of the EDCTP2 programme supported by the European Union. DB was awarded a pump-priming grant for this project (Grant Number: P002). The funders had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Declaration of competing interest
None.
Appendix A. Search strategies
MEDLINE search strategy:
-
1.
(Psychosocial or anxi* or depress* or stress*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
2.
Psychosocial deprivation/
-
3.
exp Anxiety/
-
4.
exp Anxiety Disorders/
-
5.
exp Depression/
-
6.
exp Stress, psychological/
-
7.
1 or 2 or 3 or 4 or 5 or 6
-
8.
(Immune OR Immunity OR Immunosuppress* OR Immunosenescence OR Immunology OR Immunological OR Psychoimmunology OR Psychoneuroimmunology OR Inflammation OR Inflammatory).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
9.
exp Immune system phenomena/
-
10.
exp Immune system/
-
11.
8 or 9 or 10
-
12.
(Infect* or communicable or epidemic or bacteri* or virus or viral).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
13.
exp Infection/
-
14.
exp Bacterial infections/
-
15.
exp Virus diseases/
-
16.
exp Parasitic diseases/
-
17.
(Tuberculosis or TB).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
-
18.
exp Tuberculosis/
-
19.
12 or 13 or 14 or 15 or 16 or 17 or 18
-
20.
7 and 11 and 19
-
21.
Limit 20 to (English language and humans)
PsycINFO/PsycEXTRA search strategy:
-
1.
(Psychosocial or anxi* or depress* or stress*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
-
2.
exp Psychosocial factors/
-
3.
exp Anxiety/
-
4.
exp Anxiety disorders/
-
5.
exp Recurrent depression/
-
6.
exp “Depression (emotion)”/
-
7.
exp Major depression/
-
8.
exp Stress/
-
9.
exp Social stress/
-
10.
exp Psychological stress/
-
11.
exp Chronic stress/
-
12.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
-
13.
(Immune OR Immunity OR Immunosuppress* OR Immunosenescence OR Immunology OR Immunological OR Psychoimmunology OR Psychoneuroimmunology OR Inflammation OR Inflammatory).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
-
14.
exp Immune system/
-
15.
exp Immunology/
-
16.
exp Inflammation
-
17.
13 or 14 or 15 or 16
-
18.
(Infect* or communicable or epidemic or bacteri* or virus or viral).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
-
19.
exp Infectious disorders/
-
20.
(Tuberculosis or TB).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
-
21.
exp Tuberculosis/
-
22.
18 or 19 or 20 or 21
-
23.
12 and 17 and 22
-
24.
Limit 20 to (English language and human)
Global Health search strategy:
-
1.
(Psychosocial or anxi* or depress* or stress*).mp. [mp=abstract, title, original title, broad terms, heading words, identifiers, cabicodes]
-
2.
exp Psychosocial aspects/
-
3.
exp Anxiety/
-
4.
exp Depression/
-
5.
exp Stress/
-
6.
1 or 2 or 3 or 4 or 5
-
7.
(Immune OR Immunity OR Immunosuppress* OR Immunosenescence OR Immunology OR Immunological OR Psychoimmunology OR Psychoneuroimmunology OR Inflammation OR Inflammatory).mp. [mp=abstract, title, original title, broad terms, heading words, identifiers, cabicodes]
-
8.
exp Immune response/
-
9.
exp Immune system/
-
10.
exp Immunity/
-
11.
7 or 8 or 9 or 10
-
12.
(Infect* or communicable or epidemic or bacteri* or virus or viral).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
-
13.
exp Infection/
-
14.
exp Infectious diseases/
-
15.
exp Bacterial diseases/
-
16.
exp Viral diseases/
-
17.
(Tuberculosis or TB).mp. [mp=abstract, title, original title, broad terms, heading words, identifiers, cabicodes]
-
18.
exp Tuberculosis/
-
19.
12 or 13 or 14 or 15 or 16 or 17 or 18
-
20.
6 and 11 and 19
-
21.
Limit 20 to English language
Appendix B. Data extraction/critical appraisal form
| Data Extraction | Critical Appraisal | |
|---|---|---|
| General Information | ||
| First author | ||
| Year | ||
| Title | ||
| Participants | ||
| Population | ||
| Setting | ||
| Sampling method | ||
| Inclusion/exclusion criteria | ||
| Sample size | ||
| Response rate | ||
| Sample characteristics | ||
| Methods | ||
| Aim of study | ||
| Study design | ||
| Description of methods | ||
| Duration of follow-up (if relevant) | ||
| Loss to follow-up (if relevant) | ||
| Statistical methods used | ||
| Variables | ||
| Exposure variables (psychosocial factors) | ||
| Measurement of exposure | ||
| Outcome variables (biomarkers of immunity) | ||
| Measurement of outcome | ||
| Mediator variables | ||
| Measurement of mediators | ||
| Confounder variables | ||
| Measurement of confounders | ||
| Application to infectious disease | ||
| Findings | ||
| Strength of findings (none, weak, mixed, strong) | ||
| Key findings | ||
| Key conclusions of authors | ||
| Notes | ||
| Study quality overall judgement: high/moderate/low | ||
| Other comments | ||
Appendix C. Vaccine response studies
Appendix D. Herpesvirus studies
Appendix E. Other infectious disease studies
References
- Adato M. IFPRI policy brief; Washington, DC. United States: 2008. Social protection: Opportunity for Africa. [Google Scholar]
- Aliabad M.H.B., Jafari E., Kakh M.K., Nosratababadi R., Bakhshi H., Sheikhha M.H.…Arababadi M.K. Anxiety leads to up-regulation of CD36 on the monocytes of chronic hepatitis B-infected patients. International Journal of Psychiatry in Medicine. 2016;51(5):467–475. doi: 10.1177/0091217416680199. [DOI] [PubMed] [Google Scholar]
- Altare F., Durandy A., Lammas D., Emile J.F., Lamhamedi S., Le Deist F.…Casanova J.L. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- Amran F.S., Kim K., Lim A., Thomson R., Lee S., Waterer G. Is pulmonary non-tuberculous mycobacterial disease linked with a high burden of latent cytomegalovirus? Journal of Clinical Immunology. 2016;36(2):113–116. doi: 10.1007/s10875-016-0233-1. [DOI] [PubMed] [Google Scholar]
- Bahramabadi R., Fathollahi M.S., Hashemi S.M., Arababadi A.S., Arababadi M.S., Yousefi-Daredor H.…Arababadi M.K. Serum levels of IL-6, IL-8, TNF-alpha, and TGF-beta in chronic HBV-infected patients: Effect of depression and anxiety. Laboratory Medicine. 2017;49(1):41–46. doi: 10.1093/labmed/lmx064. [DOI] [PubMed] [Google Scholar]
- Bartley M. 2nd ed. Polity Press; Cambridge: 2017. Health inequality: An introduction to concepts, theories and methods. [Google Scholar]
- Bastagli F., Hagen-Zanker J., Harman L., Barca V., Sturge G., Schmidt T. 2016. Cash transfers: What does the evidence say? A rigorous review of programme impact and of the role of design and implementation features. London. [Google Scholar]
- Batuman O.A., Sajewski D., Ottenweller J.E., Pitman D.L., Natelson B.H. Effects of repeated stress on T cell numbers and function in rats. Brain, Behavior, and Immunity. 1990;4(2):105–117. doi: 10.1016/0889-1591(90)90013-g. [DOI] [PubMed] [Google Scholar]
- Bekker V., Bronke C., Scherpbier H.J., Weel J.F., Jurriaans S., Wertheim-van Dillen P.M.…Kuijpers T.W. Cytomegalovirus rather than HIV triggers the outgrowth of effector CD8+CD45RA+CD27- T cells in HIV-1-infected children. AIDS. 2005;19(10):1025–1034. doi: 10.1097/01.aids.0000174448.25132.ad. [DOI] [PubMed] [Google Scholar]
- Benschop R.J., Jabaaij L., Oostveen F.G., Vingerhoets A.J.J.M., Ballieux R.E. The influence of psychological stress on immunoregulation of latent Epstein-Barr virus. Stress Medicine. 1998;14(1):21–29. [Google Scholar]
- Boccia D., Hargreaves J., Lonnroth K., Jaramillo E., Weiss J., Uplekar M.…Evans C.A. Cash transfer and microfinance interventions for tuberculosis control: Review of the impact evidence and policy implications. International Journal of Tuberculosis & Lung Disease. 2011;15(Suppl 2):37–49. doi: 10.5588/ijtld.10.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G.H. Smoking and tuberculosis: A chance or causal association? Thorax. 2005;60(7):527. doi: 10.1136/thx.2004.036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W.T., Chesney M., Alkon A., Tschann J.M., Adams S., Chesterman B. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57(5):411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Brook M.J., Christian L.M., Hade E.M., Ruffin M.T. The effect of perceived stress on Epstein-Barr virus antibody titers in Appalachian Ohio women. Neuroimmunomodulation. 2017;24(2):67–73. doi: 10.1159/000478658. [DOI] [PubMed] [Google Scholar]
- Brunner E. 2017. Lecture at LSHTM “health inequalities: The psychosocial model”. February 2017. [Google Scholar]
- Burns V.E., Carroll D., Drayson M., Whitham M., Ring C. Life events, perceived stress and antibody response to influenza vaccination in young, healthy adults. Journal of Psychosomatic Research. 2003;55(6):569–572. doi: 10.1016/s0022-3999(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Burns V.E., Carroll D., Ring C., Drayson M. Antibody response to vaccination and psychosocial stress in humans: Relationships and mechanisms. Vaccine. 2003;21(19/20):2523–2534. doi: 10.1016/s0264-410x(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Burns V.E., Carroll D., Ring C., Harrison L.K., Drayson M. Stress, coping, and hepatitis B antibody status. Psychosomatic Medicine. 2002;64(2):287–293. doi: 10.1097/00006842-200203000-00012. [DOI] [PubMed] [Google Scholar]
- Burns V.E., Drayson M., Ring C., Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosomatic Medicine. 2002;64(6):963–970. doi: 10.1097/01.psy.0000038936.67401.28. [DOI] [PubMed] [Google Scholar]
- Carter D.J., Glaziou P., Lönnroth K., Siroka A., Floyd K., Weil D.…Boccia D. The impact of social protection and poverty elimination on global tuberculosis incidence: A statistical modelling analysis of sustainable development goal 1. Lancet. 2018;6(5):e514–e522. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta M.T., O'Connor T.G., Wyman P.A., Wang H., Moynihan J., Cross W.…Jin X. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain, Behavior, and Immunity. 2008;22(6):933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielski J.P., McMurray D.N. The relationship between malnutrition and tuberculosis: Evidence from studies in humans and experimental animals. International Journal of Tuberculosis & Lung Disease. 2004;8(3):286–298. [PubMed] [Google Scholar]
- Cheng K.C., Liao K.F., Lin C.L., Lai S.W. Increased risk of pulmonary tuberculosis in patients with depression: A cohort study in taiwan. Frontiers in Psychiatry. 2017;8:235. doi: 10.3389/fpsyt.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian L.M., Iams A.D., Porter K., Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: Effects of race and racial discrimination. Brain, Behavior, and Immunity. 2012;26(8):1280–1287. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clays E., De Bacquer D., Delanghe J., Kittel F., Van Renterghem L., De Backer G. Associations between dimensions of job stress and biomarkers of inflammation and infection. Journal of Occupational and Environmental Medicine. 2005;47(9):878–883. doi: 10.1097/01.jom.0000171056.22917.ad. [DOI] [PubMed] [Google Scholar]
- Cobelens F., Nagelkerke N., Fletcher H. The convergent epidemiology of tuberculosis and human cytomegalovirus infection [version 2; referees: 2 approved] F1000Research. 2018;7:280. doi: 10.12688/f1000research.14184.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care (EPOC) 2017. Data collection form. EPOC Resources for review authors.http://epoc.cochrane.org/epoc-specific-resources-review-authors Available from: [Accessed 28th March 2018] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S., Tyrrell D.A., Smith A.P. Psychological stress and susceptibility to the common cold. New England Journal of Medicine. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme . 2018. CASP case control study checklist.https://casp-uk.net/casp-tools-checklists/ Available from: Accessed 28th March 2018. [Google Scholar]
- Critical Appraisal Skills Programme . 2018. CASP cohort study checklist.https://casp-uk.net/casp-tools-checklists/ Available from: Accessed 28th March 2018. [Google Scholar]
- Curtin N.M., Boyle N.T., Mills K.H., Connor T.J. Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: Reversal by the anxiolytic chlordiazepoxide. Brain, Behavior, and Immunity. 2009;23(4):535–547. doi: 10.1016/j.bbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Darwin E., Murni A.W., Nurdin A.E. The effect of psychological stress on mucosal IL-6 and Helicobacter pylori activity in functional dyspepsia. Acta Med Indones. 2017;49(2):99–104. [PubMed] [Google Scholar]
- Deinzer R., Kleineidam C., Stiller-Winkler R., Idel H., Bachg D. Prolonged reduction of salivary immunoglobulin A (sIgA) after a major academic exam. International Journal of Psychophysiology. 2000;37(3):219–232. doi: 10.1016/s0167-8760(99)00112-9. [DOI] [PubMed] [Google Scholar]
- Dowd J.B., Aiello A.E. Socioeconomic differentials in immune response. Epidemiology. 2009;20(6):902–908. doi: 10.1097/EDE.0b013e3181bb5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J.B., Aiello A.E., Alley D.E. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiology and Infection. 2009;137(1):58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J.B., Fletcher H.A., Boccia D. Social determinants and BCG efficacy: A call for a socio-biological approach to TB prevention [version 1; referees: 2 approved] F1000Research. 2018;(224):7. doi: 10.12688/f1000research.14085.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J.B., Palermo T., Chyu L., Adam E., McDade T.W. Race/ethnic and socioeconomic differences in stress and immune function in the National Longitudinal Study of Adolescent Health. Social Science & Medicine. 2014;115:49–55. doi: 10.1016/j.socscimed.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Drummond P.D., Hewson-Bower B. Increased psychosocial stress and decreased mucosal immunity in children with recurrent upper respiratory tract infections. Journal of Psychosomatic Research. 1997;43(3):271–278. doi: 10.1016/s0022-3999(97)00002-0. [DOI] [PubMed] [Google Scholar]
- Dubos R., Dubos J. Rutgers University Press; New Brunswick, N.J.: 1996. The white plague: Tuberculosis, man, and society. [Google Scholar]
- Elwenspoek M.M.C., Sias K., Hengesch X., Schaan V.K., Leenen F.A.D., Adams P.…Turner J.D. T cell immunosenescence after early life adversity: Association with cytomegalovirus infection. Frontiers in Immunology. 2017;8 doi: 10.3389/fimmu.2017.01263. 1263-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E.S., Crosswell A.D., Mayer S.E., Prather A.A., Slavich G.M., Puterman E. More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology. 2018;49:146–169. doi: 10.1016/j.yfrne.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EpiNet AXIS - appraisal tool. http://www.epinet.net/AXIS-Appraisal-Tool Available from: Accessed 25th March 2018.
- Esterling B.A., Antoni M.H., Kumar M., Schneiderman N. Defensiveness, trait anxiety, and Epstein-Barr viral capsid antigen antibody titers in healthy college students. Health Psychology. 1993;12(2):132–139. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- Fletcher H.A., Snowden M.A., Landry B., Rida W., Satti I., Harris S.A.…McShane H. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nature Communications. 2016;7:11290. doi: 10.1038/ncomms11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K., Hesse C., Boren J.P., Veksler A.E. Affectionate communication can suppress immunity: Trait affection predicts antibodies to latent Epstein-Barr virus. Southern Communication Journal. 2014;79(1):2–13. [Google Scholar]
- Fülöp T., Larbi A., Pawelec G. Human T cell aging and the impact of persistent viral infections. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00271. 271-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterman A.D., Wellisch D.K., Zighelboim J., Luna-Raines M., Weiner H. Psychological and immunological reactions of family members to patients undergoing bone marrow transplantation. Psychosomatic Medicine. 1996;58(5):472–480. doi: 10.1097/00006842-199609000-00009. [DOI] [PubMed] [Google Scholar]
- Gallagher S., Phillips A.C., Ferraro A.J., Drayson M.T., Carroll D. Psychosocial factors are associated with the antibody response to both thymus-dependent and thymus-independent vaccines. Brain, Behavior, and Immunity. 2008;22(4):456–460. doi: 10.1016/j.bbi.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Gallagher S., Phillips A.C., Ferraro A.J., Drayson M.T., Carroll D. Social support is positively associated with the immunoglobulin M response to vaccination with pneumococcal polysaccharides. Biological Psychology. 2008;78(2):211–215. doi: 10.1016/j.biopsycho.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Linares M., Sanchez-Lorente S., Coe C., Martinez M. Intimate male partner violence impairs immune control over herpes simplex virus type 1 in physically and psychologically abused women. Psychosomatic Medicine. 2004;66(6):965–972. doi: 10.1097/01.psy.0000145820.90041.c0. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Annals of Behavioral Medicine. 1997;19(2):78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K., Stout J.C., Tarr K.L., Speicher C.E., Holliday J.E. Stress-related impairments in cellular immunity. Psychiatry Research. 1985;16(3):233–239. doi: 10.1016/0165-1781(85)90111-8. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Uldrich A.P., McCluskey J., Rossjohn J., Moody D.B. The burgeoning family of unconventional T cells. Nature Immunology. 2015;16:1114. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- Goldberg N. 2005. Measuring the impact of microfinance: Taking stock of what we know. Washington DC, USA. [Google Scholar]
- Groër M.W., Yolken R.H., Xiao J.C., Beckstead J.W., Fuchs D., Mohapatra S.S.…Postolache T.T. Prenatal depression and anxiety in Toxoplasma gondii-positive women. American Journal of Obstetrics and Gynecology. 2011;204(5) doi: 10.1016/j.ajog.2011.01.004. 433.e431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen-Zanker J., Bastagli F., Harman L., Barca V., Sturge G., Schmidt T. 2016. Briefing: Understanding the impact of cash transfers: The evidence. London. [Google Scholar]
- Hargreaves J.R., Boccia D., Evans C.A., Adato M., Petticrew M., Porter J.D.H. The social determinants of tuberculosis: From evidence to action. American Journal of Public Health. 2011;101(4):654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries A.D., Kumar A.M.V., Satyanarayana S., Lin Y., Zachariah R., Lönnroth K. Addressing diabetes mellitus as part of the strategy for ending TB. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2016;110(3):173–179. doi: 10.1093/trstmh/trv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayney M.S., Love G.D., Buck J.M., Ryff C.D., Singer B., Muller D. The association between psychosocial factors and vaccine-induced cytokine production. Vaccine. 2003;21(19/20):2428–2432. doi: 10.1016/s0264-410x(03)00057-4. [DOI] [PubMed] [Google Scholar]
- He Y., Gao H., Li X., Zhao Y. Psychological stress exerts effects on pathogenesis of hepatitis B via type-1/type-2 cytokines shift toward type-2 cytokine response. PLoS ONE [Electronic Resource] 2014;9(8) doi: 10.1371/journal.pone.0105530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn E.E., Turkheimer E., Strachan E. Psychological distress, emotional stability, and emotion regulation moderate dynamics of herpes simplex virus type 2 recurrence. Annals of Behavioral Medicine. 2015;49(2):187–198. doi: 10.1007/s12160-014-9640-9. [DOI] [PubMed] [Google Scholar]
- Hsu P.C., Yolken R.H., Postolache T.T., Beckie T.M., Munro C.L., Groer M.W. Association of depressed mood with herpes simplex virus-2 immunoglobulin-G levels in pregnancy. Psychosomatic Medicine. 2016;78(8):966–972. doi: 10.1097/PSY.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaaij L., Grosheide P.M., Heijtink R.A., Duivenvoorden H.J., Ballieux R.E., Vingerhoets A.J. Influence of perceived psychological stress and distress on antibody response to low dose rDNA hepatitis B vaccine. Journal of Psychosomatic Research. 1993;37(4):361–369. doi: 10.1016/0022-3999(93)90138-6. [DOI] [PubMed] [Google Scholar]
- Jabaaij L., vanHattum J., Vingerhoets A., Oostveen F.G., Duivenvoorden H.J., Ballieux R.E. Modulation of immune response to rDNA hepatitis B vaccination by psychological stress. Journal of Psychosomatic Research. 1996;41(2):129–137. doi: 10.1016/0022-3999(96)00123-7. [DOI] [PubMed] [Google Scholar]
- Jani N. Poverty & stress. Berkeley Scientific Journal. 2013;18(1):42–45. [Google Scholar]
- Janicki-Deverts D., Cohen S., Doyle W.J., Marsland A.L., Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain, Behavior, and Immunity. 2014;40:174–181. doi: 10.1016/j.bbi.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmott J.B., 3rd, Borysenko J.Z., Borysenko M., McClelland D.C., Chapman R., Meyer D. Academic stress, power motivation, and decrease in secretion rate of salivary secretory immunoglobulin A. Lancet. 1983;1(8339):1400–1402. doi: 10.1016/s0140-6736(83)92354-1. [DOI] [PubMed] [Google Scholar]
- Jemmott J.B., 3rd, Magloire K. Academic stress, social support, and secretory immunoglobulin A. Journal of Personality and Social Psychology. 1988;55(5):803–810. doi: 10.1037//0022-3514.55.5.803. [DOI] [PubMed] [Google Scholar]
- Jenkins F.J., Baum A. Stress and reactivation of latent herpes simplex virus: A fusion of behavioral medicine and molecular biology. Annals of Behavioral Medicine. 1995;17(2):116–123. doi: 10.1007/BF02895060. [DOI] [PubMed] [Google Scholar]
- Karalian M.A. Chronic virus infections in combination with tubercular process. Georgian Medical News. 2008;159:40–43. [PubMed] [Google Scholar]
- Kemeny M.E. An interdisciplinary research model to investigate psychosocial cofactors in disease: Application to HIV-1 pathogenesis. Brain, Behavior, and Immunity. 2003;17(1):S62–S72. doi: 10.1016/s0889-1591(02)00069-7. [DOI] [PubMed] [Google Scholar]
- Kemeny M.E., Cohen F., Zegans L.S., Conant M.A. Psychological and immunological predictors of genital herpes recurrence. Psychosomatic Medicine. 1989;51(2):195–208. doi: 10.1097/00006842-198903000-00008. [DOI] [PubMed] [Google Scholar]
- Kemeny M.E., Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: A stepwise progression. Brain, Behavior, and Immunity. 2007;21(8):1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Karkowski L.M., Prescott C.A. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Fisher L.D., Ogrocki P., Stout J.C., Speicher C.E., Glaser R. Marital quality, marital disruption, and immune function. Psychosomatic Medicine. 1987;49(1):13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Garner W., Speicher C., Penn G.M., Holliday J., Glaser R. Psychosocial modifiers of immunocompetence in medical students. Psychosomatic Medicine. 1984;46(1):7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Glaser R., Shuttleworth E.C., Dyer C.S., Ogrocki P., Speicher C.E. Chronic stress and immunity in family caregivers of Alzheimer's disease victims. Psychosomatic Medicine. 1987;49(5):523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kilburn K., Handa S., Angeles G., Tsoka M., Mvula P. Paying for happiness: Experimental results from a large cash transfer program in Malawi. Journal of Policy Analysis and Management. 2018;37(2):331–356. doi: 10.1002/pam.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut M.L., Cooper M.M., Nickolaus M.S., Russell D.R., Cunnick J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57(9):M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- Koh D., Yong Y., Ng V., Chia S.E. Stress, mucosal immunity, upper respiratory tract infections, and sickness absence. Journal of Occupational and Environmental Medicine. 2002;44(11):987–988. doi: 10.1097/00043764-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Lalor M.K., Ben-Smith A., Gorak-Stolinska P., Weir R.E., Floyd S., Blitz R.…Dockrell H.M. Population differences in immune responses to Bacille Calmette-Guérin vaccination in infancy. The Journal of Infectious Diseases. 2009;199(6):795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S.D., Myer L., Edwards D., Bekker L.G., Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner B.H. Can stress cause disease? Revisiting the tuberculosis research of Thomas Holmes, 1949-1961. Annals of Internal Medicine. 1996;124(7):673–680. doi: 10.7326/0003-4819-124-7-199604010-00008. [DOI] [PubMed] [Google Scholar]
- Levy S.M., Herberman R.B., Lee J., Whiteside T., Beadle M., Heiden L. Persistently low natural killer cell activity, age, and environmental stress as predictors of infectious morbidity. Natural Immunity and Cell Growth Regulation. 1991;10(6):289–307. [PubMed] [Google Scholar]
- Liu W.J., Zhang Y.H., Jiang H.Y. Relationship of anxiety state with lymphocyte subsets and the effect of Chinese medical treatment on anxiety in patients with chronic hepatitis B. Chinese Journal of Integrative Medicine. 2011;17(4):302–306. doi: 10.1007/s11655-011-0708-0. [DOI] [PubMed] [Google Scholar]
- Lönnroth K., Jaramillo E., Williams B.G. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science & Medicine. 2009;68(12):2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Lu L.L., Chung A.W., Rosebrock T.R., Ghebremichael M., Yu W.H., Grace P.S.…Alter G. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–443. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C., Breen A., Flisher A.J., Kakuma R., Corrigall J., Joska J.A.…Patel V. Poverty and common mental disorders in low and middle income countries: A systematic review. Social Science & Medicine. 2010;71(3):517–528. doi: 10.1016/j.socscimed.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicklal S., Emery V.C., Lazzarotto T., Boppana S.B., Gupta R.K. The “silent” global burden of congenital cytomegalovirus. Clinical Microbiology Reviews. 2013;26(1):86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M., Wilkinson R.G. Psychosocial and material pathways in the relation between income and health: A response to Lynch et al. BMJ. 2001;322(7296):1233–1236. doi: 10.1136/bmj.322.7296.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen P., Bartley M., Lahelma E. Psychosocial determinants of health in social epidemiology. International Journal of Epidemiology. 2002;31(6):1091–1093. doi: 10.1093/ije/31.6.1091. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McKinnon W., Weisse C.S., Reynolds C.P., Bowles C.A., Baum A. Chronic stress, leukocyte subpopulations, and humoral response to latent viruses. Health Psychology. 1989;8(4):389–402. doi: 10.1037//0278-6133.8.4.389. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Cohen S., Pressman S., Barkin A., Rabin B.S., Treanor J.J. Psychological stress and antibody response to influenza vaccination: When is the critical period for stress, and how does it get inside the body? Psychosomatic Medicine. 2004;66(2):215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., The Prisma Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan J.A., Larson M.R., Treanor J., Duberstein P.R., Power A., Shore B. Psychosocial factors and the response to influenza vaccination in older adults. Psychosomatic Medicine. 2004;66(6):950–953. doi: 10.1097/01.psy.0000140001.49208.2d. [DOI] [PubMed] [Google Scholar]
- Muller J., Matsumiya M., Snowden M.A., Landry B., Satti I., Harris S.A., Tanner R. bioRxiv; 2017. Cytomegalovirus infection is a risk factor for TB disease in infants. 222646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Tanner R., Matsumiya M., Snowden M.A., Landry B., Satti I., Harris S.A. JCI Insight; 2019. Cytomegalovirus infection is a risk factor for TB disease in infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali L., Handa S., Peterman A., Seidenfeld D., Tembo G. Does money buy happiness? Evidence from an unconditional cash transfer in Zambia. SSM - Population Health. 2018;4:225–235. doi: 10.1016/j.ssmph.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoskelainen J., Hannuksela M., Palva T. Antibodies to Epstein-Barr virus and some other herpesviruses in patients with sarcoidosis, pulmonary tuberculosis and erythema nodosum. Scandinavian Journal of Infectious Diseases. 1974;6(3):209–216. doi: 10.3109/inf.1974.6.issue-3.01. [DOI] [PubMed] [Google Scholar]
- O'Connor T.G., Winter M.A., Hunn J., Carnahan J., Pressman E.K., Glover V.…Caserta M.T. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain, Behavior, and Immunity. 2013;32:21–28. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.H., Choi H., Kim E.J., Kim H.J., Cho S.I. Depression and risk of tuberculosis: A nationwide population-based cohort study. International Journal of Tuberculosis & Lung Disease. 2017;21(7):804–809. doi: 10.5588/ijtld.17.0038. [DOI] [PubMed] [Google Scholar]
- Olaleye O.D., Omilabu S.A., Baba S.S. Cytomegalovirus infection among tuberculosis patients in a chest hospital in Nigeria. Comparative Immunology, Microbiology and Infectious Diseases. 1990;13(2):101–106. doi: 10.1016/0147-9571(90)90522-u. [DOI] [PubMed] [Google Scholar]
- Oxlade O., Murray M. Tuberculosis and poverty: Why are the poor at greater risk in India? PLoS ONE [Electronic Resource] 2012;7(11) doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E.J., Fernald L.C.H., Weber A., Flynn E.P., VanderWeele T.J. Does alleviating poverty affect mothers' depressive symptoms? A quasi-experimental investigation of Mexico's oportunidades programme. International Journal of Epidemiology. 2011;40(6):1565–1576. doi: 10.1093/ije/dyr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett D.A., Glaser R. How stress influences the immune response. Trends in Immunology. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C., Eggerman M., Mojadidi A., McDade T.W. Social stressors, mental health, and physiological stress in an urban elite of young Afghans in Kabul. American Journal of Human Biology. 2008;20(6):627–641. doi: 10.1002/ajhb.20797. [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham L., Clay E., Braunholz T. 2011. Natural disasters: What is the role for social safety nets? Social Protection Discussion Paper No. 1102. [Google Scholar]
- Peltzer K., Naidoo P., Matseke G., Louw J., McHunu G., Tutshana B. Prevalence of psychological distress and associated factors in tuberculosis patients in public primary care clinics in South Africa. BMC Psychiatry. 2012;12(1):89. doi: 10.1186/1471-244X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry L.J., Weems L.B., Livingstone J.N. Relationship of stress, distress, and the immunological response to a recombinant hepatitis-B vaccine. Journal of Family Practice. 1991;32(5):481–486. [PubMed] [Google Scholar]
- Phillips A.C., Burns V.E., Carroll D., Ring C., Drayson M. The association between life events, social support, and antibody status following thymus-dependent and thymus-independent vaccinations in healthy young adults. Brain, Behavior, and Immunity. 2005;19(4):325–333. doi: 10.1016/j.bbi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips A.C., Carroll D., Bums V.E., Ring C., Macleod J., Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain, Behavior, and Immunity. 2006;20(3):279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Phillips A.C., Carroll D., Khan N., Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain, Behavior, and Immunity. 2008;22(1):52–55. doi: 10.1016/j.bbi.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Powell-Jackson T., Pereira S.K., Dutt V., Tougher S., Haldar K., Kumar P. Cash transfers, maternal depression and emotional well-being: Quasi-experimental evidence from India's Janani Suraksha Yojana programme. Social Science & Medicine. 2016;162:210–218. doi: 10.1016/j.socscimed.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Pressman S.D., Cohen S., Miller G.E., Barkin A., Rabin B.S., Treanor J.J. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24(3):297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Rector J.L., Dowd J.B., Loerbroks A., Burns V.E., Moss P.A., Jarczok M.N.…Bosch J.A. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain, Behavior, and Immunity. 2014;38:133–141. doi: 10.1016/j.bbi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lorente S., Blasco-Ros C., Coe C.L., Martinez M. Recovery of immune control over herpes simplex virus type 1 in female victims of intimate partner violence. Psychosomatic Medicine. 2010;72(1):97–106. doi: 10.1097/PSY.0b013e3181c5080a. [DOI] [PubMed] [Google Scholar]
- Schaubroeck J., Jones J.R., Xie J.J. Individual differences in utilizing control to cope with job demands: Effects on susceptibility to infectious disease. Journal of Applied Psychology. 2001;86(2):265–278. doi: 10.1037/0021-9010.86.2.265. [DOI] [PubMed] [Google Scholar]
- Schmitt V., De L. 2013. Social protection assessment-based national dialogue: A good practices guide. Bangkok. [Google Scholar]
- Segerstrom S.C., Hardy J.K., Evans D.R., Greenberg R.N. Vulnerability, distress, and immune response to vaccination in older adults. Brain, Behavior, and Immunity. 2012;26(5):747–753. doi: 10.1016/j.bbi.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Coe C.L., Pollak S.D. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroka A., Ponce N.A., Lonnroth K. Association between spending on social protection and tuberculosis burden: A global analysis. The Lancet Infectious Diseases. 2016;16(4):473–479. doi: 10.1016/S1473-3099(15)00401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., McLaughlin K.A., Dunn E.C., Koenen K.C. Childhood adversity and cell-mediated immunity in young adulthood: Does type and timing matter? Brain, Behavior, and Immunity. 2013;28:63–71. doi: 10.1016/j.bbi.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyker J.A., Rowland-Jones S.L., Dong T., Reilly M., Richardson B., Emery V.C. Acute cytomegalovirus infection is associated with increased frequencies of activated and apoptosis-vulnerable T cells in HIV-1-infected infants. Journal of Virology. 2012;86(20):11373–11379. doi: 10.1128/JVI.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale L., Nash S., Farmer R., Raynes J., Malikaarjun S., Newton R., Fletcher H.A. The Journal of Infectious Diseases; 2019. Cytomegalovirus antibody responses associated with increased risk of TB disease in Ugandan adults. [DOI] [PubMed] [Google Scholar]
- Stockdale L., Nash S., Nalwoga A., Painter H., Asiki G., Fletcher H. Human cytomegalovirus epidemiology and relationship to tuberculosis and cardiovascular disease risk factors in a rural Ugandan cohort. PLoS ONE [Electronic Resource] 2018;13(2) doi: 10.1371/journal.pone.0192086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C.C., Lai C.L., Tsao S.M., Lin M.N., Hsieh T.C., Lu J.J. High prevalence of human herpesvirus type 8 infection in patients with pulmonary tuberculosis in Taiwan. Clinical Microbiology and Infections. 2015;21(3) doi: 10.1016/j.cmi.2014.10.015. 266.e265-267. [DOI] [PubMed] [Google Scholar]
- Sweetland A.C., Kritski A., Oquendo M.A., Sublette M.E., Norcini Pala A., Silva L.R.B.…Wainberg M.L. Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. International Journal of Tuberculosis & Lung Disease. 2017;21(8):852–861. doi: 10.5588/ijtld.16.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rood Y.R., Bogaards M., Goulmy E., Van Houwelingen H.C. The effects of stress and relaxation on the in vitro immune response in man: A meta-analytic study. Journal of Behavioral Medicine. 1993;16(2):163–181. doi: 10.1007/BF00844891. [DOI] [PubMed] [Google Scholar]
- Webster J.I., Tonelli L., Sternberg E.M. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- White D.W., Beard R.S., Barton E.S. Immune modulation during latent herpesvirus infection. Immunological Reviews. 2012;245(1):189–208. doi: 10.1111/j.1600-065X.2011.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.E., Fineberg H.V., Colditz G.A. Geographic latitude and the efficacy of bacillus Calmette-Guerin vaccine. Clinical Infectious Diseases. 1995;20(4):982–991. doi: 10.1093/clinids/20.4.982. [DOI] [PubMed] [Google Scholar]
- World Bank . 2015. The state of social safety nets 2015. Washington, DC. [Google Scholar]
- World Health Organization BCG vaccines: WHO position paper – february 2018. WHO Weekly Epidemiological Record. 2018;8(93):73–96. [Google Scholar]
- World Health Organization . WHO; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- Wouters M.M., Van Wanrooy S., Nguyen A., Dooley J., Aguilera-Lizarraga J., Van Brabant W.…Boeckxstaens G. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65(8):1279–1288. doi: 10.1136/gutjnl-2015-309460. [DOI] [PubMed] [Google Scholar]
- Wu R.F., Zimmerman R.K., Lin C.J. The effect of perceived psychological stress on the immunogenicity of the quadrivalent human papillomavirus vaccine in males. Human Vaccines & Immunotherapeutics. 2017;13(3):676–679. doi: 10.1080/21645515.2016.1236880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Koh D., Ng V., Lee C.Y., Chan G., Dong F.…Chia S.E. Self perceived work related stress and the relation with salivary IgA and lysozyme among emergency department nurses. Occupational and Environmental Medicine. 2002;59(12):836. doi: 10.1136/oem.59.12.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak D.E., Penn-Nicholson A., Scriba T.J., Thompson E., Suliman S., Amon L.M.…teams, G C., s c. A prospective blood RNA signature for tuberculosis disease risk. Lancet. 2016;387(10035):2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Okutsu M., Kanemi O., Gametchu B., Nagatomi R. Repeated stress suppresses interferon-gamma production by murine intestinal intraepithelial lymphocytes. Tohoku Journal of Experimental Medicine. 2005;206(3):203–212. doi: 10.1620/tjem.206.203. [DOI] [PubMed] [Google Scholar]
- Zorrilla E.P., Luborsky L., McKay J.R., Rosenthal R., Houldin A., Tax A.…Schmidt K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain, Behavior, and Immunity. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]