Abstract
Objective
The aim of this study was to investigate the effects of human amniotic membrane (HAM) on fracture healing in an animal model.
Methods
Standard tibial diaphysial fractures were created in twenty-eight Wistar-Albino rats and treated with intramedullary Kirschner wire (K-wire) and HAM (HAM (+) group) or K-wire only (HAM (–) group). Fracture healing was evaluated by histological analysis, radiologic X-ray views and callus diameter measurements at 3rd and 6th weeks postoperatively.
Results
Fracture healing was histologically better in the HAM (+) group and the difference was statistically significant at both 3rd and 6th weeks postoperatively (p < 0.05). The highest histologic scores and entire woven bone formation (Huo Stage 8–9) were obtained at 6th weeks postoperatively in the HAM (+) group. Histological examination also revealed predominant fibrous tissue and partial cartilage formation (Huo Stage 2) at the postoperative 3rd week in the HAM (-) group. Equal amounts of woven bone and cartilage formation (Huo Stage 6–7) were observed at 3rd weeks postoperatively in the HAM (+) group and at 6th weeks postoperatively in the HAM (-) group. The callus diameters were greater in the HAM (+) group and the difference was statistically significant (p < 0.05) at 3rd and 6th weeks postoperatively. Although there was only a statistically significant difference (p < 0.05) at the postoperative 3rd week, radiological scores tended to be higher in the HAM (+) group at both the 3rd and 6th weeks postoperatively.
Conclusion
HAM is a cheap and easily accessible alternative biological material. HAM may be used to support surgical treatment of fractures, particularly where bone healing is expected to last longer.
Keywords: Amniotic membrane, Amnion, Fracture healing, Bone union, Animal study
Introduction
Bone healing is a natural biological process which is completed without any problems if the appropriate environment is maintained. In the modern world, the expectations are getting higher from the healthcare providers in terms of decreasing the medical costs and labor losses. Enhancing and/or accelerating the bone regeneration is currently becoming a new goal for orthopedic procedures to meet these challenging demands. Innovative developments in fracture treatment and regenerative medicine are promising in terms of shortening the fracture healing duration.
Human amniotic membrane (HAM) has anti-inflammatory, immunomodulatory, hypoimmunogenic, multipotency and non-tumorigenic properties.1, 2 Human amnion-derived products (cells, fluid or membrane) have been used by various surgical disciplines for different purposes such as corneal defect reconstruction, periodontal surgical applications, treatment of burn wounds or diabetic ulcers and the repair of tendon and nerve ruptures (as well as the prevention of adhesions after repair).3, 4, 5, 6
There are several reports in which acellular amniotic membranes (AAM) are used as scaffolds, showing that osteogenic and chondrogenic differentiation occur in stem cells loaded on amniotic membranes.7, 8, 9 There are several studies in the literature that evaluated the effects of amniotic fluid on fracture healing.10, 11 Studies examining the effects of amnion-based products on bone regeneration, such as the treatment of bone defects and spinal fusion surgery, have focused on the 'cellular' effects of the cells from amniotic or non-amniotic sources.10, 11, 12 However, to the best of our knowledge there is no study that examined the effects of amniotic membrane (AM) which was directly applied on the fracture site by means of fracture healing.
The AM is composed of many essential elements, biologically active mediators and growth factors including collagen, laminin, fibronectin, elastin, proteoglycans, hyaluronic acid, epidermal growth factor (EGF), fibroblast growth factor (FGF), and transforming growth factors (TGF).2, 4, 8 This composition of the AM is thought to have paracrine effects, such as protection of the damaged tissue from external factors, optimization of the micro-environment, and stimulation of tissue repair.1, 2, 8 There are studies suggesting that AM supports epithelial tissue healing.13 Also, positive results were reported with the use of AM in large bone defects.2, 8 Despite these reports mentioning that AM supports soft tissue and bony regeneration with paracrine properties, there is no study in the literature about the positive effects of AM on fracture healing with direct application on fracture site.
The aim of this study is to evaluate the potential positive effects of cryopreserved HAM on fracture healing by means of radiological, histological and metric measurements. Another aim of this study is to define a cheap, easily accessible and lesser invasive biomaterial that can be used in surgical fracture treatment.
Materials and methods
This research has been approved by the ethics committee of the authors' affiliated institution. The placenta to be used in the production of HAM was taken under sterile conditions during a routine caesarean section with approval of a pregnant woman. All pregnant women are routinely evaluated for HIV, Hepatitis B, Hepatitis C and Syphilis in our institution before delivery as a surgical protocol. The placenta donor of this study had negative test results. The amniotic membrane was washed 4 times with a combination of antibiotics (penicillin G, streptomycin and amphotericin B) and Medium 199 (Sigma–Aldrich, USA) to remove the blood clots and residual tissue in a Class II laminar flow chamber under sterile conditions. Then, the AM was spread on a 45 micrometers nitrocellulose porous membrane (Whatman, Germany) and cut into 5 × 5 cm pieces. Each piece was put in a bottle containing 50% glycerol and 50% Dulbecco's Modified Eagle Medium (DMEM) (Sigma–Aldrich, USA) and stored at −80 °C in a freezer.
A single rat was used as a preliminary test subject to observe potential tissue rejection reactions. The application of HAM on rat tibia did not show any signs of early or late tissue rejection. The animal was sacrificed at 6th weeks postoperatively and excluded from the study. Twenty-eight male Wistar-Albino rats with an average weight of 450 g (±50 g) and an average age of 5 months (±15 days) were used in our study. Intraperitoneal ketamine and xylazine combination were used for sedation. After subperiosteal elevation of the soft tissues around the tibia in both groups, a standard tibia shaft fracture was created using a low-speed drill in all rats. The rat tibia has an anterior bowing in the midshaft region. A 2 mm drill bit was used to drill the center of this bowing, and anterior and posterior cortex were then cut with a fine bonecutter. The animals were then divided into two groups: half of the animals were treated with intramedullary fixation (HAM (-) group) using only Kirschner wire (K-wire), whereas the other half was treated with K-wire intramedullary fixation and HAM wrapping around tibia (HAM (+) group). A 1.5 mm K-wire was inserted into distal fragment's intramedullary cavity. Proximal end of the K-wire was then inserted into proximal fragment's intramedullary cavity and the fracture was reduced. After the fracture fixation was completed in HAM (+) group, HAM wrapped circumferentially around the tibia and fixed to the bone with proximal and distal fixation sutures (Fig. 1). Skin was closed with continuous stitches in both groups. Radiological imaging was performed at 3rd and 6th weeks postoperatively for both the HAM (+) and HAM (-) groups. Radiological fracture healing was evaluated according to the scoring system described by Lane et al.14 Half of the animals in both the HAM (+) and HAM (-) groups were sacrificed by cervical dislocation under ketamine and xylazine sedation at 3rd weeks postoperatively and the other half at 6th weeks postoperatively. The tibias of the animals were excised and fracture healing in the excised tibia was evaluated histologically according to the scoring system described by Huo et al.15 The callus diameters of all excised tibias were also measured with a microscope.
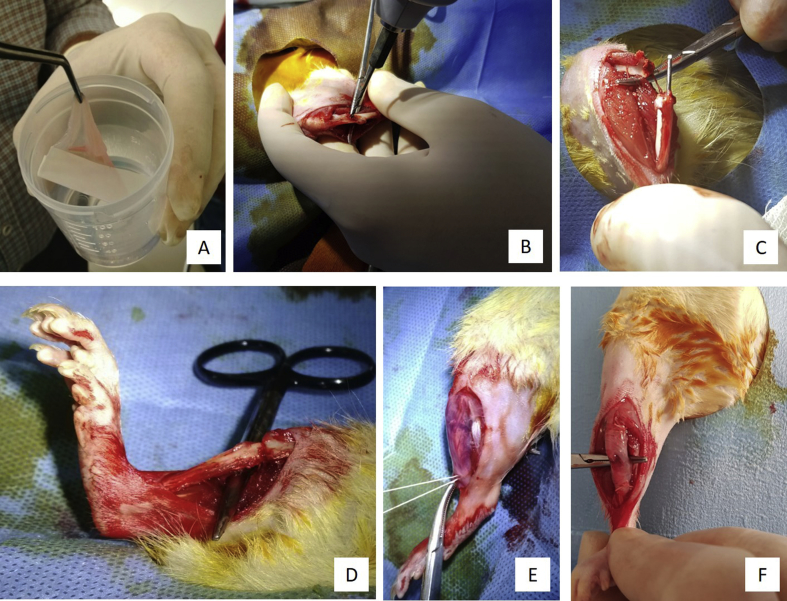
Fig. 1.
Phases of surgery: (A) HAM. (B) Standard tibial shaft fracture is created by using a drill bit in rat tibia. (C) Intramedullary nail (K-wire) insertion. (D) Fracture reduction. (E) HAM is wrapped circumferentially around tibia. (F) Final status: HAM is fixed with proximal and distal sutures.
The bone was surgically removed, dissected, and fixed in 10% neutral-buffered formalin solution. The specimens were processed at the institution's Laboratory of Medical Pathology. The samples were demineralized with 5% nitric acid (pH 7.4), and routinely embedded in paraffin. Paraffin blocks containing the bone were serially sectioned with an average thickness of 4–5 mm. Sections were stained with hematoxylin-eosin and examined under light microscopy. Two histologic slides (4 fields in each) were evaluated for every bone. Callus diameters were also measured under microscope.
The distribution of the variables was measured by the Kolmogorov–Simirnov test. The Mann–Whitney U test was used in the analysis of the quantitative independent data. The paired Friedman test was used for the analysis of the dependent quantitative data. In-class correlation analysis was used in the evaluation of interobserver reliability. The SPSS 22.0 program was used in the analyses.
Results
Three orthopaedic surgeons (MY, BP, ES) with 7, 5 and 4 years of experience respectively, evaluated all radiological images independently according to the scoring system described by Lane et al.14 The authors were blinded during the evaluation. There was a significant correlation between the radiologic score measures of the researchers (p = 0.000, r = 0.803 (0.671–0.887). Although there was a strong correlation in the majority of the evaluation, there also was a significant (p < 0.05) difference between the measurements in some images. While there was a disagreement between the authors a reevaluation was made until a consensus was reached.
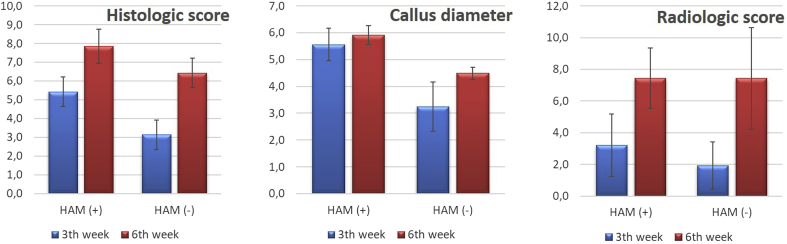
Fracture healing was histologically better in the HAM (+) group and the difference was statistically significant at both 3rd and 6th weeks postoperatively (p < 0.05) (Table 1). The highest histologic scores and entire woven bone formation (Huo Stage 8–9) were obtained at 6th weeks postoperatively in the HAM (+) group (Fig. 2A). Histological examination also revealed predominant fibrous tissue and partial cartilage formation (Huo Stage 2) at the postoperative 3rd week in the HAM (-) group (Fig. 2B). Equal amounts of woven bone and cartilage formation (Huo Stage 6–7) were observed at 3rd weeks postoperatively in the HAM (+) group and at 6th weeks postoperatively in the HAM (-) group (Fig. 2C). The callus diameters were greater in the HAM (+) group and the difference was statistically significant (p < 0.05) at 3rd and 6th weeks postoperatively. Although there was only a statistically significant difference (p < 0,05) at the postoperative 3rd week, radiological scores tended to be higher in the HAM (+) group at both the 3rd and 6th weeks postoperatively (Fig. 3, Fig. 4). There were two cases of superficial wound infections (1 in HAM (+) group and 1 in HAM (-) group), but no osteomyelitis or bone healing problems were detected.
Table 1.
Statistical analysis of data.
| HAM+ |
HAM− |
p | |||||
|---|---|---|---|---|---|---|---|
| Mean ± s.d. | Median | Min-Max | Mean ± s.d | Median | Min-Max | ||
| 3rd week | |||||||
| Radiologic score | 3.2 ± 2.0 | 2.5 | 1.0–6.0 | 1.9 ± 1.5 | 1.5 | 0.0–5.0 | 0.042a |
| Histologic score | 5.4 ± 0.8 | 6.0 | 4.0–6.0 | 3.1 ± 1.2 | 3.0 | 2.0–5.0 | 0.005a |
| Callus diameter | 5.6 ± 0.6 | 5.9 | 4.4–6.1 | 3.2 ± 0.9 | 2.8 | 2.4–4.7 | 0.003a |
| 6th week | |||||||
| Radiologic score | 7.4 ± 1.9 | 6.0 | 6.0–10.0 | 7.4 ± 3.2 | 8.0 | 2.0–12.0 | 0.840a |
| Histologic score | 7.9 ± 0.9 | 8.0 | 6.0–9.0 | 6.4 ± 0.8 | 6.0 | 6.0–8.0 | 0.015a |
| Callus diameter | 5.9 ± 0.3 | 5.9 | 5.4–6.4 | 4.5 ± 0.2 | 4.4 | 4.2–4.9 | 0.002a |
Mann-whitney u test.
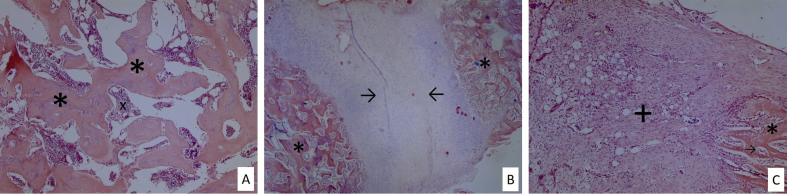
Fig. 2.
(A) Predominant fibrous tissue and partial cartilage formation (x20 H&E). (B) Equal amounts of woven bone and cartilage tissue (x10 H&E). (C) Entire woven bone formation (x20 H&E). + fibrotic tissue, * woven bone, → cartilage tissue, x haematopoetic cells.
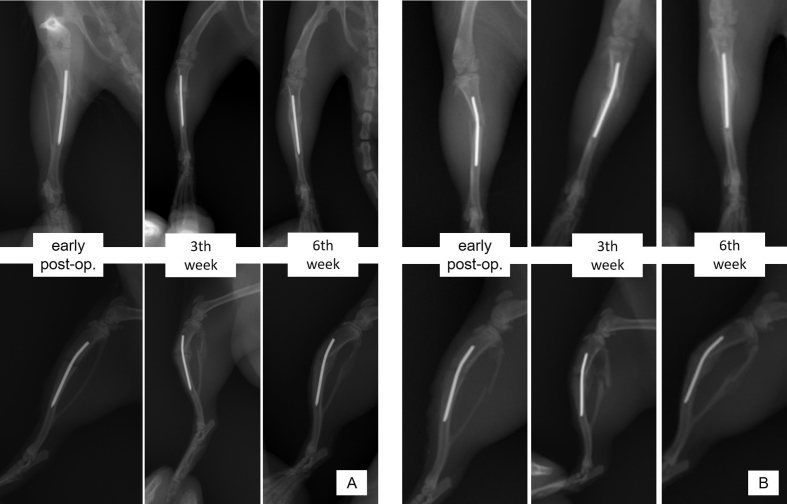
Fig. 3.
Radiographic images of HAM (-) (A) and HAM (+) (B) subjects just after the operation, and at postoperative 3rd and 6th weeks.
Fig. 4.
Graphics demonstrating the histologic scores, callus diameters and radiologic scores both in HAM (+) and HAM (-) groups.
Discussion
The most important finding of this study is that the cryopreserved HAM had positive effect on early fracture healing by means of callus diameter measurements, and radiologic and histologic scores. Even though radiological scores did not reach statistical significance in the late phases of fracture regeneration, HAM continued to promote bony union according to histologic scores and callus diameter measurements at sixth weeks postoperatively.
There are several studies in vitro and in vivo on the use of amniotic membrane in the orthopedic field. Peister et al showed that amniotic fluid-derived cells produce approximately 5-fold more mineralized matrix than bone-marrow mesenchymal stem cells (BMSCs).16 Amniotic fluid (AF) was reported to enhance bone healing by subperiosteal application in rabbit calvarial defects due to high hyaluronic acid, hyaluronic acid stimulated activator, and other bioactive molecular content.10 There are also other studies that have reported that AF has positive effects on fracture healing and posterolateral spinal fusion in animal models.11, 17 In an animal study by Ghanmi et al, it was stated that fresh HAM has an enhancing effect on bone regeneration in the treatment of large bone defects in tibia diaphysis, but this effect is lesser than in the natural periosteum.2 In the same study, it was emphasized that fresh HAM is an absorbable scaffold, supporting bone regeneration by functioning as a donor of healing stimulator cells and growth factors. Amniotic tissue also has chondrogenic properties.18, 19 Zhang et al reported that BMSCs implanted on the human acellular amniotic membrane (HAAM) have positive effects on chondral defects formed in the load bearing surface of rabbit femur.7 In this study, it was also stated that HAAM-BMSCs accelerated the repair of damaged tissue by increasing the number of chondrocytes and type II collagen production. A human in vitro osteoarthritis study has shown that the use of cryopreserved HAM stimulated the repair of damaged joint cartilage.20 Namba et al observed that articular cartilage defects in a fetal sheep model heal without scar formation or inflammatory response.21
In our study, histologic scores and callus diameters were higher in the HAM (+) group at both postoperative 3rd and 6th weeks. These results in HAM (+) group indicate that the cryopreserved HAM supports callus formation and maturation, thus accelerating bone regeneration. Although there was only a statistically significant difference at the postoperative 3rd week, radiological scores tended to be higher in the HAM (+) group at both the 3rd and 6th weeks postoperatively. These results show that direct application of the cryopreserved HAM on fracture site has positive effect on bone regeneration and this effect was more prominent in the preliminary stages of the healing process. We may interpret that the positive effects of HAM may take role in the early steps of fracture union like inflammation and callus formation phases and has limited effect on consolidation phase.
In studies investigating the therapeutic use of amnion-derived products, the general focus was on the ‘cellular’ effects of cells from amniotic tissue (from AM or AF) or stem cells obtained from various sources loaded on AM.1, 8, 9 Additionally, the properties of these cells, such as the capability of osteogenic differentiation and synthesizing bone structural products, were also examined in these studies.7, 8 On the other hand, not only the cellular properties but also the paracrine effects of AM and AF may prevent further tissue damage, may stimulate tissue repair or may serve as a vector for the exogenous factors that play an active role in the healing process.9, 22 We assume that HAM has positive effects on fracture healing with two different mechanisms. These are: 1. HAM serves as a source for essential biological factors that are important in early fracture healing, acts as a mechanical barrier to prevent the loss of fracture hematoma and provides an ideal microenvironment for fracture healing (paracrine effect); 2. Amnion-derived cells may transform into osteogenic progenitors or may produce biologic factors that supports bone regeneration (cellular effect). We think that the positive effects of HAM on fracture healing are a combination of paracrine and cellular features.
In the light of current study, it may be advocated that HAM has several advantages to be used in orthopaedic field as a biomaterial. Besides being commercially available on the market in different forms, HAM also may easily be produced and safely be preserved.1, 23 Placenta is a residual tissue after delivery. Ethical concerns are limited because there is no potential harm for the donor or the baby. This might be a superiority over the autografts because of avoiding the donor site complications. HAM acts as a scaffold and components of HAM promotes cell proliferation, angiogenesis and osteogenesis.2, 8 HAM also has antimicrobial and anti-inflammatory properties.24 Thus, HAM may promote bone healing as an osteoinductive and osteoconductive agent while inhibiting excessive inflammation and serving as a barrier for infection. This could be beneficial in the treatment of open fractures or in patients with immune deficiency. There are several grafts and/or bone substitutes which are already in use in orthopaedic practice including allografts, BMP (bone morphogenic protein), DBM (demineralized bone matrix), etc. These materials are mostly used for nonunion treatment or filling bony defects. HAM may also be used in combination with allografts and/or bone substitutes to maximize their osteoinductive and osteoconductive properties. Further studies in which conventional allografts or HAM-allograft combinations are compared with HAM may give valuable information.
This present study has several limitations. We used only one placenta for this experimental study. Comparative studies are needed in which the placenta is obtained from different donors to show the consistency of the positive effects of HAM. Although we evaluated the radiologic, histologic and metric parameters of fracture healing, we did not assess the mechanical strength of the bone union. Further biomechanical studies are needed in this manner. We also used digital X-rays for radiological evaluation. Micro-computerized tomography may be used for more sensitive evaluation. The underlying mechanisms that enhances the bone regeneration is still not understood. Further clinical and experimental studies are required to clarify the effects of HAM on bone healing in more detail.
Conclusions
HAM is a cheap and easily accessible alternative biological material. There is also no risk of donor morbidity because the HAM is obtained after birth. We think that HAM may be used to support surgical treatment of fractures, particularly where bone healing is expected to last longer.
Conflicts of interest
None.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Atilla H.A., Stubbs A.J. Amniyotik membran ve sıvı kaynaklı tedaviler – kök hücre tedavisi. TOTBID Derg. 2017;16(3):259–265. [Google Scholar]
- 2.Ghanmi S., Trigui M., Baya W. The periosteum-like effect of fresh human amniotic membrane on bone regeneration in a rabbit critical-sized defect model. Bone. 2018;110:392–404. doi: 10.1016/j.bone.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y., Borrelli M., Reichl S., Schrader S., Geerling G. Review of alternative carrier materials for ocular surface reconstruction. Curr Eye Res. 2014;39(6):541–552. doi: 10.3109/02713683.2013.853803. [DOI] [PubMed] [Google Scholar]
- 4.DiDomenico L.A., Orgill D.P., Galiano R.D. Aseptically processed placental membrane improves healing of diabetic foot ulcerations. Plast Reconstr Surg – Glob Open. 2016;4(10):e1095. doi: 10.1097/GOX.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özgenel G.Y. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301–307. doi: 10.1302/0301-620x.86b2.14435. [DOI] [PubMed] [Google Scholar]
- 6.Mohan R., Bajaj A., Gundappa M. Human amnion membrane: potential applications in oral and periodontal field. J Int Soc Prev Community Dent. 2017;7(1):15–21. doi: 10.4103/jispcd.JISPCD_359_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Zeng L., Yang J., Guo L., Hou Q., Zhu F. Amniotic membrane-derived stem cells help repair osteochondral defect in a weight-bearing area in rabbits. Exp Ther Med. 2017;14(1):187–192. doi: 10.3892/etm.2017.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang K., Wu J., Xiong Z., Ji Y., Sun T., Guo X. Human acellular amniotic membrane: a potential osteoinductive biomaterial for bone regeneration. J Biomater Appl. 2018;32(6):754–764. doi: 10.1177/0885328217739753. [DOI] [PubMed] [Google Scholar]
- 9.Liu P.F., Guo L., Zhao D.W. Study of human acellular amniotic membrane loading bone marrow mesenchymal stem cells in repair of articular cartilage defect in rabbits. Genet Mol Res. 2014;13(3):7992–8001. doi: 10.4238/2014.September.29.12. [DOI] [PubMed] [Google Scholar]
- 10.Karaçal N., Koşucu P., Çobanǵl^u Ü., Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283–287. doi: 10.1016/j.jss.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Kerimoǧlu S., Livaoǧlu M., Sönmez B. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281–287. doi: 10.1016/j.jss.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Oner M., Dulgeroglu T.C., Karaman I., Guney A., Kafadar I.H., Erdem S. The effects of human amniotic fluid and different bone grafts on vertebral fusion in an experimental rat model. Curr Ther Res – Clin Exp. 2015;77:35–39. doi: 10.1016/j.curtheres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblum B.I. A retrospective case series of a dehydrated amniotic membrane allograft for treatment of unresolved diabetic foot ulcers. J Am Podiatr Med Assoc. 2016;106(5):328–337. doi: 10.7547/15-139. [DOI] [PubMed] [Google Scholar]
- 14.Lane J.M., Sandhu H.S. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213–225. [PubMed] [Google Scholar]
- 15.Huo M.H., Troiano N.W., Pelker R.R., Gundberg C.M., Friedlaender G.E. The influence of ibuprofen on fracture repair: biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res. 1991;9(3):383–390. doi: 10.1002/jor.1100090310. [DOI] [PubMed] [Google Scholar]
- 16.Peister A., Woodruff M.A., Prince J.J., Gray D.P., Hutmacher D.W., Guldberg R.E. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17–27. doi: 10.1016/j.scr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aydın H., Saraçoğlu M., Kerimoğlu G., Kerimoğlu S., Topbaş M. İnsan amniyon sıvısının posterolateral spinal füzyon üzerindeki etkileri: deneysel bir ön çalışma Effects of human amniotic fluid on posterolateral spinal fusion: an experimental preliminary study. Eklem Hast Cerrahisi. 2011;22(3):166–171. [PubMed] [Google Scholar]
- 18.Krishnamurithy G., Shilpa P.N., Ahmad R.E., Sulaiman S., Ng C.L.L., Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res Part A. 2011;99A(3):500–506. doi: 10.1002/jbm.a.33184. [DOI] [PubMed] [Google Scholar]
- 19.Garcia D., Longo U.G., Vaquero J. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2015;10(1):77–83. doi: 10.2174/1574888x09666140710120012. [DOI] [PubMed] [Google Scholar]
- 20.Díaz-Prado S., Rendal-Vázquez M.E., Muiños-López E. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183–195. doi: 10.1007/s10561-009-9144-1. [DOI] [PubMed] [Google Scholar]
- 21.Namba R.S., Meuli M., Sullivan K.M., Le A.X., Adzick N.S. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rennie K., Gruslin A., Hengstschläger M. Applications of amniotic membrane and fluid in stem cell biology and regenerative medicine. Stem Cell Int. 2012;2012:1–13. doi: 10.1155/2012/721538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanselman A.E., Lalli T.A., Santrock R.D. Use of fetal tissue in foot and ankle surgery. Foot Ankle Spec. 2015;8(4):297–304. doi: 10.1177/1938640015578513. [DOI] [PubMed] [Google Scholar]
- 24.Heckmann N., Auran R., Mirzayan R. Application of amniotic tissue in orthopedic surgery. Am J Orthop (Belle Mead NJ) 2016;45(7):E421–E425. [PubMed] [Google Scholar]