Abstract
Objective
Glucose-dependent insulinotropic polypeptide is an intestinally derived hormone that is essential for normal metabolic regulation. Loss of the GIP receptor (GIPR) through genetic elimination or pharmacological antagonism reduces body weight and adiposity in the context of nutrient excess. Interrupting GIPR signaling also enhances the sensitivity of the receptor for the other incretin peptide, glucagon-like peptide 1 (GLP-1). The role of GLP-1 compensation in loss of GIPR signaling to protect against obesity has not been directly tested.
Methods
We blocked the GIPR and GLP-1R with specific antibodies, alone and in combination, in healthy and diet-induced obese (DIO) mice. The primary outcome measure of these interventions was the effect on body weight and composition.
Results
Antagonism of either the GIPR or GLP-1R system reduced food intake and weight gain during high-fat feeding and enhanced sensitivity to the alternative incretin signaling system. Combined antagonism of both GIPR and GLP-1R produced additive effects to mitigate DIO. Acute pharmacological studies using GIPR and GLP-1R agonists demonstrated both peptides reduced food intake, which was prevented by co-administration of the respective antagonists.
Conclusions
Disruption of either axis of the incretin system protects against diet-induced obesity in mice. However, combined antagonism of both GIPR and GLP-1R produced additional protection against diet-induced obesity, suggesting additional factors beyond compensation by the complementary incretin axis. While antagonizing the GLP-1 system decreases weight gain, GLP-1R agonists are used clinically to target obesity. Hence, the phenotype arising from loss of function of GLP-1R does not implicate GLP-1 as an obesogenic hormone. By extension, caution is warranted in labeling GIP as an obesogenic hormone based on loss-of-function studies.
Keywords: Glucose-dependent insulinotropic polypeptide receptor, Glucagon-like peptide 1 receptor, Food intake, Energy expenditure, GIPR antagonist, GLP-1R antagonist
Highlights
-
•
Acute administration of either GIP or GLP-1 reduces food intake inmice, which is blocked by antagonizing antibodies.
-
•
Chronic antagonism of the GIPR limits weight gain, improves glucose tolerance, and enhances sensitivity to GLP-1R agonists.
-
•
Chronic antagonism of the GLP-1R reduces weight gain and enhances sensitivity to GIPR agonists.
-
•
Chronic antagonism of both GIPR and GLP-1R provides additive protections against weight gain when mice are fed a HFD.
-
•
Incretin receptor antagonism reduces food intake but does not change energy expenditure.
1. Introduction
The incretins, glucose-dependent insulinotropic polypeptide (GIP), and glucagon-like peptide 1 (GLP-1) are gut-derived peptides released in response to ingested nutrients that mediate important actions on nutrient metabolism [1]. In addition to the regulation of insulin and glucagon secretion from pancreatic islets, both peptides exert numerous extrapancreatic actions. Notably, GLP-1 limits appetite, decreases gut motility and chylomicron secretion, and exerts mild effects on energy expenditure [[2], [3], [4]]. These actions have supported the development of GLP-1 receptor (GLP-1R) agonists for the treatment of obesity independent of type 2 diabetes (T2D) [5].
The role of GIP in the regulation of body weight is less clear. In addition to islet endocrine cells, the GIP receptor (GIPR) is expressed in white and brown adipocytes [6] as well as discrete regions of the brain [7], all tissues intimately involved in the regulation of body weight. Global germ-line deletion of Gipr produces protection against diet-induced obesity in mice [8], which has generated the widely held belief that GIPR stimulation contributes to the development of obesity [9]. Increased GIPR activity in the brain has been recently proposed to induce leptin resistance and drive obesity [10]. In addition, GIPR agonists potently stimulate insulin secretion and enhance insulin sensitivity in adipose tissue [11], suggesting that enhanced β-cell activity of GIPR may drive adipose tissue expansion. Supporting this, selective elimination of Gipr from β-cells produced a mild decrease in adiposity in aged mice on low-fat diets, but did not confer any changes in body composition when the mice were fed a high-fat diet [12]. The importance of adipocyte GIPR for body composition is unclear. Although the rescue of GIPR expression exclusively in the adipose tissue of Gipr−/− mice restored body weight to WT levels, this was the result of elevated lean mass, not gain of fat mass [13]. Furthermore, complete adipose tissue knockout of Gipr [14] or selective knockout in brown adipocytes [6] did not alter body weight or fat mass in high-fat fed mice. Consequently, the mechanism of action or tissue location that accounts for protection against diet-induced obesity in the Gipr−/− mice remains unresolved.
The clinical success of GLP-1R agonists has clearly demonstrated the glucose-lowering and weight-reducing effects of enhancing GLP-1R activity. Because of this, the observation that Glp1r−/− mice are protected against diet-induced obesity has been largely overlooked [4,15,16]. The paradoxical findings that both loss of function and gain of function in the GLP-1R system reduces diet-induced obesity complicates understanding the role of the GLP-1 system in energy balance. One explanation for these discrepant results is developmental compensation in germ-line gene deletion models. Indeed, there seems to be a balanced interplay between the incretin receptors in germ-line knockout mice [12,[17], [18], [19]], with the elimination of one system enhancing activity of the alternative system. Whether hyperactivity of one incretin compensates for loss of the other to mitigate diet-induced obesity in Gipr−/− or Glp1r−/− mice is plausible but untested.
In the studies described herein, we tested the role of incretin antagonism on weight loss using a pharmacologic approach in adult mice, obviating any developmental compensations that might be present in animals with germ-line deletions of the incretin receptors. We hypothesized that this acute intervention would not affect body weight in fully developed animals challenged with a high-fat diet.
2. Methods
2.1. Animals
Eight-week-old male C57BL/6J mice were purchased from Jackson Labs (#000664) and allowed to acclimate until the beginning of the study at 12 weeks of age. The mice were housed under a 12 h light/dark cycle and provided free access to food and water. All of the mice were fed a high-fat diet consisting of 45% calories from fat purchased from Research Diets (#D12451). Throughout the study protocol, the mice were group housed (5 mice/cage) with treatment groups represented in each cage. All animal experiments were approved and performed in accordance with the Duke University Institutional Animal Care and Use Committee.
2.2. Treatment protocols
Antagonizing antibodies for GIPR (Gipg013 [20]), GLP-1R (Glp1R0017 [21]), and IgG controls were generated at AstraZeneca. The mice were treated once weekly at doses determined to provide sustained antagonism for 7 days (Gipg013, 30 mg/kg; Glp1R0017, 19.2 mg/kg). No adverse effects were noted in response to any of the antibodies used. The GLP-1R agonist (dulaglutide, 1 mg/kg) was given twice weekly based on previous results [22].
2.3. In vivo measurements
Body composition was determined by nuclear magnetic resonance (Bruker). Intraperitoneal (IP) and oral glucose tolerance was determined in response to 1.5 g/kg glucose (Sigma). Insulin tolerance was determined in response to 0.5 U/kg Humalog (Lilly). Exendin-4 (Ex4, 0.1 nmol/kg) and GIP (D-ala2GIP, 4 nmol/kg) were administered 10 min prior to IP glucose. Energy expenditure, respiratory exchange ratio, and activity were determined using a comprehensive lab animal monitoring system (Columbus Instruments).
2.4. Plasma hormone analysis
Whole blood was collected in EDTA-coated tubes following a 5 h fast and 10 min after the oral administration of glucose and stored on ice until centrifuged. Plasma was collected and assayed for insulin (Mercodia), glucagon (Mercodia), and total GLP-1 (Millipore).
2.5. Food intake studies
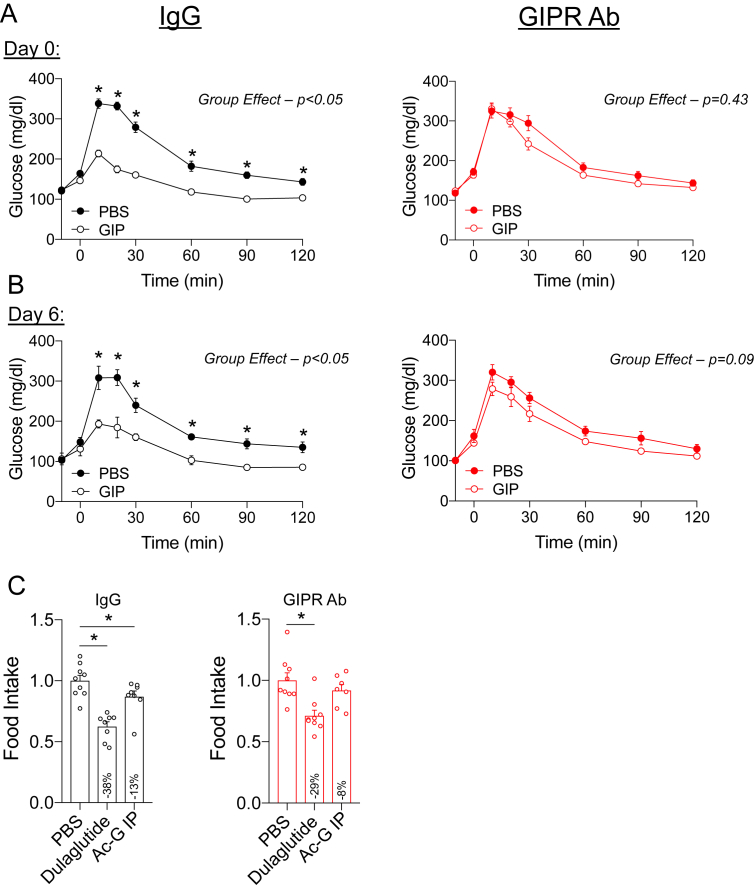
Food intake in the mice chronically treated with antagonists was determined after 19 weeks of research immediately prior to the end of the study to allow for single housing of the mice. The mice were fasted overnight in clean cages and pre-determined amounts of high-fat diet were given at 7 AM. Cumulative food intake was monitored for the next 72 h. For acute food intake studies in response to incretin peptide treatment, the mice were fasted overnight and their food intake was monitored from 7 to 11 AM in response to a predetermined amount of high-fat diet. These studies were conducted after 3 weeks of treatment with the respective antagonist (Gipg013, Glp1R0017, or Gipg013 + Glp1R0017) administered weekly at the previously described doses. Incretin receptor agonists (GLP-1-dulaglutide (1 mg/kg)), 10 mg/ml; GIP-acylated GIP analog (mGIP Aib2 [23]; (Ac-GIP)) were administered 30 min prior to refeeding and initiation of the study. Acute food intake studies were conducted in a cross-over fashion over a period of 2 weeks, with each animal undergoing all of the treatment protocols (PBS, dulaglutide, Ac-GIP). To account for variance between experimental time points, all the data were expressed relative to the PBS conditions for the given experiment.
2.6. Statistical analysis
All the data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 7. A two-tailed Student's t-test or one- or two-way ANOVA was performed depending on the experimental design, with a Bonferroni post-hoc analysis. P < 0.05 was used to determine statistically significant differences.
3. Results and discussion
3.1. Chronic antagonism of GIPR limits weight gain in high-fat fed mice
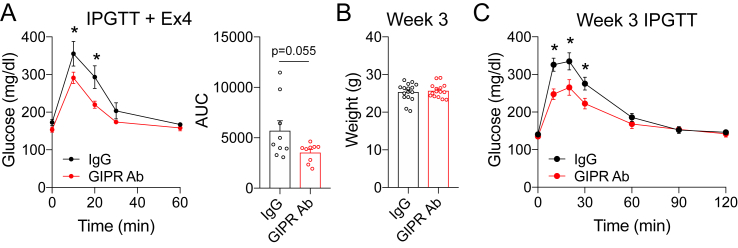
Prior to the initiation of the metabolic studies, we confirmed that weekly dosing of the GIPR Ab (GIPR Ab; Gipg013 [20]) provides sustained antagonism of the GIPR in this dosing regimen. A separate cohort of wild-type (WT) mice received a single dose of GIPR Ab at 7 AM, with the beginning of a 5 h fast. The animals pretreated with GIP had significantly improved glucose tolerance after challenge with intraperitoneal glucose (IPGTT), which was completely blocked by treatment with the GIPR Ab (Figure 1A). Glucose lowering in response to exogenous GIP is due to GIPR activity in β-cells and insulin secretion [12], indicating that the GIPR Ab antagonized GIPR activity in β-cells. Repeating the IPGTT 6 days following the single treatment with the GIPR Ab confirmed sustained antagonism over a 1-week period (Figure 1B), providing evidence for the sustained effectiveness of this treatment over 1 week. We then assessed GIPR antagonism using a food intake assay using an assay protocol. A GIPR agonist has been shown to induce modest decreases in food intake when administered peripherally [23]. Following the same protocol, we found that GIPR agonism acutely reduced food intake in the IgG control treated mice, which was prevented by pretreatment with the GIPR Ab (Figure 1C). Thus, two independent assays conducted in vivo demonstrated efficient GIPR antagonism.
Figure 1.
Validation of the GIPR antagonist. A) WT mice were administered a single dose of the GIPR Ab (30 mg/kg) at 7 AM coinciding with the initiation of the fast. Following a 5-h fast, glucose tolerance was assessed in response to PBS or GIP (4 nmol/kg) during an IPGTT. (n = 4) B) The IPGTT protocol was repeated in the mice receiving the GIPR Ab 6 days prior to the test for sustained antagonism. (n = 4) C) Food intake measured over a 4-h period in mice acutely treated with either PBS, dulaglutide, or GIP. Values are mean ± SEM, *p < 0.05 vs control. Statistical tests used: two-way ANOVA with a Bonferroni post-hoc test.
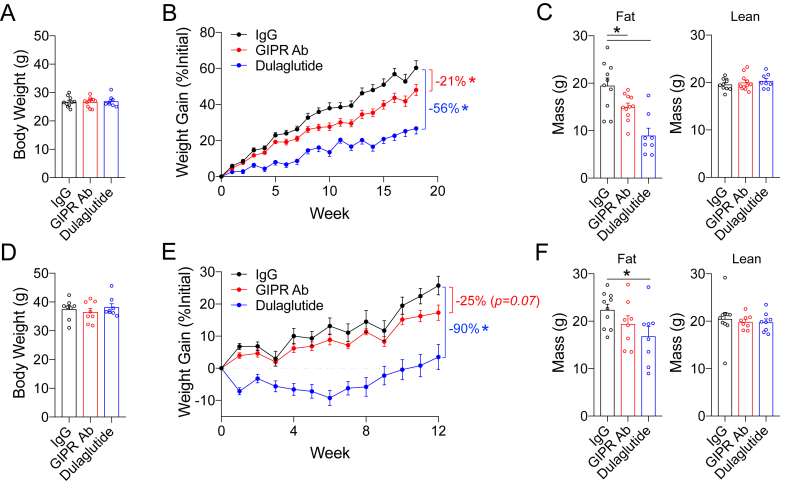
To determine the impact of GIPR antagonism on high-fat diet-induced weight gain, the 12-week-old WT mice were divided into 3 groups with comparable starting body weights (BW) (Figure 2A). Prior to starting the high-fat diet, the mice were treated with weekly: i) IgG control, ii) GIPR Ab, or iii) GLP-1 receptor agonist (dulaglutide), which continued for 18 weeks. Dulaglutide was used as a positive control because it is known to reduce body weight and fat mass. The GIPR Ab and dulaglutide significantly reduced body weight gain by 21% and 56%, respectively (Figure 2B), with parallel decreases in the fat mass and no changes in the lean mass (Figure 2C). An IPGTT with GIP at the end of the study demonstrated persistent antagonism of GIPR (Supplementary Fig. 1). Thus, chronic antagonism of the GIPR blunted diet-induced gain of body weight and fat mass, similar to a previous report of results with a different GIPR-blocking antibody [22].
Figure 2.
Antagonism of GIPR prevents weight gain in response to a high-fat diet, but not in mice already obese. A) Body weight of the mice at 12 weeks of age, immediately prior to the treatment intervention and commencement of high-fat feeding. B) Body weight gain over the treatment protocol. C) Fat and lean mass determined by MRI at the end of the study (week 18). D) Body weight of the mice at 24 weeks of age, following 12 weeks of high-fat diet without any drug intervention. E) Changes in body weight over the treatment protocol. F) Fat and lean mass determined by MRI at the end of the study (week 12). Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: one-way ANOVA (A, C, D, and F) and two-way ANOVA (B and D).
To determine the impact of the GIPR Ab in established obesity, the mice were fed a HFD for 3 months to increase their weight to >35 g prior to dividing them into three groups of equal BW (Figure 2D). Administration of the GIPR Ab to the obese mice produced a trend toward BW reduction relative to the controls that did not achieve statistical significance over 12 weeks, while dulaglutide significantly reduced body weight (Figure 2E). Similarly, the GIPR Ab produced only a trend toward significant lowering of fat mass, while the mice given dulaglutide had significantly reduced fat mass (Figure 2F). There were no changes in lean mass between the groups (Figure 1F). These data were consistent with the effects of GIPR Ab to prevent weight gain in our first experiment, albeit with lesser effects in the animals who were already obese. While this cohort of mice was observed over a shorter treatment period with the GIPR antagonist, we cannot exclude the possibility that a level of resistance to the effects of GIPR antagonism on body weight develops in obese mice. Indeed, the observation that GIPR antagonism was less impactful on body weight in the setting of obesity is consistent with previous work utilizing a separate GIPR-blocking antibody [22] or GIPR peptide antagonist [23].
3.2. Chronic effects of GIPR antagonism on food intake and energy expenditure
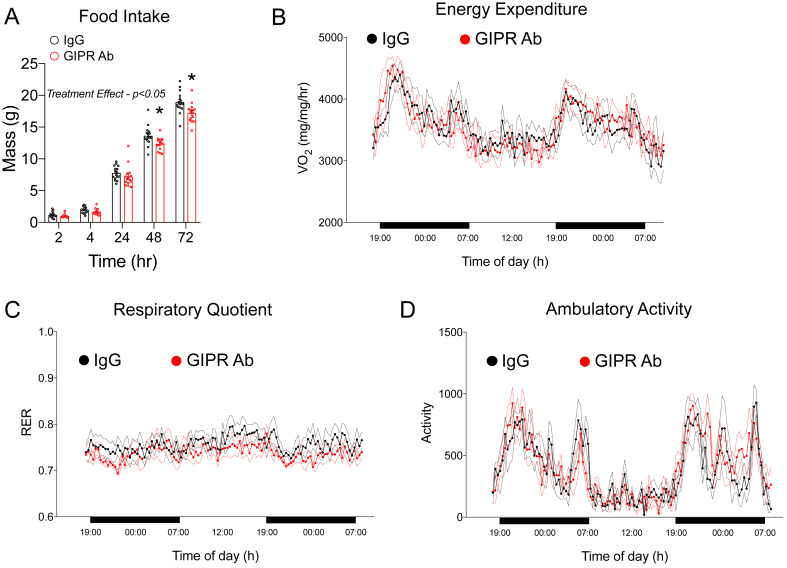
To identify the mechanism by which chronic GIPR antagonism reduces body weight gain and adiposity (Figure 2B,C), we conducted a food intake assay in the mice treated with the GIPR Ab for 18 weeks. Monitoring their food intake for 72 h revealed a subtle decrease in the GIPR Ab treated mice vs the controls (Figure 3A). To assess potential differences in energy expenditure, the mice were then placed in metabolic cages for ∼48 h. We found no differences in energy expenditure determined by oxygen consumption (Figure 3B), no changes in the respiratory quotient (Figure 3C), and no differences in activity levels (Figure 3C).
Figure 3.
Impact of chronic GIPR antagonism of food intake and energy expenditure at the end of the weight gain protocol. A) Cumulative food intake was recorded over a 72-h period following an overnight fast in the control (n = 9) and GIPR Ab (n = 8) groups at the end of the study. B) Oxygen consumption, C) respiratory exchange ratio, and D) activity levels were measured in the control and GIPR Ab groups at the end of the study (n = 6/group). Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: two-way ANOVA with a Bonferroni post-hoc test.
3.3. Chronic effects of GIPR antagonism on parameters of glucose tolerance
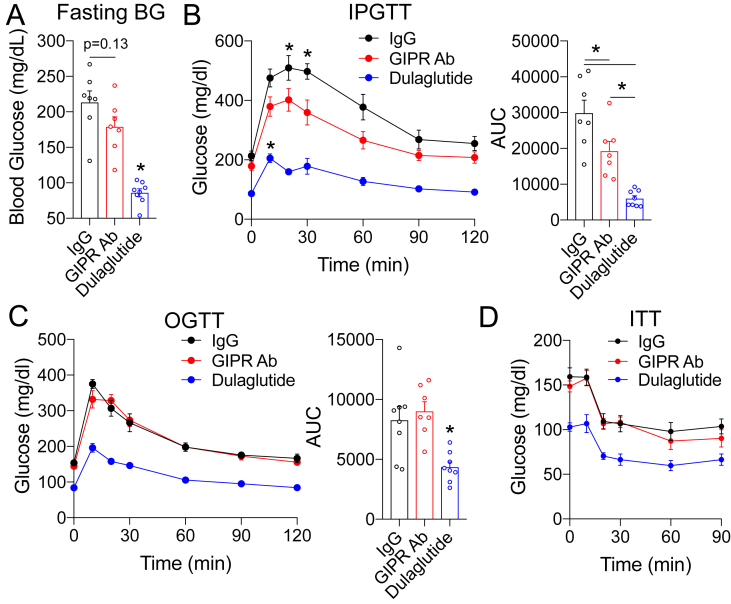
We next assessed whether the decreased body weight and fat mass observed in the mice treated with the GIPR Ab during weight gain (Figure 2B,C) led to meaningful improvements in glucose metabolism. After 18 weeks, fasting glycemia was lower in the GIPR Ab group, but did not reach statistical significance (p = 0.13, Figure 4A). However, fasting glycemia in the dulaglutide cohort was markedly lower than that in the other groups (Figure 4A). The mice treated with the GIPR Ab had ∼30% lower glycemic excursions during an IPGTT than the control mice (Figure 4B), indicating improved glucose tolerance, while the group chronically treated with dulaglutide displayed even better glucose tolerance (Figure 4B). Interestingly, oral glucose tolerance (Figure 4C) and insulin sensitivity (Figure 4D) did not differ between the control and GIPR Ab groups, while dulaglutide treatment improved both outcomes (Figure 4C,D). We infer from these observations that decreased body weight and fat mass induced by GIPR antagonism was insufficient to produce meaningful increases in insulin sensitivity measured by these physiological tests. It is possible that a more sensitive insulin sensitivity test, for example, a glucose clamp, would have revealed differences in insulin sensitivity in the GIPR Ab group. Nonetheless, it is possible that the improved IPGTT in the GIPR Ab group was due to a mechanism independent of changes in insulin sensitivity.
Figure 4.
GIPR antagonism improves intraperitoneal glucose tolerance at the end of the weight gain protocol. A) Glycemia following a 5-h fast in the mice at the end of the study (week 18). (B–D) B) IPGTT (1.5 g/kg), C) OGTT (1.5 g/kg), and D) ITT (0.5 U/kg) conducted in the mice at the end of the study (week 18). Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: 1-way ANOVA (A and B) (AUC), C (AUC), and two-way ANOVA with a Bonferroni post-hoc test (B, C, and D).
3.4. GIPR antagonism enhances glucose lowering in response to GLP-1R agonists
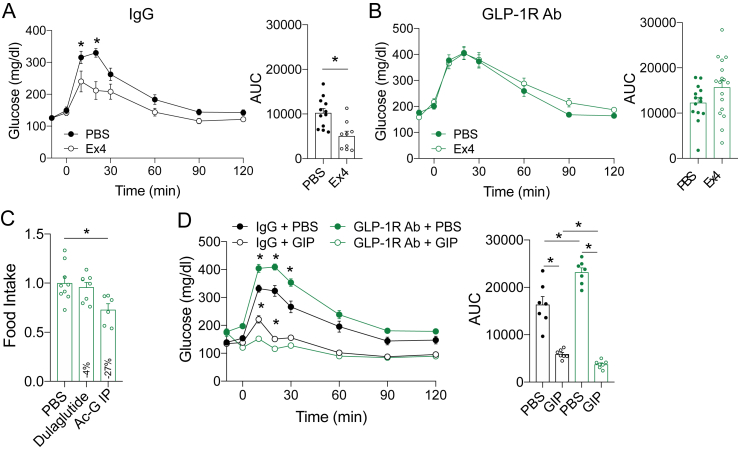
We recently reported that IP glucose tolerance, but not oral glucose tolerance, is directly related to β-cell GLP-1R function [18,24]. We also previously demonstrated that β-cell GLP-1R or β-cell GIPR are dispensable for normal oral glucose tolerance in mice, with OGTT comparable to controls in β-cell knockout of either Glp1r [25] or Gipr [12]. However, mice with selective deletion of β-cell Glp1r have impaired intraperitoneal glucose tolerance [25]. GLP-1R activity sets the β-cell tone in an islet, facilitating α-cell proglucagon input to drive insulin secretion [18]. Consequently, the improved IP glucose tolerance but normal oral glucose tolerance prompted us to hypothesize that GIPR antagonism increased the sensitivity of the GLP-1R to ligand activation. Indeed, previous studies showed that genetic elimination [12,19] or chronic antagonism [22] of GIPR enhances GLP-1R sensitivity. To test this possibility in our experimental paradigms, we administered the GLP-1R agonist exendin-4 (Ex4) prior to an IPGTT in the control and GIPR Ab groups after 18 weeks of treatment. Ex4 significantly reduced the glycemic response in the GIPR Ab mice relative to the controls, indicating enhanced GLP-1R sensitivity (Figure 5A). To further highlight that the improved IPGTT was independent of changes in body weight, we treated a separate group of mice with GIPR Ab for only 3 weeks. Body weight was not different between the groups (Figure 5B), yet the IPGTT was significantly improved in the mice treated with GIPR Ab (Figure 5C). These data support the concept that antagonism of GIPR enhances GLP-1R sensitivity, which is in agreement with previous reports [22].
Figure 5.
GIPR antagonism enhances GLP-1R activity. A) IPGTT in the mice pretreated with the GLP-1R agonists Ex4 (0.1 nmol/kg) at the end of the study. B) Body weight of the mice fed a high-fat diet starting at 12 weeks of age and treated for 3 weeks with the GIPR Ab. C) IPGTT (1.5 g/kg) conducted after 3 weeks of treatment. Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: Student's t-test (A) (AUC), (B), and 2-way ANOVA with a Bonferroni post-hoc test (A and C).
3.5. GLP-1R antagonism increases the activity of GIPR
Our findings, along with previous reports describing enhanced GLP-1R activity following loss of GIPR signaling [22], led us to hypothesize that the reductions in body weight and fat mass induced by GIPR antagonism are attributable to a compensatory increase in GLP-1R activity. The anorexic effects of GLP-1R agonists are well-established [3], and it stands to reason that increasing the activity of the endogenous GLP-1 system could produce modest decreases in weight gain observed with GIPR antagonism (Figure 2B). To test this, we established a protocol for chronically antagonizing GLP-1R. Glp1R0017 is a blocking antibody (GLP-1R Ab) specific for GLP-1R that does not impact GIPR or glucagon receptor activity [21]. To demonstrate the antagonistic properties of the GLP-1R Ab, we acutely treated mice and conducted IPGTTs with Ex4. In the control treated mice, Ex4 decreased the glycemic excursion (Figure 6A), but this was completely blocked in the mice treated with the GLP-1R Ab (Figure 6B). Similar to our validation of the GIPR Ab, we conducted an acute food intake assay to determine whether the GLP-1R Ab was capable of preventing the anorexic effects of exogenously delivered GLP-1R agonists. We used dulaglutide, which potently reduced food intake in the IgG treated control mice (Figure 1C). Pretreatment with the GLP-1R Ab completely mitigated the ability of dulaglutide to reduce food intake in this assay (Figure 6C). The GLP-1R Ab did not prevent the reduction in food intake in response to the GIPR agonists and potentially even increased the reduction in food intake relative to the IgG control animals (13% vs 27%, IgG vs GLP-1R Ab, respectively). Together, these data demonstrate that the GLP-1R Ab is an effective antagonist of the GLP-1R.
Figure 6.
Validation of the GLP-1R antagonist. A) WT mice were administered a single dose of the GLP-1R Ab (19.2 mg/kg) at 7 AM coinciding with the initiation of the fast. Following a 5-hr fast, glucose tolerance was assessed in response to PBS or Ex4 (0.1 nmol/kg) during an IPGTT. C) Food intake measured over a 4-h period in the mice acutely treated with either PBS, dulaglutide, or GIP. D) An IPGTT +/− GIP (4 nmol/kd). Values are mean ± SEM, *p < 0.05 vs control. Statistical tests used: Student's t-test (A) (AUC), (B) (AUC), one-way ANOVA (C), and two-way ANOVA with a Bonferroni post-hoc test (A, B, and D).
To test the hypothesis that GLP-1R Ab treatment would increase the sensitivity to GIP [17,18], we treated the mice with either IgG control or GLP-1R Ab for 3 weeks, the same time needed to enhance IP glucose tolerance in response to the GIPR Ab (Figure 5B,C). We then conducted IPGTTs with GIP to test for changes in GIPR sensitivity. Glucose tolerance was impaired during IPGTT in the mice treated with the GLP-1R Ab (Figure 6D; PBS conditions), while oral glucose was unaffected (Supplemental Fig. 3), recapitulating the phenotype of the mice with β-cell specific deletion of Glp1r [25] and consistent with our previous reports that GLP-1R activity is important for IP glucose tolerance [18,24]. Interestingly, the GLP-1R Ab improved the glycemic response when the mice were administered GIP (Figure 6D), showing that pharmacological antagonism of GLP-1R improves GIPR sensitivity.
3.6. Antagonism of GLP-1R mitigates weight gain
GLP-1R agonists are well established to reduce body weight, allowing us to reason that chronic antagonism of the GLP-1R would increase body weight in the high-fat fed animals. Thus, we repeated the chronic antagonism protocol previously carried out with the GIPR antagonist (Figure 2). The 12-week-old mice with equal starting body weights (Figure 7A) were started on a high-fat diet concurrent with weekly treatment with the GLP-1R Ab; dulaglutide was used as a positive control (Figure 7B). Surprisingly, chronic antagonism of the GLP-1R reduced body weight gain, with the GLP-1R Ab group weighing less at the end of the study (Figure 7C). This reduction in body weight was due to a modest decrease in fat mass, not lean mass (Figure 7D,E).
Figure 7.
Antagonism of GLP-1R prevents weight gain in response to a high-fat diet. A) Body weight of the mice at 12 weeks of age, immediately prior to the treatment intervention and commencement of high-fat feeding. B) Body weight gain over the treatment protocol. C) Body weights at the end of the study. D) Lean and E) fat mass determined by MRI at the end of the study (week 18). Values are mean ± SEM, *p < 0.05 vs IgG control or GLP-1R Ab (as indicated by color). Statistical tests used: one-way ANOVA (A, C, D, and E) and two-way ANOVA with a Bonferroni post-hoc test (B).
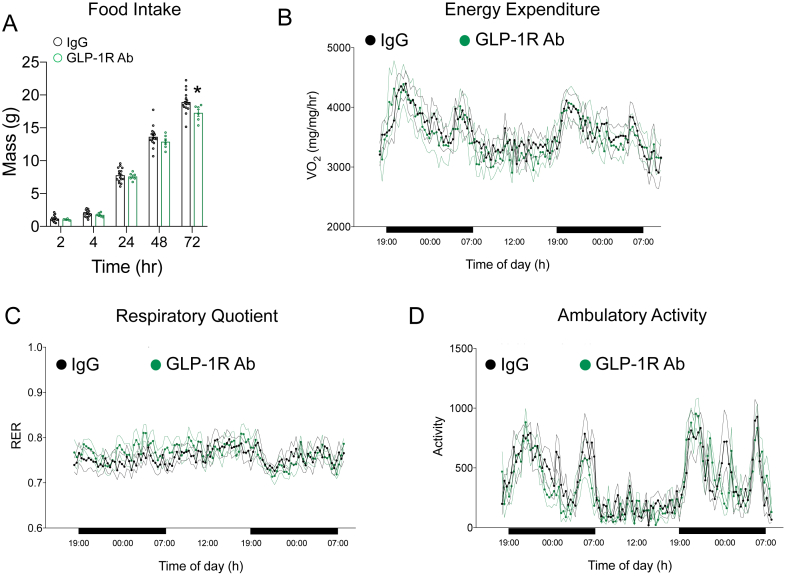
To assess the potential mechanisms by which chronic GLP-1R antagonism prevents weight gain, we assessed food intake and energy expenditure at the end of the study. Similar to the mice treated with the GIPR Ab (Figure 3A), chronic GLP-1R antagonism reduced food intake over a 72 h period (Figure 8A). No differences were observed in energy expenditure (Figure 8B), respiratory quotient (Figure 8C), or activity levels (Figure 8D).
Figure 8.
Impact of chronic GLP-1R antagonism of food intake and energy expenditure at the end of the weight gain protocol. A) Cumulative food intake was recorded over a 72-h period following an overnight fast in the control and GLP-1R Ab groups at the end of the study. B) Oxygen consumption, C) respiratory exchange ratio, and D) activity levels were measured in the control and GLP-1R Ab groups at the end of the study (n = 6/group). Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: two-way ANOVA with a Bonferroni post-hoc test.
3.7. Combined antagonism of both GIPR and GLP-1R produces a greater effect on body weight
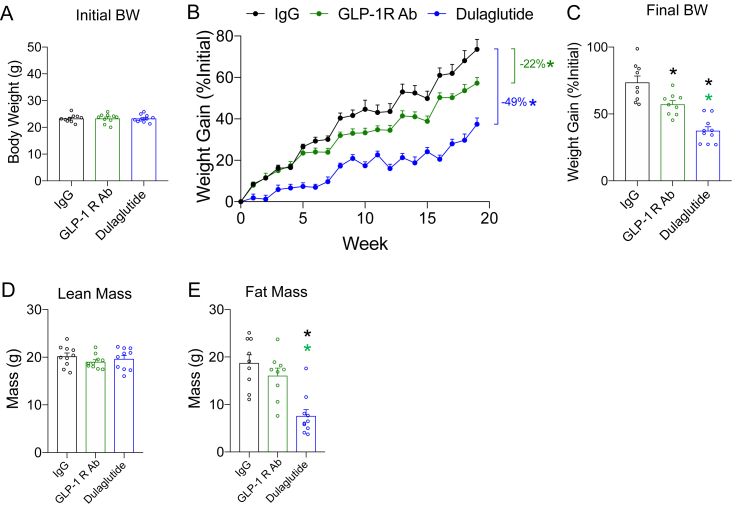
GLP-1R agonists reduce body weight, while emerging data have demonstrated that GIPR agonists are also able to produce decreases in body weight [23]. However, GIPR antagonism reduces body weight [22], although by unclear mechanisms. Our findings demonstrate that chronic GLP-1R antagonism prevents weight gain (Figure 7B) similar to what is observed with chronic GIPR antagonism (Figure 2B). We also noted that antagonism of either receptor produces an increase in the sensitivity of the complementary receptor (Figure 5, Figure 6D), which is in agreement with the phenotype of genetic knockouts for either Gipr [12,19] or Glp1r [17,18,24]. Moreover, a recent report utilizing a different GIPR antagonist alluded to enhanced GLP-1R activity when the GIPR is chronically antagonized [22]. Thus, we hypothesized that the decrease in body weight gain in response to and incretin receptor antagonist is due to enhanced signaling of the complementary receptor rather than antagonism per se. To test this hypothesis, we fed the mice a high-fat diet while chronically antagonizing both the GIPR and GLP-1R, anticipating that dual antagonism would mitigate the compensatory actions of a single antagonism and produce no changes, or perhaps elevations, in body weight gain. Finally, we also co-treated the mice with both the GIPR Ab and dulaglutide to test for enhanced GLP-1R sensitivity on body weight, following a similar experimental protocol that demonstrated robust weight loss with GIPR antagonism plus GLP-1R agonism in high-fat fed mice [22].
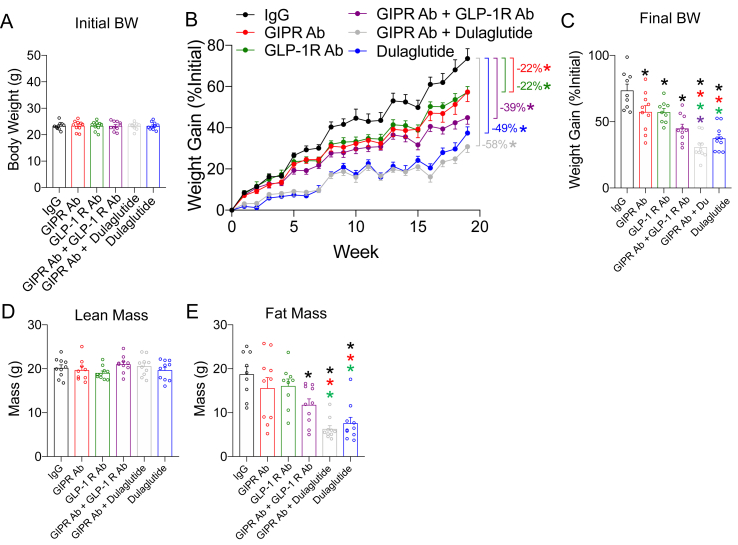
Six groups of 12-week-old WT C57BL/6J mice were evenly distributed to ensure equal starting BW (Figure 9A), including the three groups presented in Figure 7 (IgG, GLP-1R Ab, and dulaglutide). The mice were started on a high-fat diet concurrent with their experimental treatment protocol and followed for 19 weeks (Figure 9B). At the end of the study, GIPR antagonism significantly reduced BW gain by 22% (Figure 3B), almost identical to the results of the first study (Figure 2B). To our surprise, the combination of GIPR and GLP-1R antagonism yielded a further decrease in BW (39%) consistent with an additive effect of the two antagonists alone (Figure 9B). However, dulaglutide treatment produced the greatest reduction in BW (49%; Figure 9B). Finally, the combination of GIPR antagonism plus dulaglutide was comparable to dulaglutide alone (58% vs 49%; p = 0.38) (Figure 9B). Any differences in body weight induced by the six interventions were attributable to lower fat and not lean mass (Figure 9D,E).
Figure 9.
Antagonism of GIPR and GLP-1R, alone or in combination, prevents weight gain in response to a high-fat diet. A) Body weight of the mice at 12 weeks of age, immediately prior to treatment intervention and commencement of high-fat feeding. B) Body weight gain over the treatment protocol. C) Final body weights at the end of the study (week 19). D) Lean and E) fat mass determined by MRI at the end of the study (week 19). Values are mean ± SEM, *p < 0.05 as indicated by color. Statistical tests used: one-way ANOVA with a Bonferroni post-hoc test (A, C, D, and E) and two-way ANOVA with a Bonferroni post-hoc test (B).
Both GIP and GLP-1 control glucose tolerance at the level of islet hormones induced changes in both insulin and glucagon secretion. To determine whether chronic antagonism of either receptor, alone or in combination, influences insulin of glucagon levels, a modified glucose tolerance test was conducted at the end of the feeding study. Antagonism of either the GIPR or GLP-1R or the combination did not change the glycemic excursion in response to oral glucose (Supplementary Fig. 3A) or the ability for oral glucose to stimulate insulin secretion (Supplementary Fig. 3B) or reduce glucagon levels (Supplementary Fig. 3C). Finally, the GIP response to oral glucose was unchanged in all of the groups (Supplementary Fig. 3D), while we did not measure GLP-1 levels.
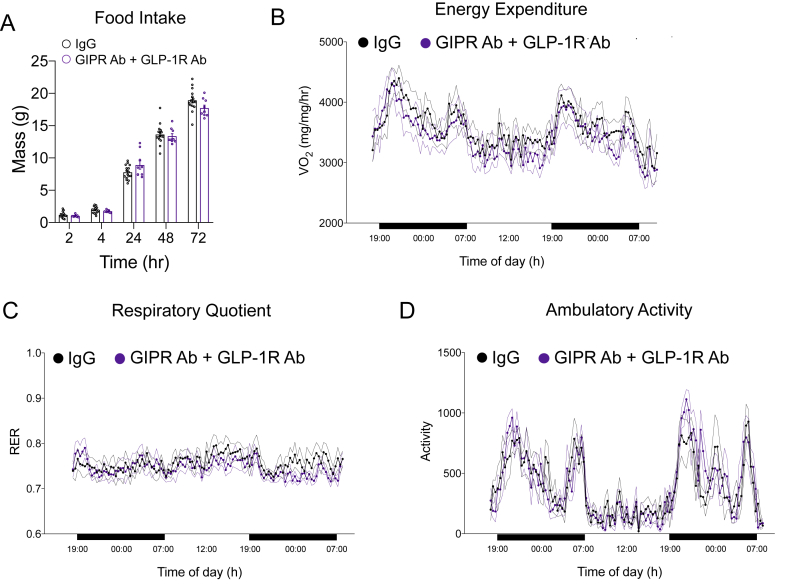
Finally, we assessed food intake and energy metabolism in the mice treated with both antagonists at the end of the study. We found no difference in food intake (Figure 10A), energy expenditure (Figure 10B), respiratory quotient (Figure 10C), or activity levels (Figure 10D) compared to the control mice. Based on these observations, it is difficult to ascertain whether the prevention of weight gain achieved by chronic antagonism of the incretin system is due to subtle changes in either metabolism or food intake that is not apparent with the assays utilized herein or due to another mechanism.
Figure 10.
Impact of chronic GIPR and GLP-1R antagonism of food intake and energy expenditure at the end of the weight gain protocol. A) Cumulative food intake was recorded over a 72-h period following an overnight fast in the control and GLP-1R/GIPR Ab groups at the end of the study. B) Oxygen consumption, C) respiratory exchange ratio, and D) activity levels were measured in the control and GLP-1R/GIPR Ab groups at the end of the study (n = 6/group). Values are mean ± SEM, *p < 0.05 vs IgG control. Statistical tests used: two-way ANOVA with a Bonferroni post-hoc test.
4. Conclusions
The results of this study confirmed that chronic GIPR antagonism caused modest decreases in body weight in high-fat fed mice. However, in contrast to our prediction, the mechanism was not mediated by increased GLP-1R sensitivity as co-antagonism of the GLP-1R did not affect reductions in body weight. In fact, combined antagonism produced a more robust decrease in body weight than single antagonism alone. This study also produced the paradoxical observation that both agonism (dulaglutide) and antagonism (GLP-1R Ab) of the GLP-1R conferred protection against diet-induced obesity. While the finding that GLP-1R antagonism decreased weight gain was initially surprising, there is precedence for this result. Mice with germ-line deletion of Glp1r were also protected against diet-induced obesity [15,16]. Therefore, one conclusion from our data is that antagonism of either, or both, arms of the incretin axis induces downstream changes in metabolic pathways that protect against diet-induced weight gain. While our initial hypothesis was that these changes would be mediated by greater efficacy of the complementary incretin system, the findings presented herein suggest that the effects extend beyond the two incretin receptor pathways to yet unidentified mechanisms. However, one firm conclusion that can be drawn from these studies is that categorizing GIP as an obesogenic peptide based on responses of Gipr knockout mice is not wholly accurate and misses the true complexity of incretin action.
The original observation that Gipr−/− mice are protected from diet-induced obesity has spurred numerous efforts to develop GIPR antagonists to reduce or prevent weight gain [26]. Genetic or pharmacological blockade of GIPR signaling has been proposed to reduce body weight and improve the metabolic profile in preclinical models of insulin resistance and obesity [8,[27], [28], [29], [30], [31], [32], [33]]. Recently, central administration of the same neutralizing antibody targeting the GIPR used in our studies reduced weight in obese mice through a leptin-sensitizing mechanism [10]. Furthermore, a separate antibody with antagonizing properties on the GIPR reduced weight in obese mice and nonhuman primates [22]. Interestingly, this reagent also markedly enhanced the efficacy of multiple GLP-1R agonists, replicating the plasticity invoked by gene deletion of Gipr. Based on these findings, it is reasonable to hypothesize that a portion of the weight effects resulting from GIPR antagonism can be attributed to enhanced GLP-1R activity. However, the combination of GLP-1R and GIPR antagonism produced an additive effect toward reducing body weight gain, refuting this hypothesis and suggesting more complicated mechanisms involved.
We also demonstrated that chronic antagonism of the GLP-1R produces similar protection against diet-induced obesity, which conforms with the phenotype of Glp1r knockout mice fed a high-fat diet [15,16]. Concluding that GLP-1 promotes obesity based on the body weight effects of the GLP-1R Ab studies presented herein would be a tenuous argument and contradict the magnitude of clinical data demonstrating the opposite effect. Instead, the collective knowledge of GLP-1R biology would indicate that reduced weight gain induced by GLP-1R antagonism is not due to a loss of GLP-1R signaling per se, but rather an indirect effect compensating for the loss of a major axis of metabolic regulation. It is clear that the incretin system exerts primary control over metabolic regulation. However, it is also likely that this system is interconnected with other factors to regulate energy balance. Because the biology of GLP-1 and GIP evolved as primary regulators of metabolism, it is not hard to envision that disruption of the incretin system would place unintended stress on these complementary systems. This inefficiency could drive the subtle decreases in weight gain following the loss of GLP-1R signaling. This unifying hypothesis reconciles the paradoxical observations that both gain and loss of GLP-1R or GIPR signaling produces decreased body weight. However, this is an untested hypothesis based on the observations presented herein, along with previous work, that requires further evaluation.
Compared to GLP-1, there is much less data to support the concept that GIPR agonism can decrease body weight. Numerous co-agonists that incorporate GIPR activity have demonstrated robust effects on decreasing body weight [2], highlighted most recently by phase 2 clinical results of tirzepatide [34]. However, the relative contribution of GIPR activity to these compounds remains unknown. Preclinical studies using GIPR monoagonists lowered body weight in obese mice [23], demonstrating the potential weight-lowering effects of GIP. It is interesting that the GIPR Ab used in these studies was much less effective in reducing body weight when administered to obese mice (Figure 2E). Recent reports demonstrated that DREADD activation of GIPR + neurons reduced food intake in mice [35], which aligns with our current data (Figure 1, Figure 6C) and supports the notion that GIPR agonism, not antagonism, would reduce body weight. Moreover, no studies have demonstrated that chronic GIPR agonism increases body weight. Consequently, the general consensus that GIP promotes obesity is solely based on loss-of-function studies. Herein we clearly show that by juxtaposing GLP-1R agonism with antagonism, phenotypes of systemic metabolism in loss-of-function models do not allow simple conclusions for the metabolic role of either incretin. Thus, concluding that GIPR activity is obesogenic based on loss-of-function studies is precarious, especially given emerging data reporting the opposite conclusion. A large gap in knowledge remains in our understanding of the metabolic role of GIP and the implications modulating the GIPR system can have for targeting obesity and type 2 diabetes.
5. Limitations
There are number of limitations to this study that warrant discussion. First, we did not identify the mechanism by which incretin receptor antagonism, alone or in combination, facilitates reductions in weight gain. Nor did we demonstrate the mechanism that explains the increase in incretin receptor activity following antagonism of the complementary incretin receptor. We were especially surprised to find that combined receptor antagonism produced enhanced reductions in weight gain, suggesting that antagonizing the incretin axis invokes an additional system, separate from the incretin receptors, to exert control over the metabolic processes normally governed by the incretin axis. However, given that the effect size of the combined antagonism was significantly lower than the dulaglutide treatment, we are not optimistic that identifying this system will produce a meaningful target to treat obesity. Second, we were unable to recapitulate the same level of enhanced GLP-1R signaling in response to GIPR Ab as previously reported with a different GIPR antagonist [22]. It is possible this was because the antagonist in the current study was developed to target human GIPR [20], whereas the Amgen compound was designed to target mouse GIPR [22]. Consequently, the level of GIPR antagonism in mice and the subsequent compensatory increase in GLP-1R signaling achieved in the current study was most likely less than that of the Amgen compound.
Author contributions
Conceptualization, B.S, P.R., and J.E.C.; methodology, B.S., B.F., J.N., P.R., and J.E.C.; investigation, B.S., M.E.C., J.N., S.H., and J.E.C.; writing original draft, J.E.C.; writing review and editing, M.E.C., P.R., D.A.D., and J.E.C.; supervision, D.A.D and J.E.C.
Acknowledgments
M.E.C. received support from the NIH/NIDDK (F32 DK116542). D.A.D. is supported by the NIH/NIDDK (R01 DK101991). J.E.C. is supported by a career development award from the American Diabetes Association (1-18-JDF-017), NIH/NIDDK (R01 DK123075), and is a Borden Scholar.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.11.018.
Conflict of interest
B.F. is an employee of Novo Nordisk. J.N. and P.R. are employees of AstraZeneca. J.E.C. has received speaker honoraria from Merck, and Duke Molecular Physiology Institute receives funding from Eli Lilly for preclinical studies in the Campbell Laboratory. The other authors have no other conflicts of interest to disclose relevant to this article.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metabolism. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Capozzi M.E., DiMarchi R.D., Tschop M.H., Finan B., Campbell J.E. Targeting the incretin/glucagon system with triagonists to treat diabetes. Endocrine Reviews. 2018;39(5):719–738. doi: 10.1210/er.2018-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Finan B., Capozzi M.E., Campbell J.E. Reappraisal of GIP pharmacology for metabolic diseases. Trends in Molecular Medicine. 2016;22(5):359–376. doi: 10.1016/j.molmed.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpem A., Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 6.Beaudry J.L., Kaur K.D., Varin E.M., Baggio L.L., Cao X., Mulvihill E.E. Physiological roles of the GIP receptor in murine brown adipose tissue. Molecular Metabolism. 2019;28:14–25. doi: 10.1016/j.molmet.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paratore S., Ciotti M.T., Basille M., Vaudry D., Gentile A., Parenti R. Gastric inhibitory polypeptide and its receptor are expressed in the central nervous system and support neuronal survival. Central Nervous System Agents in Medicinal Chemistry. 2011;11(3):210–222. doi: 10.2174/187152411798047771. [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 9.Paschetta E., Hvalryg M., Musso G. Glucose-dependent insulinotropic polypeptide: from pathophysiology to therapeutic opportunities in obesity-associated disorders. Obesity Reviews. 2011;12(10):813–828. doi: 10.1111/j.1467-789X.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K., Fu Y., Lin H.Y., Cordonier E.L., Mo Q., Gao Y. Gut-derived GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. Journal of Clinical Investigation. 2019:130. doi: 10.1172/JCI126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammad S., Ramos L.S., Buck J., Levin L.R., Rubino F., McGraw T.E. Gastric inhibitory peptide controls adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110beta isoform of phosphatidylinositol 3-kinase. Journal of Biological Chemistry. 2011;286(50):43062–43070. doi: 10.1074/jbc.M111.289009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell J.E., Ussher J.R., Mulvihill E.E., Kolic J., Baggio L.L., Cao X. TCF1 links GIPR signaling to the control of beta cell function and survival. Nature Medicine. 2016;22(1):84–90. doi: 10.1038/nm.3997. [DOI] [PubMed] [Google Scholar]
- 13.Ugleholdt R., Pedersen J., Bassi M.R., Fuchtbauer E.M., Jorgensen S.M., Kissow H.L. Transgenic rescue of adipocyte glucose-dependent insulinotropic polypeptide receptor expression restores high fat diet-induced body weight gain. Journal of Biological Chemistry. 2011;286(52):44632–44645. doi: 10.1074/jbc.M111.311779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo E., Harada N., Yamane S., Fukushima T., Taura D., Iwasaki K. Inhibition of gastric inhibitory polypeptide receptor signaling in adipose tissue reduces insulin resistance and hepatic steatosis in high-fat diet-fed mice. Diabetes. 2017;66(4):868–879. doi: 10.2337/db16-0758. [DOI] [PubMed] [Google Scholar]
- 15.Hansotia T., Maida A., Flock G., Yamada Y., Tsukiyama K., Seino Y. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. Journal of Clinical Investigation. 2007;117(1):143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scrocchi L.A., Drucker D.J. Effects of aging and a high fat diet on body weight and glucose tolerance in glucagon-like peptide-1 receptor -/- mice. Endocrinology. 1998;139(7):3127–3132. doi: 10.1210/endo.139.7.6092. [DOI] [PubMed] [Google Scholar]
- 17.Ali S., Lamont B.J., Charron M.J., Drucker D.J. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. Journal of Clinical Investigation. 2011;121(5):1917–1929. doi: 10.1172/JCI43615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capozzi M.E., Svendsen B., Encisco S.E., Lewandowski S.L., Martin M.D., Lin H. beta-Cell tone is defined by proglucagon peptides through cyclic AMP signaling. JCI Insight. 2019;4(5) doi: 10.1172/jci.insight.126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamir N., Lynn F.C., Buchan A.M., Ehses J., Hinke S.A., Pospisilik J.A. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. American Journal of Physiology. Endocrinology and Metabolism. 2003;284(5):E931–E939. doi: 10.1152/ajpendo.00270.2002. [DOI] [PubMed] [Google Scholar]
- 20.Ravn P., Madhurantakam C., Kunze S., Matthews E., Priest C., O'Brien S. Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. Journal of Biological Chemistry. 2013;288(27):19760–19772. doi: 10.1074/jbc.M112.426288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biggs E.K., Liang L., Naylor J., Madalli S., Collier R., Coghlan M.P. Development and characterisation of a novel glucagon like peptide-1 receptor antibody. Diabetologia. 2018;61(3):711–721. doi: 10.1007/s00125-017-4491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killion E.A., Wang J., Yie J., Shi S.D., Bates D., Min X. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Science Translational Medicine. 2018;10(472) doi: 10.1126/scitranslmed.aat3392. [DOI] [PubMed] [Google Scholar]
- 23.Mroz P.A., Finan B., Gelfanov V., Yang B., Tschop M.H., DiMarchi R.D. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Molecular Metabolism. 2019;20:51–62. doi: 10.1016/j.molmet.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capozzi M.E., Wait J.B., Gordon A.N., Koech J., Coch R.W., Svendsen B. Glucagon lowers glycemia when β-cells are active. JCI Insight. 2019;5 doi: 10.1172/jci.insight.129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith E.P., An Z., Wagner C., Lewis A.G., Cohen E.B., Li B. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metabolism. 2014;19(6):1050–1057. doi: 10.1016/j.cmet.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasbjerg L.S., Gabe M.B.N., Hartmann B., Christensen M.B., Knop F.K., Holst J.J. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides. 2018;100:173–181. doi: 10.1016/j.peptides.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Irwin N., McClean P.L., O'Harte F.P., Gault V.A., Harriott P., Flatt P.R. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia. 2007;50(7):1532–1540. doi: 10.1007/s00125-007-0692-2. [DOI] [PubMed] [Google Scholar]
- 28.McClean P.L., Irwin N., Cassidy R.S., Holst J.J., Gault V.A., Flatt P.R. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(6):E1746–E1755. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- 29.McClean P.L., Irwin N., Hunter K., Gault V.A., Flatt P.R. (Pro(3))GIP[mPEG]: novel, long-acting, mPEGylated antagonist of gastric inhibitory polypeptide for obesity-diabetes (diabesity) therapy. British Journal of Pharmacology. 2008;155(5):690–701. doi: 10.1038/bjp.2008.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proceedings of the National Academy of Sciences of the U S A. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T., Tanimoto H., Mizuno Y., Okamoto M., Takeuchi M., Tsubamoto Y. Gastric inhibitory polypeptide receptor antagonist, SKL-14959, suppressed body weight gain on diet-induced obesity mice. Obesity Science & Practice. 2018;4(2):194–203. doi: 10.1002/osp4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasteska D., Harada N., Suzuki K., Yamane S., Hamasaki A., Joo E. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63(7):2332–2343. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- 33.Pathak V., Gault V.A., Flatt P.R., Irwin N. Antagonism of gastric inhibitory polypeptide (GIP) by palmitoylation of GIP analogues with N- and C-terminal modifications improves obesity and metabolic control in high fat fed mice. Molecular and Cellular Endocrinology. 2015;401:120–129. doi: 10.1016/j.mce.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 35.Adriaenssens A.E., Biggs E.K., Darwish T., Tadross J., Sukthankar T., Girish M. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metabolism. 2019;30(5):987–996. doi: 10.1016/j.cmet.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.