Abstract
Introduction
A systematic review and meta-analysis was performed regarding the diagnostic performance of neurofilament light chain (NfL) in CSF and blood.
Methods
A database search was conducted for NfL biomarker studies in the context of Alzheimer's disease (AD), frontotemporal dementia (FTD), and amyotrophic lateral sclerosis (ALS) compared with controls (i.e., cognitively unimpaired, mild cognitive impairment, or disease mimics).
Results
In groups with a sufficient number of studies, the performance of NfL in blood and CSF was similar. Compared with disease mimics, we observed that CSF NfL had strong discriminatory power for ALS, modest discriminatory power for FTD, and no discriminatory power for AD. NfL provided the greatest separation between ALS and cognitively unimpaired controls in both the blood and CSF, followed by FTD (CSF and blood), then AD (blood and CSF).
Discussion
Comparable performance of CSF and blood NfL in many groups demonstrates the promise of NfL as a noninvasive biomarker of neurodegeneration; however, its utility in clinically meaningful scenarios requires greater scrutiny. Toward clinical implementation, a more comprehensive understanding of NfL concentrations in disease subtypes with overlapping phenotypes and at defined stages of disease, and the development of a harmonization program, are warranted.
Keywords: Alzheimer's disease, Amyotrophic lateral sclerosis, Biomarker, Dementia, Frontotemporal dementia, Meta-analysis, Neurodegeneration, Neurofilament light chain
1. Introduction
Neurofilaments are intracellular intermediate filaments found in the central and peripheral nervous systems. Neurofilament protein assemblies can include the following subunits: neurofilament light chain (NfL) of ∼68 kDa, Nf medium chain of ~150 kDa, and Nf heavy chain of ~190–210 kDa [1]. All three subunits have relatively conserved head and rod domains, but their tail domains differ in sequence length and composition, contributing to their molecular weight differences. Neurofilaments function as elastic assemblies that help maintain cell shape [2]. In neurons, this action controls axonal diameter, which is correlated with nerve conduction velocity, thus modulating the neurons response to stimuli [3]. After axonal injury, intracellular neurofilaments can leak into the extracellular space, leading to an increased concentration in the CSF [4]. As such, neurofilament subunits have been proposed as nonspecific biomarkers of axonal injury and have been extensively studied in the context of neurodegenerative diseases. Of the three subunits, NfL has been the focus of most clinical biomarker studies, which likely reflects the relative abundance and solubility of NfL in CSF and blood, compared with Nf medium chain and Nf heavy chain [1].
The established core fluid biomarkers for Alzheimer's disease (AD) include CSF amyloid-β, phospho-tau and total-tau. Of these biomarkers, total-tau has the lowest specificity for AD and is considered a general marker of any cause of neuronal damage or injury [5]. A challenge with the core biomarker system for AD is that total-tau and phospho-tau are highly correlated [5], and therefore alternate/additional biomarkers for total-tau are being sought. While the recent AT(N) biomarker classification system emphasizes the three core biomarkers (i.e., amyloid-β for “A”, phospho-tau for “T” and total-tau for “N”), it makes provisions for the addition of new disease-specific biomarkers categories [ATX(N)] for non-AD dementias and the addition of nonspecific markers of neurodegeneration or neuronal injury [5], [6]. NfL represents one of the candidate biomarkers, along with neurogranin, for addition to the “N” category [5], [6]. Given its nonspecificity, NfL has been explored in related neurodegenerative disorders that do not yet have a biomarker classification system, such as frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), Parkinson's disease, and prion disease [7], [8], [9].
With the potential of NfL as a biomarker of axonal damage, there has been an exponential growth over the past decade in the number of studies exploring this protein in the context of neurodegeneration (Supplementary Fig. A1). The systematic review and meta-analysis herein explores quantification of NfL in both CSF and blood (i.e., plasma and serum), in not only AD but also FTD and ALS, with comparison to cognitively unimpaired controls, mild cognitive impairment (MCI) and disease mimics, as well as, an examination of the analytical approaches used for quantification of NfL. The goal of this review was an assessment and synthesis of the diagnostic performance of NfL across these various biofluids, diseases, and clinical contexts.
2. Methods
2.1. Literature search and study selection criteria
To inform our formal database search, a preliminary review of the literature on biomarker studies for NfL identified numerous studies pertaining to its diagnostic performance for AD and FTD, but no recent comprehensive meta-analysis. Owing to the pathological overlap with FTD, ALS was also considered. Owing to the small number of studies of NfL in Lewy body dementia, this disease group was not considered for further evaluation. The formal literature search was conducted on March 1, 2019, following the PRISMA guidelines [10]. The PubMed search string was (neurofilament proteins [MH] OR neurofil*[tiab] OR nfl [tiab]) AND (dementia [MH] OR neurodegenerat*[tiab] OR alzheimer disease [MH] alzheimer*[tiab] OR AD [tiab] OR frontotemporal dementia [MH] OR frontotemporal lobar degeneration [MH] OR frontotemp*[tiab] OR FTD [tiab] OR amyotrophic lateral sclerosis [MH] OR amyotrophic lateral sclerosis [tiab] OR als [tiab] OR motor neuron disease [tiab]) AND (plasma [MH] OR serum [MH] OR cerebrospinal fluid [MH] OR cerebral spinal [tiab] OR cerebrospinal [tiab] OR CSF [tiab] OR blood [tiab] OR serum [tiab] OR plasma [tiab] OR biofluid [tiab] or biomark*[tiab] OR marker*[tiab] OR level*[tiab] OR concentration*[tiab]) NOT (systematic review [pt] OR review [pt] OR case reports [pt] OR clinical conference [pt] OR editorial [pt] OR meta-analysis [pt]).
The titles of the resulting articles (n = 587) were then independently assessed for relevance by two investigators (L.M.F. and M.M.). The two lists of potentially relevant articles generated through this process were then compared. Any discrepancies between articles included in the two lists were resolved by examination of their abstracts. The articles deemed potentially relevant were then reviewed for inclusion in the meta-analysis using the following criteria: the study (1) was available in English; (2) reported primary data; (3) included individuals diagnosed with AD, FTD, or ALS with comparison to a control group (i.e., cognitively unimpaired, MCI, or disease mimics); (4) quantified NfL in human CSF, plasma, or serum; and (5) reported NfL concentration as mean and standard deviation, or the median and interquartile range (IQR) or range. This search and inclusion criteria resulted in 65 articles selected for the meta-analysis (Fig. 1). Data extracted (L.M.F. and M.M.) from the included studies is summarized in Supplementary Material B. To capture the analytical aspects, the assay used, including antibody pairs and detection method, were recorded for each study.
Fig. 1.
Retrieval process of peer-reviewed studies that included NfL quantitation in CSF, plasma, and serum from individuals with AD, ALS, FTD, and controls. Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; FTD, frontotemporal dementia; NfL, neurofilament light chain.
2.2. Data analysis
To address variabilities in reported biomarker concentrations not due to true differences in NfL concentration, this meta-analysis utilized ratio of means (ROM) between the disease and control group for each study. Thus, the data from each study is represented as the fold change in NfL concentration between the two groups. A ROM of 1 indicates no difference between groups; a ROM greater than one indicates that the NfL concentration measured was higher in the disease group compared with the control group; a ROM less than one indicates that the NfL concentration measured was higher in the control group compared with the disease group.
The cognitively unimpaired control group was defined as having no evidence of a neurological disorder or cognitive impairment. In the included studies, this group was referred to using terms such as “cognitively healthy”, “healthy volunteers”, “normal controls”, “asymptomatic controls”, “cognitively normal”, and “healthy controls”. The MCI group was defined as individuals with clinical evidence of reduced or impaired cognitive function that did not meet the diagnostic criteria for dementia. The disease mimic group was defined as individuals with diseases that had plausible overlapping phenotypes with the comparator disease. The disease mimic group was not strictly defined due to the likely heterogeneity of this group. In studies with more than one distinct disease group, a ROM was calculated for each of the disease groups separately; in studies with more than one subgroup within a given disease (e.g., multiple ALS subgroups), subgroups were combined using weighted means prior to calculation of the ROM. For studies using more than one method to quantify NfL, the data from the more commonly used assay was included. Standard error of the ROM was calculated using the delta method [11].
Because ROM calculations require the data inputted to be mean and standard deviation, data from studies using other summary statistics were converted to mean and standard deviation according the method by Hozo et al [12]. In studies reporting the median and range values, data were converted into mean estimates using equation 1 and into standard deviation estimates using equation 2. For studies reporting median and interquartile range, standard deviations were estimated from interquartile range using equation 3.
Equation 1:
where a, m, and b are the lowest value in the data set, the median value, and the highest value in the data set, respectively.
Equation 2:
Equation 3:
where IQR is the interquartile range of the data set.
Random-effects meta-analyses were conducted using inverse variance weighting to pool across studies. To reduce small-study bias, models-specified restricted maximum likelihood estimation of between-study heterogeneity (τ2) and the Hartung-Knapp adjustment to the 95% confidence interval (CI) and to the statistical test of the pooled ratio of means [13], [14]. Models were constructed in R version 3.5.3 (R-project.org) using the package “meta” version 4.9-5 [15]. Meta-analytic models were constructed separately for each disease type and control group comparison, and separately for CSF and blood. Within blood, an overall meta-analytic effect was calculated, and for serum and plasma separately where possible. The term “blood” is used herein to refer to plasma and serum. Publication bias was assessed visually using funnel plots.
3. Results
3.1. Methods for quantification of NfL
Quantification of NfL in human biofluids was performed exclusively by sandwich immunoassay in the studies examined; more specifically, by enzyme-linked immunosorbent assay (ELISA) with either single-well-based read (ELISA) or array-based “digital” read (ELISA-D), or by electrochemiluminescent (ECL) assay. The ratio of studies using ELISA to ELISA-D to ECL was 50:1:6 for CSF, 1:7:7 for serum, and 1:4:1 for plasma. All methods reported were two-site noncompetitive immunoassays, which captured NfL from the biofluid of interest using either monoclonal or polyclonal anti-NfL antibodies bound to a stationary phase (i.e., well or magnetic bead). For detection, all methods reported used an anti-NfL monoclonal primary antibody and a conjugated/labeled secondary antibody, with absorbance or chemiluminescence detection. Of the 65 studies reviewed, 52 reported the antibodies used for capture and detection of NfL; 45 studies used a pair of mouse monoclonal anti-NfL IgGs (Uman Diagnostics, 27016 anti NF-L mAb 47:3, UD1 and 27017 anti NF-L mAb 2:1, UD2) with anti-mouse IgG (Uman Diagnostics), 5 studies used hen anti-NfL IgG and rabbit anti-NfL IgG with donkey anti-rabbit IgG (sources not specified), 1 study used a noncommercial mouse anti-NfL monoclonal antibody pair (NfL21/NfL23) with rabbit anti-mouse IgG (GE Healthcare BR-1008-38), 1 study used a noncommercial rabbit anti-NfL polyclonal antibody (R61d) and anti-NfL mouse monoclonal IgG (Dako, NR-4) with (presumed) anti-mouse IgG, and 13 studies did not report the antibodies used. Owing to the variety of capture and detection antibodies used, the assays analyzed cannot be standardized and there exists no current harmonization program for NfL. As such, the absolute concentration of NfL reported between different immunoassays and between different laboratories using the same assay cannot be directly compared. To enable such an analysis, all data were converted to the ROM statistic.
3.2. NfL in AD
Across 29 studies [4], [9], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], CSF NfL concentration was compared between 3138 AD cases and 1230 cognitively unimpaired controls, with an average AD to control NfL concentration ratio of 2.12 (95% CI 1.85–2.42, P < 0.0001; Fig. 2A). In eight studies comparing a total of 442 AD cases with 545 MCI controls [9], [19], [22], [24], [30], [41], [43], [44], the evidence suggested a modest difference in CSF NfL concentration (average ratio 1.18, 95% CI 1.11–1.25, P = 0.0003; Fig. 2B). In the remaining 11 studies, with 2404 AD cases and 1647 disease mimic controls, CSF NfL concentration was not statistically distinguishable between AD cases and disease mimic controls (average ratio 0.87, 95% CI 0.70–1.08, P = 0.175; Fig. 2C) [16], [23], [27], [28], [33], [35], [40], [43], [45], [46].
Fig. 2.
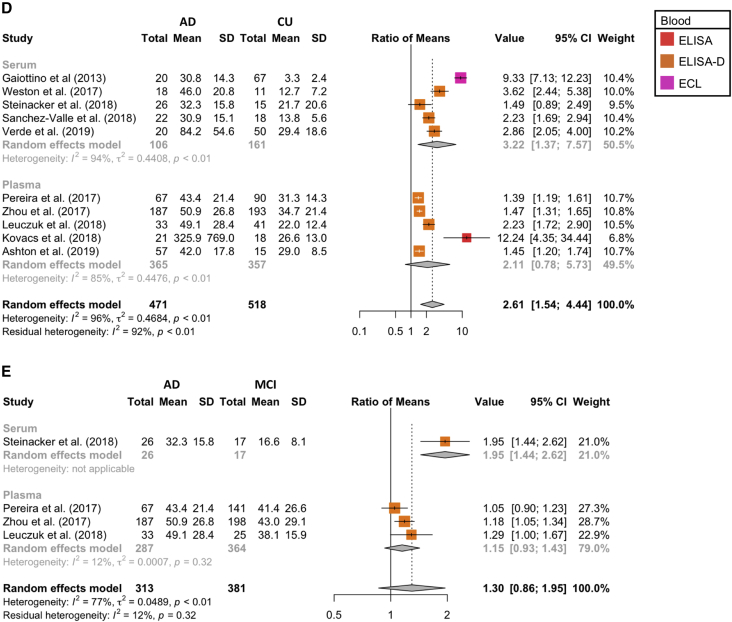
Comparison of the average ratio of NfL concentration in AD to (A) cognitively unimpaired (CU) controls in CSF, (B) MCI controls in CSF, (C) disease mimic (DM) controls in CSF, (D) cognitively unimpaired controls in blood, and (E) MCI controls in blood. Abbreviations: AD, Alzheimer's disease; NfL, neurofilament light chain; MCI, mild cognitive impairment.
Twelve studies compared blood (i.e., plasma and serum) NfL concentration in AD cases and cognitively unimpaired controls [4], [9], [25], [30], [42], [47], [48], [49], [50], [51], [52], [53]; however, three of these studies used largely overlapping data from the ADNI database [49], [50], [53]. To avoid bias, the largest of the three overlapping studies was selected for inclusion in the meta-analysis [53]; thus, 10 studies comparing 471 AD cases to 518 cognitively unimpaired controls were included in the meta-analysis. The average AD to control blood NfL concentration ratio was 2.61 (95% CI 1.54–4.44, P = 0.003; Fig. 2D). Of these studies, 5 studies (365 AD cases and 357 cognitively unimpaired controls) compared NfL concentration in plasma, with an average ratio of 2.11 (95% CI 0.78–5.73) [25], [30], [47], [48], [53]. The remaining 5 studies (106 AD cases and 161 cognitively unimpaired controls) compared serum NfL concentration [4], [9], [42], [51], [52], with an average ratio of 3.22 (95% CI 1.37–7.57). A between-subgroup comparison indicated that the NfL concentration ratio for AD to cognitively unimpaired controls was statistically indistinguishable in serum and plasma (Q (1) = 0.80, P = 0.371). Four studies compared blood NfL concentration in AD cases (n = 313) and MCI controls (n = 381) [9], [30], [48], [53], with an average ratio of 1.30 (95% CI 0.86–1.95, P = 0.136; Fig. 2E). Three of these studies (287 AD cases and 364 MCI controls) compared plasma concentrations [30], [48], [53], with an average ratio of 1.15 (95% CI 0.93–1.43). A single study investigated the serum NfL concentration of 26 AD cases compared with 17 MCI controls with a ratio of 1.95 (95% CI 1.44–2.62) [9]. A between-subgroup comparison indicated that the NfL concentration ratio for AD to MCI controls was larger in serum in comparison with plasma (Q (1) = 10.75, P = 0.001); however, with only one serum study, this difference should be interpreted cautiously.
Visual inspection of funnel plots for these various comparisons did not suggest that publication bias meaningfully skewed these effect sizes (Supplementary Figs. A2 and A3).
3.3. NfL in FTD
Twenty-six studies compared CSF NfL concentration in 1827 FTD cases and 1113 cognitively unimpaired controls, with a ROM of 3.41 (95% CI 2.96–3.93, P < 0.0001; Fig. 3A) [16], [17], [18], [20], [21], [22], [26], [27], [28], [31], [32], [35], [36], [38], [44], [45], [46], [54], [55], [56], [57], [58], [59], [60], [61], [62]. Four studies investigated 113 FTD cases and 110 MCI controls [9], [22], [43], [44], with no observable difference in CSF NfL concentration (ROM = 1.87, 95% CI 0.88–3.98, P = 0.077; Fig. 3B). The remaining nine studies compared 534 FTD cases and 1547 disease mimic controls and demonstrated higher CSF NfL concentrations in FTD cases (mean ratio of 1.69, 95% CI 1.39–2.05, P = 0.0003; Fig. 3C) [16], [21], [27], [28], [35], [43], [45], [46], [57].
Fig. 3.
Comparison of average ratio of NfL concentration in FTD to (A) cognitively unimpaired controls in CSF, (B) MCI controls in CSF, (C) disease mimic controls in CSF (D) cognitively unimpaired controls in blood, and (E) MCI controls in blood. Abbreviations: FTD, frontotemporal dementia; NfL, neurofilament light chain; MCI, mild cognitive impairment.
Five studies investigated blood NfL concentration in FTD cases compared with controls [4], [9], [51], [59], [63]. Four of these studies compared serum NfL concentration in 202 FTD cases and 139 cognitively unimpaired controls, with an average mean ratio of 2.65 (95% CI 1.59–4.43, P = 0.009; Fig. 3D) [9], [51], [59], [63]. The remaining study investigated 74 FTD cases compared with 17 MCI controls [9], with an NfL concentration ratio of 2.95 (95% CI 2.22–3.92; Fig. 3E).
Visual inspection of funnel plots for these various comparisons did not suggest that publication bias meaningfully skewed these effect sizes (Supplementary Figs. A4 and A5).
3.4. NfL in ALS
Sixteen studies investigated CSF NfL concentration in 930 ALS cases compared with 593 cognitively unimpaired controls [4], [27], [34], [41], [57], [58], [59], [64], [65], [66], [67], [68], [69], [70], [71], with an average CSF NfL concentration ratio of 9.64 (95% CI 6.65–13.99, P < 0.0001; Fig. 4A). In addition, 11 studies investigated CSF NfL concentration of 1239 ALS cases compared with 806 disease mimic controls, with an average concentration ratio of 3.35 (95% CI 2.19–5.12, P < 0.0001; Fig. 4B) [4], [8], [27], [57], [65], [68], [69], [72], [73], [74], [75].
Fig. 4.
Comparison of average ratio of NfL concentration in ALS to (A) cognitively unimpaired controls in CSF, (B) disease mimic controls in CSF, (C) cognitively unimpaired controls in blood, and (D) disease mimic controls in blood. Abbreviations: ALS, amyotrophic lateral sclerosis; NfL, neurofilament light chain; MCI, mild cognitive impairment.
Eleven studies compared blood NfL concentration from ALS cases (n = 796) and cognitively unimpaired controls (n = 455) [4], [51], [59], [64], [65], [66], [67], [71], [74], [76], with an average ratio of 8.92 (95% CI 4.85–16.43, P < 0.0001; Fig. 4C). Ten of these studies compared serum NfL concentration (693 ALS cases and 413 cognitively unimpaired controls) [4], [51], [64], [65], [66], [67], [74], [76], with an average mean ratio of 9.80 (95% CI 5.14–18.69). A single study also investigated plasma NfL concentration in 103 ALS cases and 42 cognitively unimpaired controls [66], with a ratio of 3.58 (95% CI 2.83–4.52). A between-subgroup comparison indicated that the NfL concentration ratio for ALS to cognitively unimpaired control was larger in serum in comparison with plasma (Q (1) = 10.61, P = 0.0011); however, with only one plasma study, this difference should be interpreted cautiously. An additional four studies investigated serum concentrations of NfL in 458 ALS cases compared with 181 disease mimic controls, with an average NfL concentration ratio of 8.15 (95% CI 3.88–17.12, P = 0.0029; Fig. 4D) [51], [65], [76], [77].
Visual inspection of funnel plots for these various comparisons did not suggest publication bias meaningfully skewed these effect sizes (Supplementary Figs. A6 and A7).
3.5. Average age
A meta-regression was used to determine whether group differences in NfL concentration varied as a linear function of the average age of the disease group in each study sample. These analyses showed little clear or consistent effect of age on the ration of means (Supplementary Table A1).
4. Discussion
This systematic review and meta-analysis provided an up-to-date quantitative analysis of NfL as a biomarker of neurodegeneration in CSF and blood for AD, FTD, and ALS as summarized in Fig. 5 and Supplementary Table A2.
Fig. 5.
Summary of the diagnostic performance of NfL concentration in CSF or blood in AD, FTD, and ALS compared to control groups (i.e., cognitively unimpaired, MCI and disease mimics) displayed as average ROM statistic and 95% CI, with the number of studies annotated. Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; FTD, frontotemporal dementia; NfL, neurofilament light chain: MCI, mild cognitive impairment; ROM, ratio of means.
4.1. Analytics
For CSF, standard ELISAs were the most commonly used methodology for quantitation of NfL, whereas in blood, the array-based digital ELISA and ECL approaches were more commonly utilized likely owing to the improved analytical sensitivity of these methodologies. Different methodological approaches to NfL quantitation are known to yield assays that differ in both analytical sensitivity and specificity [78], making direct comparisons challenging. Moreover, several studies were observed to misreport units for NfL; fortunately, use of the ratio means in this meta-analysis corrected for these errors. Observation of large differences in absolute NfL concentrations (correcting for unit misreporting) suggests a need for assay harmonization to facilitate comparisons between studies and between laboratories, and to support the establishment of meaningful and widely applicable reference intervals or cut-points.
4.2. Alzheimer's disease
In CSF, the best performance of NfL concentration to distinguish AD cases from controls was noted in comparison to cognitively unimpaired controls, where there was an approximately two-fold elevation in NfL concentration (Fig. 5). Elevated CSF NfL concentration appears particularly robust in light of the considerable number of relevant studies (k = 30).
Unfortunately, the performance of CSF NfL comparing AD with MCI (k = 8) and disease mimics (k = 12) was less impressive and there were far fewer studies including these group. NfL was modestly increased in AD compared with MCI, and thus not of clear utility in this setting. In future studies, it would be helpful to further differentiate the MCI group into those with MCI due to AD and those with stable MCI or MCI not due to AD—this was not possible in the current analysis because of the number of studies with MCI groups (n = 8) and the manner in which this group was reported in the literature.
Strikingly, in the CSF NfL concentration comparison of AD with disease mimics, the ROM was 0.87, indicating a decreased NfL concentration in AD compared with disease mimics, and the 95% CI spanned 1.0, substantially decreasing confidence in the discriminatory power of this biomarker in this clinical context. Thus, the currently available evidence does not support the ability of NfL to differentiate AD from disease mimics. In the studies examined, disease mimics for AD included vascular dementia, Lewy body dementia, Parkinson's disease dementia, idiopathic normal pressure hydrocephalus, and posterior cortical atrophy (Supplementary Material B) [16], [23], [27], [28], [33], [35], [40], [43], [45], [46].
In consideration of the appropriate use criteria for lumbar puncture and CSF testing in the diagnosis of AD [79], the importance of this general comparator group—disease mimics—cannot be over emphasized. The clinical indications deemed appropriate for CSF biomarker testing all involve documentation of some degree of cognitive impairment or subjective cognitive impairment in an individual at increased risk for AD. Therefore, although measurement of NfL in these groups and comparison with a reference interval of cognitively unimpaired individuals is helpful, interpretation of an NfL result in the context of clinical care must consider change in NfL concentration due to relevant non-AD causes. This highlights a need for ongoing biomarker research in diseases with phenotypic overlap with AD to better understand the potential implications for biomarker interpretation in routine clinical use. The results herein further demonstrate the need to subtype disease groups, including AD, into more clinically meaningful categories to determine if there is any utility to NfL in this diagnostic context.
There has been a strong demand to develop blood-based biomarkers for AD as a less-invasive collection method and more facile (and cost-effective) procedure relative to a lumbar puncture or administration of an imaging tracer. Reflecting this growing body of research, of the studies included in this review, 20 included evaluation of NfL in blood. This is particularly notable as there were no studies of NfL in blood in a meta-analysis published only 3 years prior, which included studies through to July 2014 [80]. The groups most studied in our cohort of blood NfL studies compared AD with cognitively unimpaired controls (k = 12) and the results were comparable, although with a greater range of uncertainty, to the performance of NfL in CSF. A similar conclusion can be drawn from studies of AD compared with MCI (k = 6). No studies were reported in disease mimics. Based on subgroup analyses, there was no clear difference between use of serum versus plasma as a sample type, and a greater number of studies would be required to assess differences based on the blood collection tube/anti-coagulant additives used.
4.3. Frontotemporal dementia
In the FTD studies, there was an over three-fold increase in CSF NfL concentration in FTD cases compared with cognitively unimpaired controls. This was a relatively consistent finding, supported by all relevant studies (k = 26) and was higher than the fold-change observed for AD (Fig. 5). The higher concentration of NfL in FTD compared with AD has been posited to be the result of prominent degeneration of the frontal and temporal lobes and increased involvement of the subcortical areas in FTD compared with AD [16], [46]. For blood NfL studies, the average fold-change suggests performance similar to CSF for differentiating FTD from cognitively unimpaired controls; however, there was a high degree of uncertainty as indicated by the wide CI.
CSF NfL concentration, while increased in FTD compared with both MCI (k = 4) and disease mimics (k = 9), was less marked than the increase observed in comparison with cognitively unimpaired controls. CSF NfL did not convincingly separate FTD from the MCI group with the 95% CI spanning 1.0. In blood, the separation was more marked; however, again the study size was small (k = 4). Without additional studies, it would be unwise to draw conclusions about the performance of NfL in these groups. In the key comparison group, FTD versus disease mimics, the discriminatory power of NfL was modest. The heterogeneity of the performance relative to disease mimics may, in part, reflect the lack of autopsy-confirmed cases in these studies (Supplementary Material B) and the high degree of heterogeneity of diseases found under the umbrella of an FTD diagnosis. As with AD, the results herein suggest the need for further subtyping of FTD by clinically meaningful phenotypes (e.g., behavioral variant, semantic variant, progressive nonfluent aphasia, presence/absence of ALS phenotype), disease duration, and severity.
4.4. Amyotrophic lateral sclerosis
In the reviewed studies, there was more than a nine-fold elevation in NfL concentration in ALS compared with cognitively unimpaired controls in both the blood and CSF. NfL in ALS compared with cognitively unimpaired controls had the highest ROM compared with the equivalent comparisons in AD and FTD, an association that has been previously attributed to the destruction of motor neurons—neurons containing the longest axons in the body—in ALS [27], [81]. Unlike the findings from the AD and FTD meta-analyses, CSF NfL was found to have good discriminatory power for ALS compared with disease mimics (Fig. 5). As with AD and FTD, a more detailed exploration of additional variables involved in such comparisons, including age, sex, and disease severity, are necessary to characterize the potential of this biomarker in routine care settings.
4.5. Variables and study limitations
Age and sex have been determined to be important considerations in the application of NfL as a biomarker. NfL correlates with age in both healthy individuals and those with most neurodegenerative diseases [41], [48], [82], [83], [84]. In addition, CSF NfL concentration is significantly higher in males compared with females in healthy individuals and several neurodegenerative diseases [19], [41]; however, mixed findings, with more modest differences, have been noted for blood NfL [66], [82], [83], [84]. Age did not moderate any of the effect sizes we observed; however, the average age could not be determined for several studies and the restricted range of ages across studies limited the ability to detect a moderating effect of age.
A few limitations of this meta-analysis should be noted. First, the definition of MCI encompassed all individuals with clinical evidence of cognitive impairment but was not further subdivided in those with stable MCI, or MCI due to an early-stage dementia. Second, the neurodegenerative disease group data were rarely subdivided/characterized into more clinically meaningful subgroups. For example, the FTD group was not further subdivided by either clinical variants or by the primary pathological finding on autopsy (e.g., tau, TDP-43, etc.). Separate analysis of these groups would allow additional conclusions to be drawn; however, information necessary to make such subdivisions was not consistently available and, where available, would have reduced the robustness of the data given the sample size understudy. Third, most studies included in the meta-analysis relied on a clinical diagnosis in classifying individuals, and therefore some error in classification of individuals must be assumed. Last, we meta-analyzed only cross-sectional data given the current paucity of longitudinal data on NfL.
We note that the random-effects meta-analysis accounts for, and estimates, between-study variation in effect sizes beyond that expected by sampling error alone. The result is estimates with larger CIs as compared with a fixed-effects meta-analysis, which assumes no variation in effect sizes across studies beyond sampling error.
5. Conclusion
NfL concentration in CSF displayed strong performance in distinguishing AD, FTD, and ALS from cognitively unimpaired controls, with NfL providing the greatest separation for ALS in both the blood and CSF, followed by FTD (CSF and blood), then AD (blood and CSF). Performance of NfL generally decreased (both in blood and CSF) when moving from comparisons with cognitively unimpaired controls, to MCI, to disease mimics. Strikingly, the performance of blood NfL (where there were sufficient studies) was similar to CSF; however, CSF NfL concentration, as of now, is a more reliable/robust marker due to the lower number of studies in blood.
In the most clinically meaningful comparisons, that is AD, FTD, and ALS compared with their respective disease mimics, we observed that CSF NfL had strong discriminatory power for ALS (as did blood NfL), modest discriminatory power for FTD and no discriminatory power for AD. With calls for implementation of NfL in routine clinical use and for clinical trials, a better understanding of the performance of this nonspecific biomarker in diseases with overlapping phenotypes is warranted. Specifically, to address use in routine care, additional variables including age, sex, phenotype, disease duration, and disease severity should be made available in NfL biomarker studies—stratifying by these variables and not adjusting for these variables, where sample size permits. As there is considerable momentum behind NfL as a candidate marker for incorporation into the AT(N) classification system for AD, and as this meta-analysis highlights, there would be great benefit to the development of a harmonization program for NfL, including the creation of an NfL reference material.
Research in context.
-
1.
Systematic review: A systematic review and meta-analysis was performed of the diagnostic performance of neurofilament light chain (NfL) in CSF and blood in the context of Alzheimer's disease (AD), frontotemporal dementia, and amyotrophic lateral sclerosis compared with controls (i.e., cognitively unimpaired, mild cognitive impairment or disease mimics).
-
2.
Interpretation: In groups with a sufficient number of studies, the performance of NfL in blood and CSF was similar. Compared with disease mimics, we observed that CSF NfL had strong discriminatory power for amyotrophic lateral sclerosis, modest discriminatory power for frontotemporal dementia, and no discriminatory power for AD.
-
3.
Future directions: Comparable performance of NfL in blood and CSF demonstrates its promise as a noninvasive biomarker of neurodegeneration. Toward clinical implementation, a more comprehensive understanding of NfL concentrations in disease subtypes with overlapping phenotypes and the development of a harmonization program are warranted.
Acknowledgments
The authors would like to acknowledge the efforts of the researchers cited herein, whose work has made this meta-analysis possible.
Footnotes
The authors have nothing to disclose.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.08.009.
Supplementary Data
References
- 1.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233:183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Yao N.Y., Broedersz C.P., Lin Y.C., Kasza K.E., Mackintosh F.C., Weitz D.A. Elasticity in ionically cross-linked neurofilament networks. Biophys J. 2010;98:2147–2153. doi: 10.1016/j.bpj.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillespie M.J., Stein R.B. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983;259:41–56. doi: 10.1016/0006-8993(83)91065-x. [DOI] [PubMed] [Google Scholar]
- 4.Gaiottino J., Norgren N., Dobson R., Topping J., Nissim A., Malaspina A. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8:e75091. doi: 10.1371/journal.pone.0075091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N., Cullen N.C., Andreasson U., Zetterberg H., Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76:791–799. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeter L.H., Dopper E.G., Jiskoot L.C., Sanchez-Valle R., Graff C., Benussi L. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol. 2016;3:623–636. doi: 10.1002/acn3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poesen K., De Schaepdryver M., Stubendorff B., Gille B., Muckova P., Wendler S. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology. 2017;88:2302–2309. doi: 10.1212/WNL.0000000000004029. [DOI] [PubMed] [Google Scholar]
- 9.Steinacker P., Anderl-Straub S., Diehl-Schmid J., Semler E., Uttner I., von Arnim C.A.F. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology. 2018;91:e1390–e1401. doi: 10.1212/WNL.0000000000006318. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich J.O., Adhikari N.K., Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. doi: 10.1186/1471-2288-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IntHout J., Ioannidis J.P., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guolo A., Varin C. Random-effects meta-analysis: the number of studies matters. Stat Methods Med Res. 2017;26:1500–1518. doi: 10.1177/0962280215583568. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 16.Abu-Rumeileh S., Capellari S., Stanzani-Maserati M., Polischi B., Martinelli P., Caroppo P. The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther. 2018;10:3. doi: 10.1186/s13195-017-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Rumeileh S., Mometto N., Bartoletti-Stella A., Polischi B., Oppi F., Poda R. Cerebrospinal fluid biomarkers in patients with frontotemporal dementia spectrum: a Single-Center Study. J Alzheimers Dis. 2018;66:551–563. doi: 10.3233/JAD-180409. [DOI] [PubMed] [Google Scholar]
- 18.Alcolea D., Vilaplana E., Suarez-Calvet M., Illan-Gala I., Blesa R., Clarimon J. CSF sAPPbeta, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology. 2017;89:178–188. doi: 10.1212/WNL.0000000000004088. [DOI] [PubMed] [Google Scholar]
- 19.Gaetani L., Hoglund K., Parnetti L., Pujol-Calderon F., Becker B., Eusebi P. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10:8. doi: 10.1186/s13195-018-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goossens J., Bjerke M., Van Mossevelde S., Van den Bossche T., Goeman J., De Vil B. Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Res Ther. 2018;10:31. doi: 10.1186/s13195-018-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall S., Ohrfelt A., Constantinescu R., Andreasson U., Surova Y., Bostrom F. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69:1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 22.Hampel H., Toschi N., Baldacci F., Zetterberg H., Blennow K., Kilimann I. Alzheimer's disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Abeta1-42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimers Dement. 2018;14:492–501. doi: 10.1016/j.jalz.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y.Y., He S.S., Wang X.C., Duan Q.H., Khatoon S., Iqbal K. Elevated levels of phosphorylated neurofilament proteins in cerebrospinal fluid of Alzheimer disease patients. Neurosci Lett. 2002;320:156–160. doi: 10.1016/s0304-3940(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 24.Kester M.I., Scheffer P.G., Koel-Simmelink M.J., Twaalfhoven H., Verwey N.A., Veerhuis R. Serial CSF sampling in Alzheimer's disease: specific versus non-specific markers. Neurobiol Aging. 2012;33:1591–1598. doi: 10.1016/j.neurobiolaging.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs G.G., Andreasson U., Liman V., Regelsberger G., Lutz M.I., Danics K. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol. 2017;24:1326-e77. doi: 10.1111/ene.13389. [DOI] [PubMed] [Google Scholar]
- 26.Magdalinou N.K., Paterson R.W., Schott J.M., Fox N.C., Mummery C., Blennow K. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2015;86:1240–1247. doi: 10.1136/jnnp-2014-309562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson B., Portelius E., Cullen N.C., Sandelius A., Zetterberg H., Andreasson U. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76:318–325. doi: 10.1001/jamaneurol.2018.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paterson R.W., Slattery C.F., Poole T., Nicholas J.M., Magdalinou N.K., Toombs J. Cerebrospinal fluid in the differential diagnosis of Alzheimer's disease: clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther. 2018;10:32. doi: 10.1186/s13195-018-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paterson R.W., Toombs J., Slattery C.F., Nicholas J.M., Andreasson U., Magdalinou N.K. Dissecting IWG-2 typical and atypical Alzheimer's disease: insights from cerebrospinal fluid analysis. J Neurol. 2015;262:2722–2730. doi: 10.1007/s00415-015-7904-3. [DOI] [PubMed] [Google Scholar]
- 30.Pereira J.B., Westman E., Hansson O., Alzheimer's Disease Neuroimaging Initiative Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer's disease. Neurobiol Aging. 2017;58:14–29. doi: 10.1016/j.neurobiolaging.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Pijnenburg Y.A., Janssen J.C., Schoonenboom N.S., Petzold A., Mulder C., Stigbrand T. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer's disease and controls. Dement Geriatr Cogn Disord. 2007;23:225–230. doi: 10.1159/000099473. [DOI] [PubMed] [Google Scholar]
- 32.Pijnenburg Y.A., Verwey N.A., van der Flier W.M., Scheltens P., Teunissen C.E. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement (Amst) 2015;1:505–512. doi: 10.1016/j.dadm.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyykko O.T., Lumela M., Rummukainen J., Nerg O., Seppala T.T., Herukka S.K. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One. 2014;9:e91974. doi: 10.1371/journal.pone.0091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosengren L.E., Karlsson J.E., Karlsson J.O., Persson L.I., Wikkelso C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67:2013–2018. doi: 10.1046/j.1471-4159.1996.67052013.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosengren L.E., Karlsson J.E., Sjogren M., Blennow K., Wallin A. Neurofilament protein levels in CSF are increased in dementia. Neurology. 1999;52:1090–1093. doi: 10.1212/wnl.52.5.1090. [DOI] [PubMed] [Google Scholar]
- 36.Scherling C.S., Hall T., Berisha F., Klepac K., Karydas A., Coppola G. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2014;75:116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjogren M., Blomberg M., Jonsson M., Wahlund L.O., Edman A., Lind K. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res. 2001;66:510–516. doi: 10.1002/jnr.1242. [DOI] [PubMed] [Google Scholar]
- 38.Sjogren M., Rosengren L., Minthon L., Davidsson P., Blennow K., Wallin A. Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology. 2000;54:1960–1964. doi: 10.1212/wnl.54.10.1960. [DOI] [PubMed] [Google Scholar]
- 39.van Eijk J.J., van Everbroeck B., Abdo W.F., Kremer B.P., Verbeek M.M. CSF neurofilament proteins levels are elevated in sporadic Creutzfeldt-Jakob disease. J Alzheimers Dis. 2010;21:569–576. doi: 10.3233/JAD-2010-090649. [DOI] [PubMed] [Google Scholar]
- 40.Wellington H., Paterson R.W., Suarez-Gonzalez A., Poole T., Frost C., Sjobom U. CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Ann Clin Transl Neurol. 2018;5:162–171. doi: 10.1002/acn3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zetterberg H., Skillback T., Mattsson N., Trojanowski J.Q., Portelius E., Shaw L.M. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73:60–67. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Valle R., Heslegrave A., Foiani M.S., Bosch B., Antonell A., Balasa M. Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer's disease. Alzheimers Res Ther. 2018;10:113. doi: 10.1186/s13195-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zerr I., Schmitz M., Karch A., Villar-Pique A., Kanata E., Golanska E. Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement. 2018;14:751–763. doi: 10.1016/j.jalz.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Niikado M., Chrem-Mendez P., Itzcovich T., Barbieri-Kennedy M., Calandri I., Martinetto H. Evaluation of cerebrospinal fluid neurofilament light chain as a routine biomarker in a memory clinic. J Gerontol A Biol Sci Med Sci. 2019;74:442–445. doi: 10.1093/gerona/gly179. [DOI] [PubMed] [Google Scholar]
- 45.de Jong D., Jansen R.W., Pijnenburg Y.A., van Geel W.J., Borm G.F., Kremer H.P. CSF neurofilament proteins in the differential diagnosis of dementia. J Neurol Neurosurg Psychiatry. 2007;78:936–938. doi: 10.1136/jnnp.2006.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skillback T., Farahmand B., Bartlett J.W., Rosen C., Mattsson N., Nagga K. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83:1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 47.Ashton N.J., Leuzy A., Lim Y.M., Troakes C., Hortobagyi T., Hoglund K. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7:5. doi: 10.1186/s40478-018-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewczuk P., Ermann N., Andreasson U., Schultheis C., Podhorna J., Spitzer P. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alzheimers Res Ther. 2018;10:71. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J.Q., Yuan X.Z., Li H.Y., Cao X.P., Yu J.T., Tan L. Genome-wide association study identifies two loci influencing plasma neurofilament light levels. BMC Med Genomics. 2018;11:47. doi: 10.1186/s12920-018-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattsson N., Andreasson U., Zetterberg H., Blennow K., Alzheimer's Disease Neuroimaging Initiative Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verde F., Steinacker P., Weishaupt J.H., Kassubek J., Oeckl P., Halbgebauer S. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:157–164. doi: 10.1136/jnnp-2018-318704. [DOI] [PubMed] [Google Scholar]
- 52.Weston P.S.J., Poole T., Ryan N.S., Nair A., Liang Y., Macpherson K. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89:2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou W., Zhang J., Ye F., Xu G., Su H., Su Y. Plasma neurofilament light chain levels in Alzheimer's disease. Neurosci Lett. 2017;650:60–64. doi: 10.1016/j.neulet.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Lista S., Toschi N., Baldacci F., Zetterberg H., Blennow K., Kilimann I. Diagnostic accuracy of CSF neurofilament light chain protein in the biomarker-guided classification system for Alzheimer's disease. Neurochem Int. 2017;108:355–360. doi: 10.1016/j.neuint.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Meeter L.H.H., Vijverberg E.G., Del Campo M., Rozemuller A.J.M., Donker Kaat L., de Jong F.J. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology. 2018;90:e1231–e1239. doi: 10.1212/WNL.0000000000005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skillback T., Mattsson N., Blennow K., Zetterberg H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:397–403. doi: 10.1080/21678421.2017.1281962. [DOI] [PubMed] [Google Scholar]
- 57.Gaiani A., Martinelli I., Bello L., Querin G., Puthenparampil M., Ruggero S. Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol. 2017;74:525–532. doi: 10.1001/jamaneurol.2016.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illan-Gala I., Alcolea D., Montal V., Dols-Icardo O., Munoz L., de Luna N. CSF sAPPbeta, YKL-40, and NfL along the ALS-FTD spectrum. Neurology. 2018;91:e1619–e1628. doi: 10.1212/WNL.0000000000006383. [DOI] [PubMed] [Google Scholar]
- 59.Wilke C., Preische O., Deuschle C., Roeben B., Apel A., Barro C. Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J Neurol Neurosurg Psychiatry. 2016;87:1270–1272. doi: 10.1136/jnnp-2015-312972. [DOI] [PubMed] [Google Scholar]
- 60.Ljubenkov P.A., Staffaroni A.M., Rojas J.C., Allen I.E., Wang P., Heuer H. Cerebrospinal fluid biomarkers predict frontotemporal dementia trajectory. Ann Clin Transl Neurol. 2018;5:1250–1263. doi: 10.1002/acn3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meeter L.H.H., Gendron T.F., Sias A.C., Jiskoot L.C., Russo S.P., Donker Kaat L. Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Ann Clin Transl Neurol. 2018;5:583–597. doi: 10.1002/acn3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sjogren M., Wallin A. Pathophysiological aspects of frontotemporal dementia--emphasis on cytoskeleton proteins and autoimmunity. Mech Ageing Dev. 2001;122:1923–1935. doi: 10.1016/s0047-6374(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 63.Rohrer J.D., Woollacott I.O., Dick K.M., Brotherhood E., Gordon E., Fellows A. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–1336. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benatar M., Wuu J., Andersen P.M., Lombardi V., Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. 2018;84:130–139. doi: 10.1002/ana.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feneberg E., Oeckl P., Steinacker P., Verde F., Barro C., Van Damme P. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. 2018;90:e22–e30. doi: 10.1212/WNL.0000000000004761. [DOI] [PubMed] [Google Scholar]
- 66.Lu C.H., Macdonald-Wallis C., Gray E., Pearce N., Petzold A., Norgren N. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menke R.A., Gray E., Lu C.H., Kuhle J., Talbot K., Malaspina A. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol. 2015;2:748–755. doi: 10.1002/acn3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scarafino A., D'Errico E., Introna A., Fraddosio A., Distaso E., Tempesta I. Diagnostic and prognostic power of CSF Tau in amyotrophic lateral sclerosis. J Neurol. 2018;265:2353–2362. doi: 10.1007/s00415-018-9008-3. [DOI] [PubMed] [Google Scholar]
- 69.Pawlitzki M., Schreiber S., Bittner D., Kreipe J., Leypoldt F., Rupprecht K. CSF neurofilament light chain levels in primary progressive MS: signs of axonal neurodegeneration. Front Neurol. 2018;9:1037. doi: 10.3389/fneur.2018.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinacker P., Huss A., Mayer B., Grehl T., Grosskreutz J., Borck G. Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: data from the German MND-net. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:112–119. doi: 10.1080/21678421.2016.1241279. [DOI] [PubMed] [Google Scholar]
- 71.Weydt P., Oeckl P., Huss A., Muller K., Volk A.E., Kuhle J. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79:152–158. doi: 10.1002/ana.24552. [DOI] [PubMed] [Google Scholar]
- 72.Reijn T.S., Abdo W.F., Schelhaas H.J., Verbeek M.M. CSF neurofilament protein analysis in the differential diagnosis of ALS. J Neurol. 2009;256:615–619. doi: 10.1007/s00415-009-0131-z. [DOI] [PubMed] [Google Scholar]
- 73.Rossi D., Volanti P., Brambilla L., Colletti T., Spataro R., La Bella V. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J Neurol. 2018;265:510–521. doi: 10.1007/s00415-017-8730-6. [DOI] [PubMed] [Google Scholar]
- 74.Steinacker P., Feneberg E., Weishaupt J., Brettschneider J., Tumani H., Andersen P.M. Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry. 2016;87:12–20. doi: 10.1136/jnnp-2015-311387. [DOI] [PubMed] [Google Scholar]
- 75.Tortelli R., Ruggieri M., Cortese R., D'Errico E., Capozzo R., Leo A. Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur J Neurol. 2012;19:1561–1567. doi: 10.1111/j.1468-1331.2012.03777.x. [DOI] [PubMed] [Google Scholar]
- 76.Wilke C., Rattay T.W., Hengel H., Zimmermann M., Brockmann K., Schols L. Serum neurofilament light chain is increased in hereditary spastic paraplegias. Ann Clin Transl Neurol. 2018;5:876–882. doi: 10.1002/acn3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gille B., De Schaepdryver M., Goossens J., Dedeene L., De Vocht J., Oldoni E. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2019;45:291–304. doi: 10.1111/nan.12511. [DOI] [PubMed] [Google Scholar]
- 78.Kuhle J., Barro C., Andreasson U., Derfuss T., Lindberg R., Sandelius A. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
- 79.Shaw L.M., Arias J., Blennow K., Galasko D., Molinuevo J.L., Salloway S. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14:1505–1521. doi: 10.1016/j.jalz.2018.07.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 81.Brown R.H., Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 82.Disanto G., Barro C., Benkert P., Naegelin Y., Schadelin S., Giardiello A. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansson O., Janelidze S., Hall S., Magdalinou N., Lees A.J., Andreasson U. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin Y.S., Lee W.J., Wang S.J., Fuh J.L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep. 2018;8:17368. doi: 10.1038/s41598-018-35766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.