Abstract
Magnesium (Mg) and its alloys as temporary medical implants with biodegradable and properly mechanical properties have been investigated for a long time. There are already three kinds of biodegradable Mg implants which are approved by Conformite Europeene (CE) or Korea Food and Drug Administration (KFDA), but not China Food and Drug Administration (CFDA, now it is National Medical Products Administration, NMPA). As we know, Chinese researchers, surgeons, and entrepreneurs have tried a lot to research and develop biodegradable Mg implants which might become other new approved implants for clinical applications. So in this review, we present the representative Mg implants of three categories, orthopedic implants, surgical implants, and intervention implants and provide an overview of current achievement in China from academic publications and Chinese patents. We would like to provide a systematic way to translate Mg and its alloy implants from experiment designs to clinical products.
Keywords: Magnesium, Implant, Device, Biomedical translation, China
Graphical abstract
Highlights
-
•
Summarized the translation of biodegradable Mg implants in China for the first time.
-
•
Including the main research groups in China.
-
•
Summarized valuable work of some representative groups.
1. Introduction
The first application of biodegradable magnesium (Mg) implants in humans dates back to over a century ago. In 1878, Edward C. Huse reported the first successful usage of pure Mg wire ligature to stop bleeding without knowledge of its unique biodegradable properties [1]. Then a lot of clinical trials using Mg-based implants followed, but they failed prematurely due to the lack of metallurgical technology to control the fast degradability. Recent years, the rapid advancement in the metallurgy field enables scientists and engineers to fabricate Mg and its alloys with much higher corrosion resistance and improved mechanical properties, which inspired more and more surgeons to reconsider the potential of biodegradable Mg for clinical applications. The first clinical application in the new century is absorbable Mg stent from Biotronik. Peeters et al. [2] studied the preclinical trial of Mg-stent in 2005. And based on this series of clinical trials, the absorbable Mg stent, named Magmaris® (Biotronik, German) became the first biodegradable coronary stent approved by Conformite Europeene (CE) in 2017 finally. And in orthopedic, Germany also is the first country to report treatment outcomes by using MAGNEZIXW® compression screw (Syntellix AG, Germany), which is classified as an MgYReZr alloy, in hallux valgus surgery [3]. And based on that clinical trial, the MgYReZr screw successfully earns a CE mark in 2013, which allowed this novel device to get access to the medical device market for its intended use. In 2015, another Mg alloy screw (K-MET, U&i, Korea) was approved in Korea for the successful fixation of knuckle in patients with no complications [4].
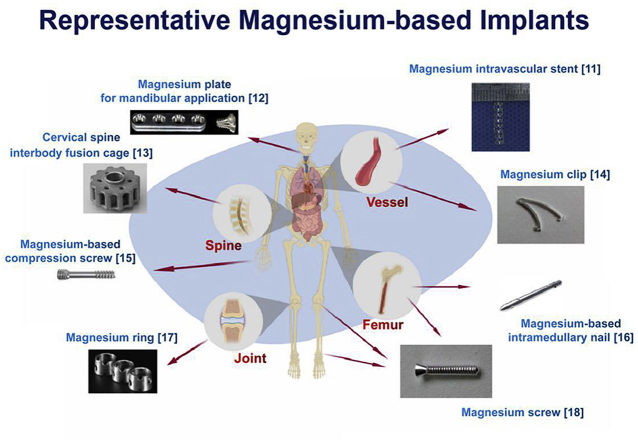
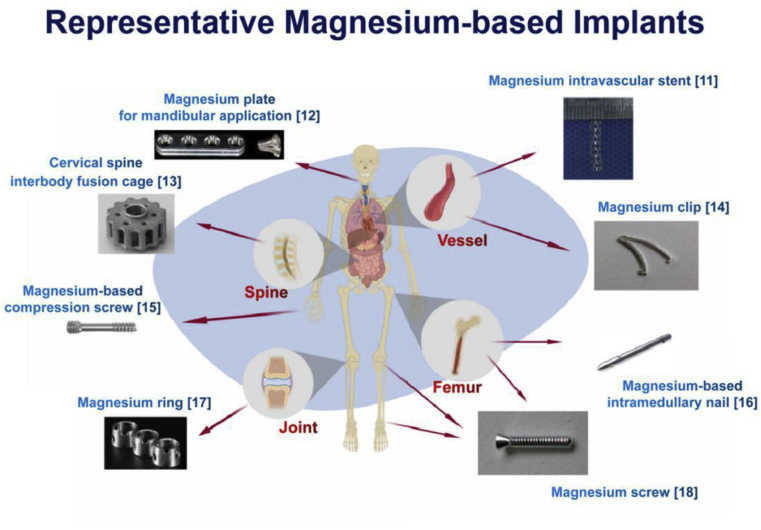
On the bright side, more and more Chinese surgeons and researchers are trying a lot in the research & development (R&D) of Mg based implants to develop products for preclinical and clinical trials. Several approaches such as optimize machining process [5], purifying, alloying with other or more metals [6,7] and surface modification [8] were taken to enhance properties of Mg-based implants. These made Mg-based biomaterials exhibit proper mechanical properties, adequate biocompatibility, favorable degradability and presented advantages in osteoconductivity and osteogenesis [[9], [10], [11]]. Fig. 1 shows some representative Mg-based implants cross the world.
Fig. 1.
Some representative Mg-based implants [[11], [12], [13], [14], [15], [16], [17], [18]].
In order to provide a systematic way to translate Mg and its alloy implants from experiment designs to marketing clinical products, we review the small-scale clinical trials of biodegradable Mg implants based on the academic publications and Chinese patents.
2. Orthopedic implants
In this century, the first paper about biodegradable Mg implants reported new bone formation post-implantation in rats [19]. After that, many papers focused on how to design a biodegradable orthopedic implant, for example, bone screw [3,20], bone plate [21], bone pin [22,23], bone scaffold [24,25], bone substitute [26] and such on. However, bone screw, bone scaffold and bone plate are the most important translational studies in China [27,28].
2.1. Bone screw
Bone screws are routinely used in fracture fixation, bone graft stabilization and osteotomies with fragment fixation. Traditional materials used as bone screw are permanent metals, such as titanium alloy and stainless steel, and biodegradable polymers, e.g. poly l-lactic acid (PLLA) and it is composite with Ca–P bone salt. However, permanent metallic screws encounter stress shielding effect and second removal surgery, while the PLLA raised several concerns including the acid products during their degradation in vivo. And these metallic screws and polymers have no potential to promote bone formation. Table 1 lists some representative case studies on Mg-based bone screws used for fixation of autologous bone grafts or bone fracture in China.
Table 1.
Representative case studies on Mg-based bone screws used for fixation of autologous bone grafts or bone fracture in China.
| Mg and Mg alloy | Animal model | Size | Implantation period | Ref | Published time |
|---|---|---|---|---|---|
| AZ31B/silicon-containing coating | rabbit femur shaft | D 2.6 mm*L 6.5 mm | 1, 4, 12, 21 W | [29] | 2014 |
| High purity Mg | rabbit femoral intracondylar fracture model |
D 2.7 mm *L 27 mm | 4, 8, 16, 24 W | [30] | 2015 |
| Mg-Nd-Zn-Zr/brushite coating (JDBM-DCPD) | rabbit mandible | D 2 mm*L 4.6 mm | 18 M | [10] | 2016 |
| Mg-Nd-Zn-Zr/uncoated (JDBM) | goat femoral condyle fracture model | D 4.5 mm*L 45 mm | 18 M | [11] | 2018 |
| JDBM-DCPD | |||||
| Mg–6Zn-0.5Sr | rabbits tendon graft fixation | D 3 mm*L 8 mm | 6, 12, 16 W | [31] | 2018 |
Interference screws are used to fix tendon graft with a bone tunnel in anterior cruciate ligament (ACL) construction. Cheng et al. [32] reported that the high-purity magnesium (HP–Mg) showed good cytocompatibility and promoted the expression of bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF), fibrocartilage markers (Aggrecan, COL2A1, and SOX-9), and glycosaminoglycan (GAG) production in vitro cell culture tests. Then they used the HP-Mg interference screw to fix the semitendinosus autograft to the femoral tunnel in a rabbit model of ACL reconstruction with titanium screw as the control. They proposed that the stimulation of BMP-2 and VEGF by Mg ions was responsible for the fibrochondrogenesis of Mg [33]. Wang et al. [34,35] also used Mg-based interference screw to promote tendon graft incorporation in rabbits bone tunnel. While in order to improve the poor mechanical strength of Mg, Wang designed one kind of Mg alloys (Mg–Zn–Sr) and tested its potential for ACL reconstruction. Both numerical and experimental analysis showed Mg–Zn–Sr has significantly higher torque and torsional stiffness [31].
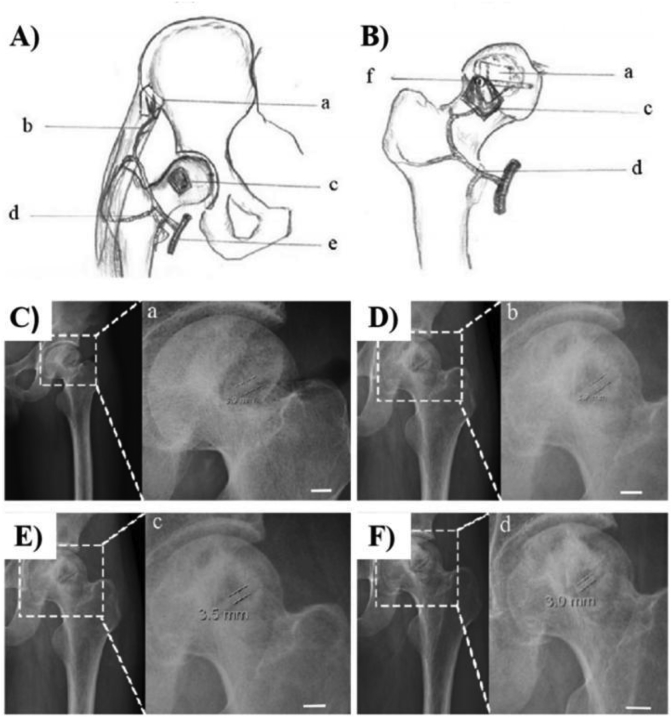
In 2013, Zhao et al. [36] designed high purity Mg screws to fix vascularized bone flaps in patients suffering from an association of research circulation osseous (ARCO) stage II/III osteonecrosis in the femoral head (ONFH) (Fig. 2). This is the first clinical trial in China. Within the 12-month follow-up period, patients treated with HP-Mg screws fixation showed significantly higher satisfactory therapeutic results (n = 23 in Mg group and n = 25 in Control group) in the Harris hip score (HHS) and bone flap displacement by using radiographic imaging. While no abnormal postoperative serum levels of Ca, Mg and P, which are relevant for liver and kidney function, were found in all patients of both groups. This innovative design of HP-Mg screw produced by Dongguan Yian Science and Technology Co., Ltd. was also the first rewarded Mg-based innovative medical device by the China food and drug administration (CFDA) in 2015 [37] and have gotten permission for official clinical trials to get registration certificate in July of 2019 [38].
Fig. 2.
A) and B) Schematic diagram of the operation and bone flap fixation with Mg screw (a: the vascularized bone graft (left: origin of bone flapefrom ilia; right: after implantation into the bone defect and removal of necrotic bone); b: the ascending branch of the circumflexa femoris lateralis; c: the excavated region of the femoral head; d: the circumflexa femoris lateralis artery; e: the femoral artery; f: the Mg screw insertion for bone flap fixation). C–F) Bone flap fixation with HP-Mg screw at 1, 3, 6, and 12 months post-surgery [36].
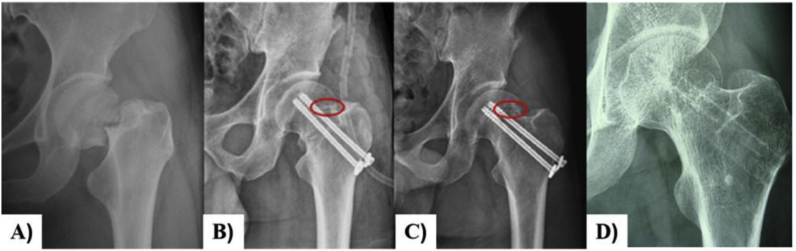
Zhao et al. [39] also used Mg screws for fixation of vascularized iliac grafting during the treatment of displaced fracture of the femoral neck in young adults (Fig. 3). 18 patients were followed up for an average of 16 months (range: 8–24 months). Only one patient experienced nonunion and was conducted to a hip replacement revision after 12 months of the operation. According to the Harris hip score (HHS) that was available for 17 hips with the satisfactory union and no patient developed avascular necrosis of femoral head after operation.
Fig. 3.
A) Preoperative radiographs of a 35-year-old male patient with Garden III fracture; B) The fracture was fixed with Mg screw (red ring) and two cannulated screws and vascularized iliac grafting; C) 12 months postoperative radiographs showing the fracture healing and Mg screw (red ring); D) 24 months postperative moving out cannulated screws and Mg screw fully absorbed [39].
2.2. Bone scaffold
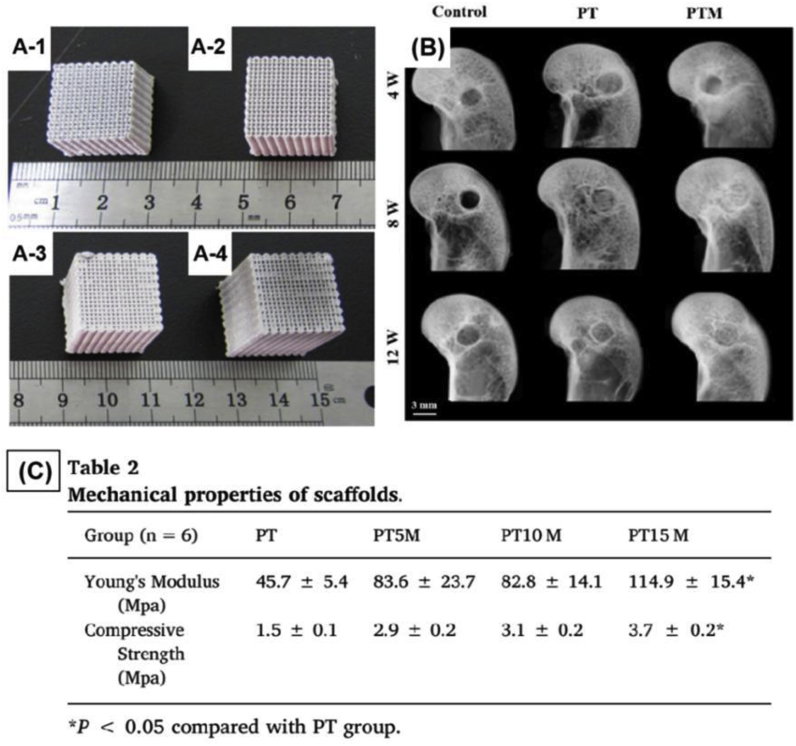
In 2015, Qin et al. [40,41] established a poly(lactic-co-glycolic acid) (PLGA)/β-calcium phosphate (β-TCP) composite scaffold using low-temperature rapid prototyping, and this scaffold exhibited good biocompatibility, osteoconductivity, and biodegradability both in vitro and in vivo. On this basis, Lai et al. [42] added Mg powder to formulate a novel porous called PLGA/TCP/Mg (PTM) scaffolds. Mg could effectively enhance the mechanical strength of the porous scaffolds. At the same time, these scaffolds have both osteogenic and angiogenic abilities which were synergistic effect in enhancing new bone formation and strengthening new formed bone quality (Fig. 4). In addition, it is worth mentioning that the novel scaffold had rewarded as an innovative medical device by CFDA in 2018 [43].
Figs. 4.
A-1) PLGA/TCP (PT) scaffold was used for comparison; A-2–4) The PLGA/TCP/Mg (PTM) with different Mg content. The Mg content of PTM was 5 wt% (PT5M, A-2), 10 wt% (PT10 M, A-3), 15 wt% (PT15 M, A-4), respectively; B) Representative radiographs of new bone formation within bone tunnel at 4, 8 and 12 weeks after surgery; C) The mechanical properties of different scaffolds [42].
2.3. Bone plate
Mao et al. [44] considered that these animal studies, which only simply inserted-based devices in the bone cavity or shaft, were too simple to demonstrate safety and efficacy. Clinical indication-oriental animal experiments should be established. So they designed an experiment to evaluate the safety and efficacy of special WE43 Mg alloy stretch plates (SPs) used as a fixation device for ACL reconstruction in beagle model. It showed WE43 SPs could provide enough and excellent primary mechanical strength for forming the secondary biological fixation during in vivo 6 months after surgery. And it was safe and efficacy for WE43 SPs being used as the fixation device for ACL reconstruction.
In the fracture repairment, rapid degradation in the early stage after implantation limits Mg used as stable fracture fixation at load-bearing sites. In order to solve this problem, Tian et al. [45] designed an Mg/Ti hybrid plate/screw system for long bone fracture fixation. While Mg co-implanted with Ti would accelerate the corrosion of Mg [46], they coated a polymer film on Mg screws to prohibit Ti and Mg contact directly. The animal experiment results showed that Mg implant used for this hybrid system not only provided enough mechanical support but promoted fracture healing through the up-regulation of local CGRP secretion and acceleration of callus mineralization and its remodeling. It has great potential as a new fixation strategy in the clinic. At the same time, some patents of Mg-based bone plates have been applied by Chinese doctors and researchers [[47], [48], [49], [50]].
3. Surgical implants
3.1. Clip
The hemostasis clip is one of the most common implants for vessels or other tubular tissue ligation in abdominal operations and especially for endoscopic operations. Mg is a potential material to manufacture hemostasis clip not only because it has excellent biocompatibility, but also because it does not exhibit artifacts in CT testing. Zhang's group from Suzhou Origin Medical Technology Co., Ltd [14]. has focused on this medical device for many years. And the HP-Mg clip designed by them has become one of the only three rewarded Mg-based innovative medical device by CFDA in 2018 [51]. They have finished 15 cases pre-clinical experiment of these clips which showed good performance. Now they are doing the official clinical trials.
Bai et al. [52] also designed an Mg–Zn–Ca alloy clip and found that this clip can maintain closure performance for 2 weeks in vitro immersion tests while in vivo tests successfully occlude the blood vessels. Fig. 5 shows the surgical procedure in which the Mg–3Zn-0.2Ca alloy clip is used for the closure of mouse renal vessels.
Fig. 5.
Representative pictures depicting closure of the renal vessel using Mg–3Zn-0.2Ca clips. A) renal veins, B) left renal vessel before cutting, C) occlusion of clip, and D) the occluded renal vessel [52].
3.2. Surgical staple
Various kinds of metallic wires are used in current surgeries. Such as K-wires, cerclage wires, tension-band wires, orthodontic archwires, ligature wires, staples and sutures [53]. Across the world, the earliest experimentally employed Mg wires were used as sutures to anchor nerves and muscle. During the first attempt, the lack of ductility limited the use of Mg wires used as sutures [54]. With the development of new biodegradable Mg alloy implants these years in China, the potential applications of biomedical Mg and its alloy fine wires are realized and explored gradually. In this section, only the Mg wires used as a surgical staple are considered.
Bai et al. [55] designed three kinds of Mg alloy fine wires, Mg-4Gd-0.4Zn, Mg-4Nd-0.4Zn, and Mg–4Y-0.4Zn, which aim to use as biomedical materials. The results indicated that Mg-4Gd-0.4Zn and Mg-4Nd-0.4Zn fine wires have similar good corrosion resistance and the uniform corrosion behavior in the SBF solution. While Mg–4Y-0.4Zn fine wires showed a poor corrosion resistance and the pitting corrosion behavior. Both Wu et al. and Qu et al. [56,57] reported that they closed the stomach of pigs successfully with the HP-Mg staples (Fig. 6). HP-Mg staples also showed good biocompatibility and limit inflammatory. These staples kept good closure to the anastomosis, no leaking and bleeding were found and exhibited no fracture or severe corrosion cracks during the degradation. Wang et al. [58] got the same conclusion with Mg–6Zn staples. Furthermore, Xia et al. [59] investigated the mechanism of HP-Mg inhibit the inflammatory response in rectal anastomoses.
Fig. 6.
Representative pictures depicting the operation process of small intestine anastomosis using HP-Mg staples. A) Liftand cut the small intestine. B) Anastomose small intestine using a side-to-side anastomosis device. C) Small intestine after end anastomosis. D) HP-Mg staples in the anastomosis. E) Closing the small intestine section. F) No bleeding and intestinal fluid exudation after intestinal anastomosis [57].
4. Intervention implants
4.1. Vascular stent
Coronary diseases remain one of the leading causes of human death in the world. Endovascular stents are the most important implantations in cardiovascular intervention. In order to reduce the long-term side effects of permanent metallic stents, such as subacute thrombosis and in-stent restenosis, a new generation of endovascular stents so-called “biodegradable metal stents” is currently being vigorously developed and considered as the most promising candidate. Biodegradable Mg-based stents (BMSs) as the most important one have aroused a lot of attention by researchers [60]. While Mg alloys are prone to fracture because of their high brittleness and poor plastic deformation. The processing of micro-tubes is one of the important factors for stent application. So, it is necessary to improve their mechanical properties before widely used [61,62]. In China, Yue et al. [63] produced an Mg alloy contained Zinc and Yttrium as stents material by cold- and warm-extrusion technologies. Simulation analysis of extension and compression showed that the strength of this alloy has reached the level of 304L and 316L stainless steel stents. Li et al. [64] tried to use Mg alloy stents as a treatment for vein graft restenosis.
Moreover, another limitation of biodegradable Mg alloy stent is its low corrosion resistance. Table 2 lists the degradation behavior of BMSs in vivo. An implanted stent is demanded to last for 6–12 months for vessel healing. Low corrosion resistance causes the BMSs to degrade rapidly. Alloying and coating are two common methods to improve the corrosion resistance of BMSs. Dong et al. [65] designed a drug delivery system (Mg/MgO/PLA-FA) which can sustainable release of ferulaic acid via anodic oxidation process and dip-coating process. And it showed superior anticorrosion behavior due to MgO-PLA composite layer. Zhang et al. [66] also investigated the long term in vivo degradation of their patented alloy JDBM in the rabbit common carotid artery for 20 months. The results showed that JDBM stents had mostly degraded after 4 months implantation and the original strut position was mostly replaced by the degradation products. While after 20 months of implantation, the degradation products tended to break into particles.
Table 2.
Degradation behavior of BMSs in vivo.
| Material | Animal model | Degradation in vivo | Ref. |
|---|---|---|---|
| Mg/PTMC coating | Subcutaneous of rats (52 W) | The homogeneous surface erosion of the PTMC coating from exterior to interior (surface-eroding behavior) and its charge neutral degradation products contribute to its excellent protective performance. | [67] |
| JDBM/MgF2 coating | abdominal aorta of rabbits (28 D) | Stents show excellent tissue compatibility of the well re-endothelialized stent with no sign of thrombogenesis and restenosis in the stent-supported vessel. | [68] |
| JDBM | common carotid artery of rabbits (20 M) | After 4 months implantation, stent has mostly degraded. | [66,69,70] |
| abdominal aorta of rabbits (16 W) | Stents well apposed to the vessel wall, with no sign of early recoil or fracture | ||
| WE43 | |||
| AZ31 | |||
| Common bile duct of rabbits (6 M) | About 60% volume loss after 3-month-implantation andno residue found afer 6-month-implantation. | [71] | |
| AZ31/silicone membrane | Oesophagus of rabbits | It provide good support for at least 2 weeks. | [72] |
| AZ91 | Coronary artery and femoral artery of dogs (28 D) | Stents were completely disappeared at 7 days after implantation. | [63] |
| Mg–6Zn | Rabbits | The in vivo corrosion rate of Mg–6Zn was ~0.107 mm year−1, which was much lower than that calculated in vitro. | [73] |
4.2. Other stents
4.2.1. Intestinal stents
Intestinal strictures are a common complication of enteral diseases, including benign strictures and malignant. Metal stents are useful for treatment of both malignant and benign strictures or fistulas throughout the intestinal tract [74]. And biodegradable Mg alloys seem to be suitable materials. However, the great challenges limit Mg alloys used as intestinal stents are probably ductility and poor workability at room temperature, which leads to difficult processing Mg wires for a small diameter to knit intestinal stent.
Chen et al. [73] evaluated the in vitro and in vivo corrosion rates of Mg–6Zn in the common bile duct (Fig. 7). The results showed that stents nearly degraded completely after 3 weeks. And in vivo corrosion rate of Mg–6Zn alloy was much lower than that calculated in vitro. And they [75] also came with the conclusion that degradation of Mg–6Zn alloy stents did not influence the healing of the bile duct in vivo. It revealed benign biosafety of Mg–6Zn alloy as a biliary stent. AZ31 was also studied by implanting into a common bile duct of rabbits for 6 months. It revealed the existence of about 30% of the original volume after 3 months [71].
Fig. 7.
Process of implant stent into rabbit common bile duct (CBD): (A) Found and ligatured CBD; (B) a 15 mm longitudinal incision was cut on the duodenum away from duodenal papilla 5 mm; (C) assembled stent introducer system and prepared to insert stent; (D) a stent introducer system with a Mg–6Zn stent was put into CBD; (E) the guide wire and plastic jacket tube were gradually withdrawn; and (F) sutured stent with CBD inner wall [73].
4.2.2. Esophageal stent
Benign stricture of the oesophagus can severely reduce the quality of life and cause major complications such as aspiration, weight loss, and malnutrition. Some researchers pay their attention to the study of Mg and its alloys as temporary esophageal stents. Zeng et al. [76] analyzed degradation production of pure Mg immersed in artificial saliva for a different time. Zhu et al. [75] investigated the feasibility of Mg-silicone stent by inserting into the oesophagus of rabbits. The Mg stent showed the same level radial force of Ni–Ti metal stent, which means Mg stent could provide enough support against lesion compression when used in vivo. And Mg stent verified that it provided good support for at least 2 weeks before biodegradation in subsequent animal experiments.
5. Summary
As novel biomaterials, Mg and its alloys have been recognized as one of the most attractive biodegradable metals for research and application in the clinic. They were also found to present multi bio-functional properties, including promoting bone formation [77], anti-inflammatory [59,78] and anti-cancer [79,80] properties, which in further enlarges the research and development fields of Mg and its alloys for biomedical applications. Though Mg and its alloys have so many merits, medical transformation is still having a long way to go. The Mg-based bone plates, for example, bone plate and screws made of the LAE442 alloy were shown to severely corrode in the bone plate/screws contact area, which is attributed to the localized corrosion induced by the compressive stress between them [81]. In China, Wu et al. [82] observed firstly the crevice corrosion morphology of the screw head without contact with the plate was different from the contact regions in the screw between the screw and the plate on the bone of rat. Based on this, they designed experiments and came up with a new corrosion mechanism of Mg inside the crevice. These found no doubt enhance the difficulty to make the medical transformation. Besides, there exist other challenges during transformation, including hard to simulate the complex environment in the body, insufficient support use in a long bone fracture as well as other critical load-bearing sites an so on. However, fortunately, many researchers have noticed these problems and trying to solve them in China [83,84]. As medical transformation is a long way, it demands us to have effective collaboration among related fields, such as material science, engineering, biology, and medicine. We are certainly sure that biomedical Mg devices will have a bright future.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (No.2018YFC1106600, No.2016YFC1102400), the Natural Science Foundation of China (NSFC No.51571142).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2019.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huse E.C. A new ligature. Chicago Med J Exam. 1878;37:117–172. [PMC free article] [PubMed] [Google Scholar]
- 2.Peeters P., Bosiers M., Verbist J., Deloose K., Heublein B. Preliminary results after application of absorbable metal stents patients with critical limb ischemia. J. Endovasc. Ther. 2005;12:1–5. doi: 10.1583/04-1349R.1. [DOI] [PubMed] [Google Scholar]
- 3.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U., Schavan R., Stukenborg-Colsman C., Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J., Han H., Han K., Park J., Jeon H., Ok M., Seok H., Ahn J., Lee K.E., Lee D., Yang S., Cho S., Cha P., Kwon H., Nam T., Han J.H.L., Rho H., Lee K., Kim Y., Mantovani D. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. P. Natl. Acad. Sci. USA. 2016;113:716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan J., Dong J., Chen J., Yang K. Development of magnesium alloys for biomedical applications: structure, process to property relationship. Mater. Technol. 2018;3:235–243. [Google Scholar]
- 6.Ding Y., Wen C., Hodgson P., Li Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: a review. J. Mater. Chem. B. 2014;2:1912–1933. doi: 10.1039/c3tb21746a. [DOI] [PubMed] [Google Scholar]
- 7.G. Yuan, X. Zhang, W. Ding. High-toughness Corrosion-Resistant Magnesium Alloy Implanted Material Capable of Being Degraded in Organism: China, CN201010204719.9. 2010-09-22.
- 8.G. Yuan, Z. Li, J. Niu, X. Zhang, W. Ding. Method for Preparing Bioactive Calcium Phosphate Coating on Magnesium Alloy Surface for Endosseous Implant: China, CN201110086203.3. 2011-04-07.
- 9.Guan X., Xiong M., Zeng F., Xu B., Yang L., Guo H., Niu J., Zhang J., Chen C., Pei J., Huang H., Yuan G. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg–Nd–Zn–Zr alloy for mandibular bone repair. ACS Appl. Matter. Inter. 2014;6:21525–21533. doi: 10.1021/am506543a. [DOI] [PubMed] [Google Scholar]
- 10.Niu J., Xiong M., Guan X., Zhang J., Huang H., Pei J., Yuan G. The in vivo degradation and bone-implant interface of Mg - Nd - Zn - Zr alloy screws: 18 months post-operation results. Corros. Sci. 2016;113:183–187. [Google Scholar]
- 11.Kong X., Wang L., Li G., Qu X., Niu J., Tang T., Dai K., Yuan G., Hao Y. Mg-based bone implants show promising osteoinductivity and controllable degradation: a long-term study in a goat femoral condyle fracture model. Mater. Sci. Eng. C. 2018;86:42–47. doi: 10.1016/j.msec.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Naujokat H., Fuff C.B., Kluter T., Seitz J.-M., Acil J., Wiltfang J. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg. 2019 doi: 10.1016/j.ijom.2019.03.966. [DOI] [PubMed] [Google Scholar]
- 13.Daentzer D., Willbold E., Kalla K., Bartsch I., Masalha W., Hallbaum M., Hurschler C., Kauth T., Kaltbeitzel D., Hopmann C., Welke B. Bioabsorbable interbody magnesium-polymer cage. Spine. 2014;39:E1220–E1227. doi: 10.1097/BRS.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 14.J. Liu, S. Zhang, Y. Zhang, M. Jiang, H. Xu, C. Zhao, X. Zhang. Metal Vascular Clamp Capable of Being Degraded and Absorbed Directionally and Manufacturing Method: China, CN201410156536.2. 2014-07-02.
- 15.Könneker S., Krockenberger K., Pieh C., von Falck C., Brandewiede B., Vogt P.M., Kirschner M.H., Ziegler A. Comparison of SCAphoid fracture osteosynthesis by MAGnesium-based headless Herbert screws with titanium Herbert screws: protocol for the randomized controlled SCAMAG clinical trial. BMC Muscoskelet. Disord. 2019;20 doi: 10.1186/s12891-019-2723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krämer M., Schilling M., Eifler R., Hering B., Reifenrath J., Besdo S., Windhagen H., Willbold E., Weizbauer A. Corrosion behavior, biocompatibility and biomechanical stability of a prototype magnesium-based biodegradable intramedullary nailing system. Mater. Sci. Eng. C. 2016;59:129–135. doi: 10.1016/j.msec.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Farraro K.F., Sasaki N., Woo S.L., Kim K.E., Tei M.M., Speziali A., McMahon P.J. Magnesium ring device to restore function of a transected anterior cruciate ligament in the goat stifle joint. J. Orthop. Res. 2016;34:2001–2008. doi: 10.1002/jor.23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.S. Zhang, X. Zhang, J. Liu, Y. Zhang, H. Xu, C. Zhao, J. Ni, X. Wang, M. Jiang. Directionally Degradable and Absorbable Magnesium Bone Screw and Preparation Method: China, CN201510496092.1. 2015-11-18.
- 19.Witte F., Kaese V., Haferkamp H., Switzer E., Meyer-Lindenberg A., Wirth C., Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Henderson S.E., Verdelis K., Maiti S., Pal S., Chung W.L., Chou D., Kumta P.N., Almarza A.J. Magnesium alloys as a biomaterials for degradable craniofacial screws. Acta Biomater. 2014;10:2323–2332. doi: 10.1016/j.actbio.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaya A., Yoshizawa S., Verdelis K., Myers N., Costello B.J., Chou D., Pal S., Maiti S., Kumta P.N., Sfeir C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015;18:262–269. doi: 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Kraus T., Fischerauer S.F., Hänzi A.C., Uggowitzer P.J., Löffler J.F., Weinberg A.M. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater. 2012;8:1230–1238. doi: 10.1016/j.actbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Lindtner R.A., Castellani C., Tangl S., Zanoni G., Hausbrandt P., Tschegg E.K., Stanzl-Tschegg S.E., Weinberg A. Comparative biomechanical and radiological characterization of osseointegration of a biodegradable magnesium alloy pin and a copolymeric control for osteosynthesis. J. Mech. Behav, Biomed. 2013;28:232–243. doi: 10.1016/j.jmbbm.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Witte F., Ulrich H., Rudert M., Willbold E. Biodegradable magnesium scaffolds: Part 1: appropriate inflammatory response. J. Biomed. Mater. Res. A. 2007;81:748–756. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 25.Witte F., Ulrich H., Palm C., Willbold E. Biodegradable magnesium scaffolds: Part II: peri-implant bone remodeling. J. Biomed. Mater. Res. A. 2007;81:757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 26.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Cui H., Li F., Guo Y., Zhao Y., Yan R., Wang W., Li Y., Wang Y., Yuan G. Intermediate analysis of magnesium alloy covered stent for a lateral aneurysm model in the rabbit common carotid artery. Eur. Radiol. 2017;27:3694–3702. doi: 10.1007/s00330-016-4715-6. [DOI] [PubMed] [Google Scholar]
- 28.Cheng P., Zhao C., Han P., Ni J., Zhang S., Zhang X., Chai Y. Site-dependent osseointegration of biodegradable high-purity magnesium for orthopedic implants in femoral shaft and femoral condyle of New Zealand rabbits. J. Mater. Sci. Technol. 2016;32:883–888. [Google Scholar]
- 29.Tan L., Wang Q., Lin X., Wan P., Zhang G., Zhang Q., Yang K. Loss of mechanical properties in vivo and bone–implant interface strength of AZ31B magnesium alloy screws with Si-containing coating. Acta Biomater. 2014;10:2333–2340. doi: 10.1016/j.actbio.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Han P., Cheng P., Zhang S., Zhao C., Ni J., Zhang Y., Zhong W., Hou P., Zhang X., Zheng Y., Chai Y. In vitro and in vivo studies on the degradation of high-purity Mg (99.99 wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials. 2015;64:57–69. doi: 10.1016/j.biomaterials.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Wu Y., Li H., Liu Y., Bai X., Chau W., Zheng Y., Qin L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials. 2018;157:86–97. doi: 10.1016/j.biomaterials.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Cheng P., Han P., Zhao C., Zhang S., Zhang X., Chai Y. Magnesium inference screw supports early graft incorporation with inhibition of graft degradation in anterior cruciate ligament reconstruction. Sci. Rep. 2016;6 doi: 10.1038/srep26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J., Hou P., Zhang Y., Liu J., Xu H., Liu S., Zhang X., Zheng Y., Chai Y. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Xu J., Fu W., Cheng W., Chan K., Yung P.S., Qin L. Biodegradable magnesium screws accelerate fibrous tissue mineralization at the tendon-bone insertion in anterior cruciate ligament reconstruction model of rabbit. Sci. Rep. 2017;7 doi: 10.1038/srep40369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Xu J., Song B., Chow D.H., Shu-hang Yung P., Qin L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Zhao D., Huang S., Lu F., Wang B., Yang L., Qin L., Yang K., Li Y., Li W., Wang W., Tian S., Zhang X., Gao W., Wang Z., Zhang Y., Xie X., Wang J., Li J. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 37.CFDA http://www.cmde.org.cn/CL0004/3139.html
- 38.NetEase News http://quotes.money.163.com/f10/ggmx_300328_5489654.html
- 39.Yu X., Zhao D., Huang S., Wang B., Zhang X., Wang W., Wei X. Biodegradable magnesium screws and vascularized iliac grafting for displaced femoral neck fracture in young adults. BMC Muscoskelet. Disord. 2015;16 doi: 10.1186/s12891-015-0790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin L., Yao D., Zheng L., Liu W., Liu Z., Lei M., Huang L., Xie X., Wang X., Chen Y., Yao X., Peng J., Gong H., Griffith J.F., Huang Y., Zheng Y., Feng J.Q., Liu Y., Chen S., Xiao D., Wang D., Xiong J., Pei D., Zhang P., Pan X., Wang X., Lee K., Cheng C. Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials. 2015;59:125–143. doi: 10.1016/j.biomaterials.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Y., Cao H., Wang X., Chen S., Zhang M., Wang N., Yao Z., Dai Y., Xie X., Zhang P., Yao X., Qin L. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Lai Y., Li Y., Cao H., Long J., Wang X., Li L., Li C., Jia Q., Teng B., Tang T., Peng J., Eglin D., Alini M., Grijpma D.W., Richards G., Qin L. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 43.CFDA https://www.cmde.org.cn/CL0050/7586.html
- 44.Mao G., Gong H., Wang Y., Li X., Lv R., Sun J., Bian W. Special biodegradable fixation device for anterior cruciate ligament reconstruction–safety and efficacy in a beagle model. ACS Biomater. Sci. Eng. 2018;4:3600–3609. doi: 10.1021/acsbiomaterials.8b00426. [DOI] [PubMed] [Google Scholar]
- 45.Tian L., Sheng Y., Huang L., Chow D.H., Chau W.H., Tang N., Ngai T., Wu C., Lu J., Qin L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. doi: 10.1016/j.biomaterials.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Hou P., Han P., Zhao C., Wu H., Ni J., Zhang S., Liu J., Zhang Y., Xu H., Cheng P., Liu S., Zheng Y., Zhang X., Chai Y. Accelerating corrosion of pure magnesium co-implanted with titanium in vivo. Sci. Rep. 2017;7 doi: 10.1038/srep41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.G. Yuan, Z. Ruan, H. Shi. Biodegradable Magnesium Alloy Claw Type Rib Coaptation Board: China, CN201520145649.2. 2015-03-13.
- 48.J. Niu, J. Zhang, G. Yuan, W. Ding, D. Dong, Q. Shen. Magnesium Alloy Sternum Plate: China, CN201810522821.X. 2018-11-06.
- 49.D. Zhao, B. Wang, H. Xie, B. Liu, Z. Ma, W. Wang. A T-Type/inclined T-type Degradable Pure Magnesium Distal Radius Bone Plate: China, CN201710906845.0. 2017-12-12.
- 50.D. Zhao, B. Wang, H. Xie, B. Liu, Z. Ma, W. Wang. Degradable Pure Magnesium Metal Bone Fracture Plate for Limb Long Bone Fracture Fixation: China, CN201710908933.4. 2017-12-22.
- 51.CFDA https://www.cmde.org.cn/CL0050/6979.html
- 52.Bai H., He X., Ding P., Liu D., Chen M. Fabrication, microstructure, and properties of a biodegradable Mg‐Zn‐Ca clip. J. Biomed. Mater. Res. B. 2019;107:1741–1749. doi: 10.1002/jbm.b.34267. [DOI] [PubMed] [Google Scholar]
- 53.Asgari M., Hang R., Wang C., Yu Z., Li Z., Xiao Y. Biodegradable metallic wires in dental and orthopedic applications: a review. Met. 2018;8:212. [Google Scholar]
- 54.Seelig M.G., M D A study of magnesium wire as an absorbable suture and ligature material. Surg. Arch. 1924;2:669–680. [Google Scholar]
- 55.Bai J., Yin L., Lu Y., Gan Y., Xue F., Chu C., Yan J., Yan K., Wan X., Tang Z. Preparation, microstructure and degradation performance of biomedical magnesium alloy fine wires. Prog. Nat. Sci. 2014;24:523–530. [Google Scholar]
- 56.Wu H., Zhao C., Ni J., Zhang S., Liu J., Yan J., Chen Y., Zhang X. Research of a novel biodegradable surgical staple made of high purity magnesium. Bioactive Materials. 2016;1:122–126. doi: 10.1016/j.bioactmat.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu S., Xia J., Yan J., Wu H., Wang H., Yi Y., Zhang X., Zhang S., Zhao C., Chen Y. In vivo and in vitro assessment of the biocompatibility and degradation of high-purity Mg anastomotic staples. J. Biomater. Appl. 2017;31:1203–1214. doi: 10.1177/0885328217692948. [DOI] [PubMed] [Google Scholar]
- 58.Wang X., Ni J., Cao N., Yu S., Chen Y., Zhang S., Gu B., Yan J. In vivo evaluation of Mg–6Zn and titanium alloys on collagen metabolism in the healing of intestinal anastomosis. Sci. Rep. 2017;7 doi: 10.1038/srep44919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia J., Chen H., Yan J., Wu H., Wang H., Guo J., Zhang X., Zhang S., Zhao C., Chen Y. High-Purity magnesium staples suppress inflammatory response in rectal anastomoses. ACS Appl. Mater. Interfaces. 2017;9:9506–9515. doi: 10.1021/acsami.7b00813. [DOI] [PubMed] [Google Scholar]
- 60.Mao L., Yuan G., Wang S., Niu J., Wu G., Ding W. A novel biodegradable Mg–Nd–Zn–Zr alloy with uniform corrosion behavior in artificial plasma. Mater. Lett. 2012;88:1–4. [Google Scholar]
- 61.Wu Q., Zhu S., Wang L., Liu Q., Yue G., Wang J., Guan S. The microstructure and properties of cyclic extrusion compression treated Mg–Zn–Y–Nd alloy for vascular stent application. J. Mech. Behav. Biomed. 2012;8:1–7. doi: 10.1016/j.jmbbm.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Wang J., Zhou Y., Yang Z., Zhu S., Wang L., Guan S. Processing and properties of magnesium alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C. 2018;90:504–513. doi: 10.1016/j.msec.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Yue Y., Wang L., Yang N., Huang J., Lei L., Ye H., Ren L., Yang S. Effectiveness of biodegradable magnesium alloy stents in coronary artery and femoral artery. J. Interv. Cardiol. 2015;28:358–364. doi: 10.1111/joic.12217. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Wang L., Chen S., Yu D., Sun W., Xin S. Biodegradable magnesium alloy stents as a treatment for vein graft restenosis. Yonsei Med. J. 2019;60:429. doi: 10.3349/ymj.2019.60.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong H., Li D., Mao D., Bai N., Chen Y., Li Q. Enhanced performance of magnesium alloy for drug-eluting vascular scaffold application. Appl. Surf. Sci. 2018;435:320–328. [Google Scholar]
- 66.Zhang J., Li H., Wang W., Huang H., Pei J., Qu H., Yuan G., Li Y. The degradation and transport mechanism of a Mg - Nd - Zn - Zr stent in rabbit common carotid artery: a 20-month study. Acta Biomater. 2018;69:372–384. doi: 10.1016/j.actbio.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Wang J., He Y., Maitz M.F., Collins B., Xiong K., Guo L., Yun Y., Wan G., Huang N. A surface-eroding poly(1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: toward better biofunction, biodegradation and biocompatibility. Acta Biomater. 2013;9:8678–8689. doi: 10.1016/j.actbio.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 68.Mao L., Shen L., Chen J., Wu Y., Kwak M., Lu Y., Xue Q., Pei J., Zhang L., Yuan G., Fan R., Ge J., Ding W. Enhanced bioactivity of Mg–Nd–Zn–Zr alloy achieved with nanoscale MgF2 surface for vascular stent application. ACS Appl. Mater. Interfaces. 2015;7:5320–5330. doi: 10.1021/am5086885. [DOI] [PubMed] [Google Scholar]
- 69.Liu F., Chen C., Niu J., Pei J., Zhang H., Huang H., Yuan G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C. 2015;48:400–407. doi: 10.1016/j.msec.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Mao L., Shen L., Niu J., Zhang J., Ding W., Wu Y., Fan R., Yuan G. Nanophasic biodegradation enhances the durability and biocompatibility of magnesium alloys for the next-generation vascular stents. Nanosc. 2013;5:9517–9522. doi: 10.1039/c3nr02912c. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y., Zheng S., Li N., Guo H., Zheng Y., Peng J. In vivo response of AZ31 alloy as biliary stents: a 6 months evaluation in rabbits. Sci. Rep. 2017;7 doi: 10.1038/srep40184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Y., Edmonds L., Wei L., Zheng R., Cheng R., Cui W., Cheng Y. Technical feasibility and tissue reaction after silicone-covered biodegradable magnesium stent insertion in the oesophagus: a primary study in vitro and in vivo. Eur. Radiol. 2017;27:2546–2553. doi: 10.1007/s00330-016-4602-1. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y., Yan J., Wang Z., Yu S., Wang X., Yuan Z., Zhang X., Zhao C., Zheng Q. In vitro and in vivo corrosion measurements of Mg–6Zn alloys in the bile. Mater. Sci. Eng. C. 2014;42:116–123. doi: 10.1016/j.msec.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z., Li N., Li R., Li Y., Ruan L. Biodegradable intestinal stents: a review. Prog. Nat. Sci. 2014;24:423–432. [Google Scholar]
- 75.Zhu Y., Yang K., Cheng R., Xiang Y., Yuan T., Cheng Y., Sarmento B., Cui W. The current status of biodegradable stent to treat benign luminal disease. Mater. Today. 2017;20:516–529. [Google Scholar]
- 76.Zeng R., Li X., Liu L., Li S., Zhang F. In vitro degradation of pure Mg for esophageal stent in artificial saliva. J. Mater. Sci. Technol. 2016;32:437–444. [Google Scholar]
- 77.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J.Q., Chow D.H.K., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H.C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22:1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan W., Lin Y., Shih P., Bow Y., Cui Q., Chang Y., Chia W., Sung H. An in situ depot for continuous evolution of gaseous H2 mediated by a magnesium passivation/activation cycle for treating osteoarthritis. Angew. Chem. Int. Ed. 2018;57:9875–9879. doi: 10.1002/anie.201806159. [DOI] [PubMed] [Google Scholar]
- 79.Ma N., Chen Y., Yang B. Magnesium metal - a potential biomaterial with antibone cancer properties. J. Biomed. Mater. Res. A. 2014;102:2644–2651. doi: 10.1002/jbm.a.34933. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y., Xiao M., Zhao H., Yang B. On the antitumor properties of biomedical magnesium metal. J. Mater. Chem. B. 2015;3:849–858. doi: 10.1039/c4tb01421a. [DOI] [PubMed] [Google Scholar]
- 81.Denkena B., Köhler J., Stieghorst J., Turger A., Seitz J., Fau D.R., Wolters L., Angrisani N., Reifenrath J., Helmecke P. Influence of stress on the degradation behavior of Mg LAE442 implant systems. Procedia CIRP. 2013;5:189–195. [Google Scholar]
- 82.Wu H., Zhang C., Lou T., Chen B., Yi R., Wang W., Zhang R., Zuo M., Xu H., Han P., Zhang S., Ni J., Zhang X. Crevice corrosion - a newly observed mechanism of degradation in biomedical magnesium. Acta Biomater. 2019;98:152–159. doi: 10.1016/j.actbio.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Yang J., Koons G.L., Cheng G., Zhao L., Mikos A.G., Cui F. A review on the exploitation of biodegradable magnesium-based composites for medical applications. Biomed. Mater. 2018;13:22001. doi: 10.1088/1748-605X/aa8fa0. [DOI] [PubMed] [Google Scholar]
- 84.Yang J., Cui F., Lee I.S. Surface modifications of magnesium alloys for biomedical applications. Ann. Biomed. Eng. 2011;7:1857–1871. doi: 10.1007/s10439-011-0300-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.