Highlights
-
•
ALE meta-analysis revealed functional activation differences in mTBI.
-
•
Reduced activation identified within the right middle frontal gyrus.
-
•
Suggests alteration of prefrontal region, associated with executive functioning.
-
•
Need for addressing subject- and task-specific variation in future studies.
Keywords: Concussion, Mild traumatic brain injury, Cognition, Functional MRI, Neuroimaging, Activation likelihood estimation
Abstract
Task-based functional magnetic resonance imaging (fMRI) has been used to examine neuroanatomical and functional changes following mild traumatic brain injury (mTBI). Prior studies have lacked consistency in identifying common regions of altered neural activity during cognitive tasks. This may be partly due to differences in task paradigm, patient heterogeneity, and methods of fMRI analysis. We conducted a meta-analysis using an activation likelihood estimation (ALE) method to identify regions of differential brain activation in patients with mTBI compared to healthy controls. We included experiments that performed scans from acute to subacute time points post-injury. The seven included studies recruited a total sample of 174 patients with mTBIs and 139 control participants. The results of our coordinate based meta-analysis revealed a single cluster of reduced activation within the right middle frontal gyrus (MFG) that differentiated mTBI from healthy controls. We conclude that the cognitive impairments in memory and attention typically reported in mTBI patients may be associated with a deficit in the right MFG, which impacts the recruitment of neural networks important for attentional control.
1. Introduction
Over the last two decades, task-based functional magnetic resonance imaging (fMRI) has been employed to investigate functional neuroanatomical networks that relate to cognition following mild traumatic brain injury (mTBI; Mayer et al., 2015). Measurement of blood oxygenation level dependent (BOLD) signal changes in mTBI during cognitive performance can reveal greater increases (i.e. hyperactivation) and decreases (i.e. hypoactivation) in neural activation across a range of brain regions when compared to a healthy control group. A reduction in neural activity compared to controls has been suggested as a disruption of functional efficiency leading to poorer task performance (Chen et al., 2004; Gosselin et al., 2011), whereas increased activation has been theorized as compensatory or due to the recruitment of additional resources to facilitate behavioral responses to cognitive demands (Scheibel et al., 2009; Turner et al., 2011).
The earliest example of fMRI research into mTBI reported greater activation in the frontal-temporal and lateral parietal regions, despite similar behavioral performance to controls, during a task of working memory (McAllister et al., 1999). More recent studies investigating the differences in brain activity between healthy controls and patients with mTBI have identified changes in several areas of the brain that serve higher-order cognitive processes, including the dorsolateral and ventrolateral prefrontal cortices (DLPFC and VLPFC), anterior cingulate cortex (ACC), supramarginal gyrus (SMG), and posterior areas such as the thalamus and cerebellum (Chen et al., 2012; Dettwiler et al., 2014; Hammeke et al., 2013; Johnson et al., 2015; Mayer et al., 2009; Slobounov et al., 2010; Witt et al., 2010; Yang et al., 2012). Conversely, other studies have shown decreased activation (i.e. hypoactivation) when tasks examining executive functions were less challenging (McAllister et al., 2001) and in pediatric samples (Hammeke et al., 2013; Keightley et al., 2014). Furthermore, some studies have observed no BOLD differences between groups (Elbin et al., 2012; Stulemeijer et al., 2010; Terry et al., 2012), highlighting issues of inter-subject variability, timing in post-injury assessment, and differences in the method of fMRI analysis (Mayer et al., 2015). Thus, the use of a quantitative meta-analysis to address experiment-specific variation and identify common mTBI-related neural activation differences across cognitive tasks is warranted.
The Activation Likelihood Estimation (ALE) meta-analysis method (Eickhoff et al., 2009) is a quantitative voxel-wise meta-analysis technique that compares the results of neuroimaging studies using reported coordinates in a standardized 3D atlas space in order to identify consistently activated neuroanatomical regions. In a meta-analysis using this methodology, Eierud et al. (2014) identified several regions of greater and lesser activation in mTBI compared with healthy controls. The pattern of brain activation suggested reduced activity in anterior regions (e.g. bilateral DLPFC and ACC) and increased activity in posterior regions (e.g. cerebellum, insula, and SMG). Additional findings from Bryer et al. (2013), which included an ALE meta-analysis on working memory experiments, suggest that the type of task and its relative cognitive difficulty may also partially explain the differential activation patterns displayed in mTBI patients.
However, a recent report of error in multiple-comparison corrections in older versions of the meta-analytic software used by the prior meta-analyses has emerged (Eickhoff et al., 2017). Correcting for multiple comparisons by controlling the (voxel-level) false discovery rate (FDR) leads to inflated positive findings (Chumbley and Friston, 2009; Eickhoff et al., 2016). Another limitation from prior meta-analyses is the inclusion of data that is derived from explicit region-of-interest (ROI) analyses. This can lead to inflated significance for the respective regions when included with experiments deriving coordinates from a whole-brain analysis (Müller et al., 2018). Furthermore, due to a small number of studies, prior meta-analyses did not have enough power to explore the effects of experimental variables (e.g. timing post-injury) and were prone to yield clusters of convergence that are almost exclusively driven by single experiments (Bryer et al., 2013; Eierud et al., 2014). Together, these factors may have resulted in a high number of spurious findings in prior meta-analyses. Therefore, the aim of the present study was to perform a fMRI meta-analysis of cognitive-related activation differences between healthy controls and patients with mTBI using the most recent ALE approach and focusing on studies that met more specific methodological inclusion criteria.
2. Methods
2.1. Data sources and search strategy
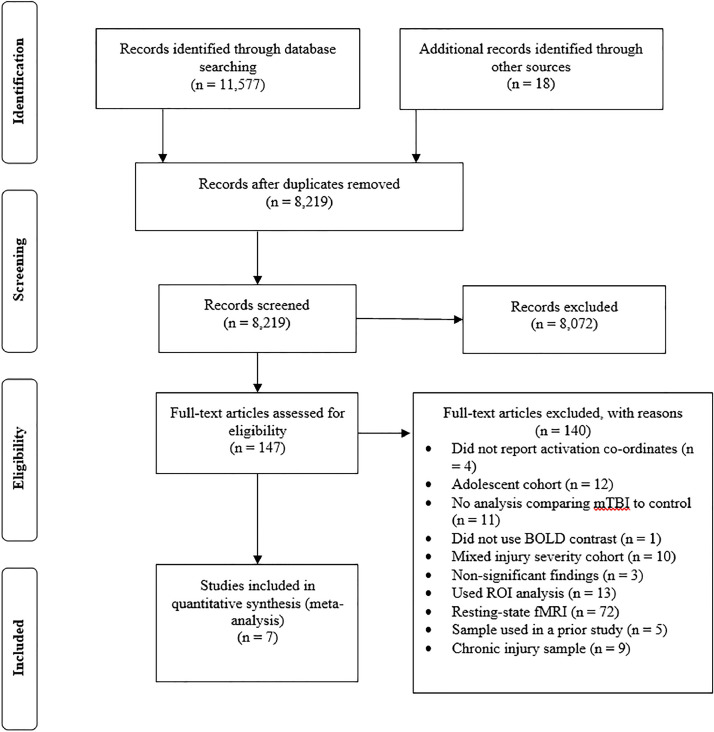
A comprehensive literature review was conducted to identify task-based fMRI studies that compared neural responses between patients with mTBI and a healthy control group (see Fig. 1 for an outline of this process). The following electronic databases were searched: Ovid (MEDLINE® Daily + Epub Ahead of Print + In-Process & Other Non-Indexed Citations, EMBASE Classic + EMBASE, PsycINFO, AMED), EBSCOhost (CINAHL Complete), Wiley Online Library (Cochrane Library), Scopus, and Informit (Health Collection). The searches were confined to full-text research manuscripts published in English and indexed from January 1990 to October 16, 2019.
Fig. 1.
Preferred reporting items of systematic reviews and meta-analyses flow diagram. BOLD, blood oxygenation level dependent; mTBI, mild traumatic brain injury; ROI, region of interest; fMRI, functional magnetic resonance imaging.
Detailed electronic search strategies and syntax were developed in collaboration with a librarian experienced in systematic reviews (see Appendix 1). The key search terms included: Glasgow coma outcome; Glasgow coma scale*; score*; unconscious*; Pneumocephalus; rancho los amigos scale; injur*; trauma*; damag*; wound*; fractur*; contusion*; haematoma*; hematoma*; haemorrhag*; hemorrhag*; pressur*; lesion*; destruction*; oedema*; edema*; contusion*; concus*; swell*; bleed*; mild*; minor; mtbi; concuss*; postconcuss*; post-concuss*; posttraum*; post-traum*; symptom*; syndrome*; complaint*; mri; neuroimag*; fmri; function*; magnetic; brain; neuro; imag*; resonance; Blood Volume; Hemoglobins; Oxygen; blood oxygen level dependent; BOLD; oxyhemoglobin; deoxyhemoglobin; memor*; cognit*; executive function*; executive; dysfunction*; Motor Skills; functionality; attention; memory; disorder*; dysfunction; impaired; impairment; difficult*; problem*; disability; organiz*; organis*; plan*; manag*; problem solving; decision making; and disorder. Additional articles were collected by hand-searching reference lists of relevant articles.
2.2. Screening and eligibility
The assessing author independently evaluated titles and abstracts of identified studies using the web-based reference management program Mendeley (Mendeley Ltd., London, UK). Studies were included if they met the eligibility criteria listed below.
-
(1)
Reported original data from a task-based fMRI (review papers excluded).
-
(2)
Included an adult sample (> 18 years) of patients diagnosed with a mTBI using criteria similar to or consistent with the World Health Organization Collaborating Centre Task Force definition (Carroll et al., 2004).
-
(3)
Included a whole brain voxel-based BOLD analysis incorporating a cognitive challenge compared with a control (less challenge or baseline) condition (excluding ROI analyses).
-
(4)
Activation coordinates reported in standardized stereotaxic space [either MNI (Collins et al., 1994) or Talairach (Talairach and Tournoux, 1988) space].
-
(5)
Included a healthy control group and presented the results of a 2nd-level (group-level) mTBI vs control contrast analysis.
We only included experiments that performed scans from acute to subacute (i.e. acute 0–7 days, subacute 8–89 days) time points post-injury (Elbin et al., 2014). There are many potential confounding factors that would need to be considered if we examined studies including people who sustained their injuries many months or years prior to imaging. If a study did not report the results of a mTBI group separately from moderate/severe TBI participants (e.g. Strangman et al., 2009), they were excluded. Results from pharmacological or psychological intervention experiments were only included if they reported either baseline between-group differences or treatment main effects involving a placebo/control condition (e.g. McAllister et al., 2011). If a study conducted an ANOVA analysis, the information equivalent to the mTBI vs. control contrast from the reported group main effects and interactions was selected (e.g. Wylie et al., 2015). Both greater and lesser (compared to controls) reported activations were included. As the ALE method attempts to identify areas of convergence across significant results, articles that did not report significant findings were unable to be included as there were no coordinates to add to the dataset (Eickhoff et al., 2012). For articles that reported results from multiple tasks using the same group (e.g. Johnson et al., 2015) or used a longitudinal design (e.g. Dettwiler et al., 2014), the first contrast of interest was selected to minimize the contribution of any one set of participants to the results from the meta-analysis. We contacted authors of studies who did not report activation coordinates in their paper or presented their results as figures for the additional information (e.g. Hsu et al., 2015). After initial screening, seven publications fulfilled the aforementioned eligibility criteria and were included in the meta-analysis (see Tables 1 and 2 for details of the studies and included contrasts).
Table 1.
Description of sample characteristics from the fMRI publications included in the meta-analysis.
| Study | mTBI | Control | Mean duration between injury and fMRI (days, SD/range) | Injury mechanism (n) | Injury classification | Symptomatic patient information | TBI history | Structural imaging pathology (CT or MRI) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N (% male) | Mean age (years, SD/range) | N (% male) | Mean age (years, SD/range) | |||||||
| Dettwiler et al. (2014) | 15 (80) | 19.8 (0.9) | 15 (80) | 19.8 (1.7) | 1st scan: 0–2 2nd scan: 2–14 3rd scan: 14–60 |
SRC (15) | 3rd ICCIS GCS: NR PTA: NR LOC: NR |
Symptoms assessed by SCAT 2. 1st scan: 15/15 symptomatic 2nd scan: 3/15 symptomatic 3rd scan: 2/15 symptomatic |
7/15 with prior mTBI | Normal |
| Johnson et al. (2015) | 9 (67) | 18–21 | 9 (67) | 20–22 | 0–7 | SRC (9) | Cantu Grade 1 GCS: NR PTA: NR No LOC |
NR | NR | NR |
| Mayer et al. (2009) | 16 (50) | 27.2 (7.6) | 15 (50) | 27.3 (7.4) | 11.9 (5.9) | Assault (1) Fall (9) MVA (5) Other (1) |
ACRM GCS: 13–15 PTA: 0–24 hrs LOC: < 30 min. |
STAI and BDI-2: emotional functioning index. NSI: cognitive and somatic complaint index. mTBI had significantly greater symptom severity scores compared with HC. |
No prior TBI | Normal |
| McAllister et al. (2011) | 26 (58) | 28.3 (11.3) | 31 (45) | 31.8 (9.7) | 39 (16.1) | NR | ACRM GCS: 14.2 (SD: 1.6) PTA: 0–24 hrs 19/26 had LOC, with mean duration of 4.8 (SD: 10.1) min. |
NR | NR | NR |
| van der Horn et al., 2016 | 52 (67) | 38 | 20 (65) | 34 | 22–69 | Assault (1) Falls (21) MVA (27) SRC (1) Other (2) |
EFNS GCS: 13–15 PTA: NR LOC: < 30 min. |
RPQ used to create two subgroups: PCC-present: 32 PCC-absent: 20 |
No prior TBI | Normal CT scans, microbleeds (≥ 2; 1–10 mm) in 40% of patients. |
| Witt et al. (2010) | 31 (67) | 33.6 (13.9) | 31 (67) | 33.2 (13.8) | 64.9 (47.7) | NR | ACRM 29/31 reported GCS 14.2 (SD: 1), others within mTBI guidelines. PTA: 0–24 hrs LOC: < 30 min. |
NR | No prior TBI | Normal |
| Wylie et al. (2015) | 25 (44) | 27.8 (11.1) | 18 (44) | 28 (9.2) | 1st scan 2 (0.9) 2nd scan: 8.7 (1.2) |
Assault (2) Fall (7) MVA (1) SRC (13) Other (2) |
Exhibiting two or more of following symptoms: any LOC, persistent headache, blurred vision, confusion, dizziness, memory problems or poor balance and GCS > 14. GCS: 15 (SD: 0) 7/25 with LOC PTA: NR |
Cognitive symptoms as assessed by ImPACT. By second scan: 8/25 complete; 10/25 minimal; and 7/25 moderate cognitive recovery. |
No prior TBI | One subject included with small area of intraparenchymal hemorrhage as shown on CT scan. |
Note: ACRM: American Congress of Rehabilitation Medicine; BDI-2: Beck Depression Inventory–Second Edition; CT: computed tomography; EFNS: European Federation of Neurological Societies; fMRI: functional magnetic resonance imaging; GCS: Glasgow Coma Scale; ImPACT: immediate post-concussion assessment and cognitive testing; ICCIS: international conference on concussion in sport; LOC: loss of consciousness; mTBI: mild traumatic brain injury; N: number; NR: not reported; NSI: Neurobehavioral Symptom Inventory; PCC: post-concussion complaints; PTA: post-traumatic amnesia; RPQ: Rivermead Post-Concussion Questionnaire; SCAT 2: Sport Concussion Assessment Tool 2; STAI: State-Trait Anxiety Inventory; SD: standard deviation.
Table 2.
Experiment and contrast specific details of the fMRI publications included in the meta-analysis.
| Study | fMRI paradigm | Domain | Experimental design: Block or event-related | Source of coordinates included in the meta- analyses | Contrast or condition | Behavioural performance between groups | Statistical significance threshold | fMRI acquisition | # Foci mTBI > Control | # Foci Control > mTBI |
|---|---|---|---|---|---|---|---|---|---|---|
| Dettwiler et al. (2014) | n-back: Three conditions (n = 1, 2, and 3) | Working memory | Block design | Table 3. Session 1. Controls vs Concussed session 1 | 2- vs. 1-back | Equivalent | AFNI, p < .005, min cluster threshold 31 voxels, p < .01 corrected | 3.0T | 11 | 0 |
| Johnson et al. (2015) | Oculomotor tasks: Saccades and smooth pursuit | Oculomotor processing and executive functioning | Block design | Table 2. Self-paced saccades | Self-paced saccade vs. reflexive saccade | Not equivalent | SPM8, p < .05 FDR corrected | 3.0T | 9 | 0 |
| Mayer et al. (2009) | Auditory orienteering task | Attention | Event-related | Table 3. | Attention task vs. visual fixation (baseline) | Equivalent | AFNI, significance threshold p < .005 uncorrected, min cluster threshold 6 contiguous voxels, p < .05 corrected | 3.0T | 0 | 6 |
| McAllister et al. (2011) | n-back: Four conditions (n = 0, 1, 2 and 3) | Working memory | Block design | Table 3. Placebo HC > mTBI | 3- vs. 0-back | Equivalent | SPM5, cluster level p < .01 uncorrected | 1.5T | 0 | 7 |
| van der Horn et al., 2016 | n-back: Three conditions (n = 0, 1, and 2) | Working memory | Block design | Results. General linear model, page 5. | 2- vs. 0-back | Equivalent | SPM12, p < .001 uncorrected, p < .05 FWE cluster corrected | 3.0T | 0 | 1 |
| Witt et al. (2010) | Auditory oddball task | Attention | Event-related | Table 3. | Target stimuli detection vs. baseline | Equivalent | SPM5, p < .05 uncorrected | 3.0T | 20 | 9 |
| Wylie et al. (2015) | n-back: Three conditions (n = 0, 1, and 2) | Working memory | Block-design | Table 7. Group x Load. | 2- vs. 0-back | Equivalent | AFNI, significance threshold p < .01, min cluster threshold 39 contiguous voxels, p < .05 corrected. | 3.0T | 1 | 0 |
Note: AFNI: analysis of functional neuroimages software package; FDR: false discovery rate; FWE: familywise error; fMRI: functional magnetic resonance imaging; HC: healthy controls; SPM: statistical parametric mapping; T: tesla; mTBI: mild traumatic brain injury.
2.3. Data Extraction and analysis
The following data was extracted from each study: first author; year of publication; demographics of mTBI and control subjects (age, number, sex); time to scan following mTBI (the mean/range time between injury and fMRI), injury specific information (mechanism, diagnostic criteria, number of symptomatic/asymptomatic patients and type of measure used, history of previous TBI, and structural imaging findings); task specific (paradigm type, cognitive domain, and if there was a significant difference in behavioral performance between groups); and ALE analysis specific information (source of stereotaxic coordinates, type and number of contrasts included, magnetic field strength (tesla) for fMRI, and whether statistical significance threshold was corrected for multiple comparisons). Independent t-tests and Mann-Whitney U tests were used for all 2-group comparisons (the latter was used when the assumptions of normality and variance heterogeneity were violated).
2.4. ALE analyses
We carried out the ALE meta-analyses using GingerALE v.2.3.6 (brainmap.org/ale). ALE assesses the spatial convergence between reported neuroimaging studies by modelling the reported foci in each study as probability distributions centered on their respective coordinates (Eickhoff et al., 2009; 2012). This version has fixed errors in both FDR and Family Wise Error (FWE) cluster-level thresholding calculation codes identified in previous iterations (Eickhoff et al., 2017). In brief, a bug in the code that incorrectly sorted P values before FDR correction found in versions prior to v.2.3.3 allowed for lenient inferences above the cut-off criterion. Additionally, the procedure for establishing the null-distribution of cluster-sizes for cluster-level FWE thresholding contained an error of including all sizes rather than recording the maximum. Prior to a fix in v2.3.2, this resulted in inference based on uncorrected cluster-level P values. We used the non-additive algorithm (Turkeltaub et al., 2012) to minimize within-experiment effects. Prior to the analyses, coordinates reported in Talairach space were converted to MNI using GingerALE's Talairach to MNI conversion tool. The resulting ALE maps were determined at a recommended cluster-level FWE rate-corrected threshold of p < .05, and a cluster-forming threshold at voxel-level p < .001 with 1000 permutations (Eickhoff et al., 2017).
Three separate analyses were conducted: (1) The first analysis (7 experiments, 64 foci, 313 subjects) pooled all comparisons between mTBI patients and healthy controls; (2) the second analysis (4 experiments, 41 foci, 153 subjects) only included contrasts that reported greater brain activity (mTBI > control) in mTBI relative to the healthy control group; and (3) the third analysis (4 experiments, 23 foci, 222 subjects) tested for areas of decreased activation in the mTBI group (mTBI < controls). For illustration, the ALE maps were displayed using MRIcron (www.mccauslandcenter.sc.edu/crnl) with anatomical labelling guided by a Colin27_T1_seg_MNI_2 × 2 × 2 template using the Mango visualization program (version 4.0.1. http://ric.uthscsa.edu/mango/).
3. Results
3.1. Description of included studies and participants
The seven included studies recruited a total sample of 174 patients with mTBIs and 139 control participants. The average age of the mTBI group was 29.1 years (Mdn = 28.1; SD = 6.2; IQR = 25–35; range = 20–38) and more than half were male (57.7%; 95% CI: 44–71%). The control group's average age was 29.0 years (Mdn = 29.9; SD = 5.3; IQR = 25–33; range = 20–34) and more than half were male (59.7%; 95% CI: 47–72%). Independent t-tests revealed no significant differences in sample size (p = .440), age (p = .977), or sex (p = .796) between groups.
Functional imaging was performed at an average of 31.1 days (range = 0–69 days) after head injury. The mechanisms of trauma in the mTBI patients included motor vehicle collisions (n = 33), falls (n = 37), assault (n = 4), sport-related concussion (n = 38), and other mechanisms not defined (n = 5). Of the five studies that incorporated Glasgow Coma Scale (GCS), post-traumatic amnesia (PTA), and loss of consciousness (LOC) into their diagnosis, three reported GCS scores (M = 14.5; SD = 0.5) and 26 individuals with mTBI were identified as having some duration of LOC. Two experiments used sport-related concussion diagnostic guidelines (Dettwiler et al., 2014; Johnson et al., 2015). Four studies included measures of somatic, emotional, and cognitive symptoms that were all greater in the mTBI group (Dettwiler et al., 2014; Mayer et al., 2009; van der Horn et al., 2016; Wylie et al., 2015). Seven individuals with a previous history of mTBI were reported in one study (Dettwiler et al., 2014). Two studies included subjects with abnormal structural imaging (van der Horn et al., 2016; Wylie et al., 2015), consisting of an intraparenchymal hemorrhage (n = 1) and microbleeds (n = 20).
Four studies used the n-back working memory paradigm, two employed attention-based tasks with auditory stimuli, and one study utilized a battery of oculomotor tasks. Block design was used in five of the studies and a 3 T magnet was used most commonly to acquire imaging data. For six out of seven studies, similar performance was reported on the cognitive tasks between the mTBI group and the control group. Only one study reported a difference in behavioral performance between groups (Johnson et al., 2015). The method of fMRI analysis was variable across the range of studies and included uncorrected (n = 2) and corrected (n = 5) thresholding.
Increased activation in the mTBI group in one or more brain areas was reported in four of seven studies, and the number of foci with increased activation was 11, 9, 20, and 1 across those studies. Decreased activation in the mTBI group in one or more brain areas was reported in four of seven studies, and the number of foci with decreased activation was 1, 6, 7, and 9 across those studies. One study reported 20 areas of increased activation and 9 areas of decreased activation (Witt et al., 2010).
3.2. ALE findings
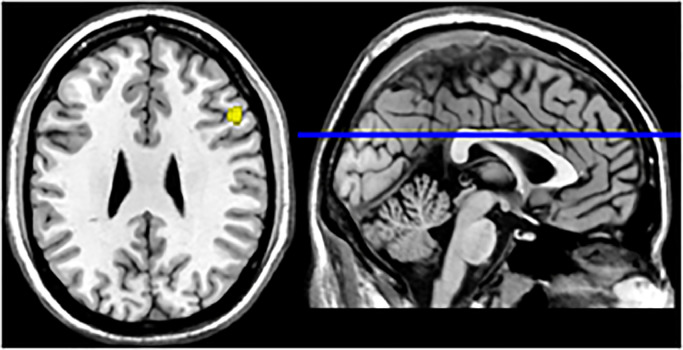
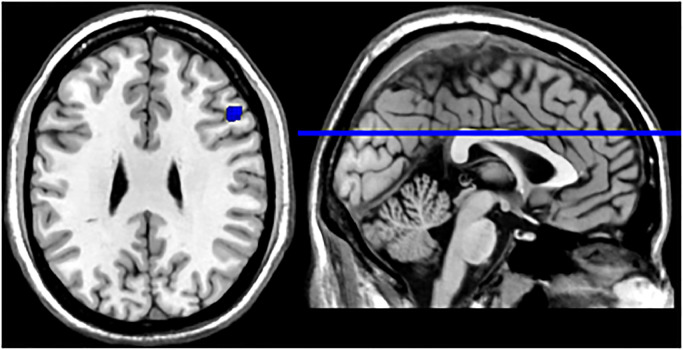
In the pooled whole-brain meta-analysis of the seven studies that reported 64 foci of activation, a single activation cluster that differentiated between patients with mTBI and healthy controls was found in the right middle frontal gyrus (MFG, BA 9; see Fig. 2 and Table 3). For the meta-analysis of the four studies reporting 41 foci of increased activation, there were no significant clusters of increased activation in patients with mTBI compared to healthy controls. For the meta-analysis of the four studies reporting 23 foci of decreased activation, the mTBI group showed reduced activation compared with controls within the right MFG (see Fig. 3 and Table 3).
Fig. 2.
Meta-analytical map of pooled activation in mTBI group. Activation was localized within the right middle frontal gyrus. Values indicate MNI-coordinates.
Table 3.
Location of differential activation clusters in the mTBI and control groups.
| Contrast | Anatomical label | BA | Cluster Size (mm3) | MNI coordinates | ALE (10−2) | Contributing experiments | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Pooled | Right middle frontal gyrus | 9 | 616 | 50 | 21 | 28 | 1.67 | Johnson et al., 2015, McAllister et al., 2011, Witt et al., 2010 |
| mTBI < control | Right middle frontal gyrus | 9 | 512 | 50 | 23 | 29 | 1.48 | McAllister et al., 2011, Witt et al., 2010 |
Note: Analyses were run with a cluster-level family wise error rate-corrected threshold of p < .05, and a cluster-forming threshold at voxel-level p < .001 with 1000 permutations. BA: Brodmann area; MNI: Montreal Neurological Institute; mTBI: mild traumatic brain injury.
Fig. 3.
Meta-analytical map of reduced activation in mTBI group. Activation was localized within the right middle frontal gyrus. Values indicate MNI-coordinates.
4. Discussion
The present meta-analysis combined peak activation coordinates from prior task-based fMRI studies to provide an overview of the neural patterns found in acute and subacute mTBI. Seven studies were included in this meta-analysis, and those studies reported inconsistent findings. Across the seven studies, 64 foci of activation, 41 areas of hyperactivation and 23 areas of hypoactivation, were identified. The result of our pooled meta-analysis revealed a single significant activation cluster in the right MFG in those with mTBI compared to controls. Secondary analyses examining the directionality of group contrasts (i.e., mTBI > controls; mTBI < controls), revealed less activation in the same cluster in the right MFG in mTBI versus controls. No significant clusters were observed when including contrasts only from experiments reporting greater activations in mTBI.
The total number of included experiments (n = 7) was slightly lower compared to previous meta-analyses. Five of the primary studies from Eierud et al. (2014) were excluded for being limited to ROI results only (Chen et al., 2007; McAllister et al., 2001; Slobounov et al., 2010), including an adolescent sample (Krivitzky et al., 2011), or being a resting-state fMRI study (Mayer et al., 2011). Similarly, seven studies from Bryer et al. (2013) were excluded for reporting on ROI results only (Chen et al., 2007, 2008; Gosselin et al., 2011; McAllister et al., 1999, 2001; Slobounov et al., 2010), or including an adolescent sample (Pardini et al., 2010).
The significant cluster activated in the right MFG during cognitive-based tasks was consistent with the results of previous meta-analyses. However, our study did not support the previous findings of significant clusters in other regions of the frontal-parietal cortex (e.g., anterior cingulate) and in more posterior areas (e.g., cerebellar tonsil). These observations could be accounted for by the differences in the studies included and/or the use of more lenient statistical thresholds used in previous meta-analyses. In the current meta-analysis, the right MFG was reported as a region of activation in five out of seven of the included studies. Examining the results from these studies, three reported a statistically significant reduction in activation in the right MFG (McAllister et al., 2011; van der Horn et al., 2016; Witt et al., 2010), and two reported statistically significant increases in activation in the right MFG (Dettwiler et al., 2014; Johnson et al., 2015). Potential sources of this variation may be explained by differences in experimental design, analytical methodology, and participant characteristics (Bramlett and Dietrich, 2015; Mayer et al., 2015; Pertab et al., 2009; Rosenbaum and Lipton, 2012).
Within the prefrontal cortex, the MFG is connected to a network of regions that are commonly co-activated during tasks requiring executive functions (Corbetta and Shulman, 2002; Duncan and Owen, 2000; Fedorenko et al., 2013). Specifically, the right MFG has been proposed to be a convergence site between the ventral attention network (VAN) and the dorsal attention network (DAN), which work in conjunction to switch between goal-directed and stimulus-driven attention by filtering out distractions and orienting attention to task-relevant information (Fox et al., 2006; Corbetta et al., 2008). This region has been suggested to be “circuit-breaker” that interrupts DAN functioning to reorient attention towards novel task-relevant stimuli (Corbetta et al., 2008; Japee et al., 2015). In the context of mTBI, previous functional neuroimaging studies have reported differential activation in the right MFG area across a variety of cognitive paradigms with the majority of tasks involving working memory (Witt et al., 2010; Dettwiler et al., 2014; van der Horn et al., 2016; Saluja et al., 2015). Taken together, altered recruitment of the right MFG after mTBI may represent an over-arching disruption in modulating attention. These findings are also consistent with resting-state fMRI studies in concussion and mTBI, which have observed altered functional connectivity in the right MFG (Meier et al., 2019) as well as other regions that mediate internally-oriented processes (Zhu et al., 2015; Militana et al., 2016; Borich et al., 2015).
As evidenced in our included experiments (see Table 2), the n-back paradigm is the most frequently used working memory task in the current mTBI literature. It is used to assess an individual's ability to hold information in mind for further processing while subject to increasing cognitive demand (Owen et al., 2005). Working memory is an area of cognition that has been found to be adversely affected by mTBI (Dean and Sterr, 2013; Kumar et al., 2013; Sinopoli et al., 2014). Further, problems with working memory also emerge during the early recovery phase rather than forming part of the initial symptomology (Meares et al., 2011). Our finding of disturbed right MFG activation in mTBI patients may therefore reflect atypical neurophysiological changes associated with cognitive impairments in memory and attention. This view is supported by functional differentiation evidence that show the right MFG as an area of focus in task demand/novelty processing (Hillary et al., 2006; Niendam et al., 2012).
A novel finding in the present work is that contrast-specific involvement of the right MFG was apparent for our analysis that incorporated hypoactivation findings and not for those that reported hyperactivation. Various authors have attempted to conceptualize the directionality of recruitment in the prefrontal region following brain injury in relation to task performance. It has been proposed that greater activation in response to a cognitively demanding task may represent a transient compensatory effect (Maruishi et al., 2007; Turner et al., 2011), permanent rewiring (Sánchez-Carrion et al., 2008a; 2008b), or the result of a native support mechanism to assist in task performance (Hillary et al., 2010; Medaglia et al., 2012). Conversely, underactivity (i.e. hypoactivation) may reflect a disruption in this network caused by pathophysiological changes which limit the capacity for focused attention and external goal directed processes (Chen et al., 2004; 2007; Gosselin et al., 2011; Keightley et al., 2014). Of note, cognitive task performance was mostly equivalent between our meta-analysis group of mTBI and healthy controls (see Table 2). In six out of seven studies, the mTBI group performed similarly to the control group on the cognitive task. One methodological constraint with imaging research is requiring near perfect accuracy on tasks to facilitate data interpretation (Hall et al., 2016). Furthermore, recruitment demand for prefrontal regions following brain injury has been shown to diminish at similar rates in both injured and healthy controls with increased task practice and familiarity (Medaglia et al., 2012). Thus, while our findings are in support of functional change in the neuroanatomical region of the MFG, the direction of change (i.e. greater or lesser activation) and the underlying cause are unclear.
From Tables 1 and 2, the description of participant characteristics highlights the scope and context of inter-subject variation in mTBI. Within our mTBI group, the number of sport-related concussions, motor vehicle accidents, and falls as injury mechanisms was relatively similar. However, biomechanical loading data reveal that primary injury strain and impact forces vary considerably depending on injury mechanism and are not uniformly distributed across grey and white matter regions of the brain (Greenwald et al., 2008; Meaney and Smith, 2011). Previous reviews into the adaptive and pathophysiological changes following mTBI (Barkhoudarian et al., 2016; Giza and Hovda, 2014) also highlight the issue of a complex and time-varied injury profile. Although we limited the scope of functional assessment post-injury to the acute-subacute phase, there is no clear consensus on how these processes affect recovery as measured by fMRI after mTBI (Kamins et al., 2017). This is evidenced in longitudinal fMRI studies which showcase varied patterns of within-subject BOLD signal changes (Dettwiler et al., 2014; Hammeke et al., 2013; Wylie et al., 2015). Finally, it is important to note the subset of subjects with complications identified on structural scans. Whereas some definitions of mTBI include patients with intracranial imaging abnormalities (Kristman et al., 2014), it is increasingly more common in current studies to reclassify mTBI into uncomplicated and complicated dichotomies based on CT/MRI findings (Iverson et al., 2012). However, symptom outcomes associated with structural findings remains inconsistent (Lee et al., 2008; Lingsma et al., 2015). Even though our activation clusters were not driven by singular studies (see Table 3), it is important to consider these inter-subject variability factors regarding the outcome of inferential analysis (Mayer et al., 2015; Rosenbaum and Lipton, 2012; Bramlett and Dietrich, 2015; Pertab et al., 2009).
A vital consideration for the current meta-analysis and for fMRI methodology in general, is that the vasculature that underlies the BOLD signal can be impaired following brain trauma. Alterations in cerebral blood flow, perfusion, and vascular reactivity have all been found following mTBI (Bailey et al., 2013; Barkhoudarian et al., 2016; Grossman et al., 2013; Romero et al., 2015; Wang et al., 2016). Alteration in the cerebrovascular dynamics can lead to subsequent change in the ratio of intravascular oxy- to deoxyhemoglobin, which contribute to the BOLD signal (Buxton, 2013; Hall et al., 2016). For example, a recent series of fMRI studies found that the shape and magnitude of the signal can differentiate between a sample of mTBI patients and controls (Astafiev et al., 2015, 2016). While the authors found signal disturbance within several areas in the mTBI group, most cortical and subcortical regions involved during the visual tracking tasks were normal. Therefore, it remains to be known whether these secondary pathophysiological changes affect the BOLD signal across the entire brain or only the most vulnerable areas (Mayer et al., 2014). Ideally, future studies should measure baseline cerebrovascular parameters (e.g. cerebral blood flow) to partial out any potential effect of neurovascular uncoupling due to alterations in vascular physiology.
Compared to previous coordinate-based meta-analyses, we used the latest version of ALE software and included several additional studies not previously incorporated (Dettwiler et al., 2014; Johnson et al., 2015; van der Horn et al., 2016; Wylie et al., 2015). Additionally, we did not include any study that used an explicit ROI analysis (Gosselin et al., 2011; McAllister et al., 1999, 2001; Slobounov et al., 2010) or adolescent population (Krivitzky et al., 2011; Pardini et al., 2010). While our study represents the most up-to-date literature search of fMRI findings in mTBI, our conservative inclusion criteria limited the total number of experiments available. Owing to the low numbers of experiments, our ALE meta-analysis may not have had enough power to completely partial out subject-specific variation (Müller et al., 2018). Separate exploratory meta-analyses investigating the effect of task domain and type, behavioral performance, time after injury, age, and corrected threshold contrast could not be performed due to strict inclusion criteria and the number of experiments available (Eickhoff et al., 2017). Future studies addressing these variations will lead to improved research outcomes and a better understanding of the impaired functional networks that may underlie cognitive dysfunction in mTBI.
5. Conclusion
In summary, the present meta-analysis employed a coordinate-based ALE approach to identify affected brain areas relating to cognitive dysfunction following mTBI. We used GingerALE v.2.3.6 (brainmap.org/ale), a version of ALE that has fixed errors in both FDR and FWE cluster-level thresholding calculation codes identified in previous versions of the program. Our results revealed neural activation differences across a variety of cognitive tasks, with the mTBI group displaying hypoactivation within the right MFG. Consistent with prior meta-analyses (Bryer et al., 2013; Eierud et al., 2014) this finding suggests that the prefrontal region may be particularly affected following mTBI. However, although these results are in support of functional change in the neuroanatomical region of the MFG, the direction of change (i.e. greater or lesser activation) and the underlying causes remain unclear and require future study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Michael J. Cook has received support through an Australian Government Research Training Program Scholarship. W. Huw Williams, and Magdalena Wojtowicz have no disclosures. Andrew J. Gardner and Peter Stanwell have been funded to conduct research into concussion in rugby league through a New South Wales Sporting Injuries Committee–Sports Research & Injury Prevention Scheme Grant, and a Brain Foundation, Australia–Brain Injury Award. Andrew J. Gardner acknowledges sport concussion research fellowship funding from Jennie Thomas, Life Governor of the Hunter Medical Research Institute, the Hunter Medical Research Institute supported by Anne Greaves, the School of Medicine and Public Health, University of Newcastle, and the National Health and Medical Council (NHMRC) Early Career Fellowship. Grant Iverson has a clinical and consulting practice in forensic neuropsychology involving individuals who have sustained mild TBIs (including athletes). He has received research funding from several test publishing companies, including ImPACT Applications, Inc., CNS Vital Signs, and Psychological Assessment Resources (PAR, Inc.). He acknowledges unrestricted philanthropic support from the Mooney-Reed Charitable Foundation, Heinz Family Foundation, and ImPACT Applications, Inc. Grant Iverson has received salary support from research grants from the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members and the National Football League.
Acknowledgements
The authors thank Debbie Booth from the University of Newcastle for her assistance with the initial database search.
Appendix 1. Search Strategies for Databases
Database (s): MEDLINE 1946 to Present with Daily Update
Search Strategy:
| # | Searches |
|---|---|
| 1 | exp Craniocerebral Trauma/ |
| 2 | Brain Edema/ |
| 3 | Glasgow Coma Scale/ |
| 4 | Glasgow Outcome Scale/ |
| 5 | exp Unconsciousness/ |
| 6 | exp Cerebrovascular Trauma/ |
| 7 | Pneumocephalus/ |
| 8 | Epilepsy, Post-Traumatic/ |
| 9 | (Glasgow adj (coma or outcome) adj (scale* or score*)).ti,ab,kw. |
| 10 | rancho los amigos scale.ti,ab,kw. |
| 11 | (diffuse axonal injury or diffuse axonal injuries).ti,ab,kw. |
| 12 | exp Cerebral Hemorrhage/ |
| 13 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*).ti,ab,kw. |
| 14 | Brain Injuries/ |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 |
| 16 | (mild* or minor).ti,ab,kw. |
| 17 | (mtbi or mhi).ti,ab,kw. |
| 18 | (concuss* adj4 (symptoms or syndrome*)).ti,ab,kw. |
| 19 | (postconcuss* or post-concuss*).ti,ab,kw. |
| 20 | ((posttraum* or post-traum*) adj4 (symptom* or complaint*)).ti,ab,kw. |
| 21 | 16 or 17 or 18 or 19 or 20 |
| 22 | exp Neuroimaging/ |
| 23 | exp Magnetic Resonance Imaging/ |
| 24 | (mri or neuroimag* or fmri).ti,ab,kw. |
| 25 | ((function* or magnetic or brain or neuro) adj3 (mri or imag* or resonance)).ti,ab,kw. |
| 26 | exp Blood Volume/ |
| 27 | exp Hemoglobins/me [Metabolism] |
| 28 | exp Oxygen/me [Metabolism] |
| 29 | exp Oxygen Consumption/ |
| 30 | Oxyhemoglobins/me [Metabolism] |
| 31 | exp Brain/bs, me, ph [Blood Supply, Metabolism, Physiology] |
| 32 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin).ti,ab,kw. |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 |
| 34 | Memory Disorders/ |
| 35 | Memory/ |
| 36 | Cognition/ |
| 37 | Executive Function/ |
| 38 | (executive adj4 dysfunction*).ti,ab,kw. |
| 39 | Cognition Disorders/ |
| 40 | Motor Skills/ |
| 41 | functionality.ti,ab,kw. |
| 42 | memor*.ti,ab,kw. |
| 43 | ((executive function* or cognit* or attention or memory) adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).ti,ab,kw. |
| 44 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).ti,ab,kw. |
| 45 | 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 |
| 46 | 15 and 21 and 33 and 45 |
| 47 | limit 46 to (english language and yr = "1990 -Current") |
Database (s): EMBASE + EMBASE Classic
Search Strategy:
| # | Searches |
|---|---|
| 1 | exp head injury/ |
| 2 | brain edema/ |
| 3 | Glasgow coma scale/ |
| 4 | Glasgow outcome scale/ |
| 5 | exp unconsciousness/ |
| 6 | exp cerebrovascular accident/ |
| 7 | pneumocephalus/ |
| 8 | traumatic epilepsy/ |
| 9 | (Glasgow adj (coma or outcome) adj (scale* or score*)).ti,ab,kw. |
| 10 | rancho los amigos scale.ti,ab,kw. |
| 11 | (diffuse axonal injury or diffuse axonal injuries).ti,ab,kw. |
| 12 | exp brain hemorrhage/ |
| 13 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*).ti,ab,kw. |
| 14 | brain injury/ |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 |
| 16 | (mild* or minor).ti,ab,kw. |
| 17 | (mtbi or mhi).ti,ab,kw. |
| 18 | (concuss* adj4 (symptoms or syndrome*)).ti,ab,kw. |
| 19 | (postconcuss* or post-concuss*).ti,ab,kw. |
| 20 | ((posttraum* or post-traum*) adj4 (symptom* or complaint*)).ti,ab,kw. |
| 21 | 16 or 17 or 18 or 19 or 20 |
| 22 | exp neuroimaging/ |
| 23 | exp nuclear magnetic resonance imaging/ |
| 24 | (mri or neuroimag* or fmri).ti,ab,kw. |
| 25 | ((function* or magnetic or brain or neuro) adj3 (mri or imag* or resonance)).ti,ab,kw. |
| 26 | blood volume/ |
| 27 | exp oxygen consumption/ |
| 28 | (exp hemoglobin/ or exp oxygen/ or oxyhemoglobin/ or exp brain/) and exp metabolism/ |
| 29 | exp brain/ and (vascularization/ or blood supply.mp. or pysiology/) [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 30 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin).ti,ab,kw. |
| 31 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 |
| 32 | exp memory disorder/ |
| 33 | memory/ |
| 34 | cognition/ |
| 35 | executive function/ |
| 36 | (executive adj4 dysfunction*).ti,ab,kw. |
| 37 | cognitive defect/ |
| 38 | motor performance/ |
| 39 | functionality.ti,ab,kw. |
| 40 | memor*.ti,ab,kw. |
| 41 | ((executive function* or cognit* or attention or memory) adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).ti,ab,kw. |
| 42 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).ti,ab,kw. |
| 43 | 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 |
| 44 | 15 and 21 and 31 and 43 |
| 45 | limit 44 to (english language and yr = "1990 -Current") |
Database (s): PsycINFO
Search Strategy:
| # | Searches |
|---|---|
| 1 | exp traumatic brain injury/ |
| 2 | Brain Edema.mp. |
| 3 | Unconscious*.mp. |
| 4 | exp head injuries/ |
| 5 | brain damage/ |
| 6 | Pneumocephalus.mp. |
| 7 | Post-Traumatic Epilepsy.mp. |
| 8 | (Glasgow adj (coma or outcome) adj (scale* or score*)).mp. |
| 9 | rancho los amigos scale.mp. |
| 10 | (diffuse axonal injury or diffuse axonal injuries).mp. |
| 11 | exp Cerebral Hemorrhage/ |
| 12 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*).mp. |
| 13 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 |
| 14 | (mild* or minor).mp. |
| 15 | (mtbi or mhi).mp. |
| 16 | (concuss* adj4 (symptoms or syndrome*)).mp. |
| 17 | (postconcuss* or post-concuss*).mp. |
| 18 | ((posttraum* or post-traum*) adj4 (symptom* or complaint*)).mp. |
| 19 | 14 or 15 or 16 or 17 or 18 |
| 20 | exp neuroimaging/ |
| 21 | (mri or neuroimag* or fmri).mp. |
| 22 | ((function* or magnetic or brain or neuro) adj3 (mri or imag* or resonance)).mp. |
| 23 | exp Blood Volume/ |
| 24 | exp HEMOGLOBIN/ |
| 25 | exp OXYGEN/ |
| 26 | Oxygen Consumption.mp. |
| 27 | Oxyhemoglobins.mp. |
| 28 | exp BRAIN/ |
| 29 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin).mp. |
| 30 | 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 |
| 31 | exp memory disorders/ |
| 32 | exp memory/ |
| 33 | cognition/ |
| 34 | exp executive function/ |
| 35 | (executive adj4 dysfunction*).mp. |
| 36 | exp Cognitive Impairment/ or Cognition Disorders.mp. |
| 37 | exp Motor Skills/ |
| 38 | functionality.mp. |
| 39 | memor*.tw. |
| 40 | ((executive function* or cognit* or attention or memory) adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 41 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 42 | 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 |
| 43 | 13 and 19 and 30 and 42 |
| 44 | limit 43 to (english language and yr = "1990 -Current") |
Database (s): AMED (Allied and Complementary Medicine)
Search Strategy:
| # | Searches |
|---|---|
| 1 | exp Brain injuries/ |
| 2 | head injuries/ |
| 3 | Brain Edema.mp. |
| 4 | exp Unconsciousness/ |
| 5 | Pneumocephalus.mp. |
| 6 | Post-Traumatic Epilepsy.mp. |
| 7 | (Glasgow adj (coma or outcome) adj (scale* or score*)).mp. |
| 8 | rancho los amigos scale.mp. |
| 9 | (diffuse axonal injury or diffuse axonal injuries).mp. |
| 10 | cerebral hemorrhage/ |
| 11 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*).mp. |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
| 13 | (mild* or minor).mp. |
| 14 | (mtbi or mhi).mp. |
| 15 | (concuss* adj4 (symptoms or syndrome*)).mp. |
| 16 | (postconcuss* or post-concuss*).mp. |
| 17 | ((posttraum* or post-traum*) adj4 (symptom* or complaint*)).mp. |
| 18 | 13 or 14 or 15 or 16 or 17 |
| 19 | exp diagnostic imaging/ |
| 20 | (mri or neuroimag* or fmri).mp. |
| 21 | ((function* or magnetic or brain or neuro) adj3 (mri or imag* or resonance)).mp. |
| 22 | Blood Volume.mp. |
| 23 | hemoglobins/ |
| 24 | Oxygen/ |
| 25 | Oxygen consumption/ |
| 26 | Oxyhemoglobin*.mp. |
| 27 | Brain/ |
| 28 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin).mp. |
| 29 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 |
| 30 | Memory disorders/ |
| 31 | Memory/ |
| 32 | Cognition/ |
| 33 | (executive adj4 (function* or dysfunction*)).mp. |
| 34 | Cognition disorders/ |
| 35 | Motor skills/ |
| 36 | functionality.mp. |
| 37 | memor*.mp. |
| 38 | ((executive function* or cognit* or attention or memory) adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 39 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 40 | 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 |
| 41 | 12 and 18 and 29 and 40 |
| 42 | limit 41 to (english and yr = "1990 -Current") |
Database (s): MEDLINE In-Process & Other Non-Indexed Citations
Search Strategy:
| # | Searches |
|---|---|
| 1 | Unconscious*.mp. |
| 2 | Pneumocephalus.mp. |
| 3 | Post-Traumatic Epilepsy.mp. |
| 4 | (Glasgow adj (coma or outcome) adj (scale* or score*)).mp. |
| 5 | rancho los amigos scale.mp. |
| 6 | (diffuse axonal injury or diffuse axonal injuries).mp. |
| 7 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*).mp. |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 9 | (mild* or minor).mp. |
| 10 | (mtbi or mhi).mp. |
| 11 | (concuss* adj4 (symptoms or syndrome*)).mp. |
| 12 | (postconcuss* or post-concuss*).mp. |
| 13 | ((posttraum* or post-traum*) adj4 (symptom* or complaint*)).mp. |
| 14 | 9 or 10 or 11 or 12 or 13 |
| 15 | (mri or neuroimag* or fmri or imag* or resonance).mp. |
| 16 | Blood Volume.mp. |
| 17 | Hemoglobin*.mp. |
| 18 | Oxygen.mp. |
| 19 | Oxyhemoglobin*.mp. |
| 20 | brain.mp. |
| 21 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin).mp. |
| 22 | Cognition.mp. |
| 23 | (executive adj4 (function* or dysfunction*)).mp. |
| 24 | Motor Skills.mp. |
| 25 | functionality.mp. |
| 26 | memor*.mp. |
| 27 | ((executive function* or cognit* or attention or memory) adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 28 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") adj3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)).mp. |
| 29 | 15 or 16 or 17 or 18 or 19 or 20 or 21 |
| 30 | 22 or 23 or 24 or 25 or 26 or 27 or 28 |
| 31 | 8 and 14 and 29 and 30 |
| 32 | limit 31 to (english language and yr = "1990 -Current") |
Database (s): CINAHL
Search Strategy:
| # | Query |
|---|---|
| S1 | (MH "Head Injuries+") |
| S2 | (MH "Cerebral Edema") |
| S3 | (MH "Glasgow Coma Scale") |
| S4 | (MH "Unconsciousness+") |
| S5 | (MH "Pneumocephalus") |
| S6 | (MH "Epilepsy, Post-Traumatic") |
| S7 | TI ((Glasgow n1 (coma or outcome) n1 (scale* or score*))) OR AB ((Glasgow n1 (coma or outcome) n1 (scale* or score*))) |
| S8 | TI (rancho los amigos scale) OR AB (rancho los amigos scale) |
| S9 | TI ((diffuse axonal injury or diffuse axonal injuries)) OR AB ((diffuse axonal injury or diffuse axonal injuries)) |
| S10 | (MH "Cerebral Hemorrhage+") |
| S11 | TI ((injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*)) OR AB ((injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*)) |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 |
| S13 | TI ((mild* or minor)) OR AB ((mild* or minor)) |
| S14 | TI ((mtbi or mhi)) OR AB ((mtbi or mhi)) |
| S15 | TI ((concuss* n4 (symptoms or syndrome*))) OR AB ((concuss* n4 (symptoms or syndrome*))) |
| S16 | TI ((postconcuss* or post-concuss*)) OR AB ((postconcuss* or post-concuss*)) |
| S17 | TI (((posttraum* or post-traum*) n4 (symptom* or complaint*))) OR AB (((posttraum* or post-traum*) n4 (symptom* or complaint*))) |
| S18 | S13 OR S14 OR S15 OR S16 OR S17 |
| S19 | (MH "Neuroradiography+") |
| S20 | (MH "Magnetic Resonance Imaging+") |
| S21 | (MH "Diagnostic Imaging") |
| S22 | TI ((mri or neuroimag* or fmri)) OR AB ((mri or neuroimag* or fmri)) |
| S23 | TI (((function* or magnetic or brain or neuro) n3 (mri or imag* or resonance))) OR AB (((function* or magnetic or brain or neuro) n3 (mri or imag* or resonance))) |
| S24 | (MH "Blood Volume+") |
| S25 | (MH "Hemoglobins+/ME") |
| S26 | (MH "Oxygen+/ME") |
| S27 | (MH "Oxygen Consumption+") |
| S28 | TI Oxyhemoglobin* OR AB Oxyhemoglobin* |
| S29 | (MH "Brain+/PH/ME/BS") |
| S30 | TI (("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin)) OR AB (("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin)) |
| S31 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 |
| S32 | (MH "Memory Disorders+") |
| S33 | (MH "Memory") |
| S34 | (MH "Cognition") |
| S35 | (MH "Executive Function") |
| S36 | TI (executive n4 dysfunction*) OR AB (executive n4 dysfunction*) |
| S37 | (MH "Cognition Disorders") |
| S38 | (MH "Motor Skills") |
| S39 | TI functionality OR AB functionality |
| S40 | TI memor* OR AB memor* |
| S41 | TI (((executive function* or cognit* or attention or memory) n3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability))) OR AB (((executive function* or cognit* or attention or memory) n3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability))) |
| S42 | TI (((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") n3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability))) OR AB (((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") n3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability))) |
| S43 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 |
| S44 | S12 AND S18 AND S31 AND S43 limited to English and 1990+ |
Database (s): COCHRANE LIBRARY
Search Strategy:
| ID | Search Hits |
|---|---|
| #1 | MeSH descriptor: [Craniocerebral Trauma] explode all trees |
| #2 | MeSH descriptor: [Brain Edema] this term only |
| #3 | MeSH descriptor: [Glasgow Coma Scale] this term only |
| #4 | MeSH descriptor: [Glasgow Outcome Scale] this term only |
| #5 | MeSH descriptor: [Unconsciousness] explode all trees |
| #6 | MeSH descriptor: [Cerebrovascular Trauma] explode all trees |
| #7 | MeSH descriptor: [Pneumocephalus] this term only |
| #8 | MeSH descriptor: [Epilepsy, Post-Traumatic] this term only |
| #9 | (Glasgow near (coma or outcome) near (scale* or score*)):ti,ab,kw |
| #10 | rancho los amigos scale:ti,ab,kw |
| #11 | (diffuse axonal injury or diffuse axonal injuries):ti,ab,kw |
| #12 | MeSH descriptor: [Cerebral Hemorrhage] explode all trees |
| #13 | (injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*):ti,ab,kw |
| #14 | MeSH descriptor: [Brain Injuries] this term only |
| #15 | {or #1-#14} |
| #16 | (mild* or minor):ti,ab,kw |
| #17 | (mtbi or mhi):ti,ab,kw |
| #18 | (concuss* near/4 (symptoms or syndrome*)):ti,ab,kw |
| #19 | (postconcuss* or post-concuss*):ti,ab,kw |
| #20 | ((posttraum* or post-traum*) near/4 (symptom* or complaint*)):ti,ab,kw |
| #21 | {or #16-#20} |
| #22 | MeSH descriptor: [Neuroimaging] explode all trees |
| #23 | MeSH descriptor: [Magnetic Resonance Imaging] explode all trees |
| #24 | (mri or neuroimag* or fmri):ti,ab,kw |
| #25 | ((function* or magnetic or brain or neuro) near/3 (mri or imag* or resonance)):ti,ab,kw |
| #26 | MeSH descriptor: [Blood Volume] explode all trees |
| #27 | MeSH descriptor: [Hemoglobins] explode all trees and with qualifier(s): [Metabolism - ME] |
| #28 | MeSH descriptor: [Oxygen] explode all trees and with qualifier(s): [Metabolism - ME] |
| #29 | MeSH descriptor: [Oxygen Consumption] explode all trees |
| #30 | MeSH descriptor: [Oxyhemoglobins] this term only and with qualifier(s): [Metabolism - ME] |
| #31 | MeSH descriptor: [Brain] explode all trees and with qualifier(s): [Blood supply - BS, Metabolism - ME, Physiology - PH] |
| #32 | ("blood oxygen level dependent" or BOLD or "BOLD effect" or "BOLD signal" or oxyhemoglobin or deoxyhemoglobin):ti,ab,kw |
| #33 | {or #22-#32} |
| #34 | MeSH descriptor: [Memory Disorders] this term only |
| #35 | MeSH descriptor: [Memory] this term only |
| #36 | MeSH descriptor: [Cognition] this term only |
| #37 | MeSH descriptor: [Executive Function] this term only |
| #38 | (executive near/4 dysfunction*):ti,ab,kw |
| #39 | MeSH descriptor: [Cognition Disorders] this term only |
| #40 | MeSH descriptor: [Motor Skills] this term only |
| #41 | functionality:ti,ab,kw |
| #42 | memor*:ti,ab,kw |
| #43 | ((executive function* or cognit* or attention or memory) near/3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)):ti,ab,kw |
| #44 | ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") near/3 (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)):ti,ab,kw |
| #45 | {or #34-#44} |
| #46 | {and #15, #21, #33, #45} |
Database (s): SCOPUS
Search Strategy:
TITLE-ABS-KEY((Glasgow w/1 (coma or outcome) w/1 (scale* or score*)) or unconscious* or Pneumocephalus or "rancho los amigos scale" or injur* or trauma* or damag* or wound* or fractur* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur* or lesion* or destruction* or oedema* or edema* or contusion* or concus* or swell* or bleed*) AND TITLE-ABS-KEY(Mild* or minor or mtbi or (concuss* w/4 (symptom* or syndrome*)) or (postconcuss* or post-concuss*) or ((posttraum* or post-traum*) w/4 (symptom* or complaint*))) AND TITLE-ABS-KEY(mri or neuroimag* or fmri or ((function* or magnetic or brain or neuro) w/3 (mri or imag* or resonance)) or Blood Volume or Hemoglobins or Oxygen or brain or ("blood oxygen level dependent" or BOLD or oxyhemoglobin or deoxyhemoglobin)) AND TITLE-ABS-KEY(memor* or cognition or Executive Function or (executive and dysfunction*) or Motor Skills or functionality or ((executive function* or cognit* or attention or memory) and (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability)) or ((organiz* or organis* or plan* or manag* or "problem solving" or "decision making") and (disorder* or dysfunction or impaired or impairment or difficult* or problem* or disability))
Database (s): INFORMIT HEALTH COLLECTION
Search Strategy:
((Glasgow AND (coma OR outcome) AND (scale* OR score*)) OR unconscious* OR Pneumocephalus OR “rancho los amigos scale” OR injur* OR trauma* OR damag* OR wound* OR fractur* OR contusion* OR haematoma* OR hematoma* OR haemorrhag* OR hemorrhag* OR pressur* OR lesion* OR destruction* OR oedema* OR edema* OR contusion* OR concus* OR swell* OR bleed*) AND (Mild* OR minor OR mtbi OR (concuss* AND (symptom* OR syndrome*)) OR (postconcuss* OR post-concuss*) OR ((posttraum* OR post-traum*) AND (symptom* OR complaint*))) AND (mri OR neuroimag* OR fmri OR ((function* OR magnetic OR brain OR neuro) AND (mri OR imag* OR resonance)) OR Blood Volume OR Hemoglobins OR Oxygen OR brain OR ("blood oxygen level dependent" OR BOLD OR oxyhemoglobin OR deoxyhemoglobin)) AND (memor* OR cognition OR Executive Function OR (executive AND dysfunction*) OR Motor Skills OR functionality OR ((executive function* OR cognit* OR attention OR memory) AND (disorder* OR dysfunction OR impaired OR impairment OR difficult* OR problem* OR disability)) OR ((organiz* OR organis* OR plan* OR manag* OR "problem solving" OR "decision making") AND (disorder* OR dysfunction OR impaired OR impairment OR difficult* OR problem* OR disability)))
References
- Astafiev S.V., Shulman G.L., Metcalf N.V., Rengachary J., MacDonald C.L., Harrington D.L., Maruta J., Shimony J.S., Ghajar J., Diwakar M., Huang M.X., Lee R.R., Corbetta M. Abnormal white matter blood-oxygen-level-dependent signals in chronic mild traumatic brain injury. J. Neurotrauma. 2015;32(16):1254–1271. doi: 10.1089/neu.2014.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev S.V., Zinn K.L., Shulman G.L., Corbetta M. Exploring the physiological correlates of chronic mild traumatic brain injury symptoms. NeuroImage Clin. 2016;11:10–19. doi: 10.1016/j.nicl.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D.M., Jones D.W., Sinnott A., Brugniaux J.V., New K.J., Hodson D., Marley C.J., Smirl J.D., Ogoh S., Ainslie P.N. Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin. Sci. (Lond). 2013;124(3):177–189. doi: 10.1042/CS20120259. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G., Hovda D.A., Giza C.C. The molecular pathophysiology of concussive brain injury - an update. Phys. Med. Rehabil. Clin. North Am. 2016;27(2):373–393. doi: 10.1016/j.pmr.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Borich M., Babul A.N., Yuan P.H., Boyd L., Virji-Babul N. Alterations in resting-state brain networks in concussed adolescent athletes. J. Neurotrauma. 2015;32(4):265–271. doi: 10.1089/neu.2013.3269. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M., Dietrich W.D. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma. 2015;32(23):1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryer E.J., Medaglia J.D., Rostami S., Hillary F.G. Neural recruitment after mild traumatic brain injury is task dependent: a meta-analysis. J. Int. Neuropsychol. Soc. 2013;19(7):751–762. doi: 10.1017/S1355617713000490. [DOI] [PubMed] [Google Scholar]
- Buxton R.B. The physics of functional magnetic resonance imaging (fMRI) Rep. Prog. Phys. 2013;76(9) doi: 10.1088/0034-4885/76/9/096601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll L.J., Cassidy J.D., Holm L., Kraus J., Coronado V.G. Methodological issues and research recommendations for mild traumatic brain injury: the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004;36(43):113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Wu C.H., Liao Y.P., Hsu H.L., Tseng Y.C., Liu H.L., Chiu W.T. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264(3):844–851. doi: 10.1148/radiol.12112154. [DOI] [PubMed] [Google Scholar]
- Chen J.K., Johnston K.M., Collie A., McCrory P., Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J. Neurol. Neurosurg. Psychiatry. 2007;78(11):1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.K., Johnston K.M., Frey S., Petrides M., Worsley K., Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen J.K., Johnston K.M., Petrides M., Ptito A. Recovery from mild head injury in sports: evidence from serial functional magnetic resonance imaging studies in male athletes. Clin. J. Sport Med. 2008;18(3):241–247. doi: 10.1097/JSM.0b013e318170b59d. [DOI] [PubMed] [Google Scholar]
- Chumbley J.R., Friston K.J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dean P.J.A., Sterr A. Long-term effects of mild traumatic brain injury on cognitive performance. Front. Hum. Neurosci. 2013;7:30. doi: 10.3389/fnhum.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettwiler A., Murugavel M., Putukian M., Cubon V., Furtado J., Osherson D. Persistent differences in patterns of brain activation after sports-related concussion: a longitudinal functional magnetic resonance imaging study. J. Neurotrauma. 2014;31(2):180–188. doi: 10.1089/neu.2013.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Fox P.M., Lancaster J.L., Fox P.T. Implementation errors in the Gingerale Software: description and recommendations. Hum. Brain Mapp. 2017;38:7–11. doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: a random‐effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Nichols T.E., Laird A.R., Hoffstaedter F., Amunts K., Fox P.T., Bzdok D., Eickhoff C.R. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., Laconte S.M. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbin R.J., Covassin T., Hakun J., Kontos A.P., Berger K., Pfeiffer K., Ravizza S. Do brain activation changes persist in athletes with a history of multiple concussions who are asymptomatic? Brain Inj. 2012;26(10):1217–1225. doi: 10.3109/02699052.2012.672788. [DOI] [PubMed] [Google Scholar]
- Elbin R.J., Schatz P., Lowder H.B., Kontos A.P. An empirical review of treatment and rehabilitation approaches used in the acute, sub-acute, and chronic phases of recovery following sports-related concussion. Curr. Treat. Options Neurol. 2014;16(11):320. doi: 10.1007/s11940-014-0320-7. [DOI] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl. Acad. Sci. U.S.A. 2013;110(41):16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U.S.A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N., Bottari C., Chen J.K., Petrides M., Tinawi S., de Guise E., Ptito A. Electrophysiology and functional MRI in post-acute mild traumatic brain injury. J. Neurotrauma. 2011;28(3):329–341. doi: 10.1089/neu.2010.1493. [DOI] [PubMed] [Google Scholar]
- Greenwald R.M., Gwin J.T., Chu J.J., Crisco J.J. Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery. 2008;62(4):789–798. doi: 10.1227/01.neu.0000318162.67472.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E.J., Jensen J.H., Babb J.S., Chen Q., Tabesh A., Fieremans E., Xia D., Inglese M., Grossman R.I. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. Am. J. Neuroradiol. 2013;34(5):951–957. doi: 10.3174/ajnr.A3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Howarth C., Kurth-Nelson Z., Mishra A. Interpreting BOLD: towards a dialogue between cognitive and cellular neuroscience. Phil. Trans. R. Soc. B. Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammeke T.A., McCrea M., Coats S.M., Verber M.D., Durgerian S., Flora K., Olsen G.S., Leo P.D., Gennarelli T.A., Rao S.M. Acute and subacute changes in neural activation during the recovery from sport-related concussion. J. Int. Neuropsychol. Soc. 2013;19(8):863–872. doi: 10.1017/S1355617713000702. [DOI] [PubMed] [Google Scholar]
- Hillary F.G., Genova H.M., Chiaravalloti N.D., Rypma B., DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum. Brain Mapp. 2006;27(11):837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary F.G., Genova H.M., Medaglia J.D., Fitzpatrick N.M., Chiou K.S., Wardecker B.M., Wang J. The nature of processing speed deficits in traumatic brain injury: is less brain more? Brain Imaging Behav. 2010;4(2):141–154. doi: 10.1007/s11682-010-9094-z. [DOI] [PubMed] [Google Scholar]
- Hsu H.L., Chen D.Y.T., Tseng Y.C., Kuo Y.S., Huang Y.L., Chiu W.T., Yan F.X., Wang W.S., Chen C.J. Sex differences in working memory after mild traumatic brain injury: a functional MR imaging study. Radiology. 2015;276(3):828–835. doi: 10.1148/radiol.2015142549. [DOI] [PubMed] [Google Scholar]
- Iverson G.L., Lange R.T., Wäljas M., Liimatainen S., Dastidar P., Hartikainen K.M., Soimakallio S., Öhman J. Outcome from complicated versus uncomplicated mild traumatic brain injury. Rehabil. Res. Prac. 2012;2012:1–7. doi: 10.1155/2012/415740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S., Holiday K., Satyshur M.D., Mukai I., Ungerleider L.G. A role of right middle frontal gyrus in reorienting of attention: a case study. Front. Syst. Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Zhang K., Hallett M., Slobounov S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2015;9(3):564–573. doi: 10.1007/s11682-014-9316-x. [DOI] [PubMed] [Google Scholar]
- Kamins J., Bigler E., Covassin T., Henry L., Kemp S., Leddy J.J., Mayer A., McCrea M., Prins M., Schneider K.J., Valovich McLeod T.C., Zemek R., Giza C.C. What is the physiological time to recovery after concussion? A systematic review. Br. J. Sports Med. 2017;51(12):935–940. doi: 10.1136/bjsports-2016-097464. [DOI] [PubMed] [Google Scholar]
- Keightley M.L., Saluja R.S., Chen J.K., Gagnon I., Leonard G., Petrides M., Ptito A. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: is it still working? J. Neurotrauma. 2014;31(5):437–451. doi: 10.1089/neu.2013.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristman V.L., Borg J., Godbolt A.K., Salmi L.R., Cancelliere C., Carroll L.J., Holm L.W., Nygren-De Boussard C., Hartvigsen J., Abara U., Donovan J., Cassidy J.D. Methodological issues and research recommendations for prognosis after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 2014;95(3):S265–S277. doi: 10.1016/j.apmr.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Krivitzky L.S., Roebuck-Spencer T.M., Roth R.M., Blackstone K., Johnson C.P., Gioia G. Functional magnetic resonance imaging of working memory and response inhibition in children with mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2011;17(6):1143–1152. doi: 10.1017/S1355617711001226. [DOI] [PubMed] [Google Scholar]
- Kumar S., Rao S.L., Chandramouli B.A., Pillai S. Reduced contribution of executive functions in impaired working memory performance in mild traumatic brain injury patients. Clin. Neurol. Neurosurg. 2013;115(8):1326–1332. doi: 10.1016/j.clineuro.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Lee H., Wintermark M., Gean A.D., Ghajar J., Manley G.T., Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J. Neurotrauma. 2008;25(9):1049–1056. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- Lingsma H.F., Yue J.K., Maas A.I., Steyerberg E.W., Manley G.T., Investigators including TRACK-TBI, Cooper S.R., Dams-O'Connor K., Gordon W.A., Menon D.K., Mukherjee P. Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J. Neurotrauma. 2015;32(2):83–94. doi: 10.1089/neu.2014.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruishi M., Miyatani M., Nakao T., Muranaka H. Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: a functional magnetic resonance imaging study. J. Neurol. Neurosurg. Psychiatry. 2007;78(2):168–173. doi: 10.1136/jnnp.2006.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Bellgowan P.S., Hanlon F.M. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev. 2015;49:8–18. doi: 10.1016/j.neubiorev.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Mannell M.V., Ling J., Elgie R., Gasparovic C., Phillips J.P., Doezema D., Yeo R.A. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Hum. Brain Mapp. 2009;30(12):4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Mannell M.V., Ling J., Gasparovic C., Yeo R.A. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011;32(11):1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Toulouse T., Klimaj S., Ling J.M., Pena A., Bellgowan P.S. Investigating the properties of the hemodynamic response function after mild traumatic brain injury. J. Neurotrauma. 2014;31(2):189–197. doi: 10.1089/neu.2013.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Flashman L.A., McDonald B.C., Ferrell R.B., Tosteson T.D., Yanofsky N.N., Grove M.R., Saykin A.J. Dopaminergic challenge with bromocriptine one month after mild traumatic brain injury: altered working memory and BOLD response. J. Neuropsychiatry Clin. Neurosci. 2011;23(3):277–286. doi: 10.1176/appi.neuropsych.23.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcallister T.W., Saykin A.J., Flashman L.A., Sparling M.B., Johnson S.C., Guerin S.J., Mamourian A.C., Weaver J.B., Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53(6):1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., Saykin A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Meaney D.F., Smith D.H. Biomechanics of concussion. Clin. Sports Med. 2011;30:19–31. doi: 10.1016/j.csm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares S., Shores E.A., Taylor A.J., Batchelor J., Bryant R.A., Baguley I.J., Chapman J., Gurka J., Marosszeky J.E. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25(4):454–465. doi: 10.1037/a0022580. [DOI] [PubMed] [Google Scholar]
- Medaglia J.D., Chiou K.S., Slocomb J., Fitzpatrick N.M., Wardecker B.M., Ramanathan D., Vesek J., Good D.C., Hillary F.G. The less BOLD, the wiser: support for the latent resource hypothesis after traumatic brain injury. Hum. Brain Mapp. 2012;33(4):979–993. doi: 10.1002/hbm.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T.B., Giraldo-Chica M., España L.Y., Mayer A.R., Harezlak J., Nencka A.S., Wang Y., Koch K.M., Wu Y.C., Saykin A.J., Giza C.C., Goldman J., DiFiori J.P., Guskiewicz K.M., Mihalik J.P., Brooks A., Broglio S.P., McAllister T., McCrea M.A. Resting-state fMRI metrics in acute sport-related concussion and their association with clinical recovery: a study from the NCAA-DOD care consortium. J. Neurotrauma. 2019 doi: 10.1089/neu.2019.6471. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militana A.R., Donahue M.J., Sills A.K., Solomon G.S., Gregory A.J., Strother M.K., Morgan V.L. Alterations in default mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging Behav. 2016;10(2):559–568. doi: 10.1007/s11682-015-9407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V.I., Cieslik E.C., Laird A.R., Fox P.T., Radua J., Mataix-Cols D., Tench C.R., Yarkoni T., Nichols T.E., Turkeltaub P.E., Wager T.D., Eickhoff S.B. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini J.E., Pardini D.A., Becker J.T., Dunfee K.L., Eddy W.F., Lovell M.R., Welling J.S. Postconcussive symptoms are associated with compensatory cortical recruitment during a working memory task. Neurosurgery. 2010;67(4):1020–1028. doi: 10.1227/NEU.0b013e3181ee33e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertab J.L., James K.M., Bigler E.D. Limitations of mild traumatic brain injury meta-analyses. Brain Inj. 2009;23(6):498–508. doi: 10.1080/02699050902927984. [DOI] [PubMed] [Google Scholar]
- Romero K., Lobaugh N.J., Black S.E., Ehrlich L., Feinstein A. Old wine in new bottles: validating the clinical utility of SPECT in predicting cognitive performance in mild traumatic brain injury. Psychiatry Res. 2015;231:15–24. doi: 10.1016/j.pscychresns.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S.B., Lipton M.L. Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging Behav. 2012;6(2):255–282. doi: 10.1007/s11682-012-9162-7. [DOI] [PubMed] [Google Scholar]
- Saluja R.S., Chen J.K., Gagnon I.J., Keightley M., Ptito A. Navigational memory functional magnetic resonance imaging: a test for concussion in children. J. Neurotrauma. 2015;32(10):712–722. doi: 10.1089/neu.2014.3470. [DOI] [PubMed] [Google Scholar]
- Sánchez-Carrion R., Fernandez-Espejo D., Junque C., Falcon C., Bargallo N., Roig T., Bernabeu M., Tormos J.M., Vendrell P. A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage. 2008;43(3):421–429. doi: 10.1016/j.neuroimage.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Sánchez-Carrión R., Gómez P.V., Junqué C., Fernández-Espejo D., Falcon C., Bargalló N., Roig-Rovira T., Enseñat-Cantallops A., Bernabeu M. Frontal hypoactivation on functional magnetic resonance imaging in working memory after severe diffuse traumatic brain injury. J. Neurotrauma. 2008;25(5):479–494. doi: 10.1089/neu.2007.0417. [DOI] [PubMed] [Google Scholar]
- Scheibel R.S., Newsome M.R., Troyanskaya M., Steinberg J.L., Goldstein F.C., Mao H., Levin H.S. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J. Neurotrauma. 2009;26(9):1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli K., Chen J.K., Wells G., Fait P., Ptito A., Taha T., Keightley M. Imaging "brain strain” in youth athletes with mild traumatic brain injury during dual-task performance. J. Neurotrauma. 2014;31(22):1843–1859. doi: 10.1089/neu.2014.3326. [DOI] [PubMed] [Google Scholar]
- Slobounov S.M., Zhang K., Pennell D., Ray W., Johnson B., Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp. Brain Res. 2010;202(2):341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G.E., Goldstein R., O'Neil-Pirozzi T.M., Kelkar K., Supelana C., Burke D., Katz D.I., Rauch S.L., Savage C.R., Glenn M.B. Neurophysiological alterations during strategy-based verbal learning in traumatic brain injury. Neurorehabil. Neural Repair. 2009;23(3):226–236. doi: 10.1177/1545968308324225. [DOI] [PubMed] [Google Scholar]
- Stulemeijer M., Vos P.E., van der Werf S., van Dijk G., Rijpkema M., Fernández G. How mild traumatic brain injury may affect declarative memory performance in the post-acute stage. J. Neurotrauma. 2010;27(9):1585–1595. doi: 10.1089/neu.2010.1298. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Terry D.P., Faraco C.C., Smith D., Diddams M.J., Puente A.N., Miller L.S. Lack of long-term fMRI differences after multiple sports-related concussions. Brain Inj. 2012;26(13–14):1684–1696. doi: 10.3109/02699052.2012.722259. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G.R., McIntosh A.R., Levine B. Prefrontal compensatory engagement in TBI is due to altered functional engagement of existing networks and not functional reorganization. Front. Syst. Neurosci. 2011;5:9. doi: 10.3389/fnsys.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horn H.J., Liemburg E.J., Scheenen M.E., Koning M.E., Spikman J.M., Naalt J. Post-concussive complaints after mild traumatic brain injury associated with altered brain networks during working memory performance. Brain Imaging Behav. 2016;10(4):1243–1253. doi: 10.1007/s11682-015-9489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Nelson L.D., LaRoche A.A., Pfaller A.Y., Nencka A.S., Koch K.M., McCrea M.A. Cerebral blood flow alterations in acute sport-related concussion. J. Neurotrauma. 2016;33(13):1227–1236. doi: 10.1089/neu.2015.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S.T., Lovejoy D.W., Pearlson G.D., Stevens M.C. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging Behav. 2010;4(3–4):232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- Wylie G.R., Freeman K., Thomas A., Shpaner M., Okeefe M., Watts R., Naylor M.R. Cognitive improvement after mild traumatic brain injury measured with functional neuroimaging during the acute period. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Yeo R.A., Pena A., Ling J.M., Klimaj S., Campbell R., Doezema D., Mayer A.R. An fMRI study of auditory orienting and inhibition of return in pediatric mild traumatic brain injury. J. Neurotrauma. 2012;29(12):2124–2136. doi: 10.1089/neu.2012.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.C., Covassin T., Nogle S., Doyle S., Russell D., Pearson R.L., Monroe J., Liszewski C.M., DeMarco J.K., Kaufman D.I. A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. J. Neurotrauma. 2015;32(5):327–341. doi: 10.1089/neu.2014.3413. [DOI] [PubMed] [Google Scholar]