Abstract
The present study carried out the optimisation of the total polyphenol content (TPC) extraction assisted by ultrasound in Ilex guayusa leaves applying response surface methodology (RSM). Also, the evaluation of the antioxidant activity of the extract obtained under the optimal extraction conditions was performed. The effect of the variables like, time of sonication, temperature, ethanol/water ratio and solid/liquid relationship and the interactions between them were analysed through the use of a factorial design 2ˆ4. The significant factors were considered for the optimisation, employing a Box-Behnken Design, and the TPC as response variables. It was found that a quadratic model was adequate, with an adjusted R2 value of 0.9367. The optimal conditions proposed, by the response surface model were: an extraction temperature of 60 °C, sonication time of 29.9 min and ethanol/water ratio of 76.8/23.2. The optimised leaves extract of I. guayusa show a TPC of 3.46 (±0.17) g gallic acid equivalents/100 g d.w. Radical scavenger activity of the obtained extract at optimum conditions, was performed through the FRAP and ABTS methods, given as result: 0.080 mmol TROLOX equivalents/100 g d.w. and 40.71 μmol TROLOX equivalents/g d.w., respectively. Due to the present findings, I. guayusa extracts can be proposed as a promising component for functional beverages, cosmetic and pharmaceutical formulation.
Keywords: Engineering, Chemistry, Food science, Natural extracts, Natural antioxidants, Design expert, ABTS, FRAP
Engineering; Chemistry; Food science; Natural extracts; Natural antioxidants; Design expert; ABTS; FRAP.
1. Introduction
Ilex guayusa Loes. (Guayusa) is a native Amazonian shrub belonging to the Aquifoliaceae family and it is widely widespread in Ecuador, Bolivia, Peru and Colombia. The species is distributed on the Amazonian piedmont and the Andean mountains, on elevation from 200 to 2000 m above sea level (Sidali et al., 2016). I. guayusa is a dioecious plant, where sexes are separated on two different plants. The shrub can reach from 6 to 10 m tall (Shemluck, 1979).
Several authors (Dueñas et al., 2016; Innerhofer and Bernhardt, 2011; Patiño, 1968; Wise and Santander, 2018) reported the history and traditional use of I. guayusa leaves as a beverage used in ritual ceremonies in the northwest Amazon region. Three recent critical review article (Gan et al., 2018; Schuster and Mitchell, 2019; Wise and Negrin, 2019), focus the phytochemical composition, the bioactivity and the nutritional content of the I. guayusa leave extracts in order to empathize the history of safe traditional use of the species.
In our previous research (Radice et al., 2017), we summarized the traditional uses of I. guayusa tea as a stimulant, diuretic and stomach tonic. The leaf infusion has also been reported as an ethnomedical remedy against diabetes, venereal diseases, flu and body pain. Local traditional knowledge emphasizes the use of guayusa tea in order to increase fertility and libido. Additionally, native people used to drink guayusa tea during an early morning ceremony that promotes body purification by drinking a large amount of beverage and then vomiting. During the ritual, people would talk about the dreams and analyse them to plan the activities of the day, based on the meanings that those dreams had revealed (Sequeda-Castañeda et al., 2016).
Several studies have been performed in the last years concerning the phytochemical composition of I. guayusa and the species has been reported as an interesting source of secondary metabolites, such as pentacyclic triterpenoid derivatives, xanthines, flavonoids, saponins and chlorogenic acid derivatives (Chianese et al., 2019; García-Ruiz et al., 2017; Pardau et al., 2017; Villacís-Chiriboga et al. 2018). In addition, preliminary preclinical assays allowed to partially confirm the traditional use of I. guayusa tea and beverages. A study performed by Chianese et al. (2019) showed the biological activity of ursolic acid, among the bioactive compounds of I. guayusa tea, as responsible for the activation of the G protein-coupled bile acid receptor (TGR5), which is responsible for the regulation of lipid and glucose metabolisms, suggesting that ursolic acid could be associated to the development of new treatments for diabetes and metabolic syndrome. In 1989, an in vivo study by Swanston-Flatt et al. (1989) performed on streptozotocin-induced diabetic mice, showed a preventive effect on hyperglycemia of the I. guayusa decoction. As reported by García-Ruiz et al. (2017), quercetin-3-O-hexose and chlorogenic acid were described as the main bioactive molecules of I. guayusa extracts obtained from green leaves and two different processed teas (blanched guayusa and fermented guayusa respectively). Chlorogenic acid and quercetin-3-O-hexose have been previously reported for their antioxidant, anticarcinogenic and anti-inflammatory activities (Santana-Gálvez et al., 2017; Sharma et al., 2018). A study performed by Gamboa et al. (2018) showed the potential of the total ethanol extract and its fractions for the treatment of chronic periodontitis, due to their inhibitory activities against P. intermedia, P. gingivalis and F. nucleatum.
Eventually, the toxicity profile of I. guayusa aqueous extracts and traditional herbal tea was shown to be safe (Bussmann et al., 2011; Wise and Santander, 2018), thus it already exists in the European Union regulations related to herbal infusions and food supplements obtained from dried guayusa leaves (EU, 2018).
The bioactivity of I. guayusa extracts can be strongly related to the phenolic compounds and also to the extraction methods. All these findings allow promoting I. guayusa derivatives as promising ingredients for functional foods, cosmetic or cosmeceutical formulations.
Several technics are used in order to extract the phenolic compounds, such as extractions performed by supercritical fluid, microwave and ultrasounds, among others (Ali et al., 2018; Cadena-Carrera et al., 2019; Cong-Cong et al., 2017; Chemat et al., 2017; Irakli et al., 2018; Rodsamran and Sothornvit, 2019; Severini et al., 2017). Especially, the ultrasound-assisted extraction (UAE) is described as an efficient, green, economically viable, easy to operate and widely applicable method, even if its industrial scale-up presents several challenges. Studies performed on phenolic compounds from fresh olives (Deng et al., 2017) and olive pomace (Goldsmith et al., 2018) demonstrated that the UAE can increase phenolic compound yield extraction compared to other methods of extraction, enhancing the antioxidant activity of the final extract. Generally, the UAE technology efficiency can be affected by ultrasonic wave frequency, temperature and sonication time, but also by solvent characteristics; and, the sample particles size also plays a role in the extraction process. The most relevant key factor in UAE is the creation of cavitation bubbles, which break plant tissues in order to release the cell content.
UAE generally enhances extraction efficiency obtaining plant derivatives richer in secondary metabolites compared to those obtained with other techniques. UAE promotes mass transfer and, possibly, rupture of cell walls through acoustic cavitation, improving the efficiency and optimising extraction yield (Jacotet-Navarro et al., 2015; Shirsath et al., 2012).
This extraction method was optimised using the response surface methodology. This approach involves several model designs like the Box Benheken design, in which the results were modelled by a polynomial equation and the coefficient of determination R2 and R2 adjusted allowed to determine the robustness of the model. With these premises, the aim of this study was to optimise the ultrasound-assisted extraction of total polyphenols from I. guayusa leaves using the response surface methodology.
2. Materials and methods
2.1. Plant material
On January 2019, I. guayusa fresh leaves were purchased in the Puyo local market, in the Amazonian region of Pastaza (Ecuador). Prior to the experiments, the fresh leaves were washed using distilled water and dried at room temperature for 48 h. The sample was crushed and sieved to obtain particles smaller than 0.5 mm. Moisture content was performed by gravimetric difference after oven drying at 105 °C and drying until constant weight (Horwitz, 2010). This value was used to determine the initial mass on a dry basis, prior to the extraction process. All chemical reagents and solvents were of analytical quality and purchased from Sigma-Aldrich.
2.2. Extracts
Ultrasound-assisted extraction (UAE) technique was applied in order to obtain the extracts, using a Branson 38000, CPXH series (Branson ultrasonic BV, Utrecht, The Netherland) ultrasound bath (with a tank capacity of 5.7 L; frequency of 40 kHz and 110 W). Ethanol was used as extraction solvent according to the methodology reported by Sun et al. (2011). For each extract, approximately 5 g of leaf sample were placed in a 100 mL Erlenmeyer flask and the corresponding solvent mixture was added. After undergoing sonication at the defined conditions for each experiment, mixtures were filtered through Whatman paper No. 4, at vacuum conditions and stored in amber glass bottles at 4 °C until use. In addition, all analysis were performed in the days after the extraction to avoid any changes in the samples due to prolonged storage, although it is well known that polyphenols are relatively stable when vegetal extracts are exposed to high temperatures (Li et al., 2013; Volf et al., 2014).
2.3. Determination of total phenolic compounds
As reported by Singleton and Rossi (1965), the determination of total phenols in the extracts was performed using the Folin-Ciocalteu method. The assay was carried out using a Genesys 10 UV scanning spectrophotometer. 40 μL of each hydroalcoholic extract and 500 μL of the Folin-Ciocalteu reagent were added in 10 mL volumetric flasks covered with aluminum foil. The mixture was allowed to stand for 10 min and then 500 μL of 10% Na2CO3 was added. The solution was completed to 10 mL with distilled water by making up to the mark and mixed thoroughly. After 2 h of rest in the dark at room temperature, the absorbance was recorded at 765 nm wavelength, measured against the blank that was prepared with 40 μL of distillated water. The content of total phenols was expressed in gram equivalents of gallic acid per 100 g of guayusa leaves based on dry mass (g gallic acid equivalents/100 g d.w.), calculated according to what was reported in previous studies by Abreu-Naranjo et al. (2018).
2.4. Antioxidant activity
The antioxidant activity was performed by the elimination tests with 2,2-azinobis (3- ethylbenzothiazoline)-6 sulfonic acid (ABTS) and the iron (III) antioxidant reduction power (FRAP), which are commonly used to measure antioxidant capacity, due to their simplicity and reliability, which means that the assays can be done quickly and the results are reproducible (Prior et al., 2005).
2.5. Free radical scavenging assay, 2,2-azinobis (3-ethylbenzothiazoline)-6 sulfonic acid
The ABTS radical cation discoloration assay, described by Re et al. (1999), was selected in order to determine the free radical scavenging activity. The ABTS radical was prepared by mixing solutions of 7 mM ABTS and 2.45 mM potassium persulfate, in equal parts. The solution was kept in the dark at room temperature for 16 h for the formation of the radical, which was diluted on ethanol to obtain a 0.873 absorbance. The preparation of potassium persulfate solution was carried out adding 0.663 g of the salt at distilled water and dilute to the mark at 100 mL. The ABTS solution was prepared dissolving 0.384 g in 10 mL of distilled water. Results were expressed in mmol TROLOX equivalents/100g of dry mass, calculated from the equation reported by Abreu-Naranjo et al. (2018).
2.6. Power of antioxidant reduction of iron (III)
The antioxidant capacity was calculated by the FRAP trial, according to Benzie and Strain (1996). 80 μL of each extract was placed in a 10 mL graduated flask, to which 5 mL of freshly prepared FRAP solution was added. After adding the reagent, distilled water was added to the flask until it completed 10 mL, and left at 37 °C for 30 min. Reading was recorded at 593 nm wavelength against the control solution. The FRAP reagent was prepared by mixing 2.5 mL of 2,4,6-pyridyl-s-triazine solution (TPTZ) with 2.5 of iron III chloride solution and 25 mL of acetate buffer. For the preparation of the TPTZ solution, 0.03 g of reagent was weighed and placed in a 10 mL graduated flask and diluted to the mark with 40 mM hydrochloric acid. Acetate buffer was prepared dissolving 0.0061 g of sodium acetate in 200 mL of distilled water, 40 Mm hydrochloric acid was added until the mixture reached a pH of 3.5, then it was diluted to the mark with distilled water to 250 mL. For the preparation of the iron (III) chloride solution, 0.1352 g were dissolved in 25 mL of distilled water. Results were expressed in μmol TROLOX equivalents/g of dried mass, calculated according to the equation reported by Abreu-Naranjo et al. (2018).
2.7. Experimental design
The experiments were carried out in two stages. The first one was through a factorial design at two levels and four factors (2ˆ4) with a total of 37 experiments, 16 tests, with 2 replicates for each one and 5 repetitions at the central point, to evaluate the curvature model. The four studied variables were sonication time, temperature, ethanol/water ratio and solid/liquid ratio on the extraction of total phenolic compounds. The significant and non-significant factors were analysed through the effects on plain paper (Daniel's chart).
In a second stage, the significant variables were analysed in order to select the optimal levels of the independent variables using a Box-Behnken Design (response surface methodology) and the effect of the independent variable interactions (Al-Dhabi et al., 2017). In Table 1 are shown the factor level and the central point of the design about the response surface methodology (low, medium, high) on independent coded and uncoded variables. The experimental data were adjusted using the following second-order polynomial equation:
| (1) |
where represents the predicted response and , and are the regression coefficients for mean, linear, interaction and quadratic terms, calculated respectively from the experimental results by the least squares method, and and are independent variables in coded values, ranging from -1 to 1. Design Expert version 10 software was used (Trial version, Stat-Ease Inc., Minneanopolis, MN, USA).
Table 1.
Level of variables selected in the factorial design and response surface methodology.
| Independent variable | Coded variable level |
|||
|---|---|---|---|---|
| Simbol | Low |
Central |
High |
|
| -1 | 0 | 1 | ||
| Sonication time (min) | A | 10 | 20 | 30 |
| Temperature (°C) | B | 30 | 45 | 60 |
| Ethanol/Water (v/v) | C | 70/30 | 80/20 | 90/10 |
| Solid/Liquid (w/v) | D | 1/20 | 1/12.5 | 1/5 |
The ANOVA analysis was applied to evaluate the relevance of independent variables' influence and interactions (p < 0.05). Additionally, the Pareto chart and the Bonferroni limit were used to improve the selection of significant factors (Anderson and Whitcomb, 2016). According to results, variables were selected for the optimisation of the extraction process. The model validity was determined by the coefficient of determination (R2), the significance (p) and the lack of adjustment tests.
2.8. Model validation
In order to validate the model, coefficient values of adjusted R2 and predicted R2 were evaluated. The validity of each experimental series was performed and the model validity was evaluated by analysis of variance (ANOVA) (DiNardo et al., 2019; Sousa et al., 2019). The extraction of phenolic compounds from I. guayusa dried leaves was optimised, considering as independent variables: ethanol/water ratio, temperature and extraction time. Optimal conditions were obtained applying the predictive equation of the surface response methodology. The antioxidant activity was performed after the polyphenolic compound extraction under optimal conditions. Finally, the Authors compared the experimental and predicted values in order to determine the validity of the model.
3. Results
3.1. Key factors affecting the ultrasound-assisted extraction of total phenolic compounds (TPC)
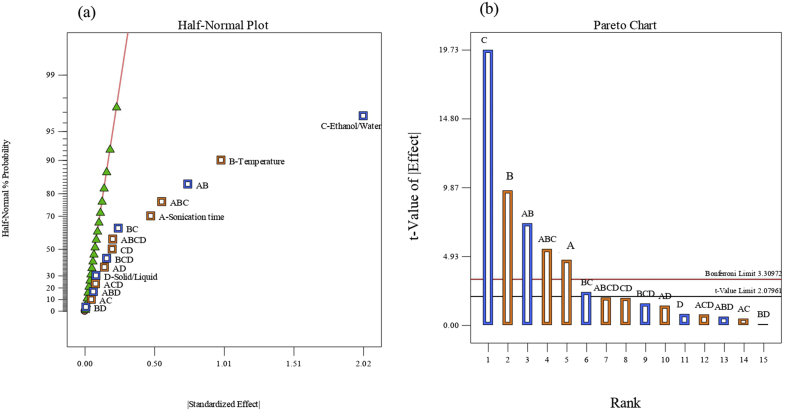
Figure 1a shows the estimation of positive and negative standardized effects on ultrasound-assisted extraction of TPC from I. guayusa leaves, considering that significant variables correspond to higher values of standardized effects (values on the right of the graph) according to (Whitcomb and Oehlert, 2007). Non-significant effects followed a normal distribution with mean equal to zero and constant variance. This means that, if effects are plotted on normal probabilistic paper, those effects that are not significant will form a straight line, while the active effects will appear far from the normality line.
Figure 1.
Standardized effects of positive (blue) and negative (orange) factors on ultrasound-assisted extraction of total phenolic compounds. Half-Normal Plot (a), Pareto Chart (b).
The Pareto graph and the Bonferroni limit (Figure 1b) strengthened the significant and non-significant variables (Anderson and Whitcomb, 2016). As shown in Figure 1, the ethanol/water ratio, temperature and time, were above the Bonferroni limit, which indicated that they were significant factors and the solid/liquid ratio was found below it, so it had no significant influence. In addition, according to results in Table 2, the significant (p < 0.05) and non-significant (p > 0.05) factors were confirmed. The interactions, time-temperature, temperature-ethanol/water ratio and time-temperature-ethanol/water ratio were significant, and the remaining interactions among factors were not. In factorial model a good adjustment was obtained with an R-Squared value of 0.9631. Moreover, the R-predicted was 0.8831 and the R-adjusted was 0.9367, with a difference of less than 0.2 that is considered as adequate, according to what exposed by Anderson and Whitcomb (2016) and Crespo et al. (2019).
Table 2.
Analysis of variance for the selected factorial model.
| Total phenolic compounds | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 50.75 | 15 | 3.38 | 36.53 | <0.0001 | Significant |
| A-Time | 1.83 | 1 | 1.83 | 19.75 | 0.0002 | |
| B-Temperature | 7.80 | 1 | 7.80 | 84.16 | <0.0001 | |
| C-Ethanol/Water | 32.53 | 1 | 32.53 | 351.19 | <0.0001 | |
| D-Solid/Liquid | 0.054 | 1 | 0.054 | 0.58 | 0.4557 | |
| AB | 4.46 | 1 | 4.46 | 48.17 | <0.0001 | |
| AC | 0.019 | 1 | 0.019 | 0.20 | 0.6590 | |
| AD | 0.16 | 1 | 0.16 | 1.78 | 0.1968 | |
| BC | 0.47 | 1 | 0.47 | 5.11 | 0.0345 | |
| BD | 5.723E-004 | 1 | 5.723E-004 | 6.180E-003 | 0.9381 | |
| CD | 0.32 | 1 | 0.32 | 3.44 | 0.0776 | |
| ABC | 2.49 | 1 | 2.49 | 26.90 | <0.0001 | |
| ABD | 0.032 | 1 | 0.032 | 0.34 | 0.5647 | |
| ACD | 0.049 | 1 | 0.049 | 0.53 | 0.4731 | |
| BCD | 0.20 | 1 | 0.20 | 2.19 | 0.1538 | |
| ABCD | 0.33 | 1 | 0.33 | 3.58 | 0.0725 | |
| Residual | 1.94 | 21 | 0.093 | |||
| Lack of Fit | 0.27 | 1 | 0.27 | 3.29 | 0.0849 | Not significant |
| Pure Error | 1.67 | 20 | 0.084 | |||
| R-Squared | 0.9631 | |||||
| Adj R-Squared | 0.9367 | |||||
| Pred R-Squared | 0.8831 | |||||
| Adeq Precision | 19.667 |
According to these results, the influential variables were selected for the optimisation of the extraction process. Other authors have found that in the extraction process of total polyphenolic compounds from the bark of Maytenus macrocarpa (Chuchuguaso), the factor solid/liquid relationship did not influence, while temperature and time were significant factors (Abreu-Naranjo et al., 2018).
It is important to remark that the positive or negative role of each factor in the mass transfer is not always obvious due to the chemical characteristics of the solvent, saturation effects and the diverse structure and composition of the natural products. Each material or solvent system shows different behaviour, which cannot be predicted (Pinelo et al., 2005).
In order to understand the effect of ethanol concentration on polyphenol extraction, in a previous study aimed at optimizing the extraction of phenolic antioxidant compounds from Ilex kudingcha (Sun et al., 2011), used a range of 50–90% ethanol concentration that directly influenced the polyphenol concentration. Increasing the ethanolic concentration, the polyphenol content was higher, until reaching a maximum of 70%, while higher concentration than 70% of ethanol caused a decrease content of TPC. It was also reported that time was a relevant factor on the extraction of this kind of compounds, with statistically significant differences in the studied range. As reported by Bazykina et al. (2002), the extraction of flavonoids and their glycosides from plant sources can be adequately performed using a polar solvent such as ethanol. Moreover, the solubility of the above mentioned compounds can be boosted mixing ethanol and water. Considering that the extraction system is a key factor, in order to optimize the whole extraction process, several hydroalcoholic mixtures have been tested as solvents to obtain phenolic compounds (Nour et al., 2016; Yu et al., 2005).
In a recent study (Zhang and Wang, 2016) aimed at optimization of TPC extraction from Vigna angularis, it was found that the optimal ethanol concentration for obtaining extracts was 40% in all the samples tested, therefore, ethanol concentrations between 20 and 60% were investigated. In fact, the authors state that the ethanol concentration was found to be the dominant factor in maximizing TPC extraction.
The extraction temperature also was a factor that influenced the process, recording significant differences between temperatures considered on this study. On the other hand, several authors such as Kim et al. (2007), Wang et al. (2008) and Trabelsi et al. (2010) have found that extraction temperature was an important factor concerning the polyphenols content obtained from plant materials.
Based on factorial experiments, the RSM design that included 17 essays was used to determine optimum levels of ethanol/water ratio, time and temperature. The optimisation objective was to find the best combination between these three independent factors, that significantly influenced the ultrasound-assisted extraction. Table 3 shows the experimental and predicted values used to build the model.
Table 3.
Box-Behnken experimental design based on independent variables (A, B and C) and experimental and predicted results of the TPC.
| Experiment | A |
B |
C |
TPC (g gallic acid equivalents/100 g d.w.) |
|
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Ethanol/Water (v/v) | Experimental∗ | Predicted | |
| 1 | 60 | 20 | 90/10 | 0.49 ± 0.01 | 0.51 |
| 2 | 30 | 20 | 90/10 | 0.85 ± 0.02 | 0.80 |
| 3 | 60 | 30 | 80/20 | 3.33 ± 0.06 | 3.21 |
| 4 | 45 | 20 | 80/20 | 2.02 ± 0.04 | 1.92 |
| 5 | 30 | 30 | 80/20 | 2.99 ± 0.06 | 2.94 |
| 6 | 30 | 20 | 70/30 | 2.34 ± 0.02 | 2.32 |
| 7 | 60 | 10 | 80/20 | 2.41 ± 0.05 | 2.46 |
| 8 | 60 | 20 | 70/30 | 2.17 ± 0.05 | 2.21 |
| 9 | 45 | 30 | 70/30 | 2.74 ± 0.03 | 2.81 |
| 10 | 45 | 20 | 80/20 | 2.05 ± 0.04 | 1.92 |
| 11 | 45 | 20 | 80/20 | 1.77 ± 0.03 | 1.92 |
| 12 | 30 | 10 | 80/20 | 3.02 ± 0.08 | 3.14 |
| 13 | 45 | 10 | 90/10 | 0.98 ± 0.02 | 0.91 |
| 14 | 45 | 20 | 80/20 | 1.82 ± 0.05 | 1.92 |
| 15 | 45 | 20 | 80/20 | 1.97 ± 0.04 | 1.92 |
| 16 | 45 | 30 | 90/10 | 1.23 ± 0.02 | 1.32 |
| 17 | 45 | 10 | 70/30 | 2.74 ± 0.05 | 2.65 |
TPC values were expressed as mean of three determinations ±SD.
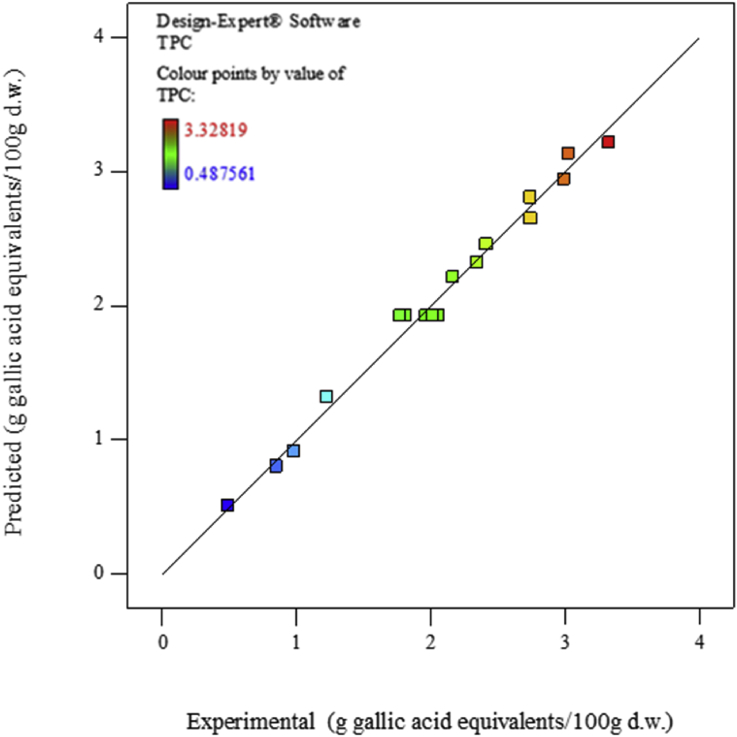
The polynomial second-order model presented the best fit with R2 value corresponding to 0.9731. The 97.31% of total variation on TPC extraction was determined by the studied factors. Predicted values of total polyphenol compounds concerning the quadratic model and those experimentally obtained were compared and are shown in Figure 2. Point distribution confirmed the ability of the model to cover the entire range of studied experiments. Point distribution verified the model suitability to cover the entire interval of analysed data, suggesting that the model can be applied successfully.
Figure 2.
Predicted and experimental relationship of TPC on I. guayusa leaves.
The Design-Expert Software generated a second-order polynomial equation to demonstrate the relationship between the three factors and the predicted response (significant terms were considered):
| (2) |
where A, B and C are coded values referring to temperature, extraction time and ethanol/water ratio respectively.
Statistical significance of regression equation, referred to quadratic polynomial model of surface response, was checked using the F test and ANOVA and data are showed in Table 4.
Table 4.
ANOVA of the response surface quadratic polynomial model.
| Total phenolic compounds | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 10.38 | 9 | 1.15 | 65.23 | <0.0001 | significant |
| A-Temperature | 0.082 | 1 | 0.082 | 4.62 | 0.0486 | |
| B-Time | 0.16 | 1 | 0.16 | 8.93 | 0.0203 | |
| C-Ethanol/Water | 5.21 | 1 | 5.21 | 294.60 | <0.0001 | |
| AB | 0.23 | 1 | 0.23 | 12.74 | 0.0091 | |
| AC | 8.388E-003 | 1 | 8.388E-003 | 0.47 | 0.5131 | |
| BC | 0.015 | 1 | 0.015 | 0.87 | 0.3818 | |
| A2 | 0.32 | 1 | 0.32 | 18.21 | 0.0037 | |
| B2 | 2.29 | 1 | 2.29 | 129.33 | <0.0001 | |
| C2 | 2.30 | 1 | 2.30 | 130.16 | <0.0001 | |
| Residual | 0.12 | 7 | 0.018 | |||
| Lack of Fit | 0.061 | 3 | 0.020 | 1.31 | 0.3867 | not significant |
| Pure Error | 0.062 | 4 | 0.016 | |||
| Cor Total | 10.50 | 16 | ||||
| R-Squared | 0.9882 | |||||
| Adj R-Squared | 0.9731 | |||||
| Pred R-Squared | 0.8972 | |||||
| Adeq Precision | 26.542 |
Fisher's F test value was 65.23, considered very high, and a low P < 0.0001, which showed that the model was highly significant. The determination coefficient (R2) was 0.9882, which suggested a satisfactory correlation between the experimental and predicted values.
The Adj R-Squared was 0.9731, which meant that most of the TPC variations (>97%) could be predicted by the model, while only 3% could not. The lack of fit of the model was not significant.
The F value of 1.31 and the P value of 0.3867 suggested that the lack of adjustment was negligible in relation to pure error due to noise.
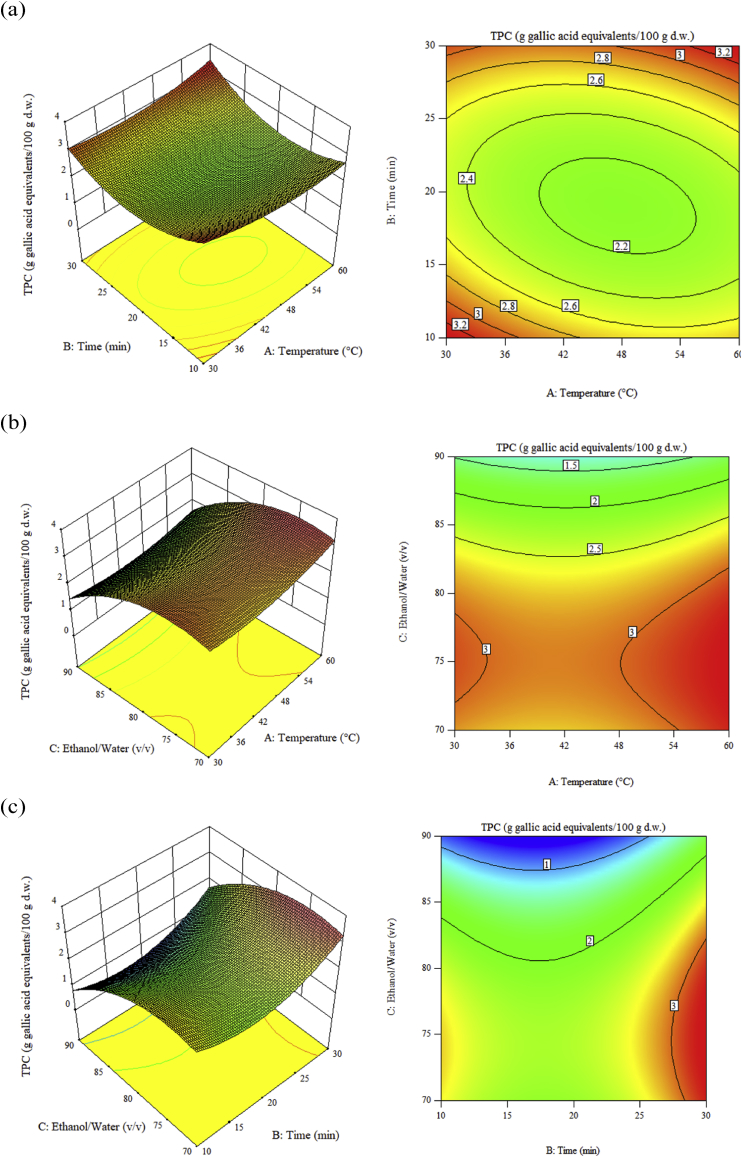
Graphical representations of regression equation simulated by the Design-Expert Software were represented through 3D response surface and 2D contour graphics.
Figure 3 graphically represents the TPC regression equation. In Table 4, both the figure and the data indicated that the extraction temperature-ethanol/water ratio and ethanol/water ratio-time interactions were not significant and only the extraction-extraction temperature interaction was.
Figure 3.
Response surface graphs (left) and contour graphs (right) show the effects of extraction time and temperature on ethanol/water ratio 76.8/23.2 v/v (a), ethanol/water ratio and temperature extraction at 29.9 min time (b), ethanol/water ratio and time at 60 °C temperature (c) total content of polyphenols.
The optimal levels of the studied variables were achieved analysing the surface contour response graphs. The optimal extraction conditions that provided the maximum TPC content (3.38 g gallic acid equivalents/100 g d.w.) were: ethanol/water ratio 76.8/23.2 v/v, extraction temperature 60 °C and extraction time 29.9 min.
At these experimental conditions, the TPC was 3.46 (±0.17) g gallic acid equivalents/100 g d.w., which corresponded to that predicted by the model. Villacís-Chiriboga et al. (2018) investigated I. guayusa extracts from adult and young leaves in Pastaza province, reported respectively 3.34 g gallic acid equivalents/100 g d.w. and 2.14 g gallic acid equivalents/100 d.w. Higher values (5.48 g gallic acid equivalents/100 g d.w.) were found by García-Ruiz et al. (2017) on fresh green leaves of the same species. These results can be explained by the stage of leaf maturity, which influences total polyphenol content.
Zhu et al. (2009) reported average values of total phenolic compounds corresponding to 10.3 g chlorogenic acid equivalents/100 g d.w. on species from the genus Ilex (I. kudingcha and I. cornuta), proceeding from different geographical regions of China.
By using the Soxhlet technique (Cadena-Carrera et al., 2019), the TPC value obtained for ethanol was 2.23 g gallic acid equivalents/100g; lower than those obtained by UAE.
Radical scavenger activity of the obtained extract at optimum conditions, measured through the FRAP and ABTS methods, was 0.080 mmol TROLOX equivalents/100 g d.w. and 40.71 μmol TROLOX equivalents/g d.w., respectively. Results were expressed on these units for a better comparison with values documented on literature available on Ilex species. Results were found to be lower than the data reported by Pardau et al. (2017) (5.44 g gallic acid equivalents/100 g d.w.). It has been shown that there is an high correlation between TPC and antioxidant activity (Sequeda-Castañeda et al., 2016).
The antioxidant activity of I. guayusa extract is scarcely documented and there are no reports on the FRAP and ABTS methods, although this bioactivity has been demonstrated through the 2,2- diphenyl-1-picrylhidrazyl radical (DPPH) and oxygen radical absorbance capacity (ORAC) methods (Dudonne et al., 2009; García-Ruiz et al. 2017; Sidali et al., 2016; Villacís-Chiriboga et al. 2018).
4. Conclusions
The ethanol/water ratio was the most influential variable in the ultrasound-assisted extraction of phenolic antioxidant compounds from I. guayusa leaves, followed by extraction temperature and sonication time, while solid/liquid ratio was not significant. Through the analysis of surface contour response graphs, the factor levels which provided the maximum theoretical content of TPC were: ethanol/water ratio 76.8/23.2 v/v, extraction temperature 60 °C and sonication time 29.9 min. The polynomial model presented the best fit, with R2 value of 0.9731. Results were validated experimentally reaching values very similar to those expected. These results provided valuable information on extraction process referred to antioxidant polyphenols in I. guayusa leaves extract. Antioxidant activity of this species was reported for the first time using the FRAP and ABTS methods.
Declarations
Author contribution statement
Yasiel Arteaga Crespo, Luis Ramon Bravo Sanchez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Matteo Radice, Yudel García Quintana, Laura Scalvenzi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Universidad Estatal Amazónica, Ecuadorian Republic.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abreu-Naranjo R., Arteaga-Crespo Y., Bravo-Sanchez L.R., Pérez-Quintana M.L., García-Quintana Y. Response surface methodology for optimisation of total polyphenol content and antioxidant activity of extracts from Maytenus macrocarpa bark by means of ultrasound-assisted extraction. Wood Sci. Technol. 2018;52:1359–1376. [Google Scholar]
- Al-Dhabi N.A., Ponmurugan K., Jeganathan P.M. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017;34:206–213. doi: 10.1016/j.ultsonch.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Ali A., Lim X.Y., Chong C.H., Mah S.H., Chua B.L. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT Food Sci. Technol. 2018;89:681–688. [Google Scholar]
- Anderson M.J., Whitcomb P.J. Productivity press; 2016. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments. [Google Scholar]
- Bazykina N., Nikolaevskii A., Filippenko T., Kaloerova V. Optimization of conditions for the extraction of natural antioxidants from raw plant materials. Pharm. Chem. J. 2002;36:46–49. [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bussmann R., Malca G., Glenn A., Sharon D., Nilsen B., Parris B., Dubose D., Ruiz D., Saleda J., Martinez M. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011;137:121–140. doi: 10.1016/j.jep.2011.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena-Carrera S., Tramontin D.P., Cruz A.B., Cruz R.C.B., Müller J.M., Hense H. Biological activity of extracts from guayusa leaves (Ilex guayusa Loes.) obtained by supercritical CO2 and ethanol as cosolvent. J. Supercrit. Fluids. 2019;152:104543. [Google Scholar]
- Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. a review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Chianese G., Golin-Pacheco S., Taglialatela-Scafati O., Collado J., Munoz E., Appendino G., Pollastro F. Bioactive triterpenoids from the caffeine-rich plants guayusa and maté. Food Res. Int. 2019;115:504–510. doi: 10.1016/j.foodres.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Cong-Cong X., Bing W., Yi-Qiong P., Jian-Sheng T., Zhang T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017;15:721–731. doi: 10.1016/S1875-5364(17)30103-6. [DOI] [PubMed] [Google Scholar]
- Crespo Y.A., Bravo Sánchez L.R., Quintana Y.G., Cabrera A.S.T., Bermúdez del Sol A., Mayancha D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Xu Z., Xiang C., Liu J., Zhou L., Li T., Yang Z., Ding C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017;37:328–334. doi: 10.1016/j.ultsonch.2017.01.023. [DOI] [PubMed] [Google Scholar]
- DiNardo A., Brar H.S., Subramanian J., Singh A. Optimization of microwave-assisted extraction parameters and characterization of phenolic compounds in Yellow European Plums. Can. J. Chem. Eng. 2019;97:256–267. [Google Scholar]
- Dudonne S., Vitrac X., Coutiere P., Woillez M., Merillon J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Dueñas J.F., Jarrett C., Cummins I., Logan–Hines E. Amazonian Guayusa (Ilex guayusa Loes.): a historical and ethnobotanical overview. Econ. Bot. 2016;70:85–91. [Google Scholar]
- EU . Official Journal of the European Union; 2018. Commission implementing regulation 2018/1023. [Google Scholar]
- Gamboa F., Muñoz C.-C., Numpaque G., Sequeda-Castañeda L.G., Gutierrez S.J., Tellez N. Antimicrobial activity of Piper marginatum Jacq and Ilex guayusa Loes on microorganisms associated with periodontal disease. Internet J. Microbiol. 2018 doi: 10.1155/2018/4147383. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan R.-Y., Zhang D., Wang M., Corke H. Health benefits of bioactive compounds from the genus Ilex, a source of traditional caffeinated beverages. Nutrients. 2018;10:1682. doi: 10.3390/nu10111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz A., Baenas N., Benítez-González A.M., Stinco C.M., Meléndez-Martínez A.J., Moreno D.A., Ruales J. Guayusa (Ilex guayusa L.) new tea: phenolic and carotenoid composition and antioxidant capacity. J. Sci. Food Agric. 2017;97:3929–3936. doi: 10.1002/jsfa.8255. [DOI] [PubMed] [Google Scholar]
- Goldsmith C.D., Vuong Q.V., Stathopoulos C.E., Roach P.D., Scarlett C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT Food Sci. Technol. 2018;89:284–290. [Google Scholar]
- Horwitz W. AOAC International; Gaithersburg (Maryland): 2010. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/edited by William Horwitz. 1997. [Google Scholar]
- Innerhofer S., Bernhardt K.-G. Ethnobotanic garden design in the Ecuadorian Amazon. Biodivers. Conserv. 2011;20:429–439. [Google Scholar]
- Irakli M., Chatzopoulou P., Ekateriniadou L. Optimization of ultrasound-assisted extraction of phenolic compounds: oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018;124:382–388. [Google Scholar]
- Jacotet-Navarro M., Rombaut N., Fabiano-Tixier A.-S., Danguien M., Bily A., Chemat F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015;27:102–109. doi: 10.1016/j.ultsonch.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Kim J.-M., Chang S.-M., Kim I.-H., Kim Y.-E., Hwang J.-H., Kim K.-S., Kim W.-S. Design of optimal solvent for extraction of bio-active ingredients from mulberry leaves. Biochem. Eng. J. 2007;37:271–278. [Google Scholar]
- Li S., Lo C.-Y., Pan M.-H., Lai C.-S., Ho C.-T. Black tea: chemical analysis and stability. Food Funct. 2013;4:10–18. doi: 10.1039/c2fo30093a. [DOI] [PubMed] [Google Scholar]
- Nour V., Trandafir I., Cosmulescu S. Optimization of ultrasound-assisted hydroalcoholic extraction of phenolic compounds from walnut leaves using response surface methodology. Pharm. Biol. 2016;54:2176–2187. doi: 10.3109/13880209.2016.1150303. [DOI] [PubMed] [Google Scholar]
- Pardau M.D., Pereira A.S., Apostolides Z., Serem J.C., Bester M.J. Antioxidant and anti-inflammatory properties of Ilex guayusa tea preparations: a comparison to Camellia sinensis teas. Food Funct. 2017;8:4601–4610. doi: 10.1039/c7fo01067b. [DOI] [PubMed] [Google Scholar]
- Patiño V.M. Guayusa, a neglected stimulant from the eastern Andean foothills. Econ. Bot. 1968;22:311–316. [Google Scholar]
- Pinelo M., Rubilar M., Jerez M., Sineiro J., Núñez M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005;53:2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Radice M., Scalvenzi L., Sablón N. Proceedings of MOL2NET 2016, International Conference on Multidisciplinary Sciences. 2017. Ilex Guayusa: a Systematic Review of its traditional uses, chemical constituents, biological activities and biotrade opportunities. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodsamran P., Sothornvit R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019;28:66–73. [Google Scholar]
- Santana-Gálvez J., Cisneros-Zevallos L., Jacobo-Velázquez D. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22:358. doi: 10.3390/molecules22030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster J., Mitchell E.S. More than just caffeine: psychopharmacology of methylxanthine interactions with plant-derived phytochemicals. Prog. Neuro Psychopharmacol. Biol. Psychiatry. 2019;89:263–274. doi: 10.1016/j.pnpbp.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Sequeda-Castañeda L., Modesti Costa G., Celis C., Gamboa F., Gutiérrez S., Luengas P. Ilex guayusa loes (Aquifoliaceae): Amazon and Andean native plant. Pharmacolog Onl. 2016;3:193–202. [Google Scholar]
- Severini C., Derossi A., Fiore A.G. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: a study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2017;243:835–847. [Google Scholar]
- Sharma P., Khan I.A., Singh R. Curcumin and quercetin ameliorated cypermethrin and deltamethrin-induced reproductive system impairment in male wistar rats by upregulating the activity of pituitary-gonadal hormones and steroidogenic enzymes. Int. J. Fertil. Steril. 2018;12:72. doi: 10.22074/ijfs.2018.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemluck M. Vol. 27. Harvard University; 1979. The Flowers of Ilex Guayusa. Botanical Museum Leaflets; pp. 155–160. [Google Scholar]
- Shirsath S., Sonawane S., Gogate P. Intensification of extraction of natural products using ultrasonic irradiations—a review of current status. Chem. Eng. Process: Process Intensification. 2012;53:10–23. [Google Scholar]
- Sidali K., Morocho P., Garrido-Pérez E. Food tourism in indigenous settings as a strategy of sustainable development: the case of Ilex guayusa Loes. in the Ecuadorian Amazon. Sustainability. 2016;8:967. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Sousa M.S.B., Júnior J.M.L., de Souza Buarque D. Optimization of the extraction of polyphenols and antioxidant capacity from Byrsonima crassifolia (L.) kunth fruit by response surface methodology. Plant Physiol. Asp. Phenolic Comp. 2019 IntechOpen. [Google Scholar]
- Sun Y., Xu W., Zhang W., Hu Q., Zeng X. Optimizing the extraction of phenolic antioxidants from kudingcha made frrom Ilex kudingcha CJ Tseng by using response surface methodology. Separ. Purif. Technol. 2011;78:311–320. [Google Scholar]
- Swanston-Flatt S.K., Day C., Flatt P.R., Gould B.J., Bailey C. Glycaemic effects of traditional European plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetes Res. 1989;10:69–73. [PubMed] [Google Scholar]
- Trabelsi N., Megdiche W., Ksouri R., Falleh H., Oueslati S., Soumaya B., Hajlaoui H., Abdelly C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT-Food Sci and Technol. 2010;43:632–639. [Google Scholar]
- Villacís-Chiriboga J., García-Ruiz A., Baenas N., Moreno D.A., Meléndez-Martínez A.J., Stinco C.M., Jerves-Andrade L., León-Tamariz F., Ortiz-Ulloa J., Ruales J. Changes in phytochemical composition, bioactivity and in vitro digestibility of guayusa leaves (Ilex guayusa Loes.) in different ripening stages. J. Sci. Food Agric. 2018;98:1927–1934. doi: 10.1002/jsfa.8675. [DOI] [PubMed] [Google Scholar]
- Volf I., Ignat I., Neamtu M., Popa V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014;68:121–129. [Google Scholar]
- Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. [Google Scholar]
- Whitcomb P., Oehlert G.W. Fall Techn Conf; 2007. Graphical Selection of Effects in General Factorials; p. 2036. [Google Scholar]
- Wise G., Negrin A. A critical review of the composition and history of safe use of guayusa: a stimulant and antioxidant novel food. Crit. Rev. Food Sci. Nutr. 2019:1–12. doi: 10.1080/10408398.2019.1643286. [DOI] [PubMed] [Google Scholar]
- Wise G., Santander D.E. Assessing the history of safe use of guayusa. J. Food Nutr. Res. 2018;6:471–475. [Google Scholar]
- Yu J., Ahmedna M., Goktepe I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005;90:199–206. [Google Scholar]
- Zhang H., Wang S. Optimization of total polyphenols extraction from Vigna angularis and their antioxidant activities. Indian J. Pharm. Sci. 2016;78:608–614. [Google Scholar]
- Zhu F., Cai Y.-Z., Sun M., Ke J., Lu D., Corke H. Comparison of major phenolic constituents and in vitro antioxidant activity of diverse Kudingcha genotypes from Ilex kudingcha, Ilex cornuta, and Ligustrum robustum. J. Agric. Food Chem. 2009;57:6082–6089. doi: 10.1021/jf901020h. [DOI] [PubMed] [Google Scholar]