Abstract
Sigma-1 receptors are ligand-regulated chaperone proteins, involved in several cellular mechanisms. The aim of this systematic review was to examine the effects that the sigma-1 receptor has on the cardiovascular system. The interaction targets and proposed mechanisms of action of sigma-1 receptors were explored, with the aim of determining if the sigma-1 receptor is a potential pharmacological target for cardiac pathologies. This systematic review was conducted according to the PRISMA guidelines and these were used to critically appraise eligible studies. Pubmed and Scopus were systematically searched for articles investigating sigma-1 receptors in the cardiovascular system. Papers identified by the search terms were then subject to analysis against pre-determined inclusion criteria. 23 manuscripts met the inclusion criteria and were included in this review. The experimental platforms, experimental techniques utilised and the results of the studies were summarised. The sigma-1 receptor is found to be implicated in cardioprotection, via various mechanisms including stimulating the Akt-eNOS pathway, and reduction of Ca2 + leakage into the cytosol via modulating certain calcium channels. Sigma-1 receptors are also found to modulate other cardiac ion channels including different subtypes of potassium and sodium channels and have been shown to modulate intracardiac neuron excitability. The sigma-1 receptor is a potential therapeutic target for treatment of cardiac pathologies, particularly cardiac hypertrophy. We therefore suggest investigating the cardioprotective mechanisms of sigma-1 receptor function, alongside proposed potential ligands that can stimulate these functions.
Keywords: Cardiac pharmacology, Sigma receptors, Ion channels, Cardioprotection
Cardiovascular disease (CVD) is considered a global issue and is the most common cause of death worldwide. In 2015, 25.2% of female and 27.4% of male deaths in the United Kingdom were caused by CVD, only second to cancer. Although incidences of CVD have reduced four-fold since 1970, it is still a current problem and an important disease which requires further intervention. CVD is also burdening the National Health Service; i2013/14, the NHS spent four billion pounds on treatment and secondary care for patients with CVD [1]. Management of CVD often involves a combination of surgical and pharmacological treatments, which have various limitations. Surgical intervention is often used to treat the primary cause of disease, which has high associated surgical, inpatient and discharge costs. Furthermore, the patient often requires chronic drug administration to manage their condition upon discharge [2].
Most drugs commonly prescribed for the treatment of CVD act on specific receptor classes within the body, albeit not specifically in the cardiovascular system. These include diuretics, β-adrenoreceptor blockers, antihypertensive drugs, anti-anginals, antiplatelet and lipid-lowering drugs [1]. Angiotensin-converting enzymes have been shown to increase within coronary endothelial cells and the valvular endothelium during vascular hypotension [3], [4]. ACE inhibitors (ACEi), such as benazepril, lower blood pressure and promote cardiac remodelling mechanisms following a myocardial infarction and acute/chronic heart failure [5]. However, prolonged ACEi usage may promote iatrogenic renal failure due to a lack of angiotensin II regulation of glomerular filtration rate, as well as angioedema due to the production of excess bradykinins [6]. Other drugs act on specific cellular receptors within the cardiomyocyte. β-blockers such as propranolol bind β-adrenoceptors, preventing the binding of adrenaline and noradrenaline to the receptor within the sino-atrial node (SAN) and therefore diminish their sympathetic effect. This impairs subsequent GsPCR activation and calcium entry into the cell, reducing heart rate and contractility of the cells [7]. However, β-blockers are contraindicated in patients with sinus bradycardia and have been related to heart failure, AV-node block and hypotension when used in high doses or for a prolonged period of time [8]. Clearly, existing treatment options are insufficient or bring about undesired side effects, warranting the need for the development of novel therapeutics.
Given the prevalence and severity of CVD, and the need for improved treatment options, an array of non-receptor channel constituents and ion channels have been screened for treatment of diseases. One such example is the sigma receptors (sigma-Rs). Sigma-Rs are ligand-gated chaperone proteins, involved in protein folding in the cell under physiological and stress conditions and binding of non-native proteins within the body. There are two distinct sub-types; Sigma-1R and Sigma-2R [9]. Sigma-1Rs are a 223 amino-acid length protein, found within the plasma membrane and intracellularly in the nuclear membrane, endoplasmic reticulum and mitochondrial-associated membrane [10]. They can translocate from the endoplasmic reticulum to other intracellular sites during increasing cellular stress. Sigma-1Rs possess two transmembrane domains and two hydrophobic steroid binding domain-like regions located on the C terminal of the receptor [11]. Sigma-1Rs have been shown to have a role in a number of cellular pathways, including ion channel activation, protein kinase A activation, neurotransmitter release, inositol-3-phosphate-mediated calcium-induced calcium release, and induction of cellular differentiation [12].
Sigma-1Rs have been widely studied in the CNS and have historically been targeted for the treatment of depression and schizophrenia with significant clinical improvements [13]. Sigma-1Rs have been shown to be neuroprotective against excess calcium currents in neurons. Specifically, they have been shown to play an important role in regulating intracellular calcium concentrations via mediation of L-type calcium channels, promoting or inhibiting the release of neurotransmitter at the neuronal synapse. When known sigma-1R agonists are applied, calcium currents via voltage-gated calcium channels (VGCC) decrease [14]. The application of such agonists with verapamil, a selective VGCC blocker, showed no overall decrease in calcium currents, illustrating a relationship between sigma-1R activation and calcium channels [14]. Similarly, sigma-1R activation also seems to inhibit sodium influx via GiPCR or the Nav1.5 channel without requiring ATP as a co-ligand. By inhibiting sodium channels, this results in a decreasing action potential (AP) firing rate, delayed AP latency and longer refractory periods. This seems to provide a protective mechanism to prevent neuronal excitability, though the exact mechanism is still unclear [15].
Recently, the endogenous molecule choline has been shown to modulate sigma-1Rs. Choline is produced in cells by phospholipase D from phosphatidylcholine and has been shown to activate sigma 1-Rs [16]. In doing so, it potentiates the calcium signals evoked by IP3Rs. Choline itself has been described as a cardioprotective agent [17] and its ability to modulate sigma-1Rs may provide a mechanism for understanding this effect.
While sigma-1Rs have been extensively studied in the CNS, they have been found to be present in multiple tissues, including the cardiovascular system and the kidneys, among many others [18]. It is interesting to note that the sigma-1R protein levels are higher in heart tissue than brain tissue in rats [18]. The first report of sigma-1R function in the heart showed that increased contractility was mediated by sigma-1R ligands in cultured cardiomyocytes from neonatal rats [19]. Subsequently, sigma-1R was detected in the membranes of adult rat ventricular cardiomyocytes [20]. Further studies using isolated rat cardiomyocyte preparations revealed that 80% of sigma-Rs in rat ventricular myocardium are sigma-1Rs, while the remaining 20% are sigma-2Rs [20]. As such, this review will mainly focus on the potential cardioprotective role of sigma-1Rs and its mechanism of action. We describe current studies considering the impact of sigma-1R agonists in treating various cardiac pathologies, including cardiac hypertrophy, and identify opportunities for further development of sigma-1R agonists as novel and safe therapeutic targets. In undertaking this critical review, we aim to (1) Establish whether sigma-1Rs are a useful area of study in cardiovascular science, (2) Describe the roles of sigma-1R in the cardiovascular system, (3) Discuss current evidence for sigma-1R as a therapeutic target in the heart, (4) Summarise whether sigma-1R is a suitable target for pharmacological studies into cardiovascular disease and suggest possible future directions.
1. Research question and search strategy
Using the PICO structure (Population, Intervention, Comparator and Outcomes), the following research question was formulated: Is the Sigma-1R a potential pharmacological target for cardiac pathologies? The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were then used to perform this systematic review [21].
Two databases (PubMed and Scopus) were searched in September 2018. The search was performed using the terms “sigma-1 receptor” AND “cardiac” to obtain the primary list of papers from each database. For PubMed, the MeSH terms applied are: (“sigma-1 receptor”[Supplementary Concept] OR “sigma-1 receptor”[All Fields] OR “sigma 1 receptor”[All Fields]) AND (“heart”[MeSH Terms] OR “heart”[All Fields] OR “cardiac”[All Fields]) AND (“1988/01/01”[PDAT] : “2018/12/31”[PDAT]). For Scopus, a similar search was performed using the search terms “sigma-1 receptor” AND “cardiac” under “Article abstract, title, keywords”.
2. Article selection and processing
Pre-defined inclusion and exclusion criteria were applied to the journal title and journal abstract. For each of the databases, papers were first excluded based on exclusion criteria of paper is not in English or is not an original research paper (i.e. it is a review article, editorial or book chapter). Next, the papers were screened for inclusion. The inclusion criteria were specified as “the article investigates Sigma-1R in relation to its interactions with pathways, organelles, channels and proteins present in the heart”. After the search methodology had been defined, two independent members of the review team independently screened the databases using the search methodology to ensure that the same papers were obtained. Full papers were then sourced from the database. If unavailable, the authors were contacted via email to obtain the full papers, or the papers were purchased from the journal publishers.
3. Quality appraisal
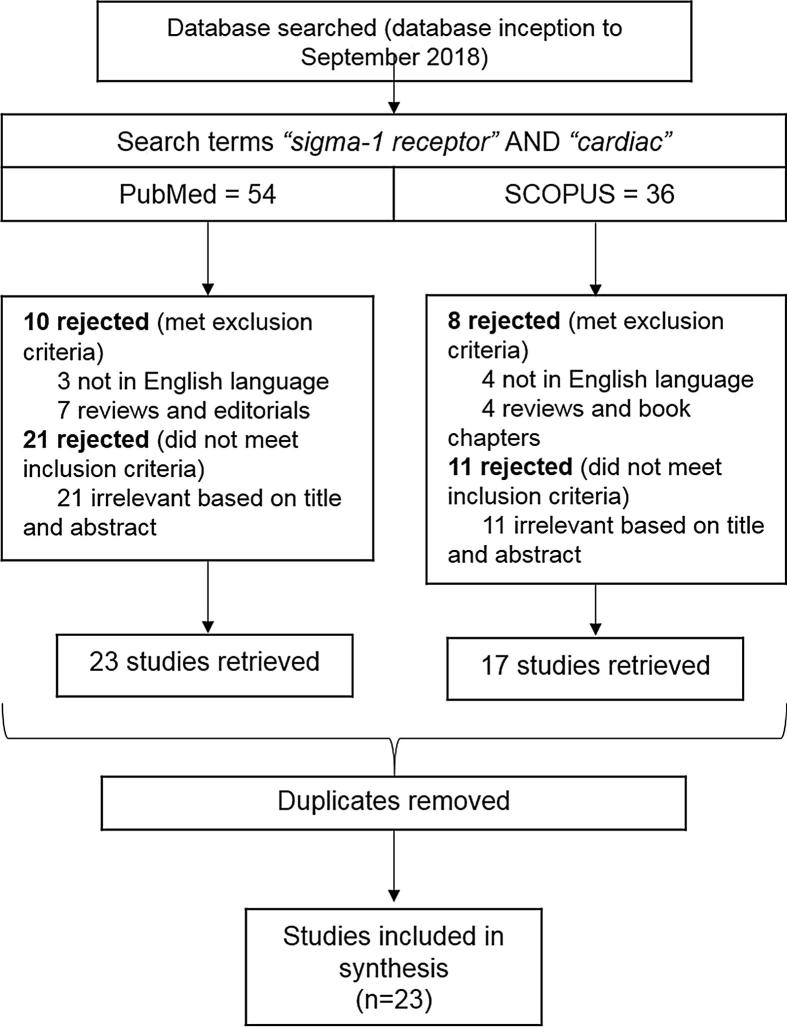
The Checklist of Review Criteria established by the Task Force of Academic Medicine and the GEA-RIME Committee [22] was used to evaluate the quality of all included studies. Relevance of the study was also a criteria in the quality appraisal, and this second screening of relevance is applied to the entire paper, while the previous screening for inclusion was applied only to the paper title and abstract. The search for “sigma-1 receptor” AND “cardiac” resulted in 54 titles in PubMed and 36 in Scopus. After screening using the inclusion and exclusion criteria, 23 papers were retrieved from PubMed and 17 were retrieved from Scopus. Subsequently, duplicates were removed, and a total of 23 papers remained that focused on the role of Sigma-1R related to the heart. The search attrition methodology and the number of papers that resulted from each screening step is summarised in Fig. 1. The 23 papers were subsequently put through the quality appraisal, with a total of 13 criteria being assessed. 22 out of 23 papers met at least 12 out of 13 criteria, reflecting adequate scientific robustness and relevance for subsequent analysis (Table 1). Subsequently, the 22 papers were included in the review, and the study characteristics of each paper has been summarised (Table 2).
Fig. 1.
Study attrition methodology Search was carried out on 2 databases: PubMed and Scopus, in September 2018.
Table 1.
. Table summarising the results of the quality appraisal of the 23 papers. A total of 13 criteria were assessed, based on the quality checklist formulated by The Checklist of Review Criteria established by the Task Force of Academic Medicine and the GEA-RIME Committee [22] was used. 22 out of 23 papers met at least 12 out of 13 criteria and were included in the study.
| Paper | Problem statement, conceptual framework, and research question | Reference to the literature and documentation | Relevance | Research design | Instrumentation, data collection, and quality control | Population and sample | Data analysis and statistics | Reporting of statistical analyses | Presentation of results | Discussion and conclusion: interpretation | Title, authors and abstract | Presentation and documentation | Scientific conduct | Total criterla met |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gao et al., 2018 [59] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Qin et al., 2018 [60] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 11 | ||
| Liu et al., 2017 [61] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Alam et al., 2017 [49] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Bao et al., 2017 [62] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Shinoda et al., 2016 [27] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Stracina et al., 2015 [63] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Balasuriya et al., 2014 [51] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Tagashira et al., 2014 [28] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Tagashira et al., 2013 [29] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Amer et al., 2013 [64] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Delaunois, et al., 2012 [65] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Tagashira et al., 2011 [24] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Crottès et al., 2011 [66] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Novakova et al., 2010 [67] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Johannessen et al., 2010 [52] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Tagashira et al., 2010 [23] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Bhuiyan et al., 2010 [18] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Bhuiyan and Fukunaga, 2009 [25] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Johannessen et al., 2009 [53] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Fontanilla et al., 2009 [68] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| Zhang and Cuevas, 2005 [58] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 13 |
| Zhang and Cuevas, 2002 [57] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 12 |
Table 2.
A summary table of the 22 research papers analysed following a systematic search protocol for papers including “sigma-1 receptor” and “cardiac”. For each paper, the methods, materials, results, limitations and further work suggested are summarised.
| Author(s) | Material used | Techniques used | Results and discussion | Limitations suggested by authors | Further work suggested by authors |
|---|---|---|---|---|---|
| Gao et al., 2018 [59] | Adult male Sprague-Dawley rats (220-250g) | Intraperitoneal injection of PRE-084 or saline, LAD ligation to induce myocardial ischaemia, followed by reperfusion, haemodynamic measurements, TUNEL assay, Western blotting | Treatment with Sigma-1R agonist PRE-084 increased recovery of haemodynamic function and reduced apoptosis, after myocardial I/R injury; cardioprotective mechanism likely via Akt-eNOS pathway and decreasing apoptosis via changing expression of apoptotic-related proteins | Animals pre-treated with PRE-084 (vs in clinical setting, they will be treated after MI). Previous studies showed PRE-084 protects the heart by stimulating Sigma-1R in brain | Sigma-1R interacts with ion channels, including Ca2+, K+, Na+, Cl− channels, potential mechanisms to be elicited; PRE-084 to treat myocardial I/R injuries |
| Liu et al., 2017 [61] | Homozygous Sigma-1R KO mice, HEK293 cells | CRISPR/Cas9, Western blotting, immunohistochemistry, electrophysiology, electroretinogram, intravitreal injection | Sigma-1R selective ligands potently inhibited Kv2.1 currents, but in a sig-R independent manner, as the ligands exerted similar effect in WT and Sigma-1R KO cells | Therapeutic opportunities – conciliating drug effects on Sig-Rs and the Kv2.1 channel | |
| Alam et al., 2017 [49] | Primary neonatal rat ventricular cardiomyocytes (NRCs) from 1 to 2-day old Sprague-Dawley rat pups | Adenovirus-mediated Sigma-1R overexpression, siRNA-mediated sigma-1R knockdown, tunicamycin, immunocytochemistry, Western blotting, immunoprecipitation, LDH release assay | Sigma-1R found to mediate the IRE1a-XBP1 response to ER stress in cardiomyocytes. Overexpression of sigma-1R led to increase in IRE1a phosphorylation, increase XBP1 expression and nuclear localisation, decrease in CHOP expression | Study the direct role of Sigma-1R in specific ER-response pathways; study pathway-specific gene activation programmes in vivo in various pathological conditions | |
| Bao et al., 2017 [62] | Neonatal rat cardiomyocytes, adult male Sprague-Dawley rats | Bioinformatic analysis, TAC, echocardiography, histological staining, transfection, dual-luciferase reporter assay, immunofluorescence, qRT-PCR, Western blotting | miR-297 negatively and directly regulates Sigma-1R expression. Increased miR-297 observed in hypertrophic models, correlated with decreasing Sigma-1R levels. miR-297 promotes CM hypertrophy by inhibiting the expression of Sigma-1R and activation of ER stress signalling, specifically XBP1 and ATF4 pathways | Precise mechanism by which Sigma-1R induces splicing of Xbp1 by IRE1a not explored; activation of ATF4 not explored in the study | Sigma-1R and inhibition of miR-297 as a potential treatment for cardiac hypertrophy |
| Shinoda et al., 2016 [27] | Neonatal rat ventricular cardiomyocytes from Wistar rats, ICR mice | TAC-induced hypertrophy, ANGII-induced hypertrophy mitochondrial staining, immunocytochemistry, Western blot, qRT-PCR, echocardiography, morphological analysis, histological analysis, ATP content assay, ratiometric dyes for intracellular and mitochondrial Ca2+ measurement, TUNEL staining for apoptosis | Treating cardiomyocytes and mice with haloperidol altered the mitochondrial calcium transport via inhibiting Sigma-1R expression. This leads to impaired mitochondrial ATP production and hence adverse cardiac remodelling and impairment of cardiac function. ATP supplementation with sodium pyruvate rescues ATP levels in haloperidol-treated TAC mice and recovers cardiac function. | Suggests supplementing schizophrenic patients treated with haloperidol with ATP supplementation (eg sodium pyruvate) may prevent adverse effects on cardiac function | |
| Stracina et al., 2015 [63] | Guinea pigs | Haloperidol administration, qRT-PCR of Sigma-1R and IP3R expression, immunohistochemical staining, Langendorff-perfusion, ECG (to study QT interval and detect arrhythmia) | Increased expression Sigma-1R in atrial cardiomyocytes in guinea pigs exposed to haloperidol. Increased signalling via Sigma-1R and IP3 receptor occurred in haloperidol-treated groups. Chronic haloperidol administration significantly decreased heart rate over time. Exposure to haloperidol increased the QT interval, suggested to be related to IKr activity. Haloperidol in guinea pig hearts increases gene expression of Sigma-1R and IP3Rs. This may lead to altered calcium handling, such as affecting the availability of calcium ions in cardiomyocytes which subsequently lowers the arrhythmogenic threshold. | Further studies need to understand how acute and chronic haloperidol treatment affects the IKr current, calcium availability and if its influences the co-localisation of Sigma-1R and IP3Rs. | |
| Balasuriya et al., 2014 [51] | tsA 201 cells (HEK-293 subclone) | Cell culture, transient transfection with DNA encoding sigma-FLAG, immunofluorescence, in situ proximity ligation assay, AFM imaging of isolated proteins, extracellular HTRF | Sigma-1R was shown to promote hERG protein expression within the plasma membrane. Sigma-1R ligands showed no effect on hERG expression. Sigma-1R likely binds to an immature hERG protein, seems to occur in the ER and its expression is enhanced with Sigma-1R is co-expressed, albeit this seems to have no effect on its activation within the cell. Sigma-1R seems to reduce expression of mature hERG in cells. | Studies into the interactions with sigma-1R to understand its mechanisms of action with selective ligands and cholesterol. With a suggestion that hERG is involved in cardiac arrhythmias, a clearer understanding of this interaction may provide potential therapeutic approaches. | |
| Tagashira et al., 2014 [28] | Adult male ICR mice, neonatal rat ventricular cardiomyocytes | TAC, ANGII-treatment to induce hypertrophy, RNAi by siRNA, morphological analysis of cells, mitochondrial staining, immunocytochemistry, Western blotting, ratiometric measurement of intracellular and mitochondrial Ca2+ (by FURA2 and pericam), measurement of ATP content in vitro and in vivo | Imbalance of Sigma-1R and IP3R2 expression was found in hypertrophic models, leading to dysregulation of IP3R2 function due to dissociation from Sigma-1R. Fluvoxamine (Sigma-1R agonist) leads to upregulation of Sigma-1R, thereby (1) stabilising the Sigma-1R/ IP3R2 complex on MAMs, allowing for Ca2+ transport into mitochondria and hence ATP production, as well as (2) suppressing intracellular Ca2+ overload by inhibiting IP3R2 and RyR-mediated Ca2+ release from SR. | Further studies to define the regulatory role of RyR-mediated Ca2+ release into cytosol by Sigma-1R, to determine potential interaction between Sigma-1R and TRPCs in CMs, explore mechanisms underlying Sigma-1R mediated inhibition of fission or enhancement of mitochondrial elongation | |
| Tagashira et al., 2013 [29] | Female adult Wistar rats, primary culture of neonatal rat ventricular cardiomyocytes | Bilateral ovariectomy, abdominal aortic banding, heart weight measurement, qRT-PCR, histological techniques (Masson’s trichome staining), haemodynamic measurements, immunohistochemistry, ANGII treatment to induce CM hypertrophy, cross-linking and immunoprecipitation, Western blot, ATP content measurement, intracellular Ca2+ measurement using FURA2 | Pentazocine, a Sigma-1R agonist, prevented downregulation of Sigma-1R in hypertrophy. Sigma-1R stimulation with pentazocine was found to be cardioprotective in inhibiting cardiac hypertrophy and dysfunction, by restoring IP3R-mediated Ca2+ entry into mitochondria and hence ATP production, as well as inhibiting RyR-mediated Ca2+ leakage into cytoplasm. | Did not define the association regions between Sigma-1R and RyR2 (- to be done in future studies) | Studies should identify the mechanism where RyR2-mediated calcium release by sigma-1R is regulated and the associated molecular regions between these two receptors. Further studies required to identify mechanisms underlying regulation of RyR2-mediated Ca2+ release into cytosol by Sigma-1R. |
| Amer et al., 2013 [64] | Endothelial cells (from human saphenous vein) and HEK-293 cells | Application of Sigma-1R agonist and antagonist, siRNA to decrease Sigma-1R expression, fluorescence microscopy, measurement of intracellular Ca2+, whole-cell patch clamp | Multiple Sigma-1R agonists and antagonists applied with different results. Some ligands were inhibitory, some were stimulatory while some did not exert effects on transient calcium channels. Specifically, BD1047/BD1063 and 4-IBP are inhibitors of receptor/chemically-activated calcium entry channels. They seem to act directly and independently of Sigma-1R. | Sigma-1R ligands exert pharmacologically effect rather independently of Sigma-1R: important information for developing therapeutic drugs | |
| Delaunois, et al., 2012 [65] | Conscious tethered Sprague-Dawley rats with intra-arterial catheter and tethers | Repeated handling and dosing with sigma-1R agonists and antagonists, HR, SBP, DBP, MAP recorded | Endogenous Sigma-1R activation may contribute to stress-induced tachycardia. 3 out of 4 agonists decrease this phenomenon; antagonists have no effect. Neither had effect on HR during and after the dosing procedure. Sigma-1Rs are involved in the stress response but not in its initiation. Findings suggest that mechanisms underlying HR regulation involving Sigma-1Rs differ according to different conditions. | Did not evaluate the anxiolytic properties of the Sigma-1R ligands | Further studies should look at the possible link between the baroreflex function of the heart and sigma-1R activation. Studies on cardiac contractility in vivo should be assessed to understand the effects of drugs acting on Sigma-1Rs. |
| Tagashira et al., 2011 [24] | Female Wistar rats | Bilateral ovariectomy, TAC (abdominal aorta) in rats, oral DHEA and subcutaneous E2 administration, haemodynamic measurements, Western blot, qRT-PCR, heart weight measurement | PO-induced hypertrophy reduced Sigma-1R expression; DHEA and E2 cardioprotective, act via increasing p-Akt, eNOS and p-eNOS. DHEA increased Sigma-1R (mRNA and protein), E2 did not. DHEA likely act via Sigma-1R, while E2 does not | DHEA(S) not found normally circulating in mice or rats. DHEA administered in the experiment is of a higher concentration that normal human circulating levels. DHEA may have acted via other Sigma-1R-independent mechanisms, such as PPARα. | To determine the localisation of Sigma-1R in cardiomyocytes after DHEA treatment (to elicit if the mechanism of action is via stabilising IP3R); DHEA as potential hormonal therapy candidate as compared to estrogens (E2) |
| Crottès et al., 2011 [66] | K562 and HEK 293 cell lines, Xenopus laevis | cDNA preparation, patch clamp, double electrode voltage clamp, adhesion experiments, Western blot (hERG, Sigma-1R), shRNA lentiviral transduction, co-immunoprecipitation, flow cytometry, transfection of HEK 293 cells with hERG1 and Sigma-1R, qRT-PCR, pulse chase | In HEK cells expressing hERG and sig1R, both proteins co-immunoprecipitate, indicating physical association. Sigma-1R expression enhances hERG protein maturation and stability. Sigma-1R controls hERG expression through the regulation of subunit trafficking activity | Sigma-1R to be considered as a new pharmacological target to reduce membrane signalling complexes activity with implications in cancer treatment | |
| Novakova et al., 2010 [67] | Cardiomyocytes | Chronic administration of haloperidol, Langendorff-perfusion, ECG, qRT-PCR, Western blot | Haloperidol treatment affects expression of IP3R1 and 2 in atria but not in ventricles. Increase in IP3R may be involved in arrhythmia, but increase is likely due to upregulation at neuronal level rather than cardiomyocyte level | Did not prove the direct link between QTc interval changes and arrhythmias | Further verification needed regarding the theory that increase in IP3R gene expression in neuronal cells rather than cardiomyocytes contribute to arrhythmogenesis |
| Johannessen et al., 2010 [52] | HEK293 cells | siRNA to knockdown Sigma-1R, electrophysiology, drug application of both agonists and antagonists, Sig-R competitive binding assays, photoaffinity labelling | Progesterone binds to Sigma-1R and Sig-2Rs, and blocks photolabeling of these 2 receptors in HEK293 cells; Progesterone inhibits the channel modulation effects of Sig-R ligands. Progesterone binding to Sig-Rs block Sig-R-mediated modulation of a voltage-gated ion channel | Since Sig-Rs modulate a wide range of ion channels, necessary to test other channels for Sig-R/progesterone interactions in the future. Furthermore, other steroids that bind to Sig-R, such as testosterone, DHEA and cholesterol should also be tested | |
| Tagashira et al., 2010 [23] | Adult male ICR mice and murine neonatal ventricular myocytes | TAC (aortic arch), oral fluvoxamine and paroxetine maleate, heart weight measurement, echocardiography, Western blot, morphological analysis, si-RNA transfection, Western blot | Fluvoxamine treatment protects against TAC-induced cardiac hypertrophy and ANG-II induced cardiomyocyte hypertrophy, via increased expression of sigma-1R, likely mediated by the Akt-eNOS signalling pathway | Mechanism in which fluvoxamine treatment causes upregulation/ stabilisation of Sigma-1R is to be elucidated; to understand how chronic fluvoxamine treatment affects Sigma-1R; potential for development of a new class of antihypertrophic drugs | |
| Bhuiyan et al., 2010 [18] | Adult male and female Wistar rats | Bilateral ovariectomy in female rats, abdominal aortic banding, oral fluvoxamine and NE-100 administration, haemodynamic measurements, Western blot, heart weight measurement, immunohistochemistry | Sigma-1R expression in the heart can attenuate PO-induced hypertrophy in ovariectomised rats; Sigma-1R agonist fluvoxamine treatment protects against PO-induced hypertrophy via upregulating Sigma-1R and stimulating Sigma-1R mediated Akt-eNOS signalling | To understand how chronic fluvoxamine treatment affects Sigma-1R; potential for development of a new class of antihypertrophic drugs | |
| Bhuiyan and Fukunaga, 2009 [25] | Female Wistar rats | Bilateral ovariectomy, abdominal aortic banding in rats, oral DHEA administration, haemodynamic measurements, Western blot, heart weight measurement | Sigma-1R expression in the heart can attenuate PO-induced hypertrophy in ovariectomised rats; Sigma-1R agonist DHEA treatment protects against PO-induced hypertrophy via upregulating Sigma-1R amd stimulating Sigma-1R mediated Akt-eNOS signalling | DHEA(S) not found normally circulating in mice or rats; administered DHEA in the experiment is also higher concentration than normal human circulating levels | Potential for development of a new class of antihypertrophic drugs |
| Johannessen et al., 2009 [53] | Neonatal mouse cardiac myocyte | siRNA to knockdown Sigma-1R, electrophysiology, drug application of both agonists and antagonists, photoaffinity labelling | Sigma-1R ligands (SKF-10047, pentazocine, haloperidol and ditoylguanidine) reversibly inhibited Nav1.5 in HEK293 and COS-7 cells, also inhibited Na+ current in neonatal mouse cardiac myocytes; Sig-Rs inhibits Nav1.5 channels and thus the INa, likely inhibit contractility and rhythmicity | Other research showed Sigma-1R to inhibit inwardly rectifying K+ and hERG K+ channels, which will stimulate contractility and rhythmicity, while inhibiting Na+ channels have the opposite effect – investigate how the opposing effects are resolved. Potential future applications for anti-arrhythmics | |
| Fontanilla et al., 2009 [68] | HEK293 cells | Competitive binding assays, electrophysiology, drug application, photoaffinity labelling | DMT bound to sigma-1R, inhibited Na+ channels in native cardiac myocytes and heterologous cells that express sigma-1Rs. DMT induced hypermobility in WT mice but not in sigma-1R knock-down mice, suggesting that DMT is an endogenous agonist for Sigma-1R | ||
| Zhang and Cuevas, 2005 [58] | Neonatal rat intracardiac ganglia | Electrophysiological techniques (whole-cell patch-clamp) | Stimulation of sigma-1Rs inhibit multiple voltage-gated K+ channel subtypes via direct coupling (delayed outwardly rectifying potassium channels, large conductance Ca2+-sensitive K+ channels, and the M-channel) and depresses excitability in intracardiac neurons | Inhibition of voltage-gated K+ channels alone cannot explain the complete block of action potential firing at high concentrations of sig ligands – sig-Rs likely to affect other channel types that affect AP firing (e.g. voltage-gated Na+ channels) | Sig-R modulation of other mechanisms of Ca2+ entry or homeostasis must be examined to confirm a direct effect of sigma-1R on Ik(Ca) |
| Zhang and Cuevas, 2002 [57] | Neonatal rat intracardiac ganglia | RT-PCR and gel electrophoresis of total RNA extracts and single-cell RNA extracts, electrophysiological studies (voltage-clamp using perforated-patch configuration of whole cell patch-clamp recording technique, electrophysiological methods), bath application of sigma-1R agonists | Sigma-1Rs found to be expressed in neonatal rat intracardiac neurons. Application of Sig-R agonists inhibited all Ca2+ channel subtypes in the neurons. Sig-R activation depressed the peak Ca2+ channel current, increased the rate of Ca2+ channel inactivation, and shifted the voltage-dependence of steady-state inactivation and activation towards more negative potentials. However, modulation of Ca2+ channel activity is likely only via the Sig-2R | Function of Sigma-1R in autonomic neurons remains to be elucidated. |
4. Experimental platforms, techniques and results
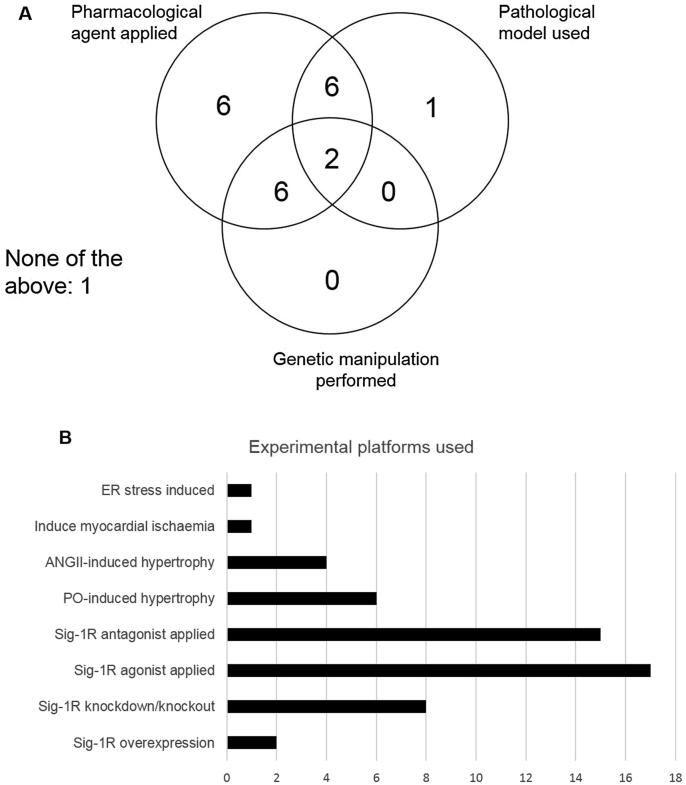
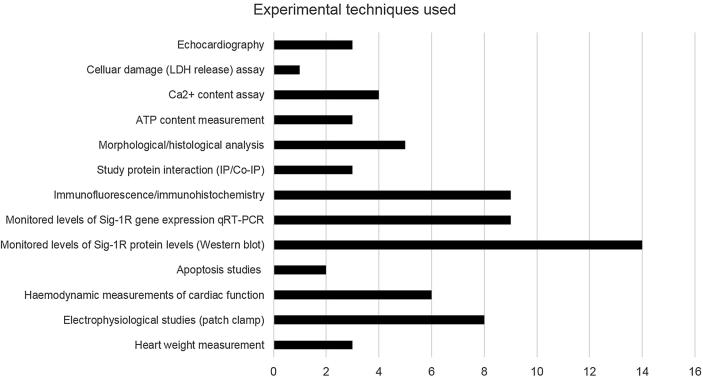
In the identified studies, various experimental platforms have been used to investigate the role of sigma-1R in relation to the heart. Common platforms included over or under-expressing sigma-1R, application of agonists or antagonists, and induction of pathology (hypertrophy, ischaemia, etc). Application of sigma-1R ligand (agonist and antagonist) was the most common platform employed to study downstream effects of sigma-1R, with 77% and 68% of papers applying a sigma-1R agonist and antagonist, respectively (Fig. 2). Various experimental techniques were used, including heart weight measurements, electrophysiological studies, haemodynamic measurements, gene and protein expression studies, protein interaction studies and Ca2+ content assays (Fig. 3). The most commonly used experimental technique was studying protein expression via Western blotting, with 64% of papers employing this technique. 11 papers demonstrated the role of sigma-1R in cardioprotection. Specifically, 8 papers discussed the cardioprotective role of sigma-1R against hypertrophy, while 2 papers discussed the cardioprotective role of sigma-1R against apoptosis and cellular toxicity. Sigma-1R is proposed to play modulatory roles in the Akt-eNOS pathway, the ER stress response pathway, to aid in restoration of IP3R-mediated mitochondrial ATP production and reduce calcium leakage into the cytosol. Sigma-1R is found to interact with various sodium, potassium and calcium channels in cardiomyocytes, with implications on arrhythmia, contractility, calcium handling and ATP production. In addition, 2 papers also studied the role of Sigma-1R in intracardiac neurons, with implications on modulating cardiac function via its effects on peripheral nervous system excitability.
Fig. 2.
Experimental platforms used Summary of the different experimental platforms used by the 22 papers to study the effect of sigma-1R in relation to the cardiovascular system. (a) Number of papers that used each of the 3 types of experimental platforms and (b) number of papers that used each of the specific experimental platforms.
Fig. 3.
Experimental techniques used Summary of the different experimental techniques used by the 22 papers to study the effect of sigma-1R in relation to the cardiovascular system.
5. Sigma-1R and cardioprotection
Sigma-1Rs have been identified as having a role in cardioprotection against (i) hypertrophy, (ii) cellular toxicity/apoptosis, and (iii) maladaptive ER stress response. The effects of sigma-1R on cardiac hypertrophy have been extensively studied in both in vivo and in vitro systems. Abdominal aortic stenosis and transverse aortic constriction were commonly used to establish pressure-overloading (PO), to induce cardiac hypertrophy in in vivo systems [18], [23], [24], [25]; while angiotensin-II stimulation was used to stimulate cardiomyocyte hypertrophy in vitro [24]. The hormones 17B-estradiol (E2) and dehydroepiandrosterone (DHEA), and drugs fluvoxamine (a selective serotonin reuptake inhibitor (SSRI)) were shown to activate sigma-1R in hypertrophic models [24], [25], [26]. It was found that sigma-1R levels are decreased in hypertrophic models, and treatment with sigma-1R agonists recovered sigma-1R mRNA and protein expression, and led to cardioprotection, as evidenced by better haemodynamic performance and reduced cardiac hypertrophy [18], [23], [24], [25]. Pre-treatment with sigma-1R siRNA abolished fluvoxamine-mediated inhibition of CM hypertrophy [23], providing further evidence of sigma-1R’s cardioprotective role against hypertrophy. The molecular mechanisms for sigma-1R-mediated cardioprotection against hypertrophy were elucidated. In the above models, Akt phosphorylation, eNOS levels and eNOS phosphorylation were decreased in cardiac hypertrophy, while sigma-1R agonists (E2, DHEA, fluvoxamine) restored the decrease [18], [23], [24], [25]. Application of the sigma-1R antagonist N, N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]-ethylamine monohydrochloride (NE-100) abolished the agonist-induced Akt and eNOS activation, further providing evidence of sigma-1R’s stimulatory role in the Akt-eNOS pathway [24]. Specifically, treatment with sigma-1R agonists promoted recovery of hypertrophy-induced decrease in Akt phosphorylation at Ser473 in rat cardiomyocytes, as well as decreased expression of eNOS and decreased eNOS phosphorylation at Ser1177 [18], [23], [24], [25]. These suggest that sigma-1R stimulation of the Akt-eNOS pathway is a key mechanism to explain its cardioprotective effects against hypertrophy in murine models and cell cultures. Sigma-1R restoration of IP3R-mediated mitochondrial ATP production was proposed as another mechanism for cardioprotection against hypertrophy. Sigma-1R directly interacts and stabilises IP3R2 channels found on the mitochondrial-associated ER (SR) membrane (MAM) [27], [28], [29]. Treatment with sigma-1R agonists such as pentazocine (an opioid receptor modulator) and fluvoxamine leads to upregulation of sigma-1R, promoting stabilisation of the sigma-1R/IP3R2 complex on MAMs, allowing for Ca2+ transport into mitochondria and hence ATP production [28]. The energy supplied by ATP production promotes SERCA2a ATPase activity [28], thereby promoting Ca2+ storage and maintenance of heart contractile function. A third mechanism for sigma-1R-mediated cardioprotection against hypertrophy is via IP3R2- and RyR-mediated Ca2+ leakage into cytosol, alleviating intracellular calcium overload. Sigma-1R inhibits IP3R2 and RyR channels found on SR membranes. Fluvoxamine and pentazocine treatment were found to be cardioprotective in inhibiting cardiac hypertrophy and dysfunction via suppressing intracellular Ca2+ overload by inhibiting IP3R2 and RyR-mediated Ca2+ release from the SR [28], [29].
Sigma-1R is also shown to regulate Ca2+ entry at both the plasma membrane level, via K+ channels and voltage-sensitive Ca2+ channels, and to modulate Ca2+ mobilisation from ER stores [30], [31]. A recent study in retinal ganglion cell line-5 showed that sigma-1R activation contributes to reduction of calcium overload by inhibiting calcium influx [31]. Taken together, a similar mechanism may be used to explain the functional recovery of angiotensin-II induced CM hypertrophy by fluvoxamine treatment [18]: fluvoxamine-mediated activation of sigma-1R may alleviate calcium overload, hence promoting cardiac remodelling and functional recovery in terms of CM contraction and relaxation.
Sigma-1R has also been shown to be cardioprotective against apoptosis after ischaemia/reperfusion injury. In a rat myocardial ischaemia/reperfusion (I/R) model administering the selective sigma-1R agonist, 2-(4-morpholinoethyl)-1-phenylcyclohexane-1-carboxylate hydrochloride (PRE-084), to I/R-injured rats led to better recovery of haemodynamic parameters such as left ventricular pressure development and left ventricular systolic pressure. Furthermore, PRE-084 treatment decreased the degree of myocardial apoptosis, by increasing Bcl-2 and decreasing Bax levels. It is proposed that this effect is due to sigma-1R activation of the PI3K/Akt/eNOS pathway, which is shown to be stimulated by sigma-1R in the heart [32], and known to be the most important pathway for apoptotic regulation [33], [34], [35]. Given that apoptosis is the major pathogenic mechanism of myocardial I/R injury [36], [37], and that myocardial cells are non-regenerative, this indicates great potential for the future treatment of myocardial I/R injuries with sigma-1R stimulation.
Lastly, sigma-1R is proposed to be cardioprotective against maladaptive ER stress. Typically, pathological development in heart disease can be a result of chronic stresses disturbing ER function, including free radicals, ischaemia, hypoxia, elevated protein synthesis and gene mutation. The result is a condition known as “ER-stress”, characterised by the accumulation of unfolded and misfolded proteins [38], [39]. While the traditional ER stress response stimulates adaptive and pro-survival pathways to restore ER homeostasis [40], [41], [42], chronic exposure to stress can lead to maladaptive expression of C/EBP-homologous protein (CHOP) [43], [44], thereby inducing apoptosis [45], [46], [47], [48]. Sigma-1R is shown to be cardioprotective under conditions of tunicamycin-induced ER stress by reducing CHOP expression in neonatal rat ventricular cardiomyocytes. The molecular interaction likely lies between Sigma-1R and IRE1α: Sigma-1R is shown to transiently and directly interact with IRE1α [49], leading to activation of IRE1α by dimerisation and phosphorylation [50]. This results in the alternative splicing of X-box binding protein 1 (XBP1) into a more stable form, XBP1 spliced (XBP1s), which localises to the nucleus and inhibits CHOP expression, suppressing ER-stress-induced cellular toxicity. It is thus worth exploring the therapeutic potential of sigma-1R-dependent activation of the IRE1α-XBP1 pathway.
6. Sigma-1R modulation of cardiac ion channels
Sigma-1R is known to interact with many ion channels in the nervous system. Various studies have thus investigated if Sigma-1R exerts modulatory effect on potassium, sodium and calcium ion channels in the cardiovascular system. Sigma-1R was shown to promote human Ether-à-go-go-Related Gene (hERG) protein expression within the plasma membrane. The hERG is a component of the rapidly-activating delayed rectifier potassium channel, which is involved in long QT syndrome, a disorder associated with increased risk of ventricular arrthymias. Co-precipitation of hERG and Sigma-1R was detected, indicating direct interaction between these two proteins. However, while Balasuriya et al., 2014 [51] proposed that Sigma-1R binds to an immature hERG in the ER and reduces expression of mature hERG, Johannessen et al, 2009, suggested that sigma-1R expression enhances hERG protein maturation and stability, by regulating subunit trafficking activity of hERG [52]. Clearly, there is conflicting evidence on the effect of sigma-1R on hERG, indicating room for further studies.
Sigma-1R ligands including N-allylnormetazocine (NANM/SKF-10047); a synthetic opioid analgesic of the benzomorphan family, pentazocine; an opioid analgesic, haloperidol; an antipsychotic drug with a strong dopamine receptor antagonism and ditoylguanidine were found to reversibly inhibit NaV1.5 in HEK293 and COS-7 cells, and inhibit sodium currents in neonatal mouse cardiac myocytes. NaV1.5 is crucial for cardiac action potential induction. The inhibition of NaV1.5 channels has subsequent effects on INa and thus cardiac contractility and rhythmicity [53], [54].
Perturbations in calcium signalling occur during cardiac hypertrophy, where cardiac myocyte phenotype changes resulting in altered electrical signalling. Calcium influx via the STIM1/Orai complex is one mechanism by which the cardiac action potential is altered in the hypertrophic heart; inhibition of this calcium entry pathway has been shown to improve cardiac function [55]. Sigma-1R provides a novel method for inhibition of this pathway, as it’s activation also inhibits store-operated calcium entry [56].
7. Sigma-1R modulation of ionic currents in intracardiac neurons
Sigma-1Rs were found to be expressed in neonatal rat intracardiac neurons [57]. Application of sigma-R agonists such as haloperidol and (+)-pentazocine inhibited all Ca2+ channel subtypes in the neurons. Calcium channel activity is critical for transduction of action potentials across the neurons. Sigma-R activation also depressed the peak Ca2+ channel current, increased the rate of Ca2+ channel inactivation, and shifted the voltage-dependence of steady-state inactivation and activation towards more negative potentials. However, pharmacological properties suggest that modulation of Ca2+ channel activity is likely only via the sigma-2R [29]. Further studies on the effect of sigma-1R on other ion channels suggested that activation of sigma-1Rs reversibly blocked numerous potassium channels in intracardiac neurons [58]. These channels include delayed outwardly rectifying potassium channels, large conductance Ca2+-sensitive K+ channels, and the M-channel. Experimental evidence suggests direct coupling of sigma-1R receptors to these K+ channels. The effect of sigma-1R activation on these K+ channels led to intracardiac neuronal depolarisation and decreased excitability. This may hence reduce parasympathetic input to the heart and impact cardiovascular function. These results have implications on drug treatments that act on sigma-1Rs, including haloperidol which is commonly used as an anti-psychotic to treat schizophrenia, and may explain the side effects of such drug treatments on cardiovascular function.
Various studies have shown sigma-1R to be cardioprotective against hypertrophy, and we suggest further work into this area. Numerous pharmacological agents including endogenous and synthetic agonists have also been identified. Anti-hypertrophic sigma-1R agonists identified in the 22 studies include: pentazocine [29], DHEA [24], [25] and fluvoxamine [18], [26], [28]. These agonists were shown to be cardioprotective against hypertrophy in TAC-induced hypertrophic murine models and in ANGII-induced hypertrophic cardiomyocyte tissue culture models. Further work into these specific ligands is encouraged for the potential development of novel drug candidates against cardiac hypertrophy. We also suggest further research into the role of sigma-1R in ER stress. Sigma-1R was found to have a role in the adaptive cardiomyocyte ER stress response by reducing CHOP expression by increasing XBP1s [49], hence reducing CHOP-induced apoptosis. However, in a separate study, Sigma-1R was found to inhibit the XBP1 pathway, resulting in cardioprotection against hypertrophy (Bao et al., 2017). The conflicting effects of sigma-1R on XBP1 expression may be due to the inability of these studies to directly investigate the molecular mechanism of sigma-1R in the ER stress response pathways. Thus, while both studies showed sigma-1R to be cardioprotective, they presented conflicting evidences regarding the effect of sigma-1R on the XBP1 ER-stress response pathway, which needs to be further explored.
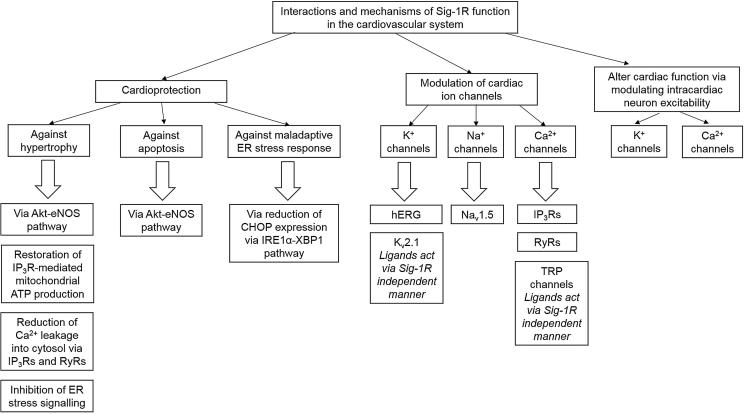
Sigma-1R has been widely studied in the brain and central nervous system, but its role in the heart and cardiovascular system has not been well elucidated. Recent studies have shown sigma-1Rs to be implicated in various pathways in the cardiovascular system. These include cardioprotection, modulation of ion channels and modulating intracardiac neuron excitability. The cardioprotective role of sigma-1R in hypertrophic models have been extensively studied, and various molecular mechanisms have been proposed to explain the anti-hypertrophic effects of administering sigma-1R agonists. These include: (1) stimulation of the Akt-eNOS pathway, (2) restoration of IP3R-mediated mitochondrial ATP production, (3) reduction of intracellular calcium by inhibition of IP3R2 and RyR channels and (4) inhibition of ER stress signalling. Furthermore, sigma-1R’s role in reducing cellular toxicity and apoptosis in reducing ER stress have also been recently investigated. Apart from cardioprotective properties, sigma-1R is shown to interact with numerous cardiac ion channels, including K+, Na+ and Ca2+ channels, such as IP3R and RyR channels that have implications in cardiomyocyte calcium handling. Studies have also looked into the role of sigma-1R in intracardiac neurons, with implications in modulating cardiovascular activity via alteration of peripheral nervous system excitability. These studies reflect the diverse functions of the sigma-1R (Fig. 4). Based on results of this systematic review, the sigma-1R appears to have a clear role in cardioprotection and should be explored as a potential drug target for cardiac pathologies.
Fig. 4.
Summary of the main roles of the sigma-1R in relation to the cardiovascular system.
References
- 1.Dicks E. BHF CVD Statistics Compendium. 2017;2017 [Google Scholar]
- 2.Wilkins E., Wilson L., Wickramasighe K., Bhatnagar P., Leal J., Luengo-Fernandez R., Burns R., Rayner M., Townsend N. European cardiovascular disease statistics 2017. Eur. Hear Netw. 2017 [Google Scholar]
- 3.Challah M., Nicoletti A., Arnal J.F., Philippe M., Laboulandine I., Allegrini J., Alhenc-Gelas F., Danilov S., Michel J.B. Cardiac angiotensin converting enzyme overproduction indicates interstitial activation in renovascular hypertension. Cardiovasc. Res. 1995;30:231–239. [PubMed] [Google Scholar]
- 4.Schunkert H., Dzau V.J., Tang S.S., Hirsch A.T., Apstein C.S., Lorell B.H. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J. Clin. Invest. 1990;86:1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Keefe J.H., Wetzel M., Moe R.R., Bronsnahan K., Lavie C.J. Should an angiotensin-converting enzyme inhibitor be standard therapy for patients with atherosclerotic disease? J. Am. Coll. Cardiol. 2001;37:1–8. doi: 10.1016/s0735-1097(00)01044-5. [DOI] [PubMed] [Google Scholar]
- 6.Valika A.A., Gheorghiade M. Ace inhibitor therapy for heart failure in patients with impaired renal function: a review of the literature. Heart Fail. Rev. 2013;18:135–140. doi: 10.1007/s10741-011-9295-6. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa J. Adverse effects of calcium antagonists and beta-blockers. Nihon Rinsho. 2007;65(Suppl 8):152–158. [PubMed] [Google Scholar]
- 8.Akbar S., Alorainy M.S. The current status of beta blockers’ use in the management of hypertension. Saudi Med. J. 2014;35:1307–1317. [PMC free article] [PubMed] [Google Scholar]
- 9.Hellewell S.B., Bowen W.D. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 10.Ortega-Roldan J.L., Ossa F., Schnell J.R. Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J. Biol. Chem. 2013;288:21448–21457. doi: 10.1074/jbc.M113.450379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brune S., Pricl S., Wünsch B. Structure of the σ1 receptor and its ligand binding site. J. Med. Chem. 2013;56:9809–9819. doi: 10.1021/jm400660u. [DOI] [PubMed] [Google Scholar]
- 12.Su T.-P., Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- 13.Cobos E., Entrena J., Nieto F., Cendan C., Pozo E. Pharmacology and therapeutic potential of sigma1 receptor ligands. Curr. Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller B.H., Park Y., Daudt D.R., Ma H.-Y., Akopova I., Stankowska D.L., Clark A.F., Yorio T. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type voltage gated calcium channels in purified retinal ganglion cells. Exp. Eye Res. 2013;107:21–31. doi: 10.1016/j.exer.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Katnik C., Cuevas J. Sigma receptor activation inhibits voltage-gated sodium channels in rat intracardiac ganglion neurons. Int. J. Physiol. Pathophysiol. Pharmacol. 2009;2:1–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Brailoiu E., Chakraborty S., Brailoiu G.C., Zhao P., Barr J.L., Ilies M.A., Unterwald E.M., Abood M.E., Taylor C.W. Choline Is an Intracellular messenger linking extracellular stimuli to IP(3)-evoked Ca(2+) signals through sigma-1 receptors. Cell Rep. 2019;26:330–337.e4. doi: 10.1016/j.celrep.2018.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Lu Y., Bi X., Xu M., Yu X., Xue R., He X., Zang W. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017;7:42553. doi: 10.1038/srep42553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhuiyan M.S., Tagashira H., Shioda N., Fukunaga K. Targeting sigma-1 receptor with fluvoxamine ameliorates pressure-overload-induced hypertrophy and dysfunctions. Expert Opin. Ther. Targets. 2010;14:1009–1022. doi: 10.1517/14728222.2010.509348. [DOI] [PubMed] [Google Scholar]
- 19.Ela C., Barg J., Vogel Z., Hasin Y., Eilam Y. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J. Pharmacol. Exp. Ther. 1994;269:1300–1309. [PubMed] [Google Scholar]
- 20.Novakova M., Ela C., Barg J., Vogel Z., Hasin Y., Eilam Y. Inotropic action of σ receptor ligands in isolated cardiac myocytes from adult rats. Eur. J. Pharmacol. 1995;286:19–30. doi: 10.1016/0014-2999(95)00424-j. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. PRISMA-P group, preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordage G., Caelleigh A.S., Steinecke A., Bland C.J., Crandall S.J., McGaghie W.C., Pangaro L.N., Penn G., Regehr G., Shea J.S. Joint task force of academic medicine and the GEA-RIME committee, review criteria for research manuscripts. Acad. Med. 2001;76:897–978. [PubMed] [Google Scholar]
- 23.Tagashira H., Bhuiyan S., Shioda N., Hasegawa H., Kanai H., Fukunaga K. σ1-Receptor stimulation with fluvoxamine ameliorates transverse aortic constriction-induced myocardial hypertrophy and dysfunction in mice. Am. J. Physiol. Circ. Physiol. 2010;299:H1535–H1545. doi: 10.1152/ajpheart.00198.2010. [DOI] [PubMed] [Google Scholar]
- 24.Tagashira H., Bhuiyan S., Shioda N., Fukunaga K. Distinct cardioprotective effects of 17β-estradiol and dehydroepiandrosterone on pressure overload-induced hypertrophy in ovariectomized female rats. Menopause. 2011;18:1317–1326. doi: 10.1097/gme.0b013e31821f915b. [DOI] [PubMed] [Google Scholar]
- 25.Bhuiyan M.S., Fukunaga K. Stimulation of Sigma-1 receptor signaling by dehydroepiandrosterone ameliorates pressure overload-induced hypertrophy and dysfunctions in ovariectomized rats. Expert Opin. Ther. Targets. 2009;13:1253–1265. doi: 10.1517/14728220903264064. [DOI] [PubMed] [Google Scholar]
- 26.Tagashira H., Bhuiyan S., Shioda N., Hasegawa H., Kanai H., Fukunaga K. Sigma1-receptor stimulation with fluvoxamine ameliorates transverse aortic constriction-induced myocardial hypertrophy and dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1535–H1545. doi: 10.1152/ajpheart.00198.2010. [DOI] [PubMed] [Google Scholar]
- 27.Shinoda Y., Tagashira H., Bhuiyan M.S., Hasegawa H., Kanai H., Fukunaga K. Haloperidol aggravates transverse aortic constriction-induced heart failure via mitochondrial dysfunction. J. Pharmacol. Sci. 2016;131:172–183. doi: 10.1016/j.jphs.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Tagashira H., Bhuiyan M.S., Shioda N., Fukunaga K. Fluvoxamine rescues mitochondrial Ca2 + transport and ATP production through σ1-receptor in hypertrophic cardiomyocytes. Life Sci. 2014;95:89–100. doi: 10.1016/j.lfs.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Tagashira H., Bhuiyan M.S., Fukunaga K. Diverse regulation of IP3 and ryanodine receptors by pentazocine through 1-receptor in cardiomyocytes. AJP Hear. Circ. Physiol. 2013;305:H1201–H1212. doi: 10.1152/ajpheart.00300.2013. [DOI] [PubMed] [Google Scholar]
- 30.Monnet F.P. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol. Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- 31.Tchedre K.T., Huang R.-Q., Dibas A., Krishnamoorthy R.R., Dillon G.H., Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Investig. Opthalmol. Vis. Sci. 2008;49:4993. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- 32.Bhuiyan M.S., Tagashira H., Fukunaga K. Sigma-1 receptor stimulation with fluvoxamine activates Akt–eNOS signaling in the thoracic aorta of ovariectomized rats with abdominal aortic banding. Eur. J. Pharmacol. 2011;650:621–628. doi: 10.1016/j.ejphar.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 33.Wu H., Ye M., Yang J., Ding J., Yang J., Dong W., Wang X. Nicorandil protects the heart from ischemia/reperfusion injury by attenuating endoplasmic reticulum response-induced apoptosis through PI3K/Akt signaling pathway. Cell. Physiol. Biochem. 2015;35:2320–2332. doi: 10.1159/000374035. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., Jiang C., Jiang J., Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clin. Exp. Pharmacol. Physiol. 2017;44:946–953. doi: 10.1111/1440-1681.12791. [DOI] [PubMed] [Google Scholar]
- 35.He F., Xu B., Chen C., Jia H., Wu J., Wang X., Sheng J., Huang L., Cheng J. Methylophiopogonanone a suppresses ischemia/reperfusion-induced myocardial apoptosis in mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol. Sin. 2016;37:763–771. doi: 10.1038/aps.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner C., Tillack D., Simonis G., Strasser R.H., Weinbrenner C. Ischemic post-conditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3β, and apoptosis. Mol. Cell. Biochem. 2010;339:135–147. doi: 10.1007/s11010-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 37.Chiong M., Wang Z.V., Pedrozo Z., Cao D.J., Troncoso R., Ibacache M., Criollo A., Nemchenko A., Hill J.A., Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 40.Glembotski C.C. Endoplasmic reticulum stress in the heart. Circ. Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 41.Bers D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. 2012;151:217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto M., Minami M., Takeda K., Sakao Y., Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–147. doi: 10.1016/0014-5793(96)01016-2. [DOI] [PubMed] [Google Scholar]
- 46.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 47.Ron D., Habener J.F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 48.Ubeda M., Wang X.Z., Zinszner H., Wu I., Habener J.F., Ron D. Stress-induced binding of the transcriptional factor CHOP to a novel DNA control element. Mol. Cell. Biol. 1996;16:1479–1489. doi: 10.1128/mcb.16.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alam S., Abdullah C.S., Aishwarya R., Orr A.W., Traylor J., Miriyala S., Panchatcharam M., Pattillo C.B., Bhuiyan M.S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170898. BSR20170898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi T., Su T.-P. Sigma-1 receptor chaperones at the ER- mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 51.Balasuriya D., D’Sa L., Talker R., Dupuis E., Maurin F., Martin P., Borgese F., Soriani O., Edwardson J.M. A direct interaction between the sigma-1 receptor and the herg voltage-gated K+channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF®) J. Biol. Chem. 2014;289:32353–32363. doi: 10.1074/jbc.M114.603506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johannessen M., Fontanilla D., Mavlyutov T., Ruoho A.E., Jackson M.B. Antagonist action of progesterone at σ-receptors in the modulation of voltage-gated sodium channels. AJP Cell Physiol. 2010;300:C328–C337. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johannessen M., Ramachandran S., Riemer L., Ramos-Serrano A., Ruoho A.E., Jackson M.B. Voltage-gated sodium channel modulation by -receptors in cardiac myocytes and heterologous systems. AJP Cell Physiol. 2009;296:C1049–C1057. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt H.R., Kruse A.C. The Molecular function of σ receptors: past, present, and future. Trends Pharmacol. Sci. 2019;40:636–654. doi: 10.1016/j.tips.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Troupes C.D., Wallner M., Borghetti G., Zhang C., Mohsin S., von Lewinski D., Berretta R.M., Kubo H., Chen X., Soboloff J., Houser S. Role of STIM1 (stromal interaction molecule 1) in hypertrophy-related contractile dysfunction. Circ. Res. 2017;121:125–136. doi: 10.1161/CIRCRESAHA.117.311094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivats S., Balasuriya D., Pasche M., Vistal G., Edwardson J.M., Taylor C.W., Murrell-Lagnado R.D. Sigma1 receptors inhibit store-operated Ca<sup>2+</sup> entry by attenuating coupling of STIM1 to Orai1. J. Cell Biol. 2016;213 doi: 10.1083/jcb.201506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J. Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H. Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J. Pharmacol. Exp. Ther. 2005;313:1387–1396. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]
- 59.Gao Q.-J., Yang B., Chen J., Shi S.-B., Yang H.-J., Liu X. Sigma-1 receptor stimulation with PRE-084 ameliorates myocardial ischemia-reperfusion injury in rats. Chin. Med. J. (Engl.) 2018;131:539. doi: 10.4103/0366-6999.226076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin J., Wang P., Li Y., Yao L., Liu Y., Yu T., Lin J., Fang X., Huang Z. Activation of sigma-1 receptor by cutamesine attenuates neuronal apoptosis by inhibiting endoplasmic reticulum stress and mitochondrial dysfunction in a rat model of asphyxia cardiac arrest. Shock. 2018;1 doi: 10.1097/SHK.0000000000001119. [DOI] [PubMed] [Google Scholar]
- 61.Liu X., Fu Y., Yang H., Mavlyutov T., Li J., McCurdy C.R., Guo L.-W., Pattnaik B.R. Potential independent action of sigma receptor ligands through inhibition of the Kv2.1 channel. Oncotarget. 2017;8:59345–59358. doi: 10.18632/oncotarget.19581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao Q., Zhao M., Chen L., Wang Y., Wu S., Wu W., Liu X. MicroRNA-297 promotes cardiomyocyte hypertrophy via targeting sigma-1 receptor. Life Sci. 2017;175:1–10. doi: 10.1016/j.lfs.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Stracina T., Slaninova I., Polanska H., Axmanova M., Olejnickova V., Konecny P., Masarik M., Krizanova O., Novakova M. Long-term haloperidol treatment prolongs QT interval and increases expression of sigma 1 and IP3 receptors in guinea pig hearts. Tohoku J. Exp. Med. 2015;236:199–207. doi: 10.1620/tjem.236.199. [DOI] [PubMed] [Google Scholar]
- 64.Amer M.S., McKeown L., Tumova S., Liu R., Seymour V.A.L., Wilson L.A., Naylor J., Greenhalgh K., Hou B., Majeed Y., Turner P., Sedo A., O’Regan D.J., Li J., Bon R.S., Porter K.E., Beech D.J. Inhibition of endothelial cell Ca 2+ entry and transient receptor potential channels by sigma-1 receptor ligands. Br. J. Pharmacol. 2013;168:1445–1455. doi: 10.1111/bph.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delaunois A., De Ron P., Detrait E., Guyaux M. Inhibitory effects of sigma-1 ligands on handling-induced tachycardia in conscious tethered rats. Fundam. Clin. Pharmacol. 2013;27:354–363. doi: 10.1111/j.1472-8206.2012.01042.x. [DOI] [PubMed] [Google Scholar]
- 66.Crottès D., Martial S., Rapetti-Mauss R., Pisani D.F., Loriol C., Pellissier B., Martin P., Chevet E., Borgese F., Soriani O. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J. Biol. Chem. 2011;286:27947–27958. doi: 10.1074/jbc.M111.226738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novakova M., Sedlakova B., Sirova M., Fialova K., Krizanova O. Haloperidol increases expression of the inositol 1,4,5-trisphosphate receptors in rat cardiac atria, but not in ventricles. Gen. Physiol. Biophys. 2010;29:381–389. doi: 10.4149/gpb_2010_04_381. [DOI] [PubMed] [Google Scholar]
- 68.Fontanilla D., Johannessen M., Hajipour A.R., Cozzi N.V., Jackson M.B., Ruoho A.E. The hallucinogen N,N-dimethyltryptamine (DMT) Is an endogenous sigma-1 receptor regulator. Science. 2009;323(80):934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]