Abstract
Purpose
The aim of the study was to test flexor tendon repair with a novel hollow mesh suture augmentation served as a centre core cable [Triple-C (Tri-C)] in an in vitro study using a turkey model.
Methods
Forty long digits from white turkey feet were divided into the following four groups based on repair techniques: Group 0, intact tendon without repair; Group 1, modified Kessler (MK) repair only (MKo); Group 2, MK repair plus Tri-C (MK + Tri-C); and Group 3, MK repair plus an additional outside knot plus Tri-C (MK-2knots + Tri-C). Mechanical evaluations were performed for all groups.
Results
The frictions of the two groups with Tri-C were not significantly different than those of the MKo group. The ultimate tensile strength of the MK + Tri-C group was not significantly different from that of the MKo group or the MK-2knots + Tri-C group. In contrast, the MK-2knots + Tri-C group had a significantly greater ultimate tensile strength compared with that of the MKo group. Forces at 2-mm gap formation in the groups with Tri-C were significantly stronger than that of MK alone.
Conclusion
Our data have demonstrated that MK repair augmented with the centre hollow mesh suture increased failure strength without inducing increased friction.
The translational potential of this article
Our study elucidates that a Tri-C augmentation designed in this study can achieve mechanical enhancements without increasing the repaired tendon friction. Hence, this novel technique has potential biological validity and clinical application.
Keywords: Centre core cable, Flexor tendon, Mechanical evaluations, Repair
Introduction
Flexor tendon injury is a common hand trauma and often leads to poor hand function after surgical repair owing to insufficient repair strength and a low intrinsic healing capacity [1], [2], [3], [4], [5], [6]. Increasing repair strength and tendon healing ability are the primary targets for improving clinical outcomes in flexor tendon research. With the progress of tissue engineering and regenerative medicine, cell-based therapy has been studied to enhance tendon healing and has yielded encouraging results [7], [8], [9], [10], [11], [12], [13], [14]. However, sufficient cell delivery for flexor tendon injury is challenging owing to complex surgical repair techniques that are required, as well as the small tendon end-to-end contact area [14].
To overcome these barriers, we developed a unique flexor tendon repair technique with a novel hollow mesh suture, as a centre cable, which not only increases repair strength but also acts as a cell delivery system. This novel mesh suture was originally designed to improve suture tissue holding strength [15], [16]. It is with an outer open mesh architecture that permits tissue ingrowth around the filaments and, thereby, increases the ultimate tensile strength (UTS) of wound closure. The hollow structure provides a stable spatial shape and forms a space longitudinally to contain and deliver the cells into the centre of the tendon, which has less healing ability compared with that of the peripheral surface region. It has been identified that the cell population and proliferative ability of central area tenocytes in flexor tendons are significantly less than those of an area close to the epitenon in vitro. The intrinsic healing of a tendon mainly relies on the epitenon cell from the tendon surface area migrating into the centre portion [17], [18]. Therefore, direct delivery of the cells into the tendon centre area may have an impact on tendon healing. In the present study, we designed a novel tendon repair technique using the mesh suture as a centre core cable [Triple-C (Tri-C)] to deliver cells combined with clinically used modified Kessler (MK) repair to enhance the healing ability of the repaired tendon. The experiment design of the present study consisted of mechanically evaluating this repair technique as the first step for future biological evaluations of this novel mesh suture as a cell delivery material.
Materials and methods
Study design
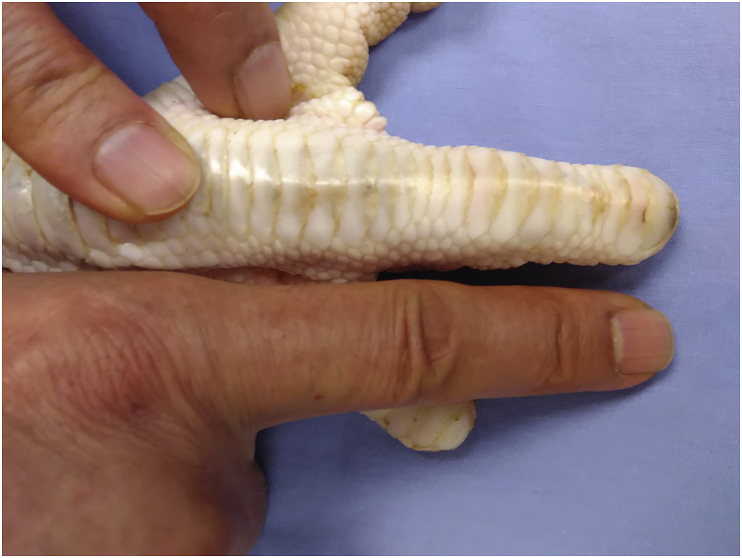
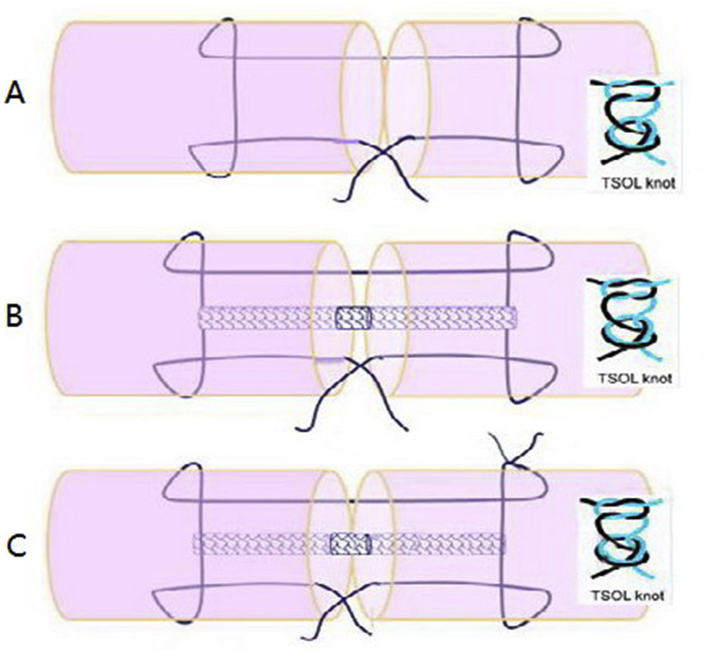
A total of 40 long digits from 40 fresh frozen white turkey feet were used in the present study. They were obtained from a commercial supplier (Jennie-O Turkey Store Sales LLC, MN, USA) who raises turkeys for meat production. The digits were kept frozen at −80 °C until they were thawed at 4 °C one day before the dissection. The turkey's long digit was chosen because it is similar to a human's finger in terms of size, structure, and biomechanical properties (Figure 1) [19]. The turkey flexor tendons were divided into the following four groups based on repair configurations: Group 0, intact tendon without repair as a control group only for frictional testing; Group 1, MK repair alone as a repaired control group (MKo) (Figure 2A); Group 2, MK repair plus Tri-C (MK + Tri-C) (Figure 2B); and Group 3, MK repair with an additional outside knot plus Tri-C (MK-2knots + Tri-C) (Figure 2C). All the techniques were used with the two-strand overhand locking (TSOL) loops (TSOL knot) to prevent knot unravelling [20]. For frictional testing, the intact flexor digitorum profundus (FDP) tendons without repair were used to serve as a normal baseline in terms of frictional force and to compare with all repaired tendon groups. A hand surgeon performed all repairs.
Figure 1.
Comparison of the human index finger with the long digits of turkeys.
Figure 2.
Three groups with different repair techniques. (A) Group 1: modified Kessler (MK) repair (MKo); (B) Group 2: MK repair plus Triple-C (MK + Tri-C); (C) Group 3: MK repair with two knots, one inside and one outside, plus Triple-C (MK-2TSOL + Tri-C). MKo = MK repair alone; Tri-C = Triple-C; TSOL = two-strand overhand locking.
Specimen preparation and repair procedure
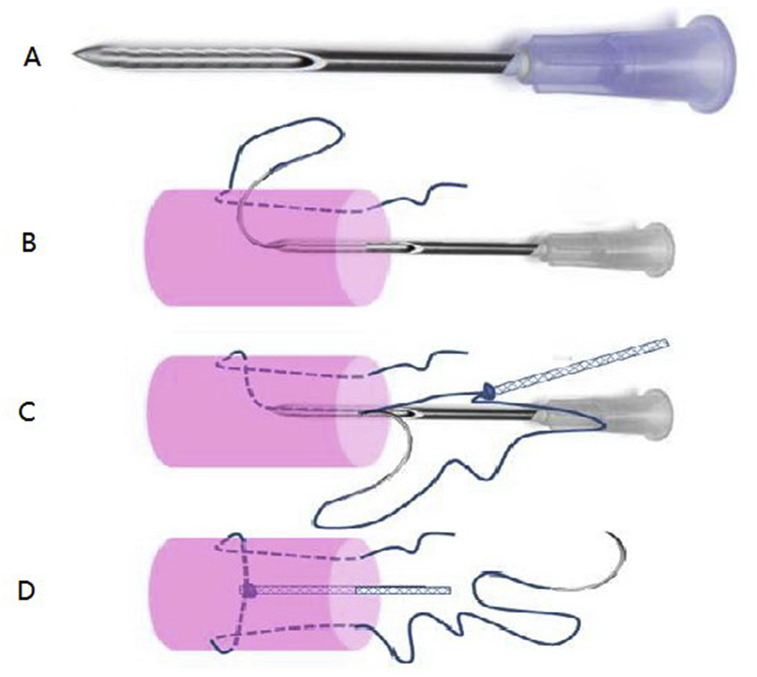
The skin, subcutaneous tissues, and two flexor digitorum superficialis tendons were removed, leaving the flexor apparatus (entire A2 pulley) intact. FDP tendons were sharply transected and repaired in the Zone II area for all groups, except for the control group. The three repair techniques are shown in Figure 2A–C. A custom-made Tri-C guiding device was modified from a 16-G needle (Figure 3A). The device was inserted into the centre of the tendon, from the tendon end, to guide the suture needle out of the tendon end (Figure 3B). The suture was tied to the end of the Tri-C, and then, the suture needle was taken back to the tendon through the guiding device (Figure 3C), thus pulling and placing the Tri-C (2 mm in diameter) into the centre of the tendon (Figure 3D). In each group, FDP tendons were repaired using the same technique. The core suture was completed using a 3-0 braided polyester suture (Ethibond; Ethicon, Inc., Somerville, USA). The transverse strand of the core suture configuration was placed at a distance of 5 mm from the margin in both tendon ends. The Tri-C mesh suture was about 1 cm in length with one knot at the each end, and the ends were smoothed with an electronic iron.
Figure 3.
(A) Repair-guiding device that is custom-made with a needle. (B and C) This device guided the suture needle out/in and in/out to connect the mesh suture. (D) This device also placed the mesh suture with the precreated tunnel in the centre of the tendon.
In Group 1 and Group 2, the MK technique was performed with a single suture and the TSOL knot buried between the ends of the repaired tendon (Figure 4A). In Group 3, one knot was between the tendon ends, and the other was outside the surface (Figure 4B). After core suture, a running epitendinous repair was performed using 6-0 Prolene (Prolene; Ethicon, Inc., Somerville, USA) for each group.
Figure 4.
The procedures and knot location of the Triple-C augmentation repair techniques. (A) MK with Triple-C; (B) MK with two knots (one within the ends of the tendon and one outside the tendon surface) with Triple-C. MK = modified Kessler.
Measurement of tendon gliding resistance
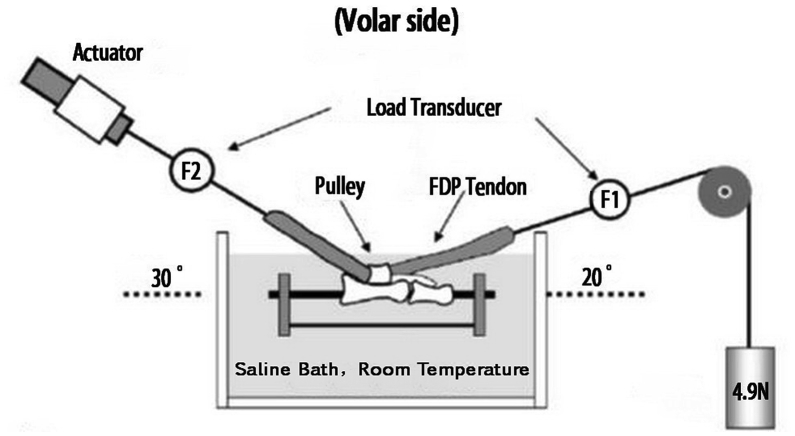
Gliding resistance between the tendons and the proximal pulley was measured in a custom-made mechanical test system [21], [22], [23] (Figure 5). The metacarpal was dissected away, and all proximal vinculums were released so that the FDP tendon moved freely within its sheath. The A2 pulley was preserved, and friction was measured at this level. The proximal and middle phalanx was fixed together with a thin K-wire. The prepared specimen was fixed in a tank full of saline, with the volar side of the pulley facing upward at room temperature. Two custom-made tensile load transducers (F1 and F2) were attached on each end of the tendon. The F1 transducer was connected to the distal tendon end with a 4.9-N weight. The F2 transducer was attached to the proximal tendon end with an actuator. The transducers were oriented to create a 20° arc, and the actuator was oriented to create a 30° arc. The tendon was pulled proximally (flexion) at a velocity of 2 mm/s over the excursion range previously established and then with reversed direction (extension), repeating for a total of three cycles. Gliding resistance between the FDP and proximal pulley was calculated, with the difference in force measured between F1 and F2 transducers in both flexion and extension over the excursion and then averaged as the tendon gliding resistance against the pulley [23].
Figure 5.
Lateral view of the testing apparatus used for measurement of gliding resistance between the FDP tendon and the pulley. F1 is the distal force transducer, and F2 is the proximal force transducer. FDP = flexor digitorum profundus.
Measurement of repair strength
After frictional testing, the repaired tendons were evaluated for mechanical strength. To measure breaking strength, the repaired tendons were fixed to a servohydraulic testing machine (MTS Systems, Eden Prairie, Minnesota, USA) and distracted to failure at a rate of 20 mm/min. A differential variable reluctance transducer (MicroStrain, Williston, Vermont, USA) was used to measure gap formation during testing; it was attached to the tendon through two barbed pins inserted perpendicularly into the tendon. The repair site was centred between the two pins. A video of each tendon with a scale was recorded in the process. Tensile force, grip-to-grip displacement, and gap displacement measured using the transducer were collected at a rate of 20 Hz. UTS was recorded. In addition, the repair stiffness was calculated from the slope of the linear region of the force versus gap-formation curve (as measured using the transducer) to measure the resistance to gap formation.
Statistical analysis
Statistical analyses were performed using one-way analysis of variance, with significance set at a level of p < 0.05. The results were expressed as mean (±standard deviation). The Tukey honestly significant difference (HSD) test was used to compare the means of variables among groups. Statistical significance was set at a level of p < 0.05.
Results
During tensile testing of the repaired tendons, failure modes of the repaired tendons included (1) suture pullout (suture cutting through the tendon without suture break), (2) MK suture break with the Tri-C core suture intact, and (3) MK suture break combined with Tri-C core suture break (Table 1). There was no significant difference in failure modes among three groups with a Fisher exact test. No suture knot unravelling occurred as a strong knot configuration (TSOL) was used for all repaired tendons [20].
Table 1.
Type of failure.
| Pullout | Rupture | Unravelling | Mesh rupture | |
|---|---|---|---|---|
| G1 | 6 | 4 | 0 | — |
| G2 | 7 | 3 | 0 | 4 |
| G3 | 8 | 2 | 0 | 3 |
G1: Group 1; G2: Group 2; G3: Group 3.
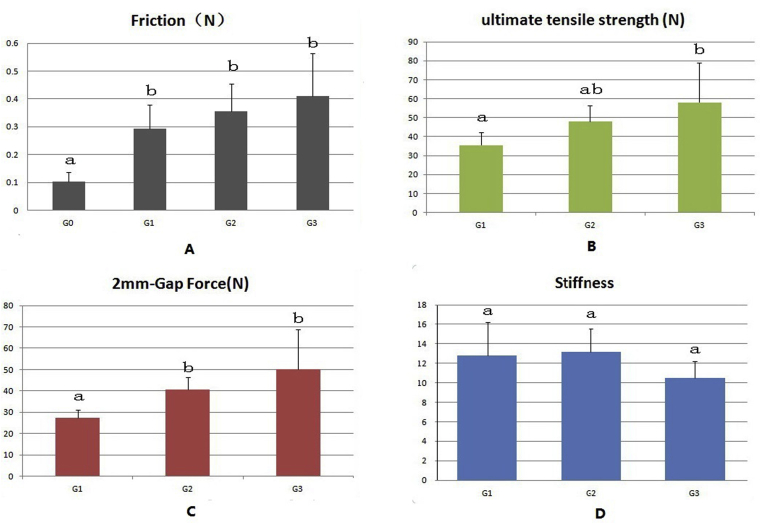
In general, the friction force, UTS, and force to form a 2-mm gap showed a trend of gradual increase from Group 1 to Group 3 (Figure 6A–C). The friction of the intact tendon group (without repair) was significantly lower than that of all three repaired groups (Figure 6A). However, there was no significant difference in friction between the three repaired groups with or without Tri-C augmentation.
Figure 6.
(A) The friction forces in all repaired tendon groups were significantly higher than those of normal tendon friction. The friction forces of the two groups with Triple-C augmentation were not significantly different from those of the MKo group. (B) The repaired tendon ultimate tensile strength of the MK + Tri-C group was not significantly different from that of the MKo group or the MK-2knots + Tri-C group, whereas the repaired tendon ultimate tensile strength of the MK-2knots + Tri-C group was significantly stronger than that of the MKo group. (C) The force to form a 2-mm gap of the groups with Tri-C was significantly higher than that of the MK group alone. (D) The repaired tendon stiffness was not significantly different among the three groups with different repair techniques. A different letter indicates a significant difference (a < b), whereas the same letter indicates no significant difference (a = ab, ab = b). MK = modified Kessler; MKo = MK repair alone; Tri-C = Triple-C.
The UTS of the MK + Tri-C group was not significantly different from that of the MKo group or the MK-2knots + Tri-C group, whereas the MK-2knots + Tri-C group had significantly stronger UTS than that of the MKo group (p < 0.05; Figure 6B).
The forces to form a 2-mm gap at the repair site of the repaired tendons using Tri-C augmentation in Groups 2 and 3 were significantly higher than those of MK alone (p < 0.05). There was no significant difference in the force for the 2-mm gap between the two groups (2 and 3) with Tri-C augmentation (Figure 6C). The stiffness was not significantly different among the three groups with different repair techniques (Figure 6D).
Discussion
Although progress in surgical techniques, suture materials, and rehabilitation have improved functional outcomes after flexor tendon repair, slow intrinsic healing and dominant extrinsic healing still cause repaired tendon rupture and severe adhesions, respectively [3], [24], [25], [26]. Cell-based therapy has recently been implemented for enhancing tendon intrinsic healing, thus reducing adhesion formation [7], [10], [11], [12], [27]. However, the effectiveness of cell-based therapy on flexor tendon healing has been limited. This inefficient improvement on tendon healing, which differs from the observations of cell therapy in other tissues such as bone [28], [29], [30], may be due to a small contact area between healing interfaces or insufficient repair strength causing a gap between healing interfaces. Therefore, developing a more effective cell delivery method that also strengthens repair would be the most ideal innovation for improving flexor tendon healing.
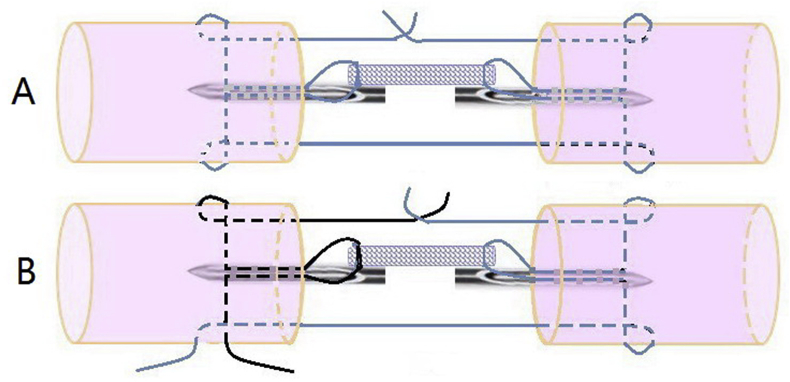
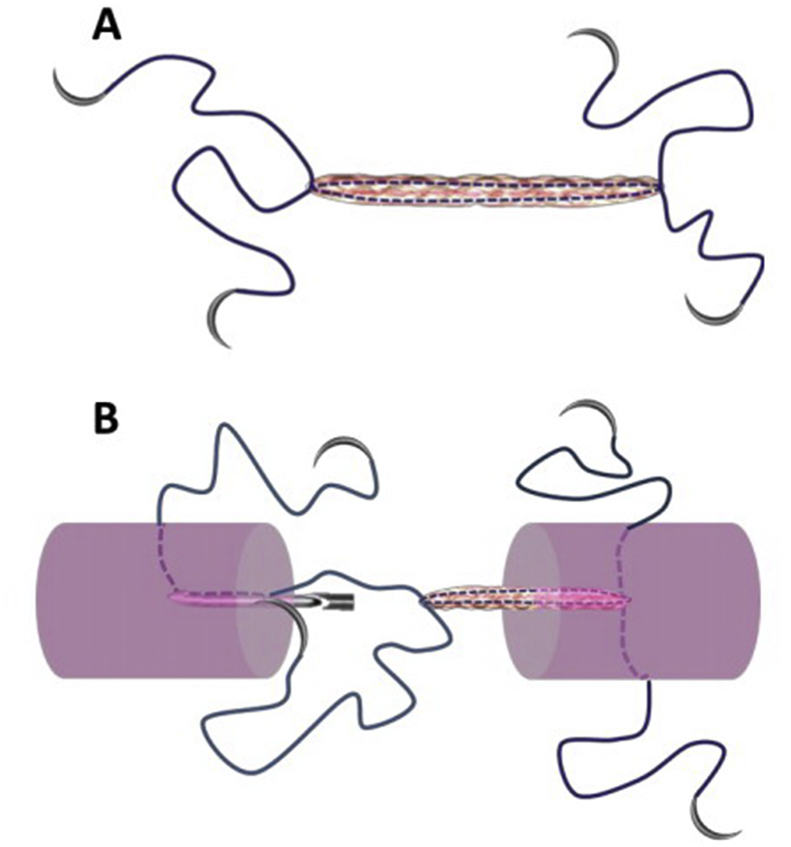
The Tri-C augmentation designed in the present study was intended to achieve biological and mechanical enhancements. The rationale for choosing the MK technique was based on this repair technique being commonly used in the clinic [31], [32], [33]. Using this technique could, indeed, increase the repair strength. A 4-needle suture with Tri-C could be designed for cell delivery and mechanical augmentation that would be easily performed in a clinical setting (Figure 7). For the Kessler repair, two knots were placed outside of the tendon surface to eliminate the potential that knots occupied the healing area, but this strategy also induced higher friction in our preliminary experiment. We chose the method of leaving one knot between the ends of the tendon and the other outside the surface in the design of our Group 3 to balance the tendon friction with the tendon end contact area. We used the TSOL knot in this experiment because it has been recently reported that it increases repair strength compared with that of a square knot [20]. Furthermore, we created a special needle to complete the surgical procedure for delivering Tri-C augmentation combined with MK repair.
Figure 7.
The concept of needle/suture material with Triple-C augmentation can be manufactured to form one unit of the device for the tendon repair. A: a schematic diagram of the special suture. B: the diagram of the principle of applying the suture to repair tendon.
Our findings suggested that the repair strength was equivalent between the MK + Tri-C and MK-2TSOL + Tri-C groups, which were stronger than that of the repaired control group (MKo). Although it was not significantly different between the MK + Tri-C and MKo groups in the UTS, the force to form a 2-mm gap was significantly stronger in the MK + Tri-C group than in the MKo group. These data demonstrated that the centre hollow mesh suture (Tri-C) strengthened the core suture (MK techniques). We also noticed that none of the TSOL knots unravelled. The TSOL knot has been reported as an alternative knotting system for tendon repair, especially during use of strong suture materials or with surgical technique in which the suture knot holding strength becomes critical. Our present study further verified that TSOL knots prevent knot unravelling that often occurred with surgical knots for tendon repair [20]. The mesh suture was 2 mm in diameter, so it is too thick to be used as a suture material to repair flexor tendon directly. However, as a centre cable system for core suture augmentation and potential cell delivery, the Tri-C concept is feasible and applicable. It is speculated that the Tri-C augmentation in the present study shared the loading that applied to the repaired tendon during mechanical failure testing as the mesh suture rupture occurred in all repaired groups with Tri-C augmentation. However, the Tri-C rupture in Group 2 (MK + Tri-C) was more than in Group 3, which might relate to the repair stiffness that was higher in Group 2 than in Group 3.
Tendon repair with Tri-C augmentation in the centre of the tendon did not increase the repaired tendon friction. Previous reports have shown that a suture knot outside the tendon surface causes the friction to increase [34]. Balancing of repair strength, healing area, and tendon gliding ability has to be considered accordingly based on clinical scenarios and rehabilitation regimes [35]. Group 3 (MK-2TSLO + Tri-C), in our present study, demonstrates an alternative way to increase repair strength without increasing friction.
Recently, we have studied flexor tendons in a turkey model and found that the turkey's digits and flexor tendon are similar to those of human fingers in terms of function, size, anatomy, and structure. Our present study using the turkey model may be useful for the translation from in vitro findings to an in vivo validation of Tri-C augmentation.
There are some limitations to our present study. First, only biomechanical performance of the Tri-C augmentation was studied. Biological augmentation with cell-based delivery was not investigated in either the ex vivo or in vivo models. Second, there was variability regarding Tri-C augmentation among the experimental groups. Based on the observation during testing, we believe that these variations were caused by manually connecting the repair suture with a knotted mesh suture, which could slip from the mesh suture. If the Tri-C is separated from the core suture, the Tri-C augmentation will fail. However, this weakness could be overcome by designing a needle suture with Tri-C in a single system (Figure 7). Finally, we only tested the MK core suture techniques for Tri-C augmentation. Other flexor tendon repair techniques were not studied.
Based on the mechanical data, we conclude that MK tendon repair with Tri-C augmentation—made of a hollow mesh suture combined with the TSOL knot (either one knot buried between ends of the tendon or an extra knot outside tendon repair)—increased flexor tendon repair strength without increasing the repaired tendon friction. These repair techniques have a potential to improve tendon healing by delivering cells through a centre cable to the repair site. Although mechanical augmentation was confirmed in the present study, the biological effects of cell-based therapy to enhance tendon healing need to be investigated in future studies.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Funding
This study was funded by a grant from NIH/NIAMS AR57745, as well as by funds provided by Mayo Clinic Orthopedic Research Program.
Author contributions
H.L., A.T., A.K., S.M., and C.Z. contributed to the conception and design of the study. H.L., A.T., and A.K. contributed to the acquisition of data. H.L., A.T., A.K., S.M., and C.Z. contributed to analysis and/or interpretation of data. H.L. contributed to drafting of the manuscript. H.L., A.T., A.K., S.M., and C.Z. contributed to revising the manuscript critically for important intellectual content. H.L., A.T., A.K., S.M., and C.Z. approved the version of the manuscript to be published.
References
- 1.Zhao C., Moran S.L., Cha S.S., Kai Nan A., Amadio P.C. An analysis of factors associated with failure of tendon repair in the canine model. J Hand Surg Am. 2007;32(4):518–525. doi: 10.1016/j.jhsa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin M., Kaiser E., Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212(3):211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltz T.S., Haddad R., Scougall P.J., Nicklin S., Gianoutsos M.P., Oliver R. Structural failure mechanisms of common flexor tendon repairs. Hand Surg. 2015;20(3):369–379. doi: 10.1142/S0218810415400092. [DOI] [PubMed] [Google Scholar]
- 4.Savage R. The search for the ideal tendon repair in zone 2: strand number, anchor points and suture thickness. J Hand Surg Eur. 2014;39(1):20–29. doi: 10.1177/1753193413508699. [DOI] [PubMed] [Google Scholar]
- 5.Branford O.A., Klass B.R., Grobbelaar A.O., Rolfe K.J. The growth factors involved in flexor tendon repair and adhesion formation. J Hand Surg Eur. 2014;39(1):60–70. doi: 10.1177/1753193413509231. [DOI] [PubMed] [Google Scholar]
- 6.Galvez M.G., Crowe C., Farnebo S., Chang J. Tissue engineering in flexor tendon surgery: current state and future advances. J Hand Surg Eur. 2014;39(1):71–78. doi: 10.1177/1753193413512432. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T., Sun Y.L., An K.N., Amadio P.C., Zhao C.F. The effect of tendon surface treatment on cell attachment for potential enhancement of tendon graft healing: an ex vivo model. Med Eng Phys. 2012;34(10):1387–1393. doi: 10.1016/j.medengphy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda J., Zhao C.F., Moran S.L., An K.N., Amadio P.C. Effects of synovial interposition on healing in a canine tendon explant culture model. J Hand Surg Am. 2010;35a(7):1153–1159. doi: 10.1016/j.jhsa.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chieh H.F., Sun Y.L., Liao J.D., Su F.C., Zhao C.F., Amadio P.C. Effects of cell concentration and collagen concentration on contraction kinetics and mechanical properties in a bone marrow stromal cell-collagen construct. J Biomed Mater Res A. 2010;93a(3):1132–1139. doi: 10.1002/jbm.a.32606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi M., Zhao C., An K.N., Amadio P.C. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Eur. 2011;36e(4):271–279. doi: 10.1177/1753193410394521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morizaki Y., Zhao C.F., An K.N., Amadio P.C. The effects of platelet-rich plasma on bone marrow stromal cell transplants for tendon healing in vitro. J Hand Surg Am. 2010;35a(11):1833–1841. doi: 10.1016/j.jhsa.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omae H., Zhao C.F., Sun Y.L., An K.N., Amadio P.C. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27(7):937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omi R., Gingery A., Steinmann S.P., Amadio P.C., An K.N., Zhao C.F. Rotator cuff repair augmentation in a rat model that combines a multilayer xenograft tendon scaffold with bone marrow stromal cells. J Shoulder Elb Surg. 2016;25(3):469–477. doi: 10.1016/j.jse.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozasa Y., Gingery A., Thoreson A.R., An K.N., Zhao C.F., Arnadio P.C. A comparative study of the effects of growth and differentiation factor 5 on muscle-derived stem cells and bone marrow stromal cells in an in vitro tendon healing model. J Hand Surg Am. 2014;39(9):1706–1713. doi: 10.1016/j.jhsa.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumanian G.A., Tulaimat A., Dumanian Z.P. Experimental study of the characteristics of a novel mesh suture. Br J Surg. 2015;102(10):1285–1292. doi: 10.1002/bjs.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza J.M., Dumanian Z.P., Gurjala A.N., Dumanian G.A. In vivo evaluation of a novel mesh suture design for abdominal wall closure. Plast Reconstr Surg. 2015;135(2) doi: 10.1097/PRS.0000000000000910. 322e-30e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelberman R.H., Khabie V., Cahill C.J. The revascularization of healing flexor tendons in the digital sheath. A vascular injection study in dogs. J Bone Joint Surg Am. 1991;73(6):868–881. [PubMed] [Google Scholar]
- 18.Gelberman R.H., Vandeberg J.S., Manske P.R., Akeson W.H. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10(6 Pt 1):776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 19.Kadar A., thoreson A., Reisdorf R., Amadio P.C., Moran S.L., Zhao C. Turkey model for flexor tendon research: in vitro comparison of human, canine, Turkey, and chicken tendons. J Surg Res. 2017;216:46–55. doi: 10.1016/j.jss.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C.F., Hsu C.C., Moriya T., Thoreson A.R., Cha S.S., Moran S.L. Beyond the square knot: a novel knotting technique for surgical use. J Bone Joint Surg Am. 2013;95a(11):1020–1027. doi: 10.2106/JBJS.K.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C.F., Amadio P.C., Berglund L., An K.N. The A3 pulley. J Hand Surg Am. 2000;25a(2):270–276. doi: 10.1053/jhsu.2000.jhsu25a0270. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C., Sun Y.L., Amadio P.C., Tanaka T., Ettema A.M., An K.N. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid - an in vivo canine model. J Bone Joint Surg Am. 2006;88a(10):2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiyama S., Amadio P.C., Coert J.H., Berglund L.J., An K.N. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg Am. 1997;79(2):219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Coats R.W., 2nd, Echevarria-Ore J.C., Mass D.P. Acute flexor tendon repairs in zone II. Hand Clin. 2005;21(2):173–179. doi: 10.1016/j.hcl.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Manske P.R., Gelberman R.H., Vande Berg J.S., Lesker P.A. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984;66(3):385–396. [PubMed] [Google Scholar]
- 26.Mass D.P., Tuel R.J. Intrinsic healing of the laceration site in human superficialis flexor tendons in vitro. J Hand Surg Am. 1991;16(1):24–30. doi: 10.1016/s0363-5023(10)80006-1. [DOI] [PubMed] [Google Scholar]
- 27.Omae H., Zhao C., Sun Y.L., Zobitz M.E., Moran S.L., Amadio P.C. The effect of tissue culture on suture holding strength and degradation in canine tendon. J Hand Surg Eur. 2009;34e(5):643–650. doi: 10.1177/1753193409104564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Barrena E., Rosset P., Lozano D., Stanovici J., Ermthaller C., Gerbhard F. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X., Zou S., Ye B., Zhu S., Liu Y., Hu J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone. 2010;46(4):1156–1161. doi: 10.1016/j.bone.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura A., Akahane M., Shigematsu H., Tadokoro M., Morita Y., Ohgushi H. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46(2):418–424. doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 31.Karlander L.E., Berggren M., Larsson M., Soderberg G., Nylander G. Improved results in zone 2 flexor tendon injuries with a modified technique of immediate controlled mobilization. J Hand Surg Br. 1993;18(1):26–30. doi: 10.1016/0266-7681(93)90189-m. [DOI] [PubMed] [Google Scholar]
- 32.Seradge H. Elongation of the repair configuration following flexor tendon repair. J Hand Surg Am. 1983;8(2):182–185. doi: 10.1016/s0363-5023(83)80012-4. [DOI] [PubMed] [Google Scholar]
- 33.Havulinna J., Leppanen O.V., Jarvinen T.L., Goransson H. Comparison of modified Kessler tendon suture at different levels in the human flexor digitorum profundus tendon and porcine flexors and porcine extensors: an experimental biomechanical study. J Hand Surg Eur. 2011;36(8):670–676. doi: 10.1177/1753193411415936. [DOI] [PubMed] [Google Scholar]
- 34.Momose T., Amadio P.C., Zhao C.F., Zobitz M.E., An K.N. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res. 2000;53(6):806–811. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C., Amadio P.C., Zobitz M.E., An K.N. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19(4):580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]