Abstract
Objective:
Recent conceptual frameworks propose anhedonia reflects abnormalities in the temporal dynamics of positive emotion in schizophrenia, characterized by intact consummatory and impaired anticipatory pleasure. A comprehensive meta-analysis can directly test this theory using self-report data.
Method:
A meta-analysis was performed on studies reporting Temporal Experience of Pleasure Scale (TEPS) data from healthy controls and schizophrenia or schizotypy groups. The TEPS was examined as it contains subscales to measure both consummatory and anticipatory pleasure separately. Statistical heterogeneity and study bias were examined. Meta-regressions evaluated moderators.
Results:
53 studies were retrieved (7,797 participants). Results revealed small effect sizes for comparisons of combined schizophrenia/schizotypy and control groups for both consummatory and anticipatory pleasure. Within-group comparisons of pleasure conditions were nonsignificant. The percentage of male schizophrenia/schizotypy participants significantly moderated anticipatory and consummatory pleasure for the combined sample and schizotypy alone; male participants were found to report reduced pleasure. There was only minor evidence of bias; sensitivity analysis confirmed result robustness. Exploratory outlier removal for schizophrenia within-group pleasure comparisons revealed a statistically significant difference between reported anticipatory and consummatory pleasure, with consummatory pleasure reduced relative to anticipatory (i.e., in the opposite direction of the majority of experimental research findings).
Conclusions:
These findings provided only modest support for the temporal dynamics of positive emotion conceptualization because they revealed no evidence for: 1) specific anticipatory pleasure deficits in schizophrenia-spectrum participants compared to controls; 2) significant reductions in anticipatory pleasure relative to consummatory pleasure in schizophrenia-spectrum participants.
Keywords: schizophrenia, schizotypy, anhedonia
Introduction
Anhedonia, traditionally defined as the diminished capacity to experience pleasure, has been considered a core component of schizophrenia since the earliest conceptualizations of the disorder (Bleuler, 1911; Kraepelin, 1919; Rado, 1953). Anhedonia in the schizophrenia spectrum was initially assumed to occur similarly to depression, reflecting a primary hedonic deficit resulting from reduced activation of reward circuitry responsible for consummatory pleasure (Meehl, 1962). However, laboratory-based tasks demonstrate that individuals with schizophrenia report experiencing as much positive emotion and subjective arousal as healthy controls (Cohen and Minor, 2010; Llerena et al., 2012). They also display robust neurophysiological response in brain regions related to reward receipt (Radua et al., 2015). These findings led some to conclude that anhedonia in schizophrenia should no longer be considered to reflect a true hedonic deficit in schizophrenia (Cohen et al., 2011; Strauss and Gold, 2012).
As evidence suggests that consummatory pleasure is intact in schizophrenia, it is unclear why hedonic experiences do not translate into motivated behaviors aimed at obtaining rewards (Barch and Dowd, 2010; Gard et al., 2007; Gold et al., 2008; Kring and Barch, 2014; Oorschot et al., 2013; Strauss et al., 2014). One framework proposed to explain this apparent disconnect, the temporal dynamics of positive emotion theory, suggests that deficits in anticipatory pleasure prevent the translation of reward information into motivated behavior (Kring and Elis, 2013). Anticipatory pleasure involves a cognitive component that entails predicting future emotion, as well as an experiential component that requires feeling positive emotion while simulating a future event. Initial evidence suggested that individuals with schizophrenia report as much pleasure as controls while performing goal-directed activities, but anticipate experiencing less pleasure than controls the next time they engage in that same activity (Gard et al., 2007).
Many subsequent investigations of anticipatory pleasure in individuals with schizophrenia have used the Temporal Experience of Pleasure Scale (TEPS: Gard et al., 2006). The TEPS is an 18-item scale designed to evaluate the experience of anticipatory and consummatory physical pleasure. The items use a hypothetical format to target either broad or situation-specifics aspects of pleasure (e.g., “I look forward to a lot of things in my life,” “I enjoy taking a deep breath of fresh air when I walk outside,”). Response format is a 6-point-Likert scale with anchors ranging from 1 (very false for me) to 6 (very true for me). Higher scores represent greater enjoyment and pleasure (i.e., less anhedonia). The anticipatory pleasure captured on this scale most closely resembles the cognitive component of anticipatory pleasure as it asks individuals to rate agreement with statements involving expectations of pleasure from future experiences. Research using the TEPS to investigate consummatory and anticipatory pleasure in schizophrenia demonstrates mixed results. Many studies find intact consummatory pleasure (Barch et al., 2014; Cassidy et al., 2012; Da Silva et al., 2017; Docherty, 2013; Fortunati et al., 2015; Gard et al., 2007; Kring et al., 2014; Lee et al., 2012; Makowski et al., 2016; Mote et al., 2014; Mucci et al., 2015; Schlosser et al., 2014; Simon et al., 2015; Subramaniam et al., 2015; Vignapiano et al., 2016; Wynn et al., 2010) while some do not (Chuang et al., 2014; Culbreth et al., 2016; Edwards et al., 2015; Gerritsen, 2015; Mann et al., 2013; Li et al., 2015; Strauss et al., 2011, 2013, 2015; Tso et al., 2014; Umesh et al., 2016). Anticipatory pleasure findings on the TEPS are even more mixed. Several investigations find reduced anticipatory pleasure in individuals with schizophrenia compared to controls (Barch et al., 2014; Chuang et al., 2014; Culbreth et al., 2016; Fortunati et al., 2015; Gard et al., 2007; Kring and Barch, 2014; Li et al., 2015; Mann et al., 2013; Mote et al., 2014; Simon et al., 2015; Tso et al., 2014; Umesh et al., 2016; Wynn et al., 2010), while others do not (Cassidy et al., 2012; Da Silva et al., 2017; Edwards et al., 2015; Gerritsen, 2015; Lee et al., 2012; Makowski et al., 2016; Mucci et al., 2015; Schlosser et al., 2014; Strauss et al., 2011, 2013, 2015; Subramaniam et al., 2015; Vignapiano et al., 2016;).
Furthermore, it is unclear whether other conditions within the schizophrenia-spectrum (e.g., psychometrically defined schizotypy, youth at clinical high-risk for psychosis [Meehl, 2001; Piskulic et al., 2012]) also display the pattern of anhedonia represented in Kring’s temporal dynamics model (i.e., intact consummatory pleasure and diminished anticipatory pleasure). For example, unlike schizophrenia patients, individuals with schizotypy and youth at clinical high-risk (CHR) for psychosis demonstrate diminished consummatory pleasure at either subjective (Cohen et al., 2012; Gruber et al., 2018; Strauss et al., 2018) or neurophysiological (Strauss et al., 2018) levels when presented with pleasurable stimuli in the laboratory. Scores on the TEPS also typically indicate reduced consummatory and anticipatory pleasure in these groups (Chan et al., 2012, 2016; Gooding and Pflum, 2012; Li et al., 2016; Loas et al., 2014; Martin et al., 2011; Schlosser et al., 2014; Shi et al., 2012; Wang et al., 2012, 2015; Wilson, 2012; Umesh et al., 2016; Xie et al., 2014; Yan et al., 2011, 2016). However, similar to the self-report literature on individuals with schizophrenia, some studies do find intact consummatory or anticipatory pleasure in individuals with schizotypy and CHR youth (Cooper et al., 2017; Docherty, 2013; Docherty et al., 2015; Fonseca-Pedrero et al., 2017; Hooker et al., 2014; McCarthy, 2013; Shi et al., 2012; Yan et al., 2016; see also Kwapil et al., 2012 for an investigation of intact pleasure in positive but not negative schizotypy in daily life).
These results point to two inherent and unresolved paradoxes in the schizophrenia spectrum anhedonia literature: The “Schizophrenia-Spectrum Anhedonia Paradox” and the “Liking-Wanting Anhedonia Paradox.”
The “Schizophrenia-Spectrum Anhedonia Paradox” (Strauss and Cohen, 2018) is defined as the paradoxical discrepancy wherein those with schizotypy or in the CHR phase of schizophrenia demonstrate reduced hedonic responses (i.e., true anhedonia; Cohen et al., 2012; Gruber et al., 2018; Strauss et al., 2018) while individuals with schizophrenia, who are considered to have a more severe form of psychopathology in nearly every way, display seemingly intact hedonic capacity. While few direct comparisons have been made between different disorders within the schizophrenia spectrum to resolve this paradox, those that have yield inconsistent results (Gooding and Pflum, 2012; Martin et al., 2011; Schlosser et al., 2014; Umesh et al., 2018; Wang et al., 2012). Several important questions need to be addressed to resolve this paradox, including which factors moderate findings.
The “Liking-Wanting Anhedonia Paradox” is defined as the apparent discrepancy wherein despite having a seemingly intact hedonic capacity, individuals with schizophrenia nevertheless engage in a reduced frequency of pleasurable activities (Frost and Strauss, 2016; Kring and Elis, 2013). Within-group differences in consummatory and anticipatory pleasure have rarely been calculated within schizophrenia and control groups, making it difficult to determine whether a selective impairment in anticipatory pleasure exists that might explain this paradox. Thus, there are many inconsistencies in the anhedonia literature in the schizophrenia-spectrum that remain to be resolved.
To address the two aforementioned “paradoxes,” a comprehensive meta-analysis was conducted that examined self-reported anticipatory and consummatory pleasure on the TEPS. Studies were selected that compared individuals with schizophrenia or psychometrically defined schizotypy to healthy controls. The TEPS was selected as the primary outcome measure because it purports to measure both anticipatory and consummatory pleasure using a comparable methodology (i.e., self-report) and it has been used in a sufficient number of studies. Other measures (e.g., the Chapman Anhedonia Scales; Chapman et al., 1976 or the Anticipatory and Consummatory Interpersonal Pleasure Scale; ACIPS: Gooding and Pflum, 2014), have also been used to investigate differences in other types of pleasure; however, they were not included as their factor structures do not support a two-factor distinction between anticipatory and consummatory pleasure (Gooding and Pflum, 2014). In addition to examining group effects, moderators of participant (e.g., sex, age, race, negative symptom severity, schizotypy presentation, medication) and study characteristics (e.g., use of a power analysis, use of a college-age sample, TEPS translation version, the scale used to measure psychometrically defined schizotypy) were also evaluated to determine their influence on self-reported consummatory or anticipatory pleasure.
Materials and Methods
Search Strategy
In accordance with PRISMA guidelines (Moher, Liberati, Tetzlaff, Altman, & the PRISMA Group, 2009), a thorough database search of articles published from January 2006 through June 2017 was conducted using the databases PubMed, PsycInfo, and ProQuest Dissertations and Theses. The protocol was preregistered with PROSPERO (Record ID: CRD42017065765, accessible at https://www.crd.york.ac.uk/PROSPERO/). See Appendix for PRISMA checklist. Searches were conducted using the following search terms: “schizophrenia,” “psychosis,” “clinical high risk,” “ultra high risk,” “schizotypal,” “schizotypy,” “anticipatory,” “consummatory,” “wanting,” “liking,” “anhedonia,” “TEPS,” “Temporal Experience of Pleasure,” “ACIPS,” and “Anticipatory and Consummatory Interpersonal Pleasure Scale.” Searches were restricted to articles that were peer-reviewed or were published dissertations, were written in English, and contained human subjects. Google Scholar was used to conduct manual searches of all articles citing the original TEPS and ACIPS papers (i.e., Gard et al., 2006, Gard et al., 2007, and Gooding and Pflum, 2014). Duplicate articles were excluded. Manual reference section citation searches of included articles were conducted1.
Selection of Studies
Inclusion criteria was comprised of peer-reviewed articles or published dissertations that included a psychometric schizotypy, clinical high risk, first episode, or schizophrenia sample and a control sample, used the TEPS, and used human subjects. In addition to the ACIPS, other measures of the experience of pleasure (e.g., Chapman Scales: Chapman et al. 1976) were excluded as physical and social consummatory and anticipatory anhedonia were the specific variables of interest. Other exclusion criteria included: 1) for published journal articles, non-peer reviewed, 2) was a review, meta-analysis, editorial, or conference abstract, 3) did not include a psychometrically defined schizotypy, high risk, first episode, genetic risk, or a schizophrenia sample, or 4) did not include a control sample. Manual searches of articles citing the TEPS were also conducted. Attempts were made to contact authors of the TEPS for any unpublished data as well as the authors for six studies that reported using the TEPS in individuals across the schizophrenia spectrum but did not report enough information to calculate effect sizes (Arrondo et al., 2015; Bedwell et al., 2016; Da Silva et al., 2017; Eisenstein et al., 2017; Sandt, 2013; Tso et al., 2014). Data were provided for all studies but two (Arrondo et al., 2015; Sandt, 2013). Accordingly, these two articles were not included in analyses. Additional unpublished data were provided from two sources (Barch and Moran; Kring).
Effect Size calculation
Means and standard deviations for anticipatory and consummatory ratings by schizophrenia spectrum and control groups were extracted for the remaining studies using a template piloted prior to retrieval. Hedges’ g was calculated by dividing the difference in patient and control means by the pooled standard deviation for each effect. In cases of longitudinal studies, baseline scores were selected (Edwards et al., 2015). Hedges’ g was chosen to account for small sample bias. In the case that means and standard deviations were not available, but other effect sizes were provided (Yan et al., 2011), Hedges’ g and the standard error were manually calculated via transformation (Lipsey and Wilson, 2001). Data were extracted and confirmed by three of the authors (KFV, HC, IR); see Appendix for further extraction and interrater agreement information.
Selection, coding of covariates, and sample characteristics
Included covariates were diagnostic group, schizotypy subtype (psychometrically defined; Perceptual Aberration/Magical Thinking, Social Anhedonia, Disorganized, and Mixed subtypes, if provided by the study), mean age, mean percentage of non-Caucasian participants, mean percentage of men (both in the spectrum sample and control samples), the percentage of first generation antipsychotics prescribed to the participants with schizophrenia, and negative symptom severity scores converted to proportions. Missing effects for all included covariates were imputed with the mean of the distribution for studies that did not provide moderator data (percentage first-generation antipsychotics, clinically rated negative symptoms, percentage of men in the sample, age, and percentage of non-Caucasian participants). See Appendix for additional information on selection and coding of covariates. See Tables 1.A–1.C for sample characteristics of all studies.
Table 1.A.
Study and participant characteristics
| Citation |
Study group/PDS subgroup |
SZ-CN n |
Mean Age |
%Non-Caucasian |
%maleSZ-%maleCN |
% 1st gen AP |
Proportion Neg. Sym. Severity |
|---|---|---|---|---|---|---|---|
| Barch and Moran, unpublished supplied data | SZ | 67-34 | 37.44 | 71.10% | 61.19%-70.59% | 18.00% | |
| Barch et al., 2014 | SZ | 59-39 | 38.35 | 64.70% | 57.60%-48.70% | 5.00% | 0.26 |
| Bedwell et al., 2016, supplied data | SZ | 10-13 | 34.99 | 48.08% | 60.00%-53.85% | ||
| Cassidy et al., 2012 | SZ | 91-91 | 24.65 | 75.00%-69.00% | 0.18 | ||
| Chan et al., 2012 | PDS | 92-85 | 19.25 | 44.57%-40.00% | |||
| Chan et al., 2016 | PDS/SocAnh | 8-20 | 19.04 | 100.00%-55.00% | |||
| Chuang et al., 2014 | SZ | 22-20 | 33.52 | 21.50% | 84.21%-75.00% | 0.33 | |
| Cooper et al., 2017 | CHR | 219-1006 | 20.47 | 38.75% | 25.30%-26.90% | ||
| Culbreth et al., 2016 | SZ | 57-36 | 36.80 | 64.05% | 66.70%-52.80% | 1.00% | 0.02 |
| Da Silva et al., 2017, supplied data | SZ | 84-81 | 34.65 | 58.33%-55.56% | 1.00% | 0.14 | |
| Docherty, 2013 | SZ | 35-29 | 43.70 | 82.00%-65.00% | |||
| Docherty, 2013 | GHR | 35-29 | 46.75 | 36.00%-65.00% | |||
| Edwards et al., 2015 | SZ | 53-52 | 41.49 | 56.00% | 74.00%-63.00% | 0.42 | |
| Eisenstein et al., 2017, supplied data | SZ | 65-34 | 35.90 | 63.19% | 43.08%-52.94% | 0.24 | |
| Fonseca-Pedrero et al., 2017 | PDS | 28-639 | |||||
| Fortunati et al., 2015 | SZ | 53-46 | 39.20 | 60.40%-50.00% | 47.20% | 0.45 | |
| Gard et al., 2007 | SZ | 51-50 | 47.34 | 51.45% | 64.71%-50.00% | 31.37% | |
| Gerritsen, 2015 | SZ | 59-65 | 45.15 | 45.85% | 69.50%-65.10% | ||
| Gooding and Pflum, 2012 | PDS/SocAnh | 68-79 | 19.32 | 54.40%-67.10% | |||
| Gooding and Pflum, 2012 | PDS/PerMag | 88-79 | 19.33 | 45.50%-67.10% | |||
| Hooker et al., 2014 | PDS/SocAnh | 15-15 | 31.14 | 46.67%-33.33% | 0.87% |
Note. SZ = Schizophrenia, PDS = Psychometric Schizotypy, CN = Control, SocAnh = Social Anhedonia cluster, PerMag = Perceptual Aberration/Magical Thinking cluster. AP = antipsychotics, PANSS = Positive and Negative Syndrome Scales. Neg. sym. = negative symptoms.
Table 1.C.
Study and participant characteristics
| Citation |
Study group/PDS subgroup |
SZ/PDS-CN n |
Mean Age |
%Non-Caucasian |
%maleSZ-%maleCN |
%1st gen AP |
Proportion Neg. Sym. Severity |
|---|---|---|---|---|---|---|---|
| Tso et al., 2014, supplied data | SZ | 39-36 | 40.95 | 58.97%-63.89% | 23.00% | 0.08 | |
| Umesh et al., 2016 | SZ | 20-20 | 29.78 | 100.00%-100.00% | 50.00% | 0.22 | |
| Umesh et al., 2016 | GHR | 20-20 | 29.80 | 100.00%-100.00% | 0.22 | ||
| Vignapiano et al., 2016 | SZ | 30-23 | 33.20 | 63.33%-43.48% | 0% | ||
| Wang et al., 2015 | PDS | 21-30 | 19.30 | 47.62%-50.00% | |||
| Wang et al., 2012 | PDS/Mixed | 231-83 | |||||
| Wang et al., 2012 | PDS/PerMag | 187-71 | |||||
| Wang et al., 2012 | PDS/SocAnh | 264-116 | |||||
| Wilson, 2012 | PDS | 79-40 | 18.96 | 43.00% | 37.50%-38.50% | ||
| Wynn et al., 2010 | SZ | 34-36 | 41.60 | 78.90%-66.70% | 14.71% | 0.58 | |
| Xie et al., 2014 | PDS/SocAnh | 28-38 | 20.74 | 39.29%-34.21% | |||
| Yan et al., 2011 | PDS | 20-20 | 22.50 | 40.00%-30.00% | |||
| Yan et al., 2016 | PDS/SocAnh | 15-22 | 19.56 | 53.33%-50.00% |
Note. SZ = Schizophrenia, PDS = Psychometric Schizotypy, CN = Control, SocAnh = Social Anhedonia cluster, PerMag = Perceptual Aberration/Magical Thinking cluster. AP = antipsychotics, PANSS = Positive and Negative Syndrome Scales. Neg. sym. = negative symptoms.
Statistical Analysis
Comprehensive Meta-Analysis version 3 (Biostat, Inc.) was used to conduct analyses. Random-effects models were used to calculate mean ES and 95% confidence intervals for consummatory pleasure and anticipatory pleasure as well as any variability due to moderators. Model heterogeneity was determined via Q and I2 (Higgins et al., 2003). Mixed-effects linear regression with restricted maximum likelihood estimation was used to determine the impact of age, percentage of men from the spectrum samples included in study samples, percentage of men from the control samples included in the study samples, percentage of non-Caucasian participants, percentage of first-generation antipsychotics prescribed to the schizophrenia participants, and the proportional severity of negative symptoms. Multivariate mixed-effects linear regression with restricted maximum likelihood estimation was used to adjust for any potential correlated effects of significant moderators within studies. Interactions between moderator variables were also assessed via univariate linear regression. Publication bias was determined via classic fail-safe N, which estimates how many unpublished effects would be needed to make the current results nonsignificant (Rosenthal, 1979). Separate funnel plots for anticipatory and consummatory pleasure by diagnostic group were graphed and analyzed as well via Egger’s test as indicators of possible sampling bias (Egger et al., 1997). Funnel plots were also used to identify outliers; outliers were determined via visual inspection of the funnel plots and identified as any score significantly more than two standard deviations above or below the mean ES. Bias related to study design was also assessed via the extraction and coding of specific study design variables, including version of the TEPS used (i.e., the Chinese version, [Chan et al., 2010]; American, [Gard et al., 2006]; or French versions, [Favrod et al., 2009]), the specific assessment used to classify schizotypy groups, and whether a college or community sample was used. Mixed-effects linear regression with restricted maximum likelihood estimation was used to assess bias related to study design.
Results
Participants
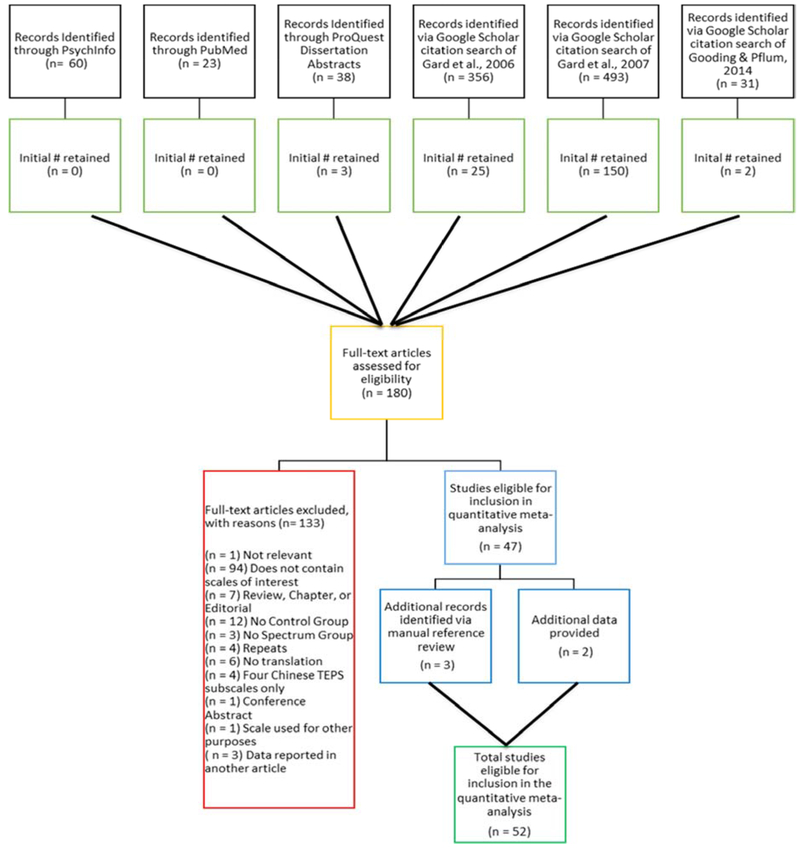
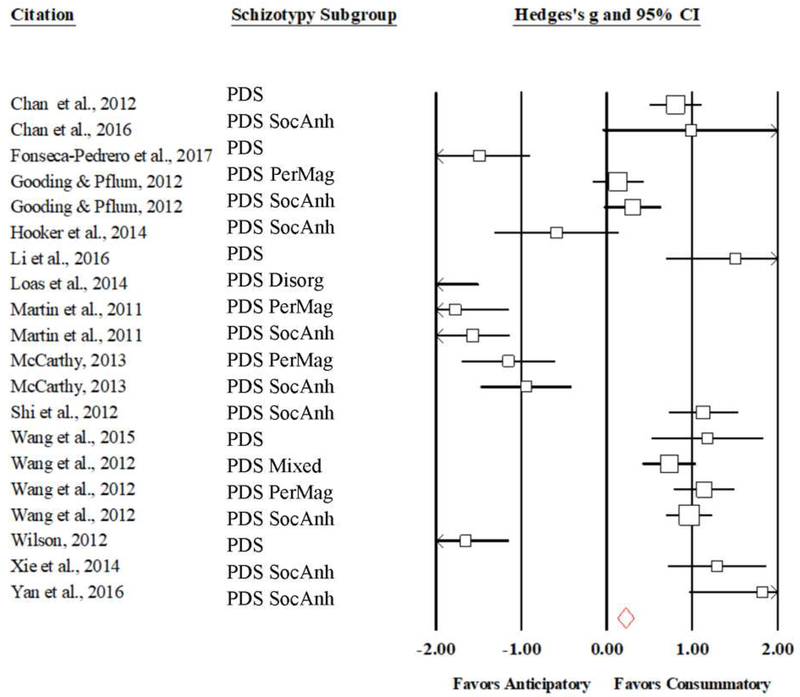
A total of 1,001 articles were reviewed (see Figure 1 for a flowchart of the screening process and eventual study selection). Overall, 110 (55 consummatory and 55 anticipatory, respectively) effects were obtained from 50 studies and extra data were provided from two research groups (Barch and Moran; Kring) for an initial inclusion of 8,164 participants (1,771 individuals with schizophrenia [SZ: both chronic and first episode, FEP], 953 individuals with psychometrically defined schizotypy [PDS; including 83 with mixed schizotypy symptoms, 39 with disorganized symptoms, 399 identified as falling within a social anhedonia cluster, and 216 falling within a perceptual/magical aberration cluster], 279 individuals at high risk [CHR], 88 individuals at genetic high risk [GHR], and 5,073 controls [CN]). CHR and GHR differences in anticipatory and consummatory pleasure were excluded from analyses due to the limited number of effects available, which in aggregate was below the 10-effect cut off determined a priori, resulting in a total inclusion of 7,797 participants (CHR = 2, GHR = 3: Cooper et al., 2017; Docherty et al., 2015; Schlosser et al., 2014; Umesh et al., 2016). Similarly, as there were fewer than 10 effects extracted for each PDS subgroup, all schizotypy types were collapsed together into one overall PDS group in the main analyses and only included as a covariate in the schizotypy-specific analyses. Mean ages of the participants ranged from 17.6 to 50.4 years. Details of each study are included in Tables 1.A – 1.C. Forest plots were constructed to visualize weighted effect size for each study, displaying both overall results and results split by spectrum subgroup (see Figures 2, 3, 4, and 5).
Figure 1.
Flowchart of Search Results and Study Selection
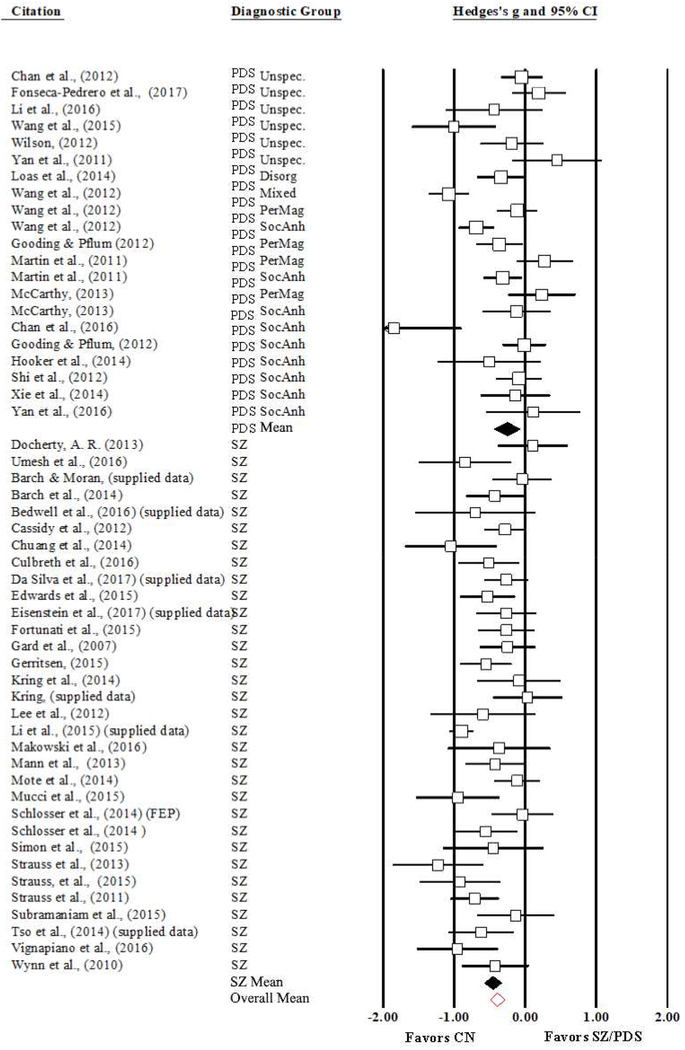
Figure 2.
Forest Plot of Consummatory Pleasure Mean Effect Sizes and Confidence Intervals, Weighted by Study Size and Divided by Diagnostic Group. SZ/PDS = Schizophrenia/Psychometrically Defined Schizotypy; CN = Control; SocAnh = Social Anhedonia cluster; PerMag = Perceptual Aberration/Magical Thinking cluster; Disorg = Disorganized cluster.
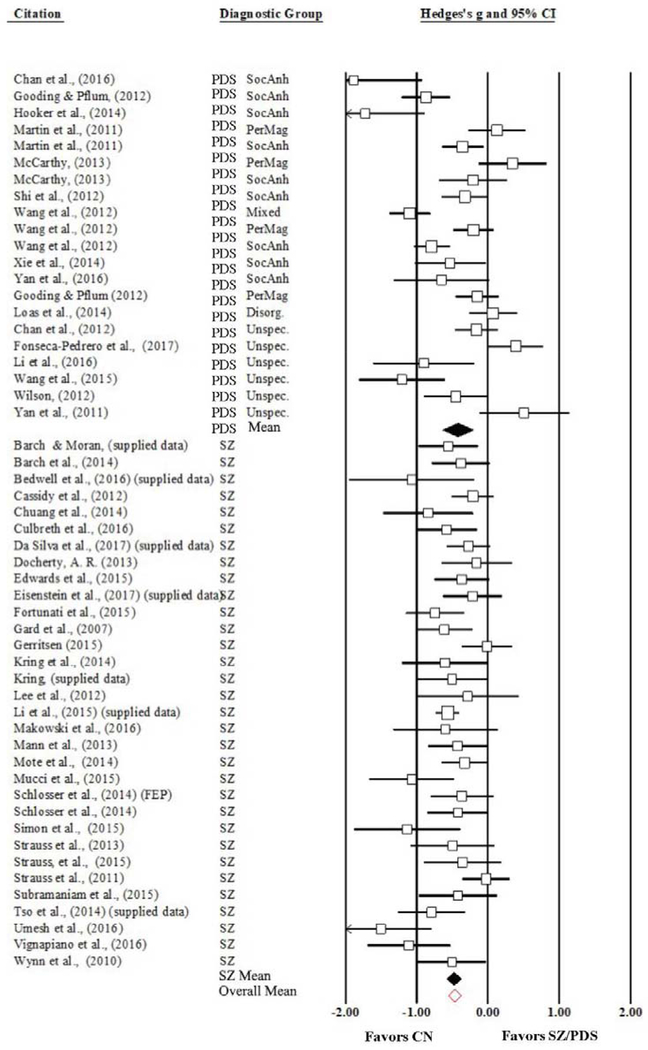
Figure 3.
Forest Plot of Anticipatory Pleasure Mean Effect Sizes and Confidence Intervals, Weighted by Study Size and Divided by Diagnostic Group. SZ/PDS = Schizophrenia/Psychometrically Defined Schizotypy; CN = Control; SocAnh = Social Anhedonia cluster; PerMag = Perceptual Aberration/Magical Thinking cluster; Disorg = Disorganized cluster.
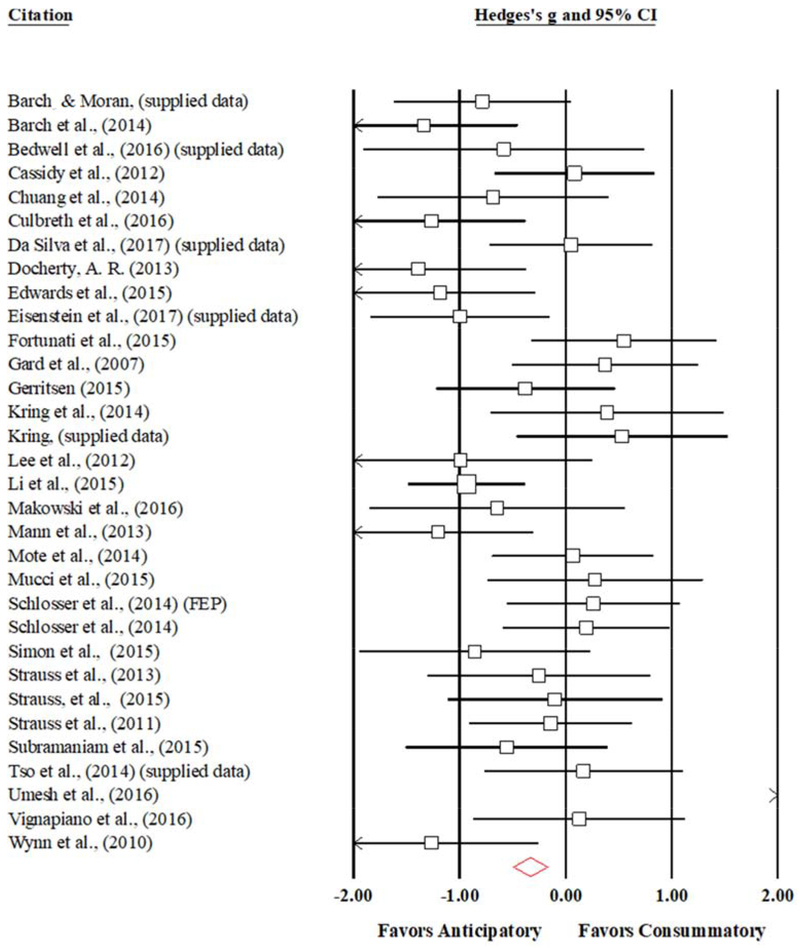
Figure 4.
Forest Plot of Anticipatory vs Consummatory Mean Effect Sizes and Confidence Intervals for Individuals with Schizophrenia, Weighted by Study Size
Figure 5.
Forest Plot of Anticipatory vs Consummatory Mean Effect Sizes and Confidence Intervals for Individuals with Psychometric Schizotypy, Weighted by Study Size. PDS = Psychometrically Defined Schizotypy; SocAnh = Social Anhedonia cluster; PerMag = Perceptual Aberration/Magical Thinking cluster; Disorg = Disorganized cluster.
Mean Effects: Schizophrenia-Spectrum Anhedonia Paradox
Initial random effects model meta-analyses were conducted across the 106 remaining effects, divided into separate analyses for consummatory and anticipatory pleasure (53 effects per analysis). See Table 2 for a full list of results.
Table 2.
Results of meta-analyses and multivariate regression covariate analyses: Schizophrenia Spectrum Anhedonia Paradox
| Schizophrenia Spectrum |
Mean ES (Hedges’ g) |
SE |
95%CI |
Q |
I2 |
p |
|---|---|---|---|---|---|---|
| Consummatory Pleasure | −0.37 | 0.06 | −0.48 | −0.27 | 183.83 | 71.71 | < 0.001 |
| Anticipatory Pleasure | −0.46 | 0.05 | −0.57 | −0.36 | 177.63 | 70.73 | < 0.001 |
| Moderators (Con|Ant) | β | SE | 95%CI Low | 95% CI Hi | p | |
| Age | −0.01|−0.01 | 0.0110.01 | −0.02|−0.02 | 0.0110.02 | 0.27|0.78 | |
| %Non-Caucasian | <−0.01|−0.01 | <0.01|0.01 | −0.01|−0.01 | 0.0110.01 | 0.94|0.83 | |
| %SZS male | −0.01|−0.02 | <0.01|0.01 | −0.02|−0.03 | <−0.01|−0.01 | 0.03|< 0.001 | |
| %CN male | 0.01|0.01 | <0.01|0.01 | <−0.01|−0.01 | 0.02|0.01 | 0.14|0.39 | |
| Group | −0.03|−0.27 | 0.16|0.18 | −0.34|−0.62 | 0.28|0.09 | 0.85|0.14 | |
| Schizotypy |
Mean ES (Hedges’ g) |
SE |
95%CI |
Q |
I2 |
p |
| Consummatory Pleasure | −0.25 | 0.09 | −0.44 | −0.07 | 90.83 | 77.98 | < 0.01 |
| Anticipatory Pleasure | −0.42 | 0.11 | −0.64 | −0.21 | 124.14 | 83.89 | < 0.001 |
| Moderators (Con|Ant) | β: | SE | 95%CI Low | 95% CI Hi | p | |
| Age | <−0.01|<0.01 | 0.02|0.02 | −0.04|−0.05 | 0.0310.05 | 0.71|0.93 | |
| %Non-Caucasian | <−0.01|<−0.01 | 0.0110.02 | −0.02|−0.03 | 0.02|0.03 | 0.97|0.99 | |
| %SZS male | −0.02|−0.02 | 0.0110.01 | −0.031–0.03 | <−0.011–0.01 | < 0.011<0.01 | |
| %CN male | 0.01|0.01 | 0.0110.01 | <−0.01|−0.01 | 0.02|0.02 | 0.13|0.49 | |
| Subgroup | −0.22|−0.54 | 0.19|0.21 | −0.15|−0.95 | 0.60|−0.13 | 0.24|<0.01 | |
| Schizophrenia | Mean ES (Hedges’ g) | SE | 95%CI | Q | I2 | p |
| Consummatory Pleasure | −0.46 | 0.06 | −0.58 | −0.33 | 77.68 | 60.09 | < 0.001 |
| Anticipatory Pleasure | −0.48 | 0.05 | −0.58 | −0.38 | 51.76 | 40.11 | < 0.001 |
| Moderators (Con|Ant) | β: | SE | 95%CI Low | 95%CI Hi | p | |
| Age | −0.01|<−0.01 | 0.0110.01 | −0.02|−0.01 | 0.01|0.02 | 0.47|0.81 | |
| %Non-Caucasian | <0.01|<0.01 | <0.01|<0.01 | −0.01|−0.01 | 0.01|0.01 | 0.92|0.93 | |
| %SZS male | <0.01|−0.01 | <0.01|0.01 | −0.02|−0.02 | 0.02|<0.01 | 0.94|0.25 | |
| %CN male | <−0.01|<0.01 | 0.0110.01 | −0.02|−0.01 | 0.01|0.02 | 0.81|0.54 | |
| %1st gen antipsychotics | <0.01|−0.01 | 0.01|0.01 | −0.01|−0.02 | 0.02|<0.01 | 0.66|0.23 | |
| Severity of neg sym. | −0.38|−0.20 | 0.52|0.43 | −1.411–1.05 | 0.65|0.65 | 0.47|0.64 |
Note. SZS = Schizophrenia Spectrum, PDS = Psychometric Schizotypy, CN = Control, Con = Consummatory, Ant = Anticipatory. Neg. sym. = negative symptoms.
Consummatory Pleasure.
The analysis for consummatory pleasure for SZ and PDS participants combined as compared to controls yielded a mean effect size (ES) of −0.37 (95% CI: −0.48 – −0.27; SE = 0.06; z = −6.79; p < 0.001), indicating that individuals with schizotypy and schizophrenia report less consummatory pleasure on average than CN. Analyses split by diagnostic group (FEP were collapsed into the chronic SZ group due to their small sample size) were conducted following full-sample analyses. Results indicated that individuals with chronic or first episode SZ report less consummatory pleasure on average than CN, (mean ES = −0.46; 95% CI: −0.58 – −0.33; SE 0.06; z = −7.25; p < 0.001). Results of PDS analyses indicated that individuals with psychometrically defined schizotypy report less consummatory pleasure on average than CN, (mean ES = −0.25; 95% CI: −0.44 – −0.07; SE 0.09; z = −2.71; p < 0.01). See Figure 2 for a forest plot of the differences in reports of consummatory pleasure overall and by group.
Anticipatory Pleasure.
The analysis for anticipatory pleasure for SZ and PDS participants indicated that individuals across the schizophrenia spectrum report less anticipatory pleasure on average than CN, (mean ES = −0.46; 95% CI: −0.57 – −0.36; SE = 0.05; Z = −8.50; p < 0.001). Analyses split by diagnostic group (FEP were collapsed into the chronic SZ group due to their small sample size) were conducted following full-sample analyses. Results indicated that individuals with chronic or first episode SZ report less anticipatory pleasure on average than CN, (mean ES = −0.48; 95% CI: −0.58 – −0.38; SE = 0.05; Z = −9.32; p < 0.001). The analysis of collapsed PDS categorizations indicated that individuals with psychometrically defined schizotypy report less anticipatory pleasure on average than CN, (mean ES = −0.42; 95% CI: −0.64 – −0.21; SE = 0.11; Z = −3.81; p < 0.001). See Figure 3 for a forest plot of the differences in reports of anticipatory pleasure overall and by group.
Heterogeneity Tests.
The Q statistic for each test was significant, suggesting heterogeneity beyond sampling error (see Table 2). I2 values for each test also indicated that less than 75% of the observed variance in each analysis was due to sampling error (see Table 2). Consequently, covariate analyses were run as planned.
Covariate analyses: Schizophrenia-Spectrum Anhedonia Paradox
Table 2 provides multivariate covariate regression results for each study. Mean age and percentage of men included in each study were each imputed in 5% of cases to the mean of the distribution. The percentage of non-Caucasian participants was imputed in 33% of cases to the mean of the distribution. The percentage of first-generation antipsychotics prescribed to the participants with SZ was imputed in 38% of cases to the mean of the distribution. Proportional negative symptom scores were imputed in 29% of cases to the mean of the distribution, respectively. Interactions for significant covariates were calculated based on continuous covariates requiring less than 50% imputation (rule determined a priori).
Consummatory pleasure.
For consummatory pleasure for the full spectrum sample, initial regression models revealed a significant effect of the percentage of men in the spectrum, group, and mean age. Percentage of men in the control sample and the percentage of non-Caucasian participants were nonsignificant. Percentage of men in the spectrum sample remained significant after controlling for mean age, group, percentage of men in the control sample, and percent of non-Caucasian participants in a multivariate regression analysis based on all 53 effects reporting all variables or containing an imputed mean, indicating that men along the schizophrenia spectrum were more likely to report less consummatory pleasure than controls. Group and age were no longer significant (see Table 2 for covariate results). A test for a two-way interaction between age and sex revealed a significant interaction, R2 = 0.24, β < −0.01, SE < 0.01, Z = −3.38, p < 0.001, suggesting that, in aggregate, younger women across the schizophrenia spectrum were more likely to report greater consummatory pleasure than older men. Interaction with race was not calculated as it required greater than 50% imputation.
For consummatory pleasure for the schizotypy sample, initial regression models revealed a significant effect of the percentage of men in the schizotypy sample. Percentage of men in the control sample, age, percentage of non-Caucasian participants, and schizotypy subgroup were nonsignificant. Percentage of men in the schizotypy sample remained significant after controlling for mean age, group, percentage of men in the control sample, and percentage of non-Caucasian participants in a multivariate regression analysis based on all 53 effects reporting all covariates or containing an imputed mean, indicating that, in aggregate, men with schizotypy were more likely to report lower consummatory pleasure than controls (see Table 2 for covariate results). A test for a two-way interaction between age and sex revealed a significant interaction, R2 = 0.14, β < −0.01, SE < 0.01, Z = −1.99, p < 0.05, indicating that, in aggregate, younger women in the schizotypy sample were more likely to report greater consummatory pleasure than older men.
For consummatory pleasure for the schizophrenia sample, initial regression models for percentage of men in the schizophrenia sample, percentage of men in the control sample, percentage of non-Caucasian participants, mean age, percentage of first generation antipsychotics prescribed to the schizophrenia sample, proportional severity of negative symptoms were all nonsignificant. Multivariate analyses were all likewise nonsignificant (see Table 2 for covariate results). Thus, no follow up analyses were conducted.
Anticipatory pleasure.
For anticipatory pleasure for the full spectrum sample, initial regression models revealed a significant effect of the percentage of men in the spectrum sample. Group, percentage of men in the control sample, mean age, and the percentage of non-Caucasian participants were nonsignificant. Percentage of men in the spectrum sample remained significant after controlling for mean age, group, percentage of men in the control sample, and percent of non-Caucasian participants in a multivariate regression analysis based on all 53 effects reporting all variables or containing an imputed mean, indicating that men along the schizophrenia spectrum were more likely to report lower anticipatory pleasure than controls (see Table 2 for covariate results). A test for a two-way interaction between age and sex was nonsignificant, R2 = 0.07, β < −0.001, SE < 0.001, Z = −1.82, p = 0.07. The interaction between men in the spectrum sample and race was not calculated as race required greater than 50% imputation.
For anticipatory pleasure for the schizotypy sample, initial regression models revealed a significant effect of the percentage of men in the schizotypy sample. Age, percentage of men in the control sample, and percentage of non-Caucasian participants were nonsignificant. Percentage of men in the schizotypy sample and schizotypy subgroup remained significant after controlling for mean age, percentage of men in the control sample, and percentage of non-Caucasian participants in a multivariate regression analysis based on all 53 effects reporting all covariates or containing an imputed mean (see Table 2 for covariate results), indicating that, in aggregate, men with schizotypy were more likely to report less anticipatory pleasure than controls and individuals endorsing symptoms of negative schizotypy were more likely to report less anticipatory pleasure than individuals endorsing symptoms of positive schizotypy. A test for a two-way interaction between age and sex was nonsignificant, R2 = 0.10, β < −0.001, SE < 0.001, Z = −1.85, p = 0.06. The interaction between men in the spectrum sample and race was not calculated as race required greater than 50% imputation.
For anticipatory pleasure for the schizophrenia sample, initial regression models were all nonsignificant. Given the nonsignificance of all analyses, no follow-up analyses were conducted; see Table 2 for metaregression results.
Mean Effects: Liking-Wanting Paradox
Random effects model meta-analyses were conducted across individuals with schizophrenia and schizotypy, separately, to investigate the Liking-Wanting Paradox. See Table 3 for a full list of results.
Table 3.
Results of meta-analyses: Liking-Wanting Anhedonia Paradox
| Mean ES (Hedges’ g) |
SE |
95%CI |
Q |
I2 |
p |
|
|---|---|---|---|---|---|---|
| Schizophrenia | −0.25 | 0.17 | −0.58 | −0.09 | 131.47 | 76.43 | 0.15 |
| Schizotypy | 0.03 | 0.25 | −0.46 | 0.52 | 448.72 | 95.77 | 0.91 |
Schizophrenia.
The consummatory-anticipatory pleasure comparison analysis yielded a mean effect size of −0.25 (95%CI: −0.58 – −0.09; SE = 0.17; Z = −1.43; p = 0.15) indicating that, in aggregate, there is no statistically significant difference between SZ reports of anticipatory and consummatory pleasure (see Table 3 and Figure 4). Accordingly, no follow-up analyses were conducted. However, one study emerged as a significant outlier (Umesh et al., 2016) (see Appendix Figure A.4 for funnel plot). Accordingly, an exploratory analysis was conducted without this outlier, which yielded a mean effect size of −0.39 (95% CI: −0.61 - −0.17; SE = 0.11; Z = −3.42; p < 0.001), indicating that, with this outlier removed, there is a statistically significant difference between SZ reports of anticipatory and consummatory pleasure, such that reports of anticipatory pleasure may be elevated in SZ relative to consummatory.
Schizotypy.
The consummatory-anticipatory comparison analysis yielded a mean effect size of 0.03 (95%CI: −0.46 – 0.52; SE = 0.25; Z = 0.12; p = 0.91) indicating that, in aggregate, there is no statistically significant difference between PDS reports of anticipatory and consummatory pleasure (see Table 3 and Figure 5). Accordingly, no follow-up analyses were conducted.
Heterogeneity Tests.
The Q statistic for each test was significant, suggesting heterogeneity in the effects beyond sampling error (see Table 3). I2 values for each test also indicated that less than 75% of the observed variance in each analysis was due to sampling error (see Table 3).
Assessment of Bias: Schizophrenia-Spectrum Anhedonia Paradox
Rosenthal’s Fail-Safe N (Rosenthal, 1979) was calculated to determine that at least 3,222 studies reporting p > 0.05 would be needed to overturn the effects of the anticipatory meta-analysis, and at least 1,129 studies would be needed to overturn the effects of the consummatory analyses.
Visual inspection of funnel plots and Egger’s test (Egger et al., 1997) did not reveal any clear asymmetry or publication bias, consummatory pleasure, t(51) = 0.82, p (2-tailed) = 0.42 and anticipatory pleasure t(51) = 1.51, p (2-tailed) = 0.14. See Appendix, Figures A.1 and A.2, for funnel plots of differences in combined SZ and PDS and control self-reports of consummatory and anticipatory pleasure, respectively. However, as random effects models were used and Egger’s tests are primarily designed to test bias in fixed effects models, the results of the Egger’s tests conducted may instead indicate the presence of true variation explainable by moderators. Thus, these results should be interpreted with caution.
Mixed-effects linear meta-regression indicated that for consummatory and anticipatory pleasure, full spectrum meta-analytic results were not biased by the use of a college-age sample (18-21), the version of the TEPS used (Chinese, or American- as only one study reported use of the French TEPS, this study was not included), or the questionnaire used to measure psychometrically defined schizotypy.
Assessment of Bias: Liking-Wanting Paradox
Schizophrenia.
Rosenthal’s Fail-Safe N (Rosenthal, 1979) was calculated to determine that at least 53 studies reportingp > 0.05 would be needed to overturn the effects of the SZ Liking-Wanting Paradox meta-analysis. Visual inspection of funnel plots and Egger’s test (Egger et al., 1997) indicated that the mean effect size of differences between consummatory and anticipatory pleasure may be subject to publication bias, t(30) = 2.08, p (2-tailed) < 0.05. See Appendix, Figure A.3 for the funnel plot.
No variables were available to use to estimate bias due to study design in the SZ Liking-Wanting Paradox analysis as they failed to meet the 10 study cut-off for inclusion.
Schizotypy.
Rosenthal’s Fail-Safe N (Rosenthal, 1979) was calculated to determine that any additional study with a p > 0.05 would influence the results of the PDS Liking-Wanting Paradox meta-analysis, indicating potential file-drawer bias. However, visual inspection of funnel plots and Egger’s test (Egger et al., 1997) indicated that the mean effect size of differences between consummatory and anticipatory pleasure may not be subject to publication bias, t(18) = 1.39, p (2-tailed) = 0.18. See Appendix, Figure A.5 for the funnel plot.
Mixed-effects linear meta-regression indicated that PDS liking-wanting meta-analytic results may not be biased by the questionnaire used to classify schizotypy but may be biased by the version of the TEPS used (Chinese or American; Chinese TEPS, R2 = 0.75, β = 2.10, SE = 0.35, Z = −5.97, p < 0.001). These results suggest that Chinese individuals may report greater consummatory pleasure than anticipatory pleasure as compared to the opposite pattern in the US (greater anticipatory pleasure than consummatory).
Discussion
A meta-analysis was conducted to examine self-reported anticipatory and consummatory pleasure in individuals with schizophrenia and psychometrically-defined schizotypy to resolve the Schizophrenia-Spectrum Anhedonia Paradox, resolve the Liking-Wanting Paradox, and determine whether discrepancies in study findings across the schizophrenia spectrum are due to moderators or specific study characteristics.
In relation to the first goal, evaluating the Schizophrenia-Spectrum Anhedonia Paradox (i.e., the paradoxical findings that individuals with schizophrenia may have intact hedonic capacity, whereas those with schizotypy or in the CHR phase of schizophrenia have diminished hedonic response (Strauss and Cohen, 2018), results indicated that both SZ and PDS reported less anticipatory and consummatory pleasure than controls. Additionally, the magnitude of these deficits did not differ between SZ and PDS. These TEPS findings are inconsistent with the notion of the Schizophrenia-Spectrum anhedonia paradox, suggesting that the paradox may be most evident in laboratory-based studies (e.g., Cohen and Minor, 2010; Llerena et al., 2012) that directly test hedonic capacity.
For the schizotypy group alone, anticipatory pleasure was moderated by schizotypy subgroup, indicating that individuals with negative schizotypy were more likely to report lower anticipatory pleasure than healthy controls or individuals endorsing negative schizotypy. These results are unsurprising given that anhedonia, as a negative symptom, is considered characteristic of psychometrically-defined negative schizotypy (Kwapil et al. 2012). However, these results may be consistent with evidence that greater severity of negative schizotypy symptoms is associated with increased risk for developing schizophrenia (Gooding et al., 2007). It is surprising, however, that negative symptom severity was not a significant moderator in the schizophrenia sample. This discrepancy may be due to limited reporting of symptoms in the schizophrenia studies, causing the analysis to be underpowered.
The Liking-Wanting paradox reflects the research findings indicating that despite intact hedonic capacity, individuals with schizophrenia still engage in fewer pleasurable activities than healthy controls (Frost and Strauss, 2016; Kring and Elis, 2013). Kring and Ellis (2013) explained this abnormality as resulting in part from impaired anticipatory pleasure (but intact consummatory pleasure). The within-group contrast comparing levels of anticipatory and consummatory pleasure were nonsignificant for both the schizophrenia and schizotypy groups. This is contrary to the notion of a greater deficit in anticipatory than consummatory pleasure. Furthermore, when the outlier study (Umesh et al., 2016) was removed, results were in the opposite direction of what would be expected based on the literature (wherein consummatory anhedonia is typically found to be reduced relative to anticipatory). The fact that the finding here is opposite to what is typically observed in laboratory and ecological momentary assessment research (i.e., more severe deficits in anticipatory pleasure relative to consummatory) serves to confirm that the anticipatory and consummatory difference does not hold in the expected direction as what would be needed for the Liking-Wanting Paradox to be supported on the TEPS. Additionally, the significant findings of the exploratory analysis without the outlier (Umesh et al., 2016), in combination with the evidence of bias due to study-specific characteristics (e.g., the version of the TEPS used), suggest that study characteristics may also be influencing results. In general, however, these findings suggest that the Liking-Wanting Paradox may be better isolated through laboratory or experience sampling studies than the TEPS.
Overall, these results suggest that the TEPS alone may not be capable of resolving the Schizophrenia-Spectrum or Liking-Wanting Anhedonia Paradoxes. This may be due to the structure of the TEPS itself, as the anticipatory and consummatory pleasure questions may examine the same underlying construct. Both the anticipatory and consummatory subscales of the TEPS rely on what the emotional self-report literature (Robinson and Clore, 2002) terms a “hypothetical” reporting format. Such formats do not allow participants to rely on experiential emotion knowledge (i.e., direct access to their feeling in the moment in relation to a mental or environmental stimulus to which they are exposed) when making self-reports, but rather rely on semantic emotion knowledge (i.e., beliefs about how one thinks they would feel if/when they were exposed to the hypothetical scenario described). Given that individuals do not access experiential emotion knowledge when completing scales using hypothetical reporting formats, by its very nature, the TEPS cannot measure consummatory pleasure as it purports. To truly test consummatory pleasure, participants would need to be directly exposed to pleasurable stimuli or real-world activities and asked how positive they feel in the moment. Understanding which types of information individuals access (i.e., experiential vs. semantic emotion knowledge) using self-reports relying on different formats helps to shed light onto what low self-reports of pleasure actually mean. On scales like the TEPS that use a hypothetical reporting format for both the anticipatory and consummatory items, lower scores do not provide any indication of an individual’s hedonic capacity (i.e., because they do not rely on experiential emotion knowledge). Rather, both subscales rely on semantic emotion knowledge and therefore most accurately reflect low pleasure beliefs that individuals hold, which may or may not be accurate representations of their true hedonic capacity (i.e., ability to respond to pleasurable stimuli in the moment). When these processes underlying emotional self-report are adequately considered (Robinson and Clore, 2002), the fact that schizophrenia-spectrum participants were lower than controls on both TEPS subscales and showed no within-group differences between anticipatory and consummatory subscales makes sense— both the anticipatory and consummatory subscales rely on the same underlying psychological processes (i.e., low pleasure beliefs) and would therefore not be expected to differ. These findings call into question the validity of scales using hypothetical reporting formats that purport to offer a distinction between consummatory and anticipatory pleasure and may explain why factor analytic studies sometimes fail to find a consummatory-anticipatory distinction using such measures (Gooding and Pflum, 2014).
Several limitations of this meta-analysis should be considered. First, only a limited number of moderators could be included. It is therefore unclear whether additional variables (e.g., depression, cognitive impairment) might account for findings. Second, despite the lack of bias detected in the results due to study design, the number of study-level variables assessed in this meta-analysis was limited. Future analyses might benefit from assessing additional potential sources of bias to explain variance. Third, analyses focused on a single measure: the TEPS. Fourth, due to the nature of the data available, the effect sizes calculated, and the scope of these analyses, we could not directly calculate the average correlation between the TEPS-ANT and TEPS-CON subscales. Future research should use confirmatory factor analysis in samples of healthy controls and schizophrenia patients to determine whether a two-factor structure is supported on the TEPS. Finally, conclusions drawn about anticipatory and consummatory pleasure are only as valid as the measure used in the meta-analysis. Our review and meta-analytic findings call into question the validity of the TEPS and other scales using hypothetical reporting formats for measuring the consummatory pleasure construct. New measures are needed that assess the anticipatory and consummatory constructs across multiple domains of pleasure. Ecological momentary assessment and laboratory-based tasks may offer a more valid means of assessing these constructs than trait questionnaires.
In summary, this meta-analysis found that individuals in the schizophrenia spectrum report lower levels of consummatory and anticipatory pleasure on the TEPS compared to controls. This was moderated by the percentage of males, who reported reduced pleasure relative to women. The distinctions supporting the schizophrenia-spectrum and liking-wanting paradoxes that were derived primarily from the laboratory-based task literature were not supported using this scale.
Supplementary Material
Table 1.B.
Study and participant characteristics
| Citation |
Study group/PDS subgroup |
SZ-CN n |
Mean Age |
%Non-Caucasian |
%maleSZ-maleCN |
% 1st gen AP |
Proportion Neg. Sym. Severity |
|---|---|---|---|---|---|---|---|
| Kring et al., 2014 | SZ | 21-24 | 44.42 | 0% | 71.40%-66.67% | 0.29% | 0.23 |
| Kring, unpublished supplied data | SZ | 32-32 | |||||
| Lee et al., 2012 | SZ | 14-16 | 30.10 | 61.30%-43.80% | 0.39 | ||
| Li et al., 2016 | PDS | 15-19 | 19.88 | 60.00%-57.89% | |||
| Li et al., 2015 | SZ | 346-268 | 33.93 | 48.56%-49.30% | 20.81% | 0.37 | |
| Loas et al., 2014 | PDS/Disorg | 39-281 | 21.82 | 16.00%-16.00% | |||
| Makowski et al., 2016 | SZ | 15-15 | 34.15 | 73.00%-60.00% | 0.16 | ||
| Mann et al., 2013 | SZ | 54–39 | 37.66 | 63.00% | 61.10%-48.70% | 7.40% | |
| Martin et al., 2011 | PDS/SocAnh | 64-304 | 18.73 | 23.50% | 35.19%-40.79% | ||
| Martin et al., 2011 | PDS/PerMag | 27-304 | 18.55 | 19.50% | 40.74%-40.79% | ||
| McCarthy, 2013 | PDS/SocAnh | 30-40 | 20.38 | 46.25% | 26.70%-27.50% | ||
| McCarthy, 2013 | PDS/PerMag | 30-40 | 19.56 | 60.40% | 23.30%-27.50% | ||
| Mote et al., 2014 | FEP | 88-66 | 21.77 | 44.50% | 77.00%-47.00% | 5.00% | 0.01 |
| Mucci et al., 2015 | SZ | 28-22 | 32.51 | 64.00%-45.00% | 0% | 0.24 | |
| Schlosser et al., 2014 | FEP | 67-29 | 19.65 | 33.50% | 70.00%-48.00% | 0.37 | |
| Schlosser et al., 2014 | CHR | 60-29 | 18.81 | 40.00% | 52.00%-48.00% | 0.09 | |
| Schlosser et al., 2014 | SZ | 78-29 | 30.25 | 41.00% | 70.00%-48.00% | 0.34 | |
| Shi et al., 2012 | PDS/SocAnh | 55-116 | 18.48 | 63.63%-64.66% | |||
| Simon et al., 2015 | SZ | 23-12 | 27.90 | 65.20%-33.30% | 0% | 0.30 | |
| Strauss et al., 2013 | SZ | 25-21 | 45.50 | 32.00% | 72.00%-62.00% | 0.42 | |
| Strauss et al., 2015 | sz | 28-25 | 44.55 | 37.65% | 67.90%-64.00% | 12.00% | 0.09 |
| Strauss et al., 2011 | SZ | 86-59 | 43.00 | 30.50% | 66.30%-55.90% | 13.00% | 0.06 |
| Subramaniam et al., 2015 | SZ | 37-20 | 44.43 | 67.57%-70.00% | 21.62% | 0.04 |
Note. SZ = Schizophrenia, PDS = Psychometric Schizotypy, CN = Control, SocAnh = Social Anhedonia cluster, PerMag = Perceptual Aberration/Magical Thinking cluster. AP = antipsychotics, PANSS = Positive and Negative Syndrome Scales. Neg. sym. = negative symptoms.
Acknowledgments:
The authors wish to thank the authors of papers included in this meta-analysis who generously responded to questions and requests related to data.
Role of Funding Sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Of note, the ACIPS was included in the pre-registration, but excluded during analyses, as it became clear during the literature review that its factor structure does not support separate two-factor consummatory and anticipatory subscales (Gooding and Pflum, 2014). Thus, though the search strategy still includes the ACIPS to be consistent with pre-registration, the articles were excluded from analyses. The references to ACIPS in the search strategy have likewise been maintained to be consistent with the pre-registration.
Conflict of Interest and Disclosures: The authors have no conflicts of interest or disclosures relevant to this paper.
References
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, et al. , 2015. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front. Psychol 6, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Dowd EC, 2010. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull 36(5), 919–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N, 2014. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J. Abnorm. Psychol 123(2), 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell JS, Potts GF, Gooding DC, Trachik BJ, Chan CC, Spencer CC, 2016. Transdiagnostic Psychiatric Symptoms and Event-Related Potentials following Rewarding and Aversive Outcomes. PLoS One. 11(6), e0157084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E,1911. Dementia Praecox or the Group of Schizophrenias. International Universities Press, New York. [Google Scholar]
- Cassidy CM, Lepage M, Harvey P-O, Malla A, 2012. Cannabis use and anticipatory pleasure as reported by subjects with early psychosis and community controls. Schizophr. Res 137(1-3), 39–44. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Li Z, Li K, Zeng Y-W, Xie W-Z, Yan C, et al. , 2016. Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology. 30(3), 274–80. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Wang Y, Huang J, Shi Y, Wang Y, Hong X, et al. , 2010. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross-cultural validation and extension. Psychiatry Res. 175(1-2), 181–3. [DOI] [PubMed] [Google Scholar]
- Chan RCK, Wang Y, Yan C, Zhao Q, McGrath J, Hsi X, et al. , 2012. A study of trait anhedonia in non-clinical Chinese samples: evidence from the Chapman Scales for Physical and Social Anhedonia. PLoS One. 7(4), e34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML, 1976. Scales for physical and social anhedonia. J. Abnorm. Psychol 85(4), 374–82. [DOI] [PubMed] [Google Scholar]
- Chuang J-Y, Murray GK, Metastasio A, Segarra N, Tait R, Spencer J, et al. , 2014. Brain structural signatures of negative symptoms in depression and schizophrenia. Front. Psychiatry. 5, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Callaway DA, Najolia GM, Larsen JT, Strauss GP, 2012. On “risk” and reward: investigating state anhedonia in psychometrically defined schizotypy and schizophrenia. J. Abnorm. Psychol 121(2), 407–15. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS, 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull 36(1), 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS, 2011. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clin. Psychol. Rev 31(3), 440–8. [DOI] [PubMed] [Google Scholar]
- Cooper S, Kring AM, Ellman LM, 2017. Attenuated positive psychotic symptoms and the experience of anhedonia. Early. Interv. Psychiatry. 12(6), 1188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Gold JM, Cools R, Barch DM, 2016. Impaired activation in cognitive control regions predicts reversal learning in schizophrenia. Schizophr. Bull 42(2), 484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva S, Saperia S, Siddiqui I, Fervaha G, Agid O, Daskalakis ZJ, et al. , 2017. Investigating consummatory and anticipatory pleasure across motivation deficits in schizophrenia and healthy controls. Psychiatry Res. 254, 112–7. [DOI] [PubMed] [Google Scholar]
- Docherty AR, 2013. Anhedonia and deficits in positive emotional experience in individuals with genetic liability for schizophrenia [Doctoral dissertation]. University of Missouri, Colombia, Missouri. [Google Scholar]
- Docherty AR, Sponheim SR, Kerns JG, 2015. Self-reported affective traits and current affective experiences of biological relatives of people with schizophrenia. Schizophr. Res 161(2-3), 340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Cella M, Tarrier N, Wykes T, 2015. Predicting the future in schizophrenia: The discrepancy between anticipatory and consummatory pleasure. Psychiatry Res. 229(1-2), 462–9. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith G, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J 315(7109), 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Bogdan R, Chen L, Moerlein SM, Black KJ, Perlmutter JS, et al. , 2017. Preliminary evidence that negative symptom severity relates to multilocus genetic profile for dopamine signaling capacity and D2 receptor binding in healthy controls and in schizophrenia. J. Psychiatr. Res 86, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrod J, Ernst F, Giuliani F, Bonsack C, 2009. [Validation of the Temporal Experience of Pleasure Scale (TEPS) in a French-speaking environment]. Encephale. 35(3), 241–8. [DOI] [PubMed] [Google Scholar]
- Fonseca-Pedrero E, Ortuño-Sierra J, de Álbeniz AP, Muñiz J, Cohen AS, 2017. A latent profile analysis of schizotypal dimensions: Associations with psychopathology and personality. Psychiatry Res. 253, 110–5. [DOI] [PubMed] [Google Scholar]
- Fortunati R, Ossola P, Camerlengo A, Bettini E, De Panfilis C, Tonna M, et al. , 2015. Anhedonia in schizophrenia: The role of subjective experiences. Compr. Psychiatry. 62, 152–60. [DOI] [PubMed] [Google Scholar]
- Frost KH, Strauss GP, 2016. A review of anticipatory pleasure in schizophrenia. Curr. Behav. Neurosci 3(3), 232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP, 2006. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J. Res. Pers 40(6), 1086–102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF, 2007. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res 93(1-3), 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen CJ, 2015. Conative dysfunction in schizophrenia: A new empirically-derived framework [Doctoral dissertation]. York University, Toronto, Canada. [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA, 2008. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr. Bull 34(5), 835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ, 2012. The nature of diminished pleasure in individuals at risk for or affected by schizophrenia. Psychiatry Res. 198(1), 172–3. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ, 2014. The assessment of interpersonal pleasure: introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Res. 215(1), 237–43. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW, 2007. Rates of avoidant, schizotypal, schizoid and paranoid personality disorders in psychometric high-risk groups at 5-year follow-up. Schizophr. Res 94(1-3), 373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Strauss GP, Dombrecht L, Mittal VA, 2018. Neuroleptic-free youth at ultrahigh risk for psychosis evidence diminished emotion reactivity that is predicted by depression and anxiety. Schizophr. Res 193, 428–34. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. BMJ. 327(7414), 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Benson TL, Gyurak A, Yin H, Tully LM, Lincoln SH, 2014. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J. Abnorm. Psychol 123(1), 190–204. [DOI] [PubMed] [Google Scholar]
- Kraepelin E, 1919. Dementia Praecox and Paraphrenia. Robertson GM, editor. Robert E. Krieger, New York. [Google Scholar]
- Kring AM, Barch DM, 2014. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur. Neuropsychopharmacol 24(5), 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Elis O, 2013. Emotion deficits in people with schizophrenia. Annu. Rev. Clin. Psychol 9, 409–33. [DOI] [PubMed] [Google Scholar]
- Kring AM, Siegel EH, Barrett LF, 2014. Unseen affective faces influence person perception judgments in schizophrenia. Clin. Psychol. Sci 2(4), 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Brown LH, Silvia PJ, Myin-Germeys I, Barrantes-Vidal N, 2012. The expression of positive and negative schizotypy in daily life: an experience sampling study. Psychol. Med 42(12), 2555–66. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chun JW, Kang JI, Kang D-I, Park H-J, Kim J-J, 2012. Hippocampus and nucleus accumbens activity during neutral word recognition related to trait physical anhedonia in patients with schizophrenia: an fMRI study. Psychiatry Res. 203(1), 46–53. [DOI] [PubMed] [Google Scholar]
- Li X, Li Z, Li K, Zeng Y-W, Shi H-S, Xie W-L, et al. , 2016. The neural transfer effect of working memory training to enhance hedonic processing in individuals with social anhedonia. Sci. Rep 6, 35481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mou X, Jiang W, Yang Z, Shen X, Jin Z, et al. , 2015. A comparative study of anhedonia components between major depression and schizophrenia in Chinese populations. Ann. Gen. Psychiatry. 14, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB, 2001. Practical meta-analysis. SAGE publications, Inc. [Google Scholar]
- Llerena K, Strauss GP, Cohen AS, 2012. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr. Res 142(1-3), 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Verrier A, Monestes JL, 2014. Relationship between anticipatory, consummatory anhedonia and disorganization in schizotypy. B.M.C. Psychiatry. 14, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski CS, Lepage M, Harvey P-O, 2016. Functional neural correlates of social approval in schizophrenia. Soc. Cogn. Affect. Neurosci 11(3), 445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM, 2013. Spared and impaired aspects of motivated cognitive control in schizophrenia. J. Abnorm. Psychol 122(3), 745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Becker TM, Cicero DC, Docherty AR, Kerns JG, 2011. Differential associations between schizotypy facets and emotion traits. Psychiatry Res. 187(1-2), 94–9. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, 2013. Motivation and effort in individuals with social anhedonia [Doctoral dissertation]. University of Maryland, College Park, Maryland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE, 1962. Schizotaxia,schizotypy, schizophrenia. American Psychologist. 17(12), 827. [Google Scholar]
- Meehl PE, 2001. Primary and secondary hypohedonia. J. Abnorm. Psychol 110(1), 188–93. [DOI] [PubMed] [Google Scholar]
- Mote J, Minzenberg MJ, Carter CS, Kring AM, 2014. Deficits in anticipatory but not consummatory pleasure in people with recent-onset schizophrenia spectrum disorders. Schizophr. Res 159(1), 76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci A, Dima D, Soricelli A, Volpe U, Bucci P, Frangou S, et al. , 2015. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med 45(8), 1765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, van Os J, et al. , 2013. Emotional experience in negative symptoms of schizophrenia--no evidence for a generalized hedonic deficit. Schizophr. Bull 39(1), 217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, et al. , 2012. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 196(2-3), 220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rado S, 1953. Dynamics and classification of disordered behavior. Am. J. Psychiatry. 110(6), 406–16. [DOI] [PubMed] [Google Scholar]
- Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, et al. , 2015. Ventral Striatal Activation During Reward Processing in Psychosis: A Neurofunctional Meta-Analysis. JAMA Psychiatry. 72(12), 1243–51. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Clore GL, 2002. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol. Bull 128(6), 934–60. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, 1979. The file drawer problem and tolerance for null results. Psychol. Bull 86(3), 638–41. [Google Scholar]
- Sandt AR, 2013. Hedonic functioning and subthreshold psychotic symptoms. [Doctoral Dissertation] Temple University, Philadelphia, Pennsylvania. [Google Scholar]
- Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S, 2014. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr. Res 158(1-3), 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang Y, Cao X, Wang Y, Wang Y, Zong J, et al. , 2012. Experience of pleasure and emotional expression in individuals with schizotypal personality features. PLoS One. 7(5), e34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Cordeiro SA, Weber M-A, Friederich H-C, Wolf RC, Weisbrod M, et al. , 2015. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophr. Bull 41(6), 1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Cohen AS, 2018. The schizophrenia spectrum anhedonia paradox. World Psychiatry. 17(2), 221–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM, 2012. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry. 169(4), 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM, 2013. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr. Bull, 39(4), 872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Ossenfort KL, Lee BG, et al. , 2015. Emotion regulation abnormalities in schizophrenia: Directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. J. Abnorm. Psychol 124(2), 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Ruiz I, Visser KH, Crespo LP, Dickinson EK, 2018. Diminished Hedonic response in neuroleptic-free youth at ultra high-risk for psychosis. Schizophr. Res. Cogn, 12, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM, 2014. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull 40 Suppl 2, S107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM, 2011. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 187(1-2), 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Hooker CI, Biagianti B, Fisher M, Nagarajan S, Vinogradov S, 2015. Neural signal during immediate reward anticipation in schizophrenia: Relationship to real-world motivation and function. Neuroimage Clin. 9:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso IF, Grove TB, Taylor SF, 2014. Differential hedonic experience and behavioral activation in schizophrenia and bipolar disorder. Psychiatry Res. 219(3), 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh S, Nizamie SH, Goyal N, Tikka S, Bose S, 2016. Social anhedonia andgamma band abnormalities as a composite/multivariate endophenotype forschizophrenia: A dense array EEG study. Early Interv. Psychiatry. doi: 10.1111/eip.12327 [DOI] [PubMed] [Google Scholar]
- Vignapiano A, Mucci A, Ford J, Montefusco V, Plescia GM, Bucci P, et al. , 2016. Reward anticipation and trait anhedonia: An electrophysiological investigation in subjects with schizophrenia. Clin. Neurophysiol 127(4), 2149–60. [DOI] [PubMed] [Google Scholar]
- Wang J, Huang J, Yang X-H, Lui SSY, Cheung EFC, Chan RCK, 2015. Anhedonia in schizophrenia: Deficits in both motivation and hedonic capacity. Schizophr. Res 168(1-2), 465–74. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu WH, Li Z, Wei XH, Jiang XQ, Geng FL, et al. , 2015. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol. Med 46(1), 125–35. [DOI] [PubMed] [Google Scholar]
- Wang Y, Neumann D, Shum DH, Chan RC, 2012. A cross-validation study of clustering of schizotypy using a non-clinical Chinese sample. Psychiatry Res. 200(1), 55–8. [DOI] [PubMed] [Google Scholar]
- Wilson A, 2012. Parsing Hedonic Capacity in Schizotypy: A Multi-Method Assessment of Consummatory and Anticipatory Pleasure. Parsing Hedonic Capacity in Schizotypy: A Multi-Method Assessment of Consummatory and Anticipatory Pleasure. [Doctoral Dissertation]. University of Maryland. [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, Green MF, 2010. Impaired anticipatory event-related potentials in schizophrenia. Int. J. Psychophysiol, 77(2), 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Yan C, Ying X, Zhu S, Shi H, Wang Y, et al. , 2014. Domain-specific hedonic deficits towards social affective but not monetary incentives in social anhedonia. Sci. Rep 4, 4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Liu WH, Cao Y, Chan RC, 2011. Self-reported pleasure experience and motivation in individuals with schizotypal personality disorders proneness. East Asian Arch. Psychiatry. 21(3), 115–22. [PubMed] [Google Scholar]
- Yan C, Wang Y, Su L, Xu T, Yin D-Z, Fan M-X, et al. , 2016. Differential mesolimbic and prefrontal alterations during reward anticipation and consummation in positive and negative schizotypy. Psychiatry Res. Neuroimaging. 254, 127–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.