Abstract
Human monocytes express known markers of dopamine synthesis, storage and clearance, including dopamine transporter (DAT), tyrosine hydroxylase (TH), all subtypes of dopamine receptors and vesicular monoamine transporter 2 (VMAT2). Immunohistochemical and immunofluorescent methodologies have traditionally been employed to determine DAT and TH expression in the CNS, their detection in the blood and specifically in the peripheral monocytes has not been studied by flow cytometry. Flow cytometry assays are widely used in medicine and in basic, preclinical or clinical research to quantify physical and chemical characteristics of target cell populations. Here, we have established a highly sensitive and reproducible flow cytometry panel to detect and quantify DAT and TH expression in freshly isolated or cryopreserved human peripheral monocytes. In healthy humans (n=41 biological replicates), we show baseline DAT and TH expressing monocytes constitute ~12% of the peripheral blood mononuclear cell (PBMC) fraction when examined in fresh isolation from whole blood. Using an identical flow cytometry panel, we found that cryopreservation of PBMCs using multiple techniques resulted in altered PBMC populations as compared to fresh isolation and relative to one another. Among these, we identified an optimum cryopreservation method for detecting TH and DAT in cryopreserved PBMCs. Our data provide a sensitive and reproducible approach to examine dopamine signaling in peripheral human immune cells. This approach can be applied to study peripheral dopamine signaling under healthy and potentially under disease conditions. The use of dopamine signaling could also be explored as a technique to monitor therapeutic interventions particularly those targeting DAT and TH in the periphery.
Keywords: dopamine transporter, tyrosine hydroxylase, peripheral blood mononuclear cell, peripheral immune cells, flow cytometry, human monocytes
1. Introduction
Peripheral immune cells of both lymphoid and myeloid lineage express known markers of the dopamine system, including proteins for synthesis, storage and release of dopamine, as well as dopamine transporter for dopamine clearance after signaling1,2,3. Two mediators of dopamine transmission, dopamine transporter (DAT) and tyrosine hydroxylase (TH) are constitutively expressed on human monocytes4,2,3. TH, the rate limiting enzyme in dopamine synthesis, and DAT, responsible for dopamine uptake, are biologically relevant targets to study dopamine transmission in these peripheral immune cells. Recent reports suggest that in neurodegenerative diseases, such as Parkinson’s diseases, dopamine transmission is altered in both the CNS and the periphery5,6. In several CNS diseases such as schizophrenia, neuroAIDS, and Parkinson’s disease, alterations in peripheral dopamine tone and markers of dopamine signaling are reported as additional readouts of disease7,3,8. Both schizophrenia and Parkinson’s disease are frequently treated with dopaminergic drugs, which alter dopamine transmission and signaling in the CNS and can also impact the peripheral immune state7. For example, the connection between psychostimulant-mediated modulation of peripheral immunity, DAT activity and peripheral dopamine levels in the patients affected by different psychotic disorders is well documented9. These studies show that DAT activity is lower in resting lymphocytes of psychotic patients when compared to healthy control subjects7. In addition, it has been shown that peripheral immune cells are dysfunctional in PD10. The inflammatory component of PD is driven by myeloid cells, such as microglia and peripheral monocytes/macrophages10. Consistent with these reports, we and others have shown that DAT, TH and other dopaminergic proteins are present in peripheral blood monocytes2,4. Therefore, our proposed flow cytometry approach provides a sensitive and non-invasive strategy for analyzing biomarkers of dopamine transmission in peripheral immune cells, which may serve as proxies in neurological conditions where dopamine transmission has gone awry.
While immunohistochemical and immunofluorescent techniques have been widely used to determine DAT and TH expression in the CNS5,11, blood-borne immune cells present a novel milieu to study dopamine transmission. To-date, immunocytochemistry and qPCR assays have been used to determine TH and DAT expression in cultured monocytes2,4. These studies have provided valuable information about DAT and TH proteins or DNA expression; however, there is no high-throughput and quantifiable methodology available to determine the levels of these proteins acutely in fresh peripheral blood samples without culturing PMBCs that is likely to affect the endogenous levels of these proteins. Importantly, there are large repositories of PBMCs at different research institutes including NINDS PDBP and Michael J Fox Foundation’s international PPMI initiative, and it is unknown if the existing preservation methodologies employed to preserve these samples alter the ratio of DAT and TH expressing PBMCs. Therefore, there is an unmet need to identify a cryopreservation methodology suitable for detection of DAT and TH in human monocytes.
Flow cytometry was originally developed for immunological detection of both surface and intracellular marker proteins to study immune cell populations in health and disease. In cell suspensions of blood-borne immune cells 12 or more markers can be distinguished depending on the capability of the flow cytometer in use12. Building on traditional immunostaining protocols, we have established a highly sensitive and reproducible multi-parameter flow cytometry method that can detect and quantify DAT and TH expression in peripheral blood monocytes of humans. After establishing a reproducible protocol in the freshly prepared samples, we compared the suitability of two different cryopreservation methods for detection of DAT and TH on peripheral monocytes. The side by side comparison revealed cryopreservation in FBS-DMSO media alters the immune phenotype of monocyte subsets, whereas cryopreservation in CryoStor10 media maintains the sample integrity. Overall in this study we have established a methodology that provides a highly sensitive and quantifiable approach to study dopamine signaling in peripheral immune cells in healthy condition, in disease states with dysregulation of dopamine signaling, and following therapeutic interventions that directly or indirectly affect DAT or TH levels in the PBMCs.
2. Materials and Methods
2.1. Materials
Whole blood was collected in K2EDTA vacutainer blood collection tubes (BD, cat. 366643) and held at room temperature for up to 2 hours prior to PBMC isolation. Primary antibodies were used as listed in Table 2. In brief, primary antibodies MAB369 and AB152 (Table 2) were used after fixation and permeabilization to detect DAT and TH respectively. Fluorochrome-conjugated anti-CD14 (MP19, BD) was used to detect CD14. Whole blood from healthy volunteers was overlaid in Leucosep tubes (Table 3) for PBMC isolation; standard 15mL polypropylene conical tubes were used for isolation from enriched leucocytes. Specific centrifuges used are listed in Table 1.
Table 2:
Antibodies
| Specificity | Clone/Species | Fluorochrome | Vendor | Catalog Number | Purpose | Optimal Titer |
|---|---|---|---|---|---|---|
| DAT | MAB369/Rat | unconjugated | Millipore | MAB369 | DA marker | 1uL |
| TH | AB152/Rabbit | unconjugated | Millipore | AB152 | DA marker | 1uL |
| CD14 | M-p19/Mouse | FITC | BD | 347493 | Mono. Sub. | 2uL |
| anti-Rat | Polyclonal/Goat | APC | BD | 551019 | Secondary | 2.5uL |
| anti-Rabbit | Polyclonal/Goat | BV421 | BD | 565014 | Secondary | 2.5uL |
| CD16 | 3G8/Mouse | APC | Biolegend | 302012 | Mono. Sub. | 2uL (mfg) |
| Mouse Isotype control | IgG2b,k/Mouse | FITC | Biolegend | 402208 | Isotype | 2uL |
| Rat Isotype control | RTK2758/Rat | unconjugated | Biolegend | 400502 | Utility | 0.1uL |
| Rabbit Isotype control | Polyclonal/Rabbit | unconjugated | Biolegend | 910801 | Utility | 0.1uL |
Table 3:
Reagents
| Name | Vendor | Catalog Number |
|---|---|---|
| NearIR Fixable Viability Dye | ThermoFisher | L34975 |
| Fetal Bovine Serum | Gemini | 100-106 |
| DMSO | Sigma | D2438-10ML |
| RPMI 1640 (−) Phenol Red | Gibco | 11835030 |
| Fix/Perm Kit | eBioscience | 88-8824-00 |
| Trypan Blue | MP Biomedicals | 1691049 |
| Ficoll-Paque PLUS | GE Healthcare | 17144003 |
| CryoStor10 | Sigma | C2874 |
Table 1:
Equipment
| Equipment | Manufacturer | Model | Utility |
|---|---|---|---|
| Microcentrifuge | Fisher | 59A | FC staining |
| Centrifuge | Sorvall | ST8 | PBMC prep |
2.2. Human samples
Human blood samples were purchased from Lifesouth Community Blood Center, Gainesville, FL from April, 2017 to April, 2018. The study was approved by the University of Florida’s Institutional Review Board (IRB). According to Lifesouth rules and regulations donors were healthy individuals aged 20-70 years-old of any gender, who were not known to have any blood borne pathogens (both self-reported, but also independently verified), and were never diagnosed with a blood disease, such as leukemia or bleeding disorders. In addition, none of the donors were using any medications for an infection, nor were they on any blood thinners (see Supplemental Questionnaire 1).
2.3. Flow cytometer
Beckton Dickenson Canto II 3 laser system with a (50 MW 488nm blue sapphire laser, 100MW 405nm violet laser and a 50MW 633 Red laser) with 8 fluorescent detectors, 2 off the violet laser, 4 off of the blue laser and 2 off the red laser (405NM filters;450/50, 510/50; 488NM filters 530/30, 585/42, 710/20, and 780/60; 633NM filters; 660/20 and 780/60) and FSC/SSC for a total of 10 parameters. Daily QC is preformed using BD FACSDiva CS&T research beads; Cat. 655051) and by running twice weekly Spherotech 8 peak rainbow Calibration beads (Cat. RCP-30-5A, 8 peaks). All QC records are kept in digital format on the instrument and available for ICBR Flow Core users to examine.
The acquisition of each sample was stopped when total events reached 100,000. Each sample data set consisted of: cell morphology and size data by sidescatter (SSC) and forwardscatter (FSC) respectively, and fluorescence data for each event in channels detecting FITC, APC, Pacific Blue/Brilliant Violet 421, and APC-Cy7/NearIR, to accurately detect each fluorochrome labeled antibody against proteins of interest (Table S1).
2.4. Cell sample preparation
2.4.1. PBMC isolation:
Briefly, enriched leucocytes/whole blood was diluted 1:1 in PBS (i.e. 15mL whole blood dilute in 15mL sterile PBS) and layered atop Ficoll (GE, 17-1440-03) using a 10mL serological pipette and centrifuged (400g, 20 minutes, 21° C, brakes off, acceleration minimum) to generate the PBMC layer. PBMCs were aspirated, centrifuged at 10Og, washed twice with sterile PBS and prepared for staining. As Ficoll density centrifugation removes red blood cells by density and improves PBMC viability, an RBC lysis step is not required.
The volume of whole blood and enriched leucocytes, dilute and layered atop Ficoll, varied depending on condition of the sample – as whole blood contains a greater percentage of red blood cells (RBCs) in contrast to enriched leucocytes, whole blood required an altered ratio of dilute blood to Ficoll (2:1) compared to enriched leucocytes (3.3:1) to compensate for increased percentage RBCs in the sample. 30mL dilute whole blood was layered atop 15mL Ficoll in a sterile 50mL Leucosep Tube (Table 3), while 10mL dilute enriched leucocytes was layered atop 3mL Ficoll in a 15mL conical tube.
2.4.2. PBMC cryopreservation:
FBS-DMSO
To compare the results of freshly isolated PBMCs to a commonly used cryopreservation method, 10 million isolated PBMCs were suspended in 900uL FBS (Table 3) and 100uL sterile DMSO added dropwise to a final volume of 1mL. Suspension was quickly transferred to a cryopreservation tube and into a cryopreservation canister pre-chilled to −20C. The canister was stored at −80C for 48 hours before moving samples into liquid nitrogen for a minimum of 24 hours. The cryopreserved PBMCs were quick-thawed at 37 degrees C for 2 minutes until a small ice crystal remained. PBMCs were suspended in 9 volumes of RPMI 1640 without phenol red, containing 10% FBS, centrifuged at 100g for 10 min, and washed with 2 volumes of sterile PBS.
CryoStor10
To compare the results of freshly isolated PBMCs to specialized cryopreservation methods, 10 million isolated PBMCs were suspended in 100uL PBS and added to a cryotube. Sterile CS10 freezing media (Table 3) at 4 degrees C was added dropwise and agitated gently by hand every 5 drops for a total volume of 1mL. Cryopreservation tube was quickly transferred to a cryopreservation canister pre-chilled to −20C. The canister was stored at −80C for 48 hours before moving samples into liquid nitrogen for a minimum of 24 hours. The cryopreserved PBMCs quick-thawed at 37 degrees C for 2 minutes until a small ice crystal remained. PBMCs were suspended in 9 volumes of sterile PBS, centrifuged at 100g for 10 min, and washed with 2 volumes of sterile PBS to remove residual freezing media.
2.5. Staining conditions and compensation:
Staining was performed in serum-free PBS. During optimization, we noted that freshly isolated PBMCs resulted in fewer than 0.5% dead cells during acquisition; therefore, viability dye was not included in fresh PBMC samples. Cryopreserved PBMC samples included incubation with NearIR fixable viability dye (Table 3) for 15 minutes at room temperature in PBS, followed by quenching with PBS containing 1% FBS (Table 3). Fc-blocking (anti-CD16/32) is often used to avoid nonspecific antibody binding in immune cells – we found no altered fluorescent signals in presence or absence of Fc-block. Every sample was stained in parallel with unstained, secondary only and isotype controls. Staining was conducted for 30 minutes on ice (live cells) and 30 minutes at room temperature (after fixation) protected from light. After testing fixation and permeabilization with freshly-prepared 4% PFA (pH 7.4) followed by a series of Triton-X100 concentrations from 0.001% to 0.5%, we found that cell morphology and staining efficiency was negatively impacted. Inclusion of Triton-X100 (at any concentration) in permeabilization step yielded too few monocytes for analysis, as identifiable by sidescatter. Therefore, we opted to use eBioscience fixation and permeabilization kit (Table 3) to fix live cells for 45 minutes at room temperature, and washed following manufacturer recommendations, to produce reliable intracellular staining.
Each set of experiments was compensated using single color compensation controls of stained PBMCs using each fluorochrome-conjugated antibody listed in Table S2 Viability dye compensation was achieved by using 1:1 live cells combined with cells heat-killed at 56C for 5 min.
2.5.1. Staining procedure – freshly isolated and cryopreserved PBMCs:
Cell pellet was resuspended in 100-400uL sterile PBS depending on qualitative pellet size, counted and density adjusted to 10,000 cells per microliter, and aliquoted 1 million cells per staining condition. Samples were incubated with 2uL anti-CD14 (BD, M-p19, mouse host) (Table S2) for 30 minutes on ice, washed twice with ice cold PBS, and suspended in 100uL fixation buffer for 45 minutes. Following two washes with permeabilization buffer, samples were stained with anti-DAT (1uL, Sigma; MAB369, rat host) and anti-TH (1uL, Sigma; AB152, rabbit host) as well as isotype controls (Table 2), followed by 2.5uL of species matched secondary antibodies (Table 2) for 30 minutes. Both DAT and TH primaries were applied following fixation and permeabilization steps, because these antibodies detect intracellular epitopes on their target protein. Samples were washed twice to remove unbound antibody and suspended in 500uL PBS; data was acquired within 2 hours of staining.
2.5.2. Staining Procedure – Step-by-step
Refer to Table 1 (equipment), 2 (antibodies) and 3 (reagents)
Suspend cell pellet in 100-400uL sterile PBS depending on pellet size. Dilute 1:200 in PBS containing 10% Trypan Blue, count, excluding dead cells and density adjust to 10,000 cells/uL. Aliquot 1 million cells into each of 4 staining tubes: Unstained, Secondary Only, Isotype control, and Stained. Repeat for each independent biological sample. Use one set of sample tubes for single color compensation controls.
Incubate on ice: stained sample with anti-CD14-FITC 1:50 dilution, Isotype control with mouse-IgG2b,K-FITC 1:50 dilution. Incubate for 30 minutes on ice protected from light.
- Wash with ice-cold PBS - centrifuge at 4C for 5 minutes at 1800 RPM in a swinging bucket microcentrifuge (Table 1), aspirate supernatant, resuspend and repeat for a total of two washes.
- For cryopreserved PBMCs – add NearIR fixable viability dye at 1:1000 final dilution, incubate at room temperature for 15 minutes protected from light. Quench unbound dye with 900uL PBS containing 1% FBS, immediately centrifuge and aspirate supernatant as above. Proceed to fixation.
Fix and permeabilize samples following kit manufacturer instructions (Table 3) except using 1mL permeabilization buffer instead of 2mL per wash step. Resuspend all samples in final volume 100uL permeabilization buffer.
Add primary antibodies against DAT and TH (Table 2) 1:100 dilution (from stock 100ug/mL antibody concentration) to appropriate tubes, and 1:1000 dilution of each isotype control (from stock 1mg/mL isotype control concentration) to appropriate tubes, for a final concentration of 0.1ug antibody per 1M cells in 100uL staining volume. Incubate 30 minutes at room temperature protected from light. Wash as above with permeabilization buffer.
Add secondary antibodies (Table 2) at 1:40 dilution to appropriate tubes and incubate as in step 5. Follow with wash steps.
Resuspend final pellet in 500uL sterile PBS. Acquire data on instrument within 2 hours of staining completion.
2.5.3. Comparing PBMCs freshly isolated to cryopreserved obtained from the same donor:
Immediately following PBMC isolation from whole blood or enriched leucocytes, 10 million cells were cryopreserved (2.4.2) while an equivalent number of PBMCs were used for immediate analysis. Cryopreserved PBMCs from the same isolation were thawed and stained as above. Direct comparisons were made following post-hoc analysis between freshly isolated and cryopreserved PBMCs from each independent biological replicate. To examine effects of cryopreservation on monocyte subsets, 2uL of an antibody against CD16 (Biolegend, cat. 302011)8,13 was added along with anti-CD14 in a separate staining tube. Post-hoc analysis was conducted in FCSExpress6 RUO.
2.5.4. Data acquisition:
All fluorochromes were compensated using BD FACSDiva automatic compensation calculation. In brief, 5,000 events of each single-color compensation control were acquired in BD FACSDiva, monocytes were gated by light scatter (shown in Fig.1b, left), and compensation gates were set on histogram plots for each fluorochrome. 1 million PBMCs in 100uL buffer were stained using the titers shown in Table 2 with primary + secondary, or a directly conjugated primary, to produce single color controls. On the instrument used in this study (BD Canto II), bandpass filters were used to detect APC, BV421 and FITC using an argon-gas red laser (555mV), and solid-state blue laser (401mV) and green laser (408mV) respectively. Spectral overlap was calculated automatically in BD FACSDiva and applied to all samples.
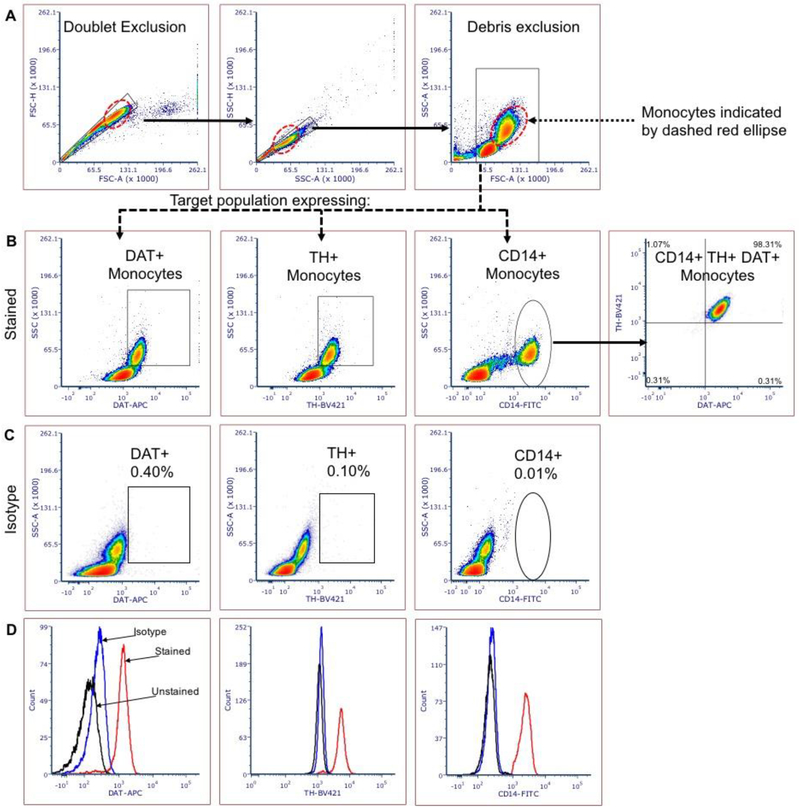
Figure 1: Flow cytometry reliably detects DAT and TH expression in human PBMCs obtained from whole blood.
Representative plots showing the gating strategy for DAT- and TH-expressing human monocytes by flow cytometry. (A) doublet discrimination and debris exclusion; (B) DAT and TH expressing monocytes can be discriminated based on sidescatter properties intrinsic to monocytes (B, 1st and 2nd panel from left) or by expression of human monocyte marker CD14 (B, 3rd and 4th panel from left). (C) Isotype controls reveal minimal nonspecific staining. (D) Histogram representations for anti-DAT, anti-TH and anti-CD14 displaying mean fluorescence intensity (MFI) for: unstained (black), isotype control (blue) and immune-stained (red). N= 41 independent biological replicates
2.5.5. Statistical analysis:
Statistical analysis was conducted in Graphpad Prism 7. T-test using the Holm-Sidak method (alpha=0.05) was used to determine the similarities and differences in monocyte subsets in freshly isolated versus cryopreserved PBMCs from the same donor.
3. Results
3.1. Step 1: PBMC preparation
Multiple lines of evidence suggest a role for blood leukocytes in dopamine transmission and thus the goal of this work was to characterize expression of key dopaminergic proteins such as dopamine transporter (DAT) and tyrosine hydroxylase (TH). Leukocytes exist as suspended cells in blood, and thus flow cytometry is a suitable method to study these dopaminergic proteins in peripheral blood-borne immune cells.
The reliability of Ficoll density centrifugation for isolation of viable PBMCs from whole blood is well established14, and provides the added benefit of excluding granulocytes from analysis which may confound analysis of monocyte populations. While flow cytometry is routinely used for detection and analysis of immune cell such as monocytes, it has not been used to detect TH and DAT in the human PBMCs; we have shown that human monocytes in the PBMC fraction express these two markers of dopamine systems both at message and protein level2. Here we report an optimized protocol to detect DAT and TH in monocytes by flow cytometry. The accuracy of DAT and TH detection was assessed by right-ward shift in the fluorescence intensity relative to isotype control (negative control); percentage monocytes expressing DAT and TH were calculated as a percentage of total PBMCs. Both freshly isolated and cryopreserved (see sections 3.6 and 3.7) samples yielded viable PBMCs, as determined by trypan blue staining. The density dot plot obtained from FSC x SSC visualization revealed two distinct cell populations in both cryopreserved and freshly prepared samples (Figure 1a, Figure 2).
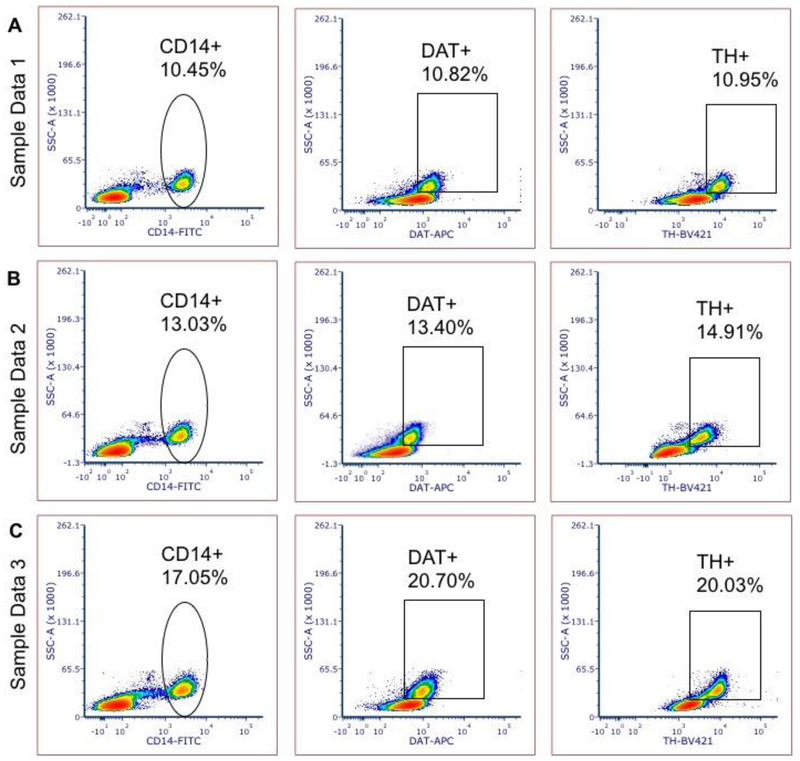
Figure 2: Sample volunteer data demonstrating sensitivity of DAT and TH detection in human PBMCs at varied expression levels.
Panels A, B and C show representative TH , DAT and CD14 expression data from three independent healthy volunteers exhibiting varied levels of DAT and TH expressing monocytes.
3.2. Step 2: Gating strategy for single cell analysis of monocytes:
As shown in Figure 1A, doublets were excluded by overlapping nested gates – single cells were first isolated by gating events on FSC-A (area) x FSC-H (height) followed by an additional overlapping gate on SSC-A x SSC-H. Less than 1% of events were excluded in the final doublet exclusion gate, indicating that only single cells were included in subordinate gates. The target population was isolated from cellular debris by including the events with FSC values greater than 45,000. The FSC value of 45,000 for debris exclusion was determined by thresholding experiments to determine the value at which intact cells were separated from debris and dead/apoptotic cells. To illustrate that antibodies are staining cells and not debris, we left the debris field to the left of FSC 45,000 is visualized (Figure 1A) as a teaching tool to illustrate the difference between debris and relevant cell populations.
Lymphoid and myeloid cells were clearly delineated in the final gate as two distinct populations with identifying FSC and SSC properties – the lymphoid population exhibits lower values on both FSC and SSC, with monocytes exhibiting higher FSC and SSC values (shown by dashed red ellipse). While lymphoid cells do express DAT and TH to varying degrees3,6,13, monocytes were the specific population of interest and studied exclusively in this analysis. Monocytes were gated from lymphocytes by sidescatter for further analysis of CD14, DAT and TH expression.
3.3. Step 3: Fixation and permeablization for intracellular staining
TH is a cytosolic protein. The DAT antibody used in this study detects an intracellular N-terminus epitope1,11,15,16; therefore, both TH and DAT staining required permeabilization step. We tested multiple fixations and permeabilization strategies to achieve reproducible results. The optimum fixation and permeabilization strategies for DAT and TH detection is described in the Materials and Methods section. While fixation using PFA proved reliable for flow cytometry detection of membrane proteins, the Triton X-100 permeabilization at any concentration affected cell viability, morphology and staining efficiency. Therefore, in this study to detect DAT and TH expressing monocytes, we used eBioscience© fixation and permeabilization kit (cat. 88-8824-00) (Table 3) which did not affect cell morphology or staining efficiency and produced reproducible staining in 41 independent staining of human samples.
3.4. Step 4: Optimization of DAT and TH detection by flow cytometry
Antibody staining conducted in a final staining volume of 100uL containing 1 million cells yielded consistent and reproducible results detecting both DAT and TH in monocytes, as measured by increased fluorescence intensity over non-immune isotype negative control of at least 1 log unit. Results shown in Figures 1, 2 and 3 represent the optimum dilution factor of 1:100 from stock. Optimum dilutions were determined by titration of antibodies at concentrations from 0.5uL/100uL staining volume to 7ul/100uL, for each primary and secondary antibody used. Isotype controls for each antibody were included as the main negative control at an identical final concentration and yielded negligible levels of nonspecific background binding (Fig. 1C).
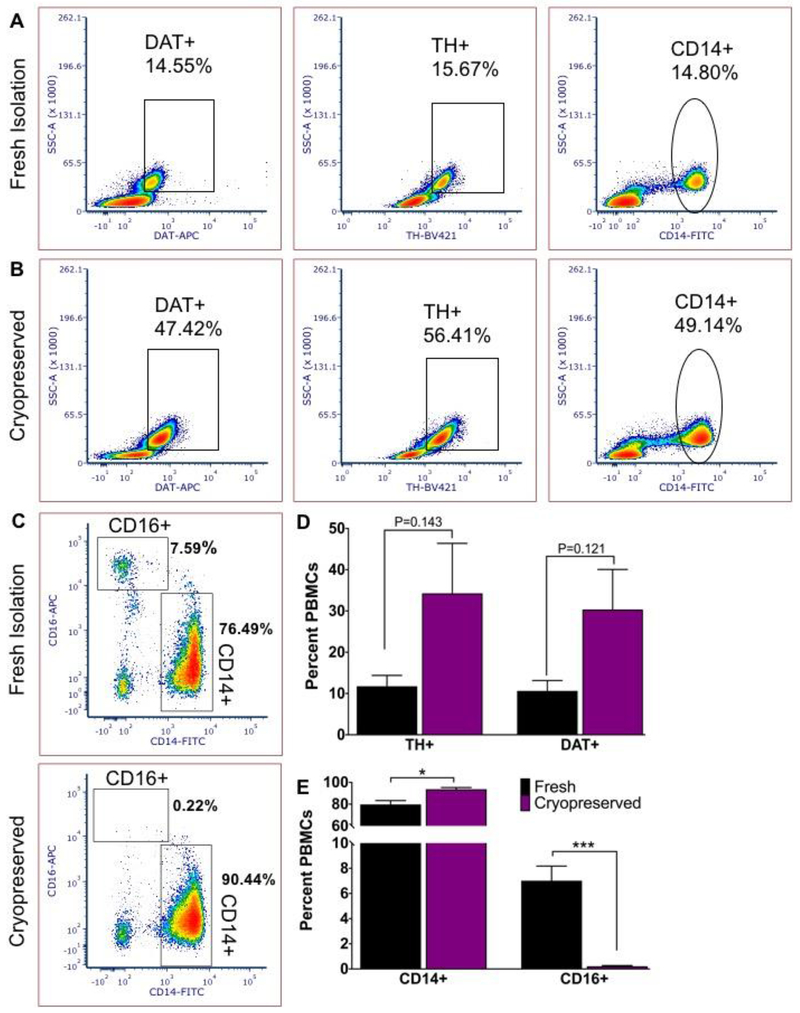
Figure 3: PBMC cryopreservation in FBS-DMSO alters flow cytometric detection of DAT and TH.
A) Freshly isolated PBMCs show DAT, TH and CD14 expressing monocytes comparable to baseline for healthy individuals. B) Cryopreserved in FBS-DMSO and quick-thawed PBMCs isolated from the same donor show dramatically altered percentage of PBMCs expressing DAT, TH and CD14. C) Cryopreserved PBMCs in FBS-DMSO show clear CD14 staining, but a significant reduction of CD16 signal. D) Altered percentage of DAT and TH expressing PBMCs before and after freeze-thaw and E) significantly altered percentage of CD14+ to CD16+ monocytes shows the consequences of cryopreservation. Statistical analysis conducted with Graphpad Prism using multiple t tests with Holm-Sidak correction for multiple comparisons; alpha =0.05. N=3 independent biological replicates.
3.5. DAT and TH expression in monocytes can be reliably detected by flow cytometry
Presence of DAT and TH in human monocytes has been demonstrated by western blot, qPCR, and immunofluorescent assays2,4. While these methodologies are reliable and proven, they are time consuming and unsuitable for use in clinical setting or the realtime comparison of large number of samples. Therefore, there is an unmet need to develop a standardized and high throughput methodology to rapidly and reliably detect these proteins at basic science laboratories and/or standard clinical facilities. Here we established a sensitive flow cytometry panel to detect DAT and TH in human monocytes. Figure 1 shows clear separation of at least 1 log unit between monocytes incubated with non-immune isotype antibody (Fig. 1C) and those incubated with anti-DAT and anti-TH (Fig. 1B). While most but not all (the majority) of human monocytes express TH and DAT, we found that ~100% of CD14+ monocytes co-express these two proteins (Fig. 1B). Therefore, in this study we assayed for CD14+ monocytes co-expressing TH and DAT to ensure consistency across samples. Applying this strategy, samples that produced less than 75% TH and DAT co-expression in CD14+ monocytes suggested under-staining and were excluded from analysis.
While Leite et al. (2016) and Gaskill et al. (2012) have reported expression of these markers in cultured monocyte-derived macrophages4,17,18, we have found that TH and DAT is expressed in multiple monocyte subsets including CD14+, CD16+ and the transitional population co-expressing CD14 and CD16. Therefore, in this study we used the number of DAT/TH expressing cells as percentages of the PBMC, rather than percentages of the monocyte population because these markers are expressed in multiple monocyte subsets beyond CD14+ monocytes. Analysis of monocytes expressing TH and DAT using a sidescatter gate allows capture of all monocyte populations expressing these markers. Future work will investigate the expression of TH and DAT on monocytes transitioning between CD14+ and CD16+ phenotypes.
We have repeated these experiments in 41 whole blood samples drawn from healthy volunteers across different ages/genders (mean age 59.74±14.35 SD) (Table S3). Our data in 41 independent experiments suggest DAT and TH expressing monocytes comprise ~12% (±7.76% SD, ±2.298%_SEM) of the PBMC fraction in the healthy human (sample data shown in Figure 2), providing a dependable methodology to analyze the expression of these two dopaminergic markers in human blood samples in health and disease.
3.6. Cryopreservation with FBS/DMSO alters the immune phenotype of monocytes.
PBMCs are routinely cryopreserved for long-term studies, transport to research labs across the world and other practical considerations. Therefore, we tested the applicability of our panel to detect DAT, TH, CD14 and CD16 in cryopreserved PBMCs. In three independent biological replicates, we compared freshly isolated PBMCs to PBMCs frozen in FBS with 10% DMSO immediately after isolation and found that in each sample the percentages of PBMCs expressing DAT and TH were altered compared to freshly isolated PBMCs (Figure 3a, b). To determine whether this change was due to a loss in a specific subset of monocytes, we assayed monocyte subsets for expression of an additional monocyte marker. Surprisingly, we found while freshly isolated PBMCs showed a stable presence of CD16+ PBMCs, this population is almost absent in the FBS-DMSO cryopreserved cells suggesting a loss of this phenotype during cryopreservation and thaw. Therefore, cryopreservation of PBMCs using FBS/DMSO alters the immune phenotype of monocyte subsets included in the PBMC fraction such that these are not equivalent to those in freshly obtained cells.
3.7. Cryopreservation with CryoStor10 maintains the immune phenotype of monocytes
Since cryopreservation in FBS-DMSO alters the immune phenotype of monocytes, we examined many other cryopreservation strategies. We identified a proprietary serum-free freezing media CryoStor10 (CS10, Sigma, Cat. C2874, Table 3), designed to manage cellular stress during freezing, to determine whether freezing in serum-free media is more suitable for DAT/TH detection by flow cytometry following PBMC cryopreservation.
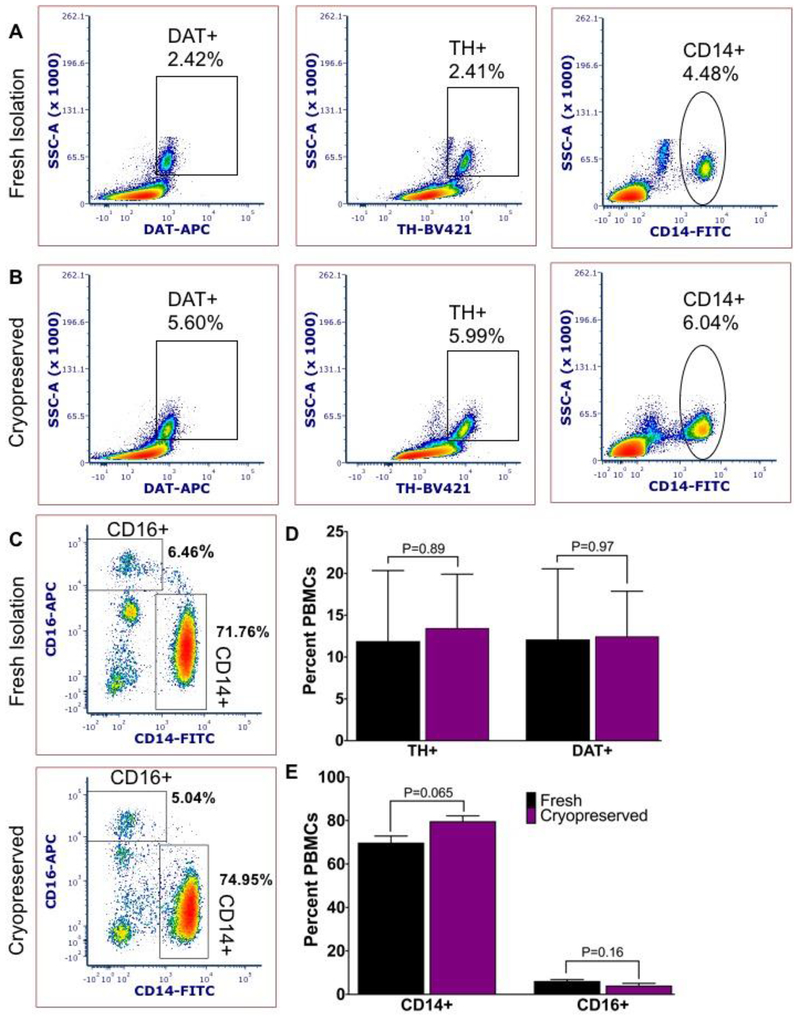
In three independent biological replicates, we compared freshly isolated PBMCs to PBMCs frozen in CS10 immediately after isolation. In each of three replicates, percentages of PBMCs expressing DAT and TH were not significantly different compared to freshly isolated PBMCs (Figure 4a, b). We assayed the monocyte subsets with CD16 as a parallel to our earlier study of cryopreserved monocytes. While CD14 monocyte populations are largely intact, CD16+ monocytes are slightly but non-significantly affected by cryopreservation in CS10, suggesting that cryopreservation with CS10 is a viable preservation method that maintains the immune phenotype integrity of monocytes required in flow cytometry assay for detection of TH and DAT.
Figure 4: Serum-free cryopreservation maintains/preserve DAT/TH detection in monocyte subsets.
Representative plots from A) freshly isolated PBMCs compared to B) serum-free cryopreservation and quick-thawed PBMCs isolated from the same donor maintains the percentage DAT+, TH+ and CD14+ PBMCs relative to fresh isolation. C) Cryopreserved PBMCs show clear CD14 staining and slightly reduced CD16 signal relative to fresh isolation. D) Similar percentage of DAT and TH expressing PBMCs before and after serum-free freeze-thaw procedure and E) there is a small, but not significant effect of cryopreservation using serum-free freezing media CryoStor10 on the percentage of CD14+ to CD16+ monocytes. Statistical analysis conducted with Graphpad Prism using multiple t tests with Holm-Sidak correction for multiple comparisons; alpha =0.05. N=3 independent biological replicates.
4. Discussion
While flow cytometry has been routinely used for immunological studies19, this report presents the first use of flow cytometry to study the dopaminergic proteins DAT and TH in peripheral monocytes. Dysregulated dopamine homeostasis is characteristic of neurodegenerative diseases, such as Parkinson’s disease2 and Alzheimer’s disease17. Increasing evidence correlate the dysregulation of peripheral dopamine signaling with dysfunction of CNS dopaminergic function17,20. For example, monocyte function and systemic dysregulation of dopamine are implicated in diseases such as neuroHIV21, addiction4, and in antigen-presenting cell function in Multiple Sclerosis22, encephalitis and Parkinson’s disease3. Our novel flow cytometry panel provides a sensitive and reproducible assay to study dysregulation of peripheral dopamine homoeostasis in immune cells that is applicable to basic and clinical research.
Multiple repositories for human blood and PBMCs, employing various cryopreservation methods, are already available. We characterized the performance of our panel in multiple cryopreservation methods and identified the most suitable method. Further, we were able to determine that cryopreservation using FBS/DMSO is unsuitable for this specific panel, as we observed a marked loss of CD16+ monocyte phenotype. Because CD16+ monocytes are involved in tissue surveillance relevant to CNS diseases21– for example, CNS-surveilling monocytes and macrophages including CD16+ monocytes exhibit altered function in presence or absence of dopamine4,23. This subset may have particular significance in the context of neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease and neuro-HIV4,21. Therefore, studies of dopamine signaling using FBS/DMSO-cryopreserved PBMCs from biorepositories may cause key immunological features to be overlooked. In future work, we anticipate incorporating our flow cytometry panel examining monocyte DAT and TH expression into pre-CLIA diagnostic tools for neurodegenerative diseases.
We identified an alternate cryopreservation method using CS10 that produced markedly better results. This disparity may be due to immune activation by serum (FBS) or as consequence of additional proprietary cryoprotectants present in CS10 that enable preservation of delicate monocyte subsets for future analysis.
The instrument used in our studies, BD Canto II, is equipped to detect up to 8 appropriately selected fluorochromes; as our panel only includes three fluorochromes, a variety of additional markers of interest can be studied in parallel expanding the potential of studying dopaminergic proteins on leukocytes included in the PBMC fraction.
The primary challenge in establishing this new flow cytometry panel was to determine the appropriate conditions for detecting DAT and TH in the PBMC fraction while maintaining cell viability and maximizing signal to background ratios to allow reliable detection of these markers in an innately heterogeneous human population. Studies applying flow cytometry in neuroscience have often relied on transgenic animal studies expressing fluorescent reporter proteins, or utilized commercially available antibodies against common immunological targets, which are typically directly fluorochrome-conjugated and therefore require minimal optimization. The absence of antibodies tested in flow cytometry for neuroscience applications has greatly limited applications of this technique. Thus, we elected to establish a protocol for detecting dopaminergic proteins in blood cells expecting that this can be used to explore mechanistic insights in CNS-associated diseases involving dysregulated dopamine homeostasis. We believe that the proposed assay can provide a sensitive tool to assess the peripheral dopaminergic proteins in various disease processes when dysregulation of peripheral dopamine transmission is implicated.
Supplementary Material
Figure S1: FMO gating template to confirm constitutive DAT and TH expression in CD14+ monocyte. Fluorescence-minus-one acquisition template setup: A) Sample lacking anti-CD14-FITC staining produces no events in DAT or TH gates, while B) lacking anti-TH-BV421 produces no events in DAT/TH double positive gate; similarly, C) lacking anti-DAT-APC produces no DAT/TH double positive events. Sample shown in D) was stained with anti-CD14, anti-DAT and anti-TH to produce a triple positive population, demonstrating a stringently controlled gating strategy to confirm constitutive DAT and TH expression in human monocytes.
Figure S2: FMO gating template confirms specific detection of CD14 and CD16 with absence of spectral overlap. Fluorescence-minus-one acquisition template setup: FMO samples used to establish positive gates for CD14 and CD16 expressing monocytes: from left to right, Unstained, CD14 only, CD16 only, and representative plot containing antibodies against CD14 and CD16.
Figure S3: Alternate anti-CD14 antibody clones and concentrations considered for this panel. Dilutions are in microliters of stock antibody as provided by the vendor, added to the final staining volume. Staining index (SI) was calculated using mean fluorescence index (MFI) and the formula: SI – [(MFI of positive cells)-(MFI of negative cells)]/(2* SD of negative cells).
Acknowledgements:
We acknowledge funding support from 2R01NS071122-07A1, 5R21NS103108-02, UF Research Developmental Fund (Moonshot Grant) and from the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards TL1TR001428 and UL1TR001427 (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Data Availability: All data will be made available upon reasonable request.
References
- 1.Eriksen J et al. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci 29, 6794–6808, doi: 10.1523/JNEUROSCI.4177-08.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackie P et al. The dopamine transporter: An unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s Disease. Brain Behav Immun 70, 21–35, doi: 10.1016/j.bbi.2018.03.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinoli M, Marino F & Cosentino M Dopaminergic Regulation of Innate Immunity: a Review. J Neuroimmune Pharmacol 12, 602–623, doi: 10.1007/s11481-017-9749-2 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Gaskill PJ, Carvallo L, Eugenin EA & Berman JW Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation 9, 203, doi: 10.1186/1742-2094-9-203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenhofer G et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82, 3864–3871, doi: 10.1210/jcem.82.11.4339 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Kustrimovic N et al. Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease. Sci Rep 6, 33738, doi: 10.1038/srep33738 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marazziti D et al. Alterations of the dopamine transporter in resting lymphocytes of patients with different psychotic disorders. Psychiatry Res 175, 54–57, doi: 10.1016/j.psychres.2009.03.009 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Boyette LB et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 12, e0176460, doi: 10.1371/journal.pone.0176460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L et al. Identification of the mRNA expression status of the dopamine D2 receptor and dopamine transporter in peripheral blood lymphocytes of schizophrenia patients. PLoS One 8, e75259, doi: 10.1371/journal.pone.0075259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raj T et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344, 519–523, doi: 10.1126/science.1249547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciliax BJ et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci 15, 1714–1723 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autissier P, Soulas C, Burdo TH & Williams KC Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A 77, 410–419, doi: 10.1002/cyto.a.20859 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee R et al. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep 5, 13886, doi: 10.1038/srep13886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fluks AJ Three-step isolation of human blood monocytes using discontinuous density gradients of Percoll. J Immunol Methods 41, 225–233, doi: 10.1016/0022-1759(81)90245-3 (1981). [DOI] [PubMed] [Google Scholar]
- 15.Hersch SM, Yi H, Heilman CJ, Edwards RH & Levey AI Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol 388, 211–227 (1997). [PubMed] [Google Scholar]
- 16.Miller GW et al. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol 41, 530–539, doi: 10.1002/ana.410410417 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Matt SM & Gaskill PJ Where Is Dopamine and how do Immune Cells See it?: Dopamine-Mediated Immune Cell Function in Health and Disease. J Neuroimmune Pharmacol, doi: 10.1007/s11481-019-09851-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leite F, Lima M, Marino F, Cosentino M & Ribeiro L Dopaminergic Receptors and Tyrosine Hydroxylase Expression in Peripheral Blood Mononuclear Cells: A Distinct Pattern in Central Obesity. PLoS One 11, e0147483, doi: 10.1371/journal.pone.0147483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossarizza A et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 47, 1584–1797, doi: 10.1002/eji.201646632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engler H et al. Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease. Brain Behav Immun 23, 518–526, doi: 10.1016/j.bbi.2009.01.018 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Veenstra M et al. Frontline Science: CXCR7 mediates CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: a potential therapeutic target for NeuroAIDS. J Leukoc Biol 102, 1173–1185, doi: 10.1189/jlb.3HI0517-167R (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, Pineda B & Sotelo J Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol 2013, 413465, doi: 10.1155/2013/413465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garris PA, Ciolkowski EL, Pastore P & Wightman RM Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14, 6084–6093 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: FMO gating template to confirm constitutive DAT and TH expression in CD14+ monocyte. Fluorescence-minus-one acquisition template setup: A) Sample lacking anti-CD14-FITC staining produces no events in DAT or TH gates, while B) lacking anti-TH-BV421 produces no events in DAT/TH double positive gate; similarly, C) lacking anti-DAT-APC produces no DAT/TH double positive events. Sample shown in D) was stained with anti-CD14, anti-DAT and anti-TH to produce a triple positive population, demonstrating a stringently controlled gating strategy to confirm constitutive DAT and TH expression in human monocytes.
Figure S2: FMO gating template confirms specific detection of CD14 and CD16 with absence of spectral overlap. Fluorescence-minus-one acquisition template setup: FMO samples used to establish positive gates for CD14 and CD16 expressing monocytes: from left to right, Unstained, CD14 only, CD16 only, and representative plot containing antibodies against CD14 and CD16.
Figure S3: Alternate anti-CD14 antibody clones and concentrations considered for this panel. Dilutions are in microliters of stock antibody as provided by the vendor, added to the final staining volume. Staining index (SI) was calculated using mean fluorescence index (MFI) and the formula: SI – [(MFI of positive cells)-(MFI of negative cells)]/(2* SD of negative cells).