Abstract
Social exclusion is associated with greater suicide risk and more needs to be known about the biological processes contributing to this association. Oxytocin, a neuropeptide that regulates social interactions, may protect against the negative effects of exclusion by motivating social engagement. Oxytocin levels and desire for social engagement increase when non-psychiatric controls experience acute social exclusion. However, among individuals with serious psychiatric illnesses, oxytocin levels decrease following exclusion. No research has examined changes in oxytocin following social exclusion among individuals at risk for suicide. This quasi-experimental study examined differences in oxytocin levels and perceptions of social connectedness following an in-laboratory, acute social exclusion task among (a) individuals with no depression or suicide attempt histories, (b) individuals with current depression symptoms, and (c) individuals with current depression symptoms and suicide attempt histories. Young adults (N=100) completed self-report measures and provided blood samples before and after an acute social exclusion task (Cyberball). Oxytocin was quantified via enzyme-linked immunosorbent assay. Mixed-design ANCOVAs were used to evaluate changes in unextracted and extracted oxytocin levels, desire for emotional support, thwarted belongingness, and perceived burdensomeness. Among suicide attempters, unextracted oxytocin levels decreased and desire for emotional support did not significantly change following exclusion. Among depressed and healthy controls, desire for emotional support increased and oxytocin levels did not significantly change. No significant changes in extracted oxytocin, thwarted belongingness and perceived burdensomeness emerged. Further research is needed to determine if dysregulated oxytocin-related processes biologically predispose individuals with suicide attempt histories to greater social disconnection and suicide risk.
Keywords: oxytocin, suicide attempt, depression, social exclusion, Cyberball
Social exclusion—a form of social disconnection due to factors outside of individual control—is associated with self-injury, suicidal thoughts, and suicide attempts (Groschwitz et al., 2016; Hames et al., 2018; McDougall and Vaillancourt, 2015; Olié et al., 2017). However, the psychological and biological impacts of social exclusion on at-risk individuals are not well-characterized. In the general population, social exclusion often results in greater self-reported desire for social interactions (Maner et al., 2007). Given the social nature of humans, this desire for affiliation is protective. Oxytocin, a modulating neuropeptide, is partly responsible for increased social interaction—oxytocin facilitates social bonds, increases social salience, and influences other prosocial processes with in-group members (Bartz et al., 2011; Shamay-Tsoory and Abu-Akel, 2016), by decreasing fear network activity and increasing attention and reward network activity (Ma et al., 2016).
Brief social separations may enhance oxytocin release and drive individuals to repair social disconnection. For example, Kojima and colleagues (2012) found increased circulating serum oxytocin levels in neonatal rats following maternal separation, and Taylor and colleagues (2006) found higher unextracted plasma oxytocin levels among socially disconnected women. This suggests that circulating oxytocin levels may rise when individuals feel disconnected to encourage affiliative behaviors and diminish social distress. Indeed, among socially excluded individuals, intranasal oxytocin administration promoted increased desires for connection (Ditzen et al., 2009). Further, Smith and Wang (2014) found that oxytocin reduced behavioral and biological social stress responses in prairie voles. Findings indicate that oxytocin may be a protective buffer that increases following exclusion to facilitate social engagement.
In contrast, social exclusion may not evoke self-protective responses among individuals at risk for suicide. Among at-risk individuals, social exclusion may increase thwarted belongingness (perceptions of not belonging) and perceived burdensomeness (perceptions of burdening others; Baumeister et al., 2007; Hames et al., 2018), which both contribute to severe suicidal ideation according to the interpersonal theory of suicide (Joiner, 2005). At-risk individuals also show diminished desires for affiliation and social engagement, particularly in the days before suicide (cf. social withdrawal; Robins, 1981). Altogether, findings suggest that among at-risk individuals, social exclusion may result in poorer social connection and diminished desire for social engagement.
Preliminary evidence also suggests that at-risk individuals may be biologically vulnerable to social disconnection. Lower cerebrospinal fluid (CSF) oxytocin levels have been found among individuals with suicide attempt histories relative to those without such histories (Lee et al., 2009). Further, attenuated unextracted CSF and plasma oxytocin levels were associated with suicide planning among suicide attempters and with higher suicidal intent specifically among male attempters (Jokinen et al., 2012). A study also found that CD38 gene SNPs, which control oxytocin release, were linked to more severe ideation and feelings of alienation (McQuaid et al., 2016). However, findings have been mixed—Deisenhammer and colleagues (2012) found no significant differences in extracted plasma oxytocin between individuals with and without past-year attempts.
Research is needed to examine psychological (diminished affiliation desire, thwarted belongingness, perceived burdensomeness) and biological (diminished oxytocin) responses to acute social exclusion in real-time to illuminate potential pathways between social exclusion and suicide risk. No research exists in this domain. However, two quasi-experimental studies found that after social exclusion (Cyberball), patients with borderline personality disorder (BPD; Jobst et al., 2014) and chronic depression (Jobst et al., 2015) showed decreased unextracted plasma oxytocin levels, while controls showed increased oxytocin. That the same pattern emerged across both studies is striking. One explanation for this pattern is that suicidal thoughts and/or behaviors are related to dysregulation in oxytocin given that suicidality is a symptom of both disorders and these disorders are associated with high suicide rates (APA, 2013). Neither study reported suicide risk; thus, investigation among at-risk individuals is needed.
Suicide is a leading cause of mortality among young adults (Heron, 2016); thus, this quasi-experimental study examined the dynamic responses of oxytocin, desires to affiliate, thwarted belongingness, and perceived burdensomeness to in vivo, acute social exclusion among young adults with mild depression symptoms and suicide attempt histories (suicide attempters). To determine whether suicidality accounts for differences in oxytocin change following social exclusion obtained in prior studies (Jobst et al., 2014, 2015), two groups were recruited to contrast findings obtained among attempters: depressed controls with mild depression symptoms and no suicidal symptom history, healthy controls with no depression or suicidal symptoms. We hypothesized that after experiencing social exclusion: (a) plasma oxytocin levels decrease more among attempters than depressed controls and plasma oxytocin levels increase among healthy controls; (b) desires to affiliate decrease more among attempters than depressed controls and desires to affiliate increase among healthy controls; and (c) thwarted belongingness and perceived burdensomeness increase more among attempters than depressed and healthy controls. Second, we examined baseline group differences in the four outcomes—we expected lower oxytocin levels and desires to affiliate, and higher thwarted belongingness and perceived burdensomeness among attempters than controls. We examined oxytocin in both unextracted and extracted samples given contrasting methodology in prior work (Deisenhammer et al., 2012; Jobst et al., 2014, 2015) and recommendations (Brandtzaeg et al., 2016; Leng and Sabatier, 2016; Szeto et al., 2011).
Method
Participants
We examined a sample of 100 young adults, primarily female (70.0%, n=70), undergraduate students (96.0%, n=96) with an average age of 20.2 years (SD=5.0, range=18–28; Table 1). Participants were recruited from a public university in the Southeastern U.S. and the surrounding community via flyers and the University’s research recruitment website. Students reporting current depression symptoms and/or prior suicide attempt(s) were targeted during recruitment via email. Eligible participants were at least 18 years old with no history of cognitive deficits; those who have (a) been pregnant or nursing in the last six months, (b) previously participated in the social exclusion paradigm, (c) a history of cognitive impairments, and (d) significant fears of blood, injections, or needles were excluded. Consistent with Jobst and colleagues (2015), all female participants reported regular menstrual cycles—those taking hormonal contraceptives were assessed between the 3rd and 18th day of their menstrual cycle, and others were assessed between the 5th and 12th menstrual cycle day, before ovulation.
Table 1.
Participant Demographic and Clinical Information (N, %).
| Variables | Total | Healthy Control | Depressed Control | Suicide Attempter | |
|---|---|---|---|---|---|
| N | 100 | 48 | 21 | 31 | -- |
| Age | F[2,96]=.99 | ||||
| Mean years (SD) | 20.2 (4.9) | 20.9 (6.1) | 19.5 (1.2) | 19.5 (4.4) | -- |
| Range | 18–28 years | 18–28 years | 18–22 years | 18–28 years | -- |
| Sex | χ 2[2]=1.79 | ||||
| Female | 70, 70% | 33, 68.8% | 13, 61.9% | 24, 77.4% | -- |
| Male | 30, 30% | 15, 31.2% | 8, 38.1% | 7, 22.6% | -- |
| Marital Status | χ 2[2]=.52 | ||||
| Single/Never Married | 90, 90% | 45, 93.8% | 19, 90.4% | 26, 83.9% | -- |
| Cohabiting/Married [ref.] | 7, 7% | 3, 6.2% | 1, 4.8% | 3, 9.7% | -- |
| Separated/ Divorced | 0, 0% | 0, 0% | 0, 0% | 0, 0% | -- |
| Widowed | 1, 1% | 0, 0% | 0, 0% | 1, 3.2% | -- |
| Missing | 2, 2% | 0, 0% | 1, 4.8% | 1, 3.2% | -- |
| Race | χ 2[2]=.44 | ||||
| Caucasian/White [ref.] | 81, 81% | 40, 83.3% | 17, 80.9% | 24, 77.4% | -- |
| African American/Black | 9, 9% | 3, 6.3% | 3, 14.3% | 3, 9.7% | -- |
| Asian/Pacific Islander | 4, 4% | 3, 6.3% | 0, 0% | 1, 3.2% | -- |
| Other | 6, 6% | 2, 4.1% | 1, 4.8% | 3, 9.7% | -- |
| Ethnicity | χ 2[2]=.92 | ||||
| Hispanic/Latino/Spanish | 27, 27% | 13, 27.1% | 4, 19.0% | 10, 32.2% | -- |
| Current Symptoms | -- | ||||
| BDI-II Depression | 10.3 (9.7) | 4.0 (3.5) | 19.2 (10.2) | 20.5 (8.0) | F[2,97]=41.67* |
| BSS Suicidal Ideation | 1.8 (2.1) | .9 (1.3) | 2.6 (1.9) | 4.7 (2.5) | F[2,97]=12.42* |
| Suicide Attempt History | -- | ||||
| Lifetime Suicide Attempt | 31, 33% | 0, 0% | 0, 0% | 31, 100% | F[2,97]=484.35* |
| 1 Previous Attempt | 23, 23% | 0, 0% | 0, 0% | 23, 74.2% | -- |
| 2+ Previous Attempts | 8, 8% | 0, 0% | 0, 0% | 8, 25.8% | -- |
| Past Year Attempt | 6, 6% | 0, 0% | 0, 0% | 6, 19.4% | -- |
| Cyberball Check (Mean[SD]) | -- | ||||
| Ignored by others? | 2.73 (.55) | 2.63 (.61) | 2.84 (.50) | 2.80 (.48) | F[2,92]=1.38 |
| Excluded by others? | 2.83 (.43) | 2.78 (.47) | 2.79 (.54) | 2.94 (.25) | F[2,93]=1.32 |
| % of throws received | 9.91% (6.98) | 9.74% (6.34) | 11.74% (7.94) | 9.00% (7.32) | F[2,93]=.92 |
Note.
p < .001
ref. = reference group. SD = standard deviation. BDI-II = Beck Depression Inventory-II. BSS = Beck Scale for Suicide Ideation. Cyberball Manipulation Check (1=Disagree, 2=Neither Agree nor Disagree, 3=Agree).
Procedures
Potential participants completed an initial phone screening to ensure eligibility. Due to the impact of food intake on oxytocin (Zhang and Cai, 2011), participants were asked to refrain from eating or drinking for two hours prior to the study; two participants who failed to meet this requirement were rescheduled. To control for hormonal variations, including circadian oxytocin changes (Zhang and Cai, 2011), this study was conducted between 08:00 and 09:30. In the lab, procedures and risks were reviewed, and informed consent was obtained. Next, participants completed a series of self-report measures and a blood sample was obtained by venipuncture performed in-person by a trained phlebotomist (pretest blood draw). Subsequently, participants played Cyberball, a social exclusion task (Williams and Jarvis, 2006). After Cyberball, approximately 10 minutes after the pretest blood draw, a second blood sample was obtained (posttest blood draw) and a battery of questionnaires was administered. Cyberball and all questionnaires were administered on a computer. Following study completion, participants were debriefed, provided with mental health resources, and compensated monetarily or with research credits. An empirically based suicide risk assessment (Chu et al., 2015) was conducted with 11 participants who scored above zero on a measure of suicidal symptom severity (Beck Suicide Scale [BSS]; Beck and Steer, 1991; described below) and reported suicide attempt histories; these participants were provided with additional resources and referrals. Procedures were in accordance with the Declaration of Helsinki and approved by the University’s Institutional Review Board (HSC.No.2015.16047).
Blood Sample Collection
Fasting blood samples, a total of 20 milliliters, were drawn for the quantification of plasma oxytocin concentrations. Samples were collected into chilled EDTA tubes, inverted to mix, and centrifuged at 1600g for 15 minutes at 4˚C. Cleared plasma was aliquoted into cryotubes and stored at −80˚C.
Oxytocin Measurement
First, consistent with others (Lancaster et al., 2015; Brandtzaeg et al., 2016), we obtained oxytocin levels from unextracted samples diluted 1:4 given that oxytocin may be discarded with plasma proteins when an extraction step is incorporated before conducting the enzyme-linked immunosorbent assay (ELISA) and our study aimed to replicate previous studies that similarly did not extract samples prior to quantification (Jobst et al., 2014, 2015). Second, given contrasting concerns among others (Leng and Sabatier, 2016; Szeto et al., 2011), an aliquot of each sample was extracted prior to quantification. Oxytocin concentrations were measured using a commercially available and sensitive ELISA kit (Enzo Life Sciences) according to the manufacturer’s protocol. The inter- and intra-assay coefficients of variation were 15.3% and 10.9%, respectively. See Supplemental Materials for details regarding quantification and extraction.
Cyberball
Cyberball is a 10-minute ball-toss game that has been used extensively to simulate acute social exclusion (Williams and Jarvis, 2006). Participants passed a virtual ball to two other players and were informed that they are playing with other university students. In reality, the two other players computerized. To bolster beliefs that they were playing with other students, participants selected a personalized display picture that would be visible to other players and display pictures for the other two players were also visible. Following the instructions, the computer program displayed three characters labeled “you,” “player A,” and “player B,” and their display pictures. Participants clicked another player’s display picture to pass the ball. Consistent with Jobst et al. (2015), the game ended after 40 throws and all participants were allocated to the exclusion condition, such that they received the ball twice at the start and were excluded for the remainder of the game. During debriefing, participants were informed about the purpose and nature of Cyberball.
Pre-Cyberball Measures
Demographics
Participants reported the following demographic information: age, sex, ethnicity/race, marital status, and, if applicable, hormonal contraceptive use and last menstrual cycle.
Interpersonal Orientation Scale (IOS; Hill, 1987)
The IOS is a 26-item measure of four dimensions underlying affiliation motivation: desire for emotional support, desire for positive (cognitive and affective) stimulation, engagement in social comparison, and desire for attention. Participants rated items a 1 (not at all true) to 5 (completely true) Likert scale. IOS subscales have good to excellent internal consistency (αs=.77-.94) and construct validity in undergraduate samples (Hill, 1987). However, Hill (1987) reported that the emotional support (IOS-ES) subscale was the most robustly correlated with other indices of affiliation motivation. Thus, we only used the IOS-ES scale to measure desire to affiliate; internal consistency was excellent at pretest (α=.94).
Interpersonal Needs Questionnaire-15 (INQ; Van Orden et al., 2012)
The INQ is a 15-item self-report measure of thwarted belongingness (INQ-TB, n=9) and perceived burdensomeness (INQ-PB, n=6), two constructs that contribute to suicidal desire (Joiner, 2005). Items were rated on a 7-point Likert scale ranging from 1 (not at all true for me) to 7 (very true for me), with positive items reverse coded and higher scores indicating greater severity. The measure has good internal consistency and construct validity in undergraduate samples (Van Orden et al., 2012). In this study, participants rated their perceptions in the moment and internal consistencies were excellent (INQ-PB α=.95; INQ-TB α=.93).
Self-Injurious Thoughts and Behaviors Inventory-Short Form (SITBI-SF; Nock et al., 2007)
The SITBI-SF assesses the nature of self-injurious thoughts and behaviors and is based on the SITBI, a structured interview (κ=.77; Nock et al., 2007). We selected 34 items from the interview for the self-report measure. Sections were preceded by screening questions (“Have you ever had thoughts of killing yourself?”; “Have you ever made an actual attempt to kill yourself in which you had at least some intent to die?”) and followed by questions about characteristics and behavior frequency and intensity. The SITBI-SF was used to determine participants’ suicide attempt histories.
Positive and Negative Affect Schedule (PANAS; Watson et al., 1988)
The PANAS is a 20-item self-report measure of global emotional states at the time of assessment. The PANAS is comprised of two, 10-item subscales that assess positive affect (PA) and negative affect (NA). Current emotion intensity was rated on a 5-point Likert scale ranging from 1 (not at all) to 5 (very much). This measure has demonstrated adequate reliability and validity as a measure of affect (Watson et al., 1988). Pretest internal consistencies were good to excellent (PANAS-PA α=.94; PANAS-NA α=.92).
Post-Cyberball Measures
The INQ-TB (α=.92), INQ-PB (α=.96), IOS-ES (α=.85), PANAS-PA (α=.94), and PANAS-NA (α=.92) were also administered following Cyberball to assess the influence of social exclusion on cognition and emotion. Consistent with previous Cyberball studies (Zadro et al., 2004), three items were used to determine whether Cyberball influenced participants’ perceptions of exclusion; participants reported the degree to which they agree with two statements (“I was ignored;” “I was excluded”) on a 4-point Likert scale and estimated the percentage of throws they received.
Data Analytic Strategy
Preliminary Analyses
Analyses were conducted using SPSS 23. The percentage of missing data for psychological measures was minimal (1–2%). However, 10% of the oxytocin data were missing, due to difficulties finding a vein, participant discomfort, and/or participant dehydration during venipuncture and measurement errors during ELISA. Skewness and outliers were examined for all variables. Only oxytocin concentrations within the range of standard curve of the assay (15.6–1500.0pg/ml and 62.5–6000.0pg/ml for undiluted and diluted samples, respectively) were included; one participant’s pretest and posttest samples were excluded.
Two self-report measures were used to create the group variable. The Beck Depression Inventory-II (BDI-II; Beck et al., 1996) is a 21-item measure of the presence and severity of past-two-weeks depression symptoms with items rated on 4-point Likert scales; the sum of all 21 items was used to indicate past-two-weeks depression severity. The BSS is a 21-item measure of past-week suicidal symptoms with items rated on 3-point Likert scales; in line with recommendations (Beck and Steer, 1991), the first 19 items were summed and used to indicate past-week suicidal ideation severity. The group variable had 3 levels: a) “Suicide Attempters” – individuals reporting one or more prior attempts (BSS item 20≥1) and current mild to moderate depression (BDI-II total≥14); b) “Depressed Controls” – individuals with current mild to moderate depression (BDI-II total≥14) and no suicidal attempt history (BSS item 20=0); and c) “Healthy Controls” – individuals with none to minimal current depression symptoms (BDI-II total<14); and no prior attempts (BSS item 20=0).
Next, we examined group differences in socio-demographic variables, clinical symptoms, and the potential covariates (age, sex, positive mood, negative mood) using one-way ANOVAs for continuous outcomes and chi-square tests for dichotomous outcomes; mixed-design ANOVAs were used to examine changes in mood following Cyberball across groups. Variables used to evaluate the effects of Cyberball were also examined descriptively.
Primary Analyses
First, multiple ANOVAs were used to evaluate baseline group differences in concentrations of plasma oxytocin, desire for emotional support, perceived burdensomeness, and thwarted belongingness. Next, multiple mixed-design ANCOVAs were used to examine group differences in changes in unextracted oxytocin level, extracted oxytocin level, desire for emotional support, perceived burdensomeness and thwarted belongingness following social exclusion, beyond covariates. Post-hoc pairwise comparisons were conducted when the omnibus test indicated statistically significant effects. Effect sizes (partial eta-squared[ηp2]) are provided.
Covariates
Six variables were evaluated as potential covariates for analyses. Given that prior research has reported sex differences in neural and behavioral responses to oxytocin injections and age-related differences in oxytocin-producing neurons (Ishunina and Swaab, 1999), age and sex were examined as potential covariates. Mood may also influence psychological responses to Cyberball (Maner et al., 2007); thus, pretest and posttest positive and negative affect were examined as potential covariates. Variables that were significantly different across groups were included as covariates.1
Statistical Power
Power analyses using G*Power based on a moderate correlation between repeated measures (r=.60) and small to moderate effect size (f=.17) indicated that a total sample size of 60 was needed for sufficient power (.80) to conduct one-way and mixed-design ANCOVAs. The estimated effect size was conservative as previous research examining changes in oxytocin levels following social exclusion in a psychiatric sample has reported a moderate effect size (d=.696; Jobst et al., 2014, 2015). This sample provided adequate power to detect small effects of group on change in biological and psychological variables.
Results
Preliminary Analyses
Study variable descriptive statistics and correlations between all variables are in Table 2. Thwarted belongingness, perceived burdensomeness, and desire for emotional support were all significantly correlated at pretest (rs=−.32-.64) and posttest (rs=−.54-.68) in expected ways. Pretest and posttest unextracted and extracted oxytocin levels were not significantly correlated with any self-reported psychological variables (rs=−.08-.14). Using the recommended procedure, we recovered 81.5% of spiked oxytocin. Despite oxytocin recovery via extraction, the unextracted and extracted oxytocin were not significantly correlated.
Table 2.
Descriptives and Correlations Between Variables.
| Pretest | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1. INQ-TB | -- | |||||||||||||
| 2. INQ-PB | .64** | -- | ||||||||||||
| 3. IOS-ES | −.32** | −.21* | -- | |||||||||||
| 4. OXT | .02 | −.10 | .02 | -- | ||||||||||
| 5. OXT EX | −.04 | −.05 | −.01 | −.05 | -- | |||||||||
| 6. PANASP | −.47** | −.23* | .37** | −.02 | .13 | -- | ||||||||
| 7. PANASN | .48** | .50** | −.16 | .14 | −.11 | −.04 | -- | |||||||
| Posttest | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 8. INQ-TB | .88** | .63** | −.42** | .07 | −.02 | −.50** | .46** | -- | ||||||
| 9. INQ-PB | .67** | .92** | −.17 | −.10 | .01 | −.27** | .56** | .68** | -- | |||||
| 10. IOS-ES | −.41** | −.34** | .87** | .01 | .04 | .46** | −.21* | −.54** | −.35** | -- | ||||
| 11. OXT | .01 | −.09 | .06 | .87** | .11 | .05 | .16 | .03 | −.09 | .02 | -- | |||
| 12. OXT EX | −.02 | −.02 | .05 | .09 | .61** | .06 | −.13 | −.03 | −.04 | .14 | .10 | -- | ||
| 13. PANASP | −.43** | −.25* | .29** | .02 | .14 | .88** | −.10 | −.49** | −.30** | .39** | .07 | .08 | -- | |
| 14. PANASN | .53** | .49** | −.13 | .10 | −.12 | −.06 | .91** | .51** | .56** | −.20* | .09 | −.07 | −.10 | -- |
| N | 100 | 100 | 100 | 89 | 90 | 100 | 100 | 99 | 99 | 99 | 89 | 90 | 99 | 99 |
| Mean | 21.9 | 9 | 19.6 | 1834.2 | 51.7 | 27.6 | 15.6 | 22.4 | 9.1 | 20.3 | 1769.0 | 52.7 | 24.3 | 15.6 |
| SD | 11.6 | 5.1 | 5.4 | 1135.5 | 38.6 | 10.3 | 7.5 | 12.0 | 5.6 | 6.0 | 1182.11 | 42.3 | 11.0 | 7.3 |
| Range | 9–54 | 6–35 | 6–30 | 292–5810 | 2.0–245.8 | 10–48 | 10–40 | 9–55 | 6–34 | 6–39 | 301–5575 | 6.6–261.6 | 10–47 | 10–42 |
| Skew | .91 | 2.39 | −.14 | 2.09 | 2.51 | .16 | 2.05 | .98 | 2.28 | −.30 | 2.35 | 2.69 | .38 | 1.93 |
Note.
p < .05
p < .01.
N = 90–100 due to missing data. INQ-TB = Interpersonal Needs Questionnaire-15, Thwarted Belongingness; INQ-PB = Interpersonal Needs Questionnaire-15, Perceived Burdensomeness; IOS-ES = Interpersonal Orientation Scale, Desire for Emotional Support subscale; OXT = oxytocin concentrations in pg/ml, diluted 1:4, unextracted samples; OXT EX = oxytocin concentrations in pg/ml, undiluted, extracted samples; Sex (1 = female, 2 = male); PANASP = Positive and Negative Affect Schedule – Positive Affect subscale; PANASN = Positive and Negative Affect Schedule – Negative Affect subscale.
Group differences in sociodemographic variables and covariates
There were no significant group differences in age, sex, marital status, race, and ethnicity (Table 1). Further, among female participants, there were no group differences in birth control use (χ 2[2]=1.51, p=.47). There was a significant effect of group on negative affect (F[2,95]=15.75, p<.001, ηp2=.25) and no significant effects of time (p=.81) or time by group (p=.11) emerged. Follow-up pairwise comparisons indicated that at pretest, attempters and depressed controls reported significantly higher negative affect than healthy controls and attempters and depressed controls were not significantly difference; at posttest, depressed controls reported higher negative affect than attempters and healthy controls and attempters also reported higher negative affect than health controls. In contrast, there was a significant effect of time on positive affect (F[2,96]=31.96, p<.001, ηp2=.25) and no significant effects of group or time by group (p=.17-.28); follow-up pairwise comparisons indicated that positive affect decreased significantly among suicide attempters and healthy controls only. Thus, pretest and posttest negative affect and change in positive affect (ΔPA=posttest PA–pretest PA) were included as covariates in all multivariate models that examined post-Cyberball changes.
Group differences in clinical symptoms
As expected, lifetime suicide attempt history (ηp2=.91), current suicidal symptom severity (ηp2=.20), and current depression symptom severity (ηp2=.46) were all significantly different across groups (Table 1). Average age of first suicide attempt was 15.2 years (SD=2.27, range=11–20); six participants reported a past-year attempt (18.2%) and of these six, two reported a past-month attempt and two sought medical treatment (e.g., at a hospital) for injuries and/or wounds related to their suicide attempt.
Cyberball manipulation (Table 1)
After playing Cyberball, all participants endorsed feelings of being ignored and excluded (mode=“Agree;” 79.1–86.8%). On average, participants estimated that they received 10.0% of throws and is consistent with previous studies (e.g., mean throws received = 8.3–9.3%; Zadro et al., 2004). No significant group differences in feelings of being ignored (F[2,93]=1.48, p=.23), feeling excluded (F[2,94]=1.29, p=.28) and estimated % of throws received (F[2,94]=1.28, p=.28) emerged.
Primary Analyses
Baseline Group Differences
There were significant group differences in perceived burdensomeness (F[2,97]=12.91, p<.001, ηp2=.21) and thwarted belongingness (F[2,97]=20.66, p<.001, ηp2=.30); in both cases, suicide attempters (TB:|mean difference|=9.44; PB:|mean difference|=3.40) and depressed controls (TB:|mean difference|=15.95; PB:|mean difference| =5.92) had significantly higher scores than healthy controls and suicide attempters and depressed controls were not significantly different. No significant group differences in unextracted and extracted oxytocin concentration and desire for emotional support emerged (ps>.10; Supplemental Table S1).
Oxytocin Concentration (Table 3, Figure 1)
Table 3.
Summary of Mixed-Design ANCOVA Results.
| Outcome Variables | Effect | df | F | p | ηp2 |
|---|---|---|---|---|---|
| Unextracted Oxytocin Concentration | Group | 2,80 | .584 | .560 | .014 |
| Time | 1,80 | 1.750 | .190 | .021 | |
| Group x Time | 2,80 | 4.877 | .010 | .109 | |
| Extracted Oxytocin Concentration | Group | 2,77 | 1.52 | .224 | .038 |
| Time | 1,77 | .34 | .560 | .004 | |
| Group x Time | 2,77 | 1.61 | .207 | .040 | |
| Desire for Emotional Support | Group | 2,96 | .253 | .777 | .005 |
| Time | 1,96 | 5.606 | .020 | .055 | |
| Group x Time | 2,96 | 4.260 | .017 | .082 | |
| Thwarted Belongingness | Group | 2,96 | 22.154 | <.001 | .316 |
| Time | 1,96 | .745 | .390 | .008 | |
| Group x Time | 2,96 | .809 | .448 | .017 | |
| Perceived Burdensomeness | Group | 2,96 | 13.802 | <.001 | .223 |
| Time | 1,96 | .264 | .608 | .003 | |
| Group x Time | 2,96 | .550 | .579 | .011 |
Note. Oxytocin concentration was measured in pg/ml. Desire for Emotional Support was measured using the Interpersonal Orientation Scale. Thwarted Belongingness and Perceived Burdensomeness were measured using the Interpersonal Needs Questionnaire-15. Significant effects in bold.
Mixed-design ANCOVA models with group as the between-subjects measure (3 levels: suicide attempters, depressed controls, healthy controls) and time as the within-subjects measure (2 levels: pre and post social exclusion) were used to evaluate changes in oxytocin concentrations, desire for emotional support, and thwarted belongingness, and perceived burdensomeness. All models included the following covariates: negative affect at pretest, negative affect at posttest, and change in positive affect (PANAS).
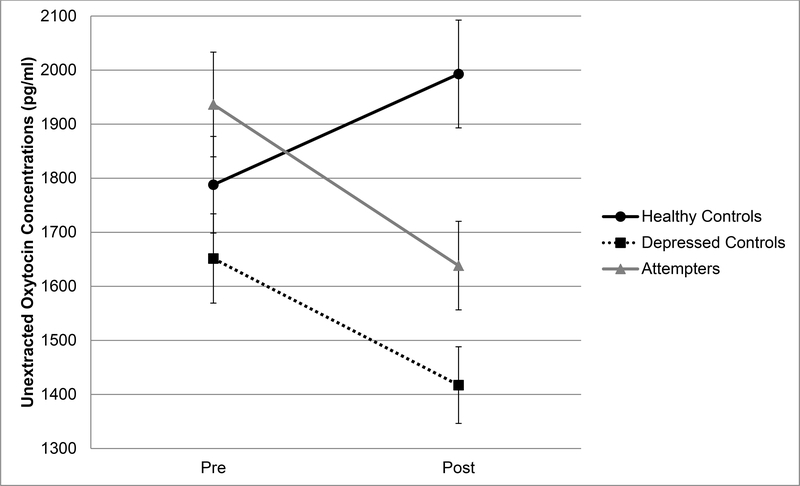
Figure 1.
Changes in plasma, unextracted oxytocin concentrations from pretest to posttest (Cyberball) among suicide attempters, depressed controls, and healthy controls. Samples were diluted 1:4 for measurement and this dilution factor was applied when calculating oxytocin concentrations in plasma from the standard curve. Time between pretest and posttest = 8–10 minutes. Error bars are standard error of the mean.
A significant effect of group by time on unextracted oxytocin concentration emerged beyond covariates (p=.010, ηp2=.11). Post-hoc pairwise comparisons indicated that oxytocin levels significantly decreased among suicide attempters (Wilks’ λ=.943, p=.030, |mean difference|=298.07pg/ml, ηp2=.057) and there were no significant changes in unextracted oxytocin levels among healthy controls (Wilks’ λ=.959, p=.068, |mean difference| =204.81pg/ml) and depressed controls (Wilks’ λ=.978, p=.185, |mean difference|=234.22pg/ml). The main effects of group and time on unextracted oxytocin concentration were not significant (ps>.39). In contrast, the main effects of group and time, and interaction effect of group by time on extracted oxytocin concentration were not significant (ps>.21).
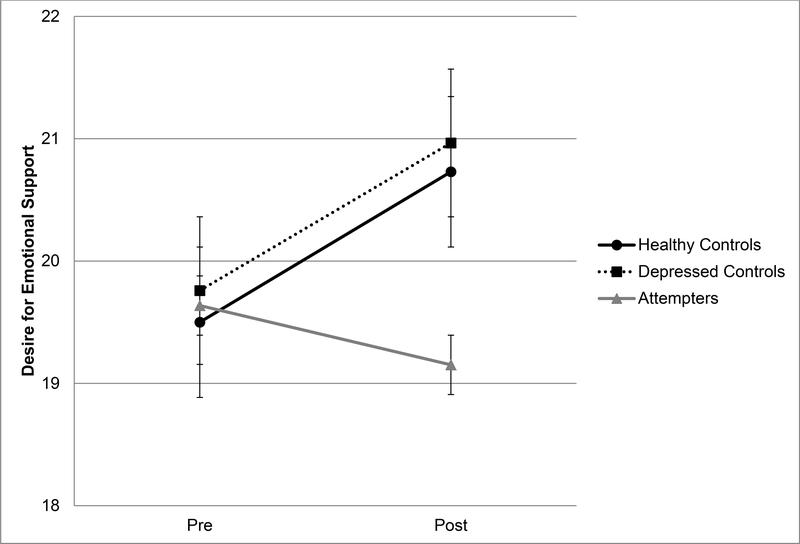
Desire for Emotional Support (Table 3, Figure 2)
Figure 2.
Changes in desire for emotional support from pretest to posttest (Cyberball) among suicide attempters, depressed controls, and healthy controls. Time between pretest and posttest = 8–10 minutes. Error bars are standard error of the mean.
Similarly, there was a significant effect of group by time on desire for emotional support (p=.017, ηp2=.08). Post-hoc pairwise comparisons indicated that desire for emotional support significantly increased among healthy controls (Wilks’ λ=.925, p=.006, |mean difference|=1.27, ηp2=.075) and depressed controls (Wilks’ λ=.946, p=.021, |mean difference|=1.21, ηp2=.054); there was no significant change in desire for emotional support among suicide attempters (Wilks’ λ=.990, p=.318, |mean difference| =.49). There was a significant main effect of time (p=.02, ηp2 =.06) and no main effect of group (p=.78).
Thwarted Belongingness and Perceived Burdensomeness (Table 3)
Significant effects of group on thwarted belongingness (p<.001, ηp2=.32) and perceived burdensomeness (p<.001, ηp2=.22) emerged. At both timepoints, follow-up pairwise comparisons indicated that relative to suicide attempters and depressed controls, healthy controls reported significantly lower levels of thwarted belongingness and perceived burdensomeness, and suicide attempters and depressed controls were not significantly different from each other. However, the main effect of time and interaction effect of group by time were not significant for both theory constructs (ps>.13).
Discussion
This quasi-experimental study examined biological and psychological responses to social exclusion among young adults with suicide attempt histories. Findings were mixed with several notable results pointing to topics for future research.
First, consistent with hypotheses, among suicide attempters, unextracted oxytocin levels decreased; however, desire for emotional support did not significantly change following social exclusion. In contrast, among non-at-risk controls, desire for emotional support increased and unextracted plasma oxytocin levels did not significantly change following social exclusion. That these findings emerged beyond covariates suggests that this pattern of responses to exclusion were not due primarily to differences in mood at the time of assessment. Increases in both unextracted oxytocin and/or desire for emotional support may lead to support-seeking behaviors and protect against the negative effects of exclusion (Bartz et al., 2011; Maner et al., 2007). Thus, decreases in oxytocin and a lack of change in desire for emotional support may negatively impact engagement in meaningful social connections and contribute to social isolation. Recent studies have reported impaired responses to social exclusion in brain regions associated with social cognition among depressed adolescents with non-suicidal self-injury (Groschwitz et al., 2016) and female attempters with depression (Olié et al., 2017). However, no studies to our knowledge have examined oxytocin changes following exclusion among individuals with suicidal symptoms. Our results, pending replication, add to the growing literature showing that at-risk individuals may lack biological and psychological buffers against negative social experiences.
Our findings suggest two probable pathways between social exclusion and suicide risk. One possibility is that at-risk individuals experience more negative social experiences (Van Orden et al., 2010, resulting in altered oxytocin functioning and a lack of change in desire for emotional support, which contributes to greater isolation and suicide risk. This also aligns with Williams’ (2009) theory that individuals who experience prolonged exclusion move to the resignation stage, which is characterized by inability to recover from threatened needs and alienation and heightened negative emotion (Riva et al., 2017). Frequent exclusion experiences, particularly when viewed as irreparable, chronic, and/or pervasive (c.f. multimotive model of interpersonal rejection; Richman and Leary, 2009), are also associated with social withdrawal (Ren et al., 2016). These consequences of prolonged exclusion could all contribute to increased suicide risk. Indeed, a large body of research has found that childhood and adolescent experiences of social exclusion are associated with greater severity of suicidal thoughts and behaviors in adulthood (cf. social rejection, peer victimization; McDougall and Vaillancourt, 2015). A second and compatible possibility is that at-risk individuals are biologically predisposed to showing disruptions in oxytocin functioning and lower desire for support, resulting in non-adaptive responses to social exclusion that contribute to greater isolation and suicide risk. Some evidence indicates genetic bases for disrupted oxytocin release that is associated with suicidal ideation and social alienation (McQuaid et al., 2016).
Importantly, we could not replicate our study findings when we explored our hypotheses using extracted oxytocin. Notably, a similar pattern has emerged in the existing literature with studies examining unextracted oxytocin obtaining significant associations with psychological symptoms (Jokinen et al., 2012; Jobst et al., 2014; 2015) and those using extracted oxytocin reporting nonsignificant results (Deisenhammer et al., 2012). Although measuring changes in oxytocin levels in response to stimuli is more reliable than quantifying oxytocin levels at a single timepoint (MacLean et al., 2019), oxytocin quantification has significant measurement error, with some reporting that unextracted samples are not viable oxytocin measures (Leng and Sabatier, 2016; Szeto et al., 2011), while others claim that extraction removes too much oxytocin (Brandtzaeg et al., 2016). Given this study’s preliminary nature and the scope of this work, we are unable to comment on the debate regarding extraction; replication and refinement of oxytocin detection methods is needed to draw further conclusions. Further, if the unextracted values are reproducible, but not reflecting changes in oxytocin levels, then it will be important for the field to examine the molecular changes associated with that assay measure, as it may reveal mechanisms underlying suicide risk.
Surprisingly, there were no significant correlations between unextracted and extracted oxytocin and self-reported desire for emotional support at either timepoint. This suggests that oxytocin changes may not immediately translate to conscious awareness and/or there may be no direct connection. Consistent with a dual-system, neurobiological model (Carballo et al., 2008), the biological and psychological impact of social exclusion may be distinct but interconnected pathways to suicide. Indeed, Franklin and colleagues’ (2016) meta-analysis reported modest effect sizes for most psychological suicide risk factors, which may be due to emphasis on one system. The nonsignificant relationship between oxytocin and desire for support may also indicate that while the direct effects of social exclusion on oxytocin and desires for support are quick, the indirect effects of oxytocin on desire for support builds over time among at-risk individuals, perhaps with regular engagement in self-injury. Larger studies are needed to evaluate whether oxytocin statistically accounts for group differences in desire for support.
In contrast to Jobst and colleagues’ (2015) study, which found that unextracted oxytocin levels decreased among patients with chronic depression, unextracted oxytocin levels did not significantly change following exclusion among depressed controls. As depressed controls in this study primarily reported mild depression, differences in symptom severity may explain this discrepancy. Additionally, blood samples were collected via catheters threaded through a wall in Jobst and colleagues’ (2014, 2015) study. We were unable to replicate this methodology and relied on in-person venipunctures performed by phlebotomists; it is possible that contact with the experimenter and phlebotomist may have impacted oxytocin levels. Alternatively, given that our results and those of Jobst and colleagues (2014, 2015) among BPD and depressed patients indicated similar oxytocin response patterns across samples often characterized by suicidality, perhaps all three studies reflect the association between suicidal symptoms and diminished oxytocin. As Jobst and colleagues’ studies did not examine suicidality, further work disentangling the relationship between depression severity, suicidality, and unextracted oxytocin is needed. It should be noted, however, that there are other factors that may underlie BPD, depression, and suicidality, and were not examined in this study, such as trauma history (APA, 2013), history of social rejection, and rejection sensitivity (Brown et al., 2019; McGuire et al., 2019; Sato et al., 2019). Given that the participants in this study were young adults, we also cannot rule out the possibility that those in the suicide attempter group later develop chronic depression and/or BPD. Longitudinal research incorporating assessments of overlapping factors and psychological diagnoses may shed light on these differing results.
Furthermore, there were no significant baseline group differences in unextracted and extracted oxytocin levels—this contrasts studies showing that suicidal behavior is associated with lower CSF oxytocin levels (Lee et al., 2009; Jokinen et al., 2012), yet is congruent with Deisenhammer and colleagues’ (2012) study showing no significant plasma oxytocin level differences among recent and non-recent attempters. This discrepancy may be due to methodological variations—this study and Deisenhammer and colleagues’ study both examined plasma oxytocin, while the other two studies measured CSF oxytocin. Interestingly, Jokinen and colleagues (2012) also quantified plasma oxytocin and found similar results between plasma and CSF oxytocin; however, they were not significantly correlated. Unlike peripheral plasma, CSF neuropeptide levels closely reflect the brain’s release patterns (Kagerbauer et al., 2013). Studies comparing plasma and CSF oxytocin among attempters are needed before biological indices of suicidality may be identified.
Consistent with the interpersonal theory of suicide, we found significant group differences in thwarted belongingness and perceived burdensomeness at both timepoints; however, there were no significant changes in these constructs following Cyberball. These constructs can and do vary; however, it is possible that these constructs were not impacted by Cyberball over a 10-minute timespan and change more slowly, with repeated social exclusion. Importantly, this theory hypothesizes that thwarted belongingness and perceived burdensomeness about meaningful social connections contribute to suicidal desire. Cyberball simulates exclusion by strangers; thus, it may not induce the distress that would be associated with exclusion by close others. This possibility has, to our knowledge, yet to be tested; however, there is evidence to suggest that thinking about the loss of deceased significant other more strongly activates pain-related neural regions than thinking about a deceased stranger (e.g. Gündel et al., 2003). Further, despite altering the INQ to evaluate present experiences, the INQ was not validated as a measure of momentary changes in these constructs. Thus, development of a measure of shorter-term changes in the theory constructs is needed.
Limitations should be noted. First, as the post-Cyberball measurement occurred 10 minutes after baseline, we were unable to investigate the long-term effects of social exclusion. Prospective studies are needed to understand the trajectory of these constructs. Second, the oxytocin concentrations for unextracted diluted samples, though within the range of standard curve of the assay, were higher in this study than in Jobst and colleagues’ (2014, 2015) studies. One explanation is that contact between the experimenter/phlebotomist and the participant (compared to obtaining blood samples via a catheter in the wall; Jobst et al., 2015) resulted in higher oxytocin concentrations. It is also possible that our samples were less diluted than Jobst and colleagues’ (2014, 2015) samples; this would place our samples at the higher end of the matrix interference curve, resulting in higher oxytocin concentrations. Unfortunately, dilution factor was not reported in their studies (Jobst et al., 2014; 2015). Additionally, oxytocin levels decrease with age (Elabd et al., 2014)—given that the average age of participants in Jobst and colleagues’ (2015) study was 46 years old, and participants in this study were 20 years old, sample age may explain why our study obtained higher oxytocin levels. Further details regarding Jobst and colleagues’ (2014; 2015) methodology are needed to determine which, if any, of these factors contributed to differences in oxytocin concentration. Third, we did not experimentally induce inclusion; thus, it is unclear whether oxytocin changes among attempters following positive social experiences. Fourth, this study sampled a narrow population with mild-to-moderate symptom severity; therefore, replication in demographically diverse and clinically severe samples is indicated. Such studies may be enhanced by measuring oxytocin and other neuropeptides that regulate social behavior (e.g., vasopressin; Heinrichs et al., 2009) in CSF and plasma. Finally, single timepoint blood draws may not have been sufficiently sensitive to capture potential pulsatile activity in oxytocin (Gan and Quinton, 2010); future studies could consider other markers of secretory dynamics, including pulsatile secretions and pulse amplitude.
Overall, in this quasi-experimental study, suicide attempters had decreased unextracted oxytocin levels and no significant changes in their desire for emotional support following exclusion; however, healthy and depressed controls had increased desire for emotional support and no significant changes in oxytocin. We were unable to replicate this pattern using oxytocin levels from extracted samples and there was no significant association between oxytocin and self-reported social engagement. Beyond improved oxytocin measurement technology, studies using large and diverse samples with measures of CSF and plasma oxytocin may be needed to determine whether biological and psychological suicide risk factors operate on distinct but interconnected pathways and dysregulated oxytocin-related processes biologically predispose individuals to greater social disconnection and suicide risk.
Supplementary Material
Highlights.
Suicide attempters, depressed, and healthy controls react differently to social exclusion
Suicide attempters showed decreased unextracted oxytocin and no changes in extracted oxytocin
Suicide attempters showed no changes in desire for emotional support
Depressed and healthy controls showed no changes in oxytocin and increased desire for emotional support
Acknowledgments
Funding/Disclosures:
This project was supported in part by the Military Suicide Research Consortium (MSRC), an effort supported by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. (W81XWH-10-2-0181, W81XWH-16-2-0003). Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the MSRC, Department of Defense, or the Department of Veterans Affairs. This manuscript was also supported, in part, by a grant from the National Institute of Mental Health (T32 MH093311-04).
Footnotes
Of note, the pattern of results remained the same when covariates were excluded from multivariate models; stronger effect sizes were observed without the inclusion of covariates.
References
- Bartz JA, Zaki J, Bolger N, Ochsner KN, 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci 15(7), 301–309. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Brewer LE, Tice DM, Twenge JM, 2007. Thwarting the need to belong: Understanding the interpersonal and inner effects of social exclusion. Soc. Personal. Psychol. Compass 1(1), 506–520. [Google Scholar]
- Beck AT, Steer RA, 1991. Manual for the Beck Scale for Suicide Ideation. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for The Beck Depression Inventory Second Edition (BDI-II). San Antonio: Psychological Corporation. [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, … Wilson SR, 2016. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci. Rep 6, 31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Mitchell SM, Roush JF, La Rosa NL, Cukrowicz KC, 2019. Rejection sensitivity and suicide ideation among psychiatric inpatients: An integration of two theoretical models. Psychiatry Res. 272, 54–60. [DOI] [PubMed] [Google Scholar]
- Carballo JJ, Akamnonu CP, Oquendo MA, 2008. Neurobiology of suicidal behavior. An integration of biological and clinical findings. Arch. Suicide Res 12(2), 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Klein KM, Buchman‐Schmitt JM, Hom MA, Hagan CR, Joiner TE, 2015. Routinized assessment of suicide risk in clinical practice: an empirically informed update. J. Clin. Psychol 71(12), 1186–1200. [DOI] [PubMed] [Google Scholar]
- Deisenhammer EA, Hofer S, Schwitzer O, Defrancesco M, Kemmler G, Wildt L, Hinterhuber H, 2012. Oxytocin plasma levels in psychiatric patients with and without recent suicide attempt. Psychiatry Res. 200(1), 59–62. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M, 2009. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry 65(9), 728–731. [DOI] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM, 2014. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Comm 5, 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan EH, Quinton R, 2010. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog. Brain. Res 181, 111–126. [DOI] [PubMed] [Google Scholar]
- Groschwitz RC, Plener PL, Groen G, Bonenberger M, Abler B, 2016. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: An fMRI study. Psychiatry Res. Neuroimaging 255, 43–49. [DOI] [PubMed] [Google Scholar]
- Gündel H, O’Connor MF, Littrell L, Fort C and Lane RD, 2003. Functional neuroanatomy of grief: an FMRI study. Am. J. Psychiatry 160(11), 1946–1953. [DOI] [PubMed] [Google Scholar]
- Hames JL, Rogers ML, Silva C, Ribeiro JD, Teale NE, Joiner TE, 2018. A social exclusion manipulation interacts with acquired capability for suicide to predict self-aggressive behaviors. Arch. Suicide Res 22(1), 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G, 2009. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrin 30(4), 548–557. [DOI] [PubMed] [Google Scholar]
- Hill CA, 1987. Affiliation motivation: people who need people… but in different ways. J. Pers. Soc. Psychol 52(5), 1008. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF (1999). Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. J. Clin. Endocrinol. Metab 84(12), 4637–4644. [DOI] [PubMed] [Google Scholar]
- Jobst A, Albert A, Bauriedl-Schmidt C, Mauer MC, Renneberg B, Buchheim A, … Padberg F, 2014. Social exclusion leads to divergent changes of oxytocin levels in borderline patients and healthy subjects. Psychother. Psychosom 83(4), 252–254. [DOI] [PubMed] [Google Scholar]
- Jobst A, Sabass L, Palagyi A, Bauriedl-Schmidt C, Mauer MC, Sarubin N, … Padberg F, 2015. Effects of social exclusion on emotions and oxytocin and cortisol levels in patients with chronic depression. J. Psychiatr. Res 60, 170–177. [DOI] [PubMed] [Google Scholar]
- Joiner T (2005). Why people die by suicide. Cambridge, MA: Harvard University Press. [Google Scholar]
- Jokinen J, Chatzittofis A, Hellström C, Nordström P, Uvnäs-Moberg K, Åsberg M, 2012. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 37(4), 482–490. [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R, 2013. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrin 25(7), 668–673. [DOI] [PubMed] [Google Scholar]
- Kojima S, Stewart RA, Demas GE, Alberts JR, 2012. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother–pup interaction that induces a filial huddling preference. J. Neuroendocrin 24(5), 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF, 2009. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology 34(10), 1567–1573. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shamay-Tsoory S, Han S, Zink CF, 2016. Oxytocin and social adaptation: insights from neuroimaging studies of healthy and clinical populations. Trends. Cogn. Sci 20(2), 133–145. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Wilson S, Martin WL, Davis JM, Nazarloo HP and Carter CS, 2019. Challenges for measuring oxytocin: The blind men and the elephant?. Psychoneuroendocrinology 107, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, DeWall CN, Baumeister RF, Schaller M, 2007. Does social exclusion motivate interpersonal reconnection? Resolving the” porcupine problem.” J. Pers. Soc. Psychol 92(1), 42. [DOI] [PubMed] [Google Scholar]
- McDougall P and Vaillancourt T, 2015. Long-term adult outcomes of peer victimization in childhood and adolescence: pathways to adjustment and maladjustment. Am. Psychol 70(4), 300. [DOI] [PubMed] [Google Scholar]
- McGuire TC, McCormick K, Koch MK, Mendle J, 2019. Pubertal Maturation and Longitudinal Trajectories of Depression During Early Adolescence. Front. Psychol 10, 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, Anisman H, 2016. Oxytocin and social sensitivity: gene polymorphisms in relation to depressive symptoms and suicidal ideation. Front. Hum. Neurosci 10, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Holmberg EB, Photos VI, Michel BD (2007). Self-Injurious Thoughts and Behaviors Interview: Development, reliability, and validity in an adolescent sample. Psychol. Assess 19(3), 309–317. [DOI] [PubMed] [Google Scholar]
- Ren D, Wesselmann E, Williams KD, 2016. Evidence for another response to ostracism: Solitude seeking. Soc. Psychol. Pers. Sci 7(3), 204–212. [Google Scholar]
- Richman SL, Leary MR, 2009. Reactions to discrimination, stigmatization, ostracism, and other forms of interpersonal rejection: a multimotive model. Psychol. Rev 116(2), 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva P, Montali L, Wirth JH, Curioni S, Williams KD, 2017. Chronic social exclusion and evidence for the resignation stage: An empirical investigation. J. Soc. Pers. Relatsh 34(4), 541–564. [Google Scholar]
- Robins E, 1981. The final months: A study of the lives of 134 persons who committed suicide. New York, NY: Oxford University Press. [Google Scholar]
- Sato M, Fonagy P and Luyten P, 2019. Rejection Sensitivity and Borderline Personality Disorder Features: The Mediating Roles of Attachment Anxiety, Need to Belong, and Self-Criticism. J. Pers. Disord 1–16. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z, 2014. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76(4), 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, … Mendez AJ, 2011. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med 73(5), 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE, 2006. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med 68(2), 238–245. [DOI] [PubMed] [Google Scholar]
- Van Orden KA, Cukrowicz KC, Witte TK, Joiner TE Jr, 2012. Thwarted belongingness and perceived burdensomeness: Construct validity and psychometric properties of the Interpersonal Needs Questionnaire. Psychol. Assess 24(1), 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Pers. Psychol 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Williams KD, 2009. Ostracism: Effects of being excluded and ignored In Zanna MP (Ed.), Advances in experimental social psychology (Vol. 41, pp. 275–314). New York: Academic Press. [Google Scholar]
- Williams KD, Jarvis B, 2006. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav. Res. Methods 38(1), 174–180. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R, 2004. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J. Exp. Soc. Psychol 40(4), 560–567. [Google Scholar]
- Zhang G, Cai D, 2011. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Phys. Endocrinol. Metab 301(5), E1004–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.