Abstract
Background:
Use of routine HIV programme data for surveillance is often limited due to inaccuracies associated with patient misclassification which can be addressed by unique patient identification. We assessed the feasibility and acceptability of integrating an iris recognition biometric identification system into routine HIV care services at 4 sites in Kenya.
Methods:
Patients who had recently tested HIV-positive or were engaged in care were enrolled. Images of the iris were captured using a dual-iris camera connected to a laptop. A prototype iris biometric identification system networked across the sites, analysed the iris patterns; created a template from those patterns; and generated a 12-digit ID number based on the template. During subsequent visits, the patients’ irises were re-scanned, and the pattern was matched to stored templates to retrieve the ID number.
Results:
Over 55 weeks 8,614 (98%) of 8,794 new patients were assigned a unique ID on their first visit. Among 6,078 return visits, the system correctly re-identified patients’ IDs 5,234 times (86%). The false match rate (a new patient given the ID of another patient) was 0·5% while the generalized false reject rate (re-scans assigned a new ID) was 4·7%. Overall, 9 (0·1%) agreed to enrol but declined to have an iris scan. The most common reasons cited for declining an iris scan were concerns about privacy and confidentiality.
Conclusion:
Implementation of an iris recognition system in routine health information systems is feasible and highly acceptable as part of routine care in Kenya. Scale-up could improve unique patient identification and tracking, enhancing disease surveillance activities.
Keywords: Biometrics, Patient identification, HIV, Implementation science, Kenya, Iris
1. Introduction
Unique patient identification is an integral part of healthcare service delivery. Correctly identifying patients is critical when undergoing clinical procedures, reporting of test and procedure results, as well as managing administrative functions such as scheduling and billing [1–3]. Unique identification of individuals is essential in case-based disease surveillance. In HIV surveillance, for example, it is impossible to know if the UNAIDS 90-90-90 targets [4] have been achieved if individuals are not uniquely identified and tracked through the care continuum.
The continuum of HIV care spans across multiple access points within the healthcare system. These include HIV counselling and testing (HTC), care and treatment, antiretroviral therapy (ART) dispensing at the pharmacy, laboratory monitoring and other supportive services. Recent evidence suggests that improving the HIV care cascade and reducing loss to follow-up at each stage is critical for control and reduction of the global HIV epidemic [5]. Patients who default from care after testing HIV-positive are unable to receive the care and treatment required to control their infections, thereby failing to achieve viral suppression. Throughout sub-Saharan Africa (SSA), estimates indicate that less than 33% of HIV-infected patients are engaged in care from their date of diagnosis till when they begin ART [6,7], and even thereafter [8–10].
Poor retention in HIV care is compounded by patient relocation or change of health facilities, where patients may change their primary healthcare facility and not be truly “lost to follow-up” [11]. In the West, almost half of HIV patients lost to follow-up had relocated and remained engaged in care [12]. In Africa, studies show that a sizeable proportion of those classified as “lost-to follow-up” can be tracked to identify their vital status [13–16]. However, these studies have not described the proportion or characteristics of those who have been found to seek care from other facilities. Access to information from these multiple care settings and the retrieval and assembly of relevant patient care information from past episodes of care across different times is required not only for provision of quality healthcare services but also for accurate and timely surveillance. Linkage of this patient care information requires the use of a unique patient identifier [17].
Globally, unique patient identification within health services is an operational challenge. Many HIV care and treatment programs use traditional text-based matching for patient identification, including personal identifiers such as name, date of birth, and clinic-issued numbers. This approach identifies patients by what they know or possess (e.g., medical ID cards) as opposed to identifying them by who they are; and is often unreliable and inaccurate [18,19]. Text-based matching is often associated with multiple registration: one patient may have multiple IDs or one ID may be associated with multiple individuals or incomplete registration. Misidentification not only compromises patient care at the individual level but also limits utilization of surveillance data from routine programme data due to inaccuracies [20]. Double-registration could lead to overestimation of HIV incidence, with HIV re-testers being counted as newly diagnosed cases. It could lead to misclassification of HIV patients as being “lost to follow up” if they have only changed their care provider; yet tracing these subjects’ demands significant amounts of effort by healthcare providers, reducing efficiency in already overburdened health facilities [21]. It is therefore imperative to develop and implement unique patient identifiers to improve both longitudinal and geographical patient information linkage [22,23].
Biometric identification is recognised as one of the six unique patient identifier options by the US Health & Human Services department [24]. Others include the social security number, personal number based on bank card method and unique identifier based on personal immutable properties. These have limitations for use in healthcare, chiefly being the need for federal privacy legislation against unauthorized access and misuse of patient information as they are used beyond healthcare. In Kenya, all adults above 18 years are expected to have a national ID number, based off finger prints. However, this administrative number is not a requisite to receiving healthcare services and not everyone obtains one.
Use of biometrics for unique identification is rapidly growing in low and middle-income countries (LMIC). According to the Centre for Global Development, a significant percentage of large-scale biometrics initiatives are in LMIC [25]. In sub-Saharan Africa as of 2013, use of biometrics for identity authentication was largely in elections, followed by social/cash transfers and thirdly in health [26]. Iris scanning, a biometric identifier, has great potential for integration with health information systems [27]. A large-scale iris identification system, especially if launched in combination with a patient registry and electronic medical record system, would allow for subjects to move more naturally through the health system, be recognized at any facility, and receive the care they need then rather than only at their “home” facility. Given the global burden and the stigma associated with HIV, understanding the uptake and performance of biometrics in this setting is important in assessing the utility of such technology.
We therefore assessed the feasibility and acceptability of integrating an iris recognition system into routine HIV testing and counselling (HTC) and linkage to HIV comprehensive care and treatment clinics (CCC) in Kenya.
2. Methods
2.1. Study design
This was a longitudinal study where iris scanning was used to uniquely identify individuals at study enrolment and at their routine clinic follow-up visits.
2.2. Study setting and population
The study was conducted in four high-volume hospital facilities in Kenya. Kenyatta National Hospital (KNH) and Kiambu District Hospital are located in Central Kenya, serving largely an urban-poor and peri-urban population respectively, while Kisumu East County Hospital and Kombewa Sub-County Hospital are located in Western Kenya with largely rural catchment populations.
We recruited two populations: individuals newly testing HIV-positive at ambulatory testing sites and individuals known to be living with HIV and engaged in care at the four health facilities. Participants were ≥ 18 years and those enrolled in other research studies were excluded. Participants were enrolled continuously from February 2015 to February 2016.
2.3. Procedures and follow-up of study participants
Potential participants were informed of the study by HTC counsellors and were taken through a scripted oral consent process. The consenting process emphasized the participants’ voluntary participation and that opting out would not affect their clinical care. An electronic version of the national HTC case report form used as part of standard care to assess patient risk factors and reasons for testing was administered by HTC counsellors. A detailed description of this case report form has been published previously [28]. Data was collected on tablets using custom-designed Open Data Kit forms [29]. Two versions of the HTC case report form were used; one for those who had just tested HIV-positive and another version for those known to be HIV-positive and already engaged in care. This information was encrypted and uploaded, via a secure connection, into a registry housed at the National AIDS & STI Control Programme (NASCOP). Once encrypted, the HTC counsellors on site no longer had access to identifiable patient information. The case report form (CRF) was only administered at intake. Only their names and telephone numbers were collected at subsequent visits as existing identifiers to validate the iris biometric ID.
2.3.1. Iris scanning and assignment of a unique identifier
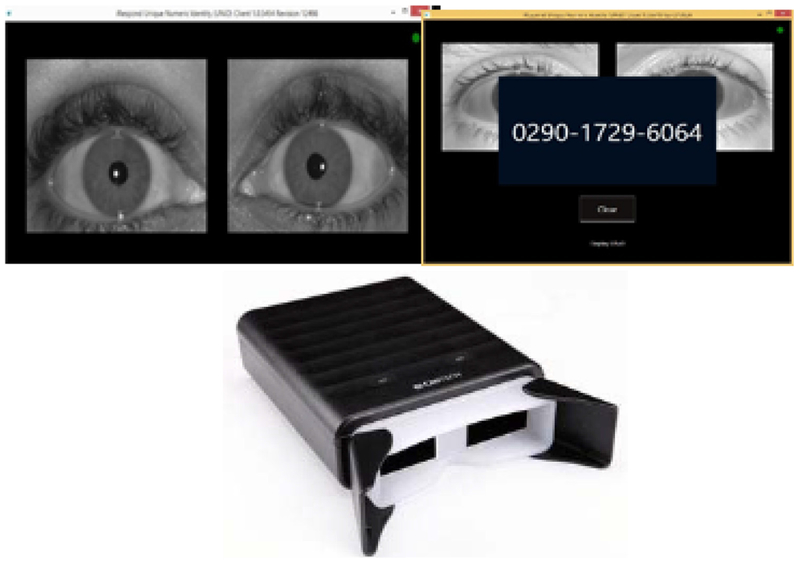
Binocular iris recognition cameras from CMITech® (model BMT-20) connected via USB to laptops were used to capture iris images. A proprietary digital identity solution by iRespond®, an international non-profit organization, was networked across the study sites to analyse the iris patterns. The software deconstructed the iris into a bit pattern and created a template. Features extracted included striation pattern, contrast ratio (between sclera and surrounding skin tone) and differences between right and left eyes. The template was then paired with a 12-digit string of randomly generated numbers to form a unique numeric ID for identification (one-to-many template matching) (Fig. 1). This template was stored on the local laptop and uploaded to a secure server. When a patient was re-scanned, the system matched the iris pattern by searching against templates stored on the laptop first. If a match was not obtained locally, the system then searched the aggregate templates from all the enrolment sites on the server for verification (one-to-one template matching). For newly tested participants, their irises were re-scanned, and the pattern matched to stored templates to retrieve the ID number if they linked to care at the CCC within the study hospital. Those who were already engaged in care were re-scanned and their ID number retrieved at any subsequent visit for routine care. This ID was then manually keyed into the electronic data collection form.
Fig. 1.
Iris Scan, Camera and ID generation as it appears on screen.
2.3.2. Assessment of feasibility and acceptability
Feasibility was assessed from two dimensions: infrastructure and human resource requirements for set-up and implementation of the system; marked by staff technological competence and average time spent per patient intake. Two biometric stations (camera, laptop, tablet and internet WiFi dongle) were set up in each hospital; one at the HIV testing service delivery point and another at the HIV care clinic. Each biometric station was staffed by one HTC counsellor. Their average education level was diploma training and they were all conversant with use of smartphones/tablets. Training on the set-up, iris scanning, and set-down process was conducted over two days. Additional training on electronic data collection was over 2 days
The second dimension was the system performance as measured by hardware failures indicated by internet failure, camera failure or laptop malfunction and system accuracy as determined by the system false acceptance and false rejection rates. False acceptance was defined as issuance of an already existent ID to a new or different patient while false rejection was defined as issuance of a new ID to a client who had already been issued with a unique ID.
Acceptability was defined as the number of participants agreeing to iris scanning as a proportion of all the individuals approached. General reasons for declining iris scanning were elicited.
2.4. Ethical approval
Ethical review and approval was obtained from the University of Washington Institutional Review Board and the Kenyatta National Hospital-University of Nairobi Ethical Review Committee.
3. Results
3.1. Demographics
The study enrolled 8794 unique HIV-infected patients who agreed to have a biometric iris scan. 1136 had newly tested HIV-positive, while 7658 knew their HIV status and were already in care. Their demographic characteristics are summarized in Table 1. Overall, 5663 (66%) were female; median age was 35 years [Interquartile Range (IQR): 29, 42]; 3.456 (39%) were from the rural facilities and 1136 (13%) were enrolled into the study at the time they received their HIV diagnosis for the first time (newly testing positive).
Table 1.
Demographic characteristics of participants by region.
| Characteristic | Rural (n = 3456) Median (IQR) or n (%) |
Urban (n = 5338) Median (IQR) or n (%) |
|---|---|---|
| Age | 33 (27-41) | 36 (30-42) |
| Sex: Female | 2,190 (65) | 3473 (66) |
| Pregnant (females) | 398 (18) | 491 (14) |
| Circumcised (males) | 362 (31) | 1554 (86) |
| Time when tested | ||
| Newly testing positive | 486 (14%) | 650 (12%) |
| Known positive | 2970 (86%) | 4688 (88%) |
| Marital Status | ||
| Single | 320 (10%) | 764 (15%) |
| Married Monogamous | 1822 (54%) | 2914 (55%) |
| Married Polygamous | 474 (14%) | 143 (3%) |
| Live-in Partner | 6 (1%) | 288 (5%) |
| Divorced | 138 (4%) | 630 (12%) |
| Widow/Widower | 590 (17%) | 546 (10%) |
| Initiated HIV test | ||
| Provider-Initiated Testing | 1940 (58%) | 3488 (66%) |
| Client-Initiated Testing | 1410 (42%) | 1797 (34%) |
| Tested for HIV previously | 697 (21%) | 2124 (40%) |
| Tested as a couple | 541 (16%) | 1026 (19%) |
| Discordant couple | 117 (26%) | 355 (41%) |
| TB Suspect | 140 (4%) | 112 (2%) |
| TB-infected | 491 (15%) | 822 (16%) |
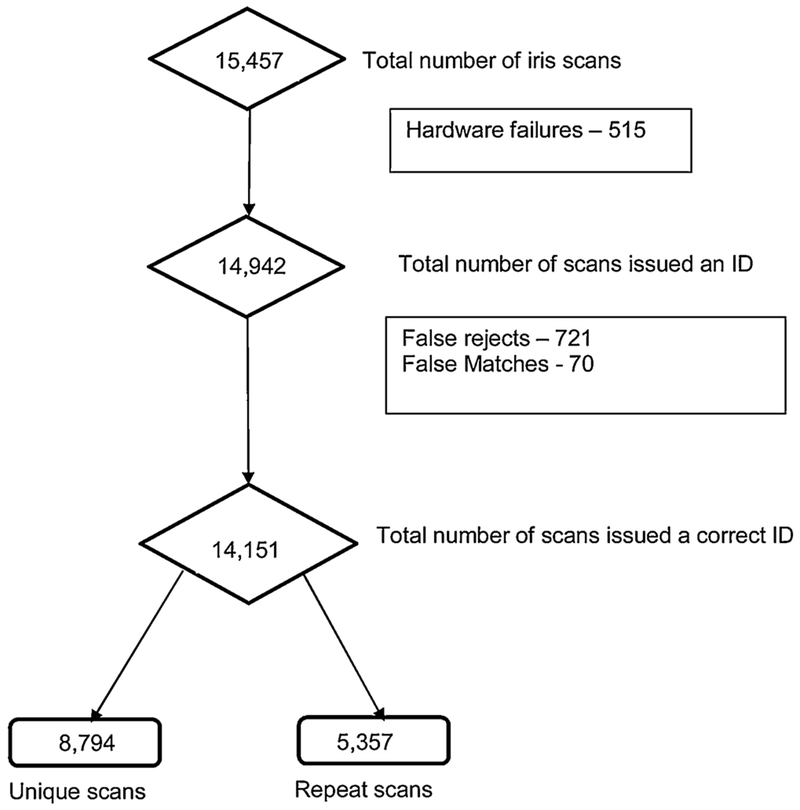
During subsequent clinic visits 4242 had one re-scan; 1262 had 2 re-scans and 574 were re-scanned 3 or more times, bringing the total number of iris scans conducted over the entire study to 15,457.
Of the 1136 newly testing, 522 (46%) linked to care at the same CCC; while 2 were identified having linked to care at a different site within the 4 study sites. Two patients presenting as newly testing for HIV were identified at the testing points as re-testers having been previously issued a unique ID at one of the 4 study sites.
3.2. Acceptability
Of the 8894 patients approached, 100 (1%) declined to be enrolled in the study. Among those enrolled, 13 (0·14%) accepted to have their data collected electronically but declined to have the iris biometric scan. Four of these individuals later agreed to have an iris scan on a subsequent visit. There was no difference in age or gender comparing those who accepted iris scanning to those who declined.
Reasons for declining enrolment into the study were privacy concerns, as some clients did not feel safe about their medical details being transmitted via the internet and others expressed concern about confidentiality and accidental disclosure if records were accessed by other people. Another reason for declining was the lack of time; some believed the entire process would take too much of their time and they were in a hurry. For those who had just tested HIV positive, some declined study enrolment as they were in shock upon learning their HIV status and requested more time to process the results.
Of the 9 who enrolled in the study and then specifically declined iris scanning, 3 declined over privacy concerns; one cited religious/cultural concerns; one did not understand the technology; two were scared of the biometric camera; and one person had an eye problem and feared that the scan would exacerbate it. One person did not have any specific reason for declining.
3.3. Implementation
3.3.1. Time to complete iris scanning
The average time taken to fill the CRF, conduct the iris scan and obtain a unique ID, fill the ID on the CRF and send the encrypted form for an enrolment visit across all the sites was 6·4 min [IQR: 4·0, 15·7]. There was improvement in time taken as the HTC counsellors grew accustomed to the procedures, as shown in Fig. 2.
Fig. 2.
Time to complete iris scanning.
The average time taken to conduct an iris scan and retrieve a previously issued unique ID (re-scan), fill the CRF and send the form for a re-visit across all the sites was 3·5 min. [IQR: 1·8, 5·8]. This retrieval time remained constant over the duration of the study despite the increase in size of the iris template database.
3.3.2. System performance
Of the 15,457 scans, the system issued a unique ID (both identification and verification) 14,942 times (96·7%). Of these instances, 14,151 were the correct IDs. The system’s sensitivity was therefore 94·7% (Fig. 3).
Fig. 3.
System Performance.
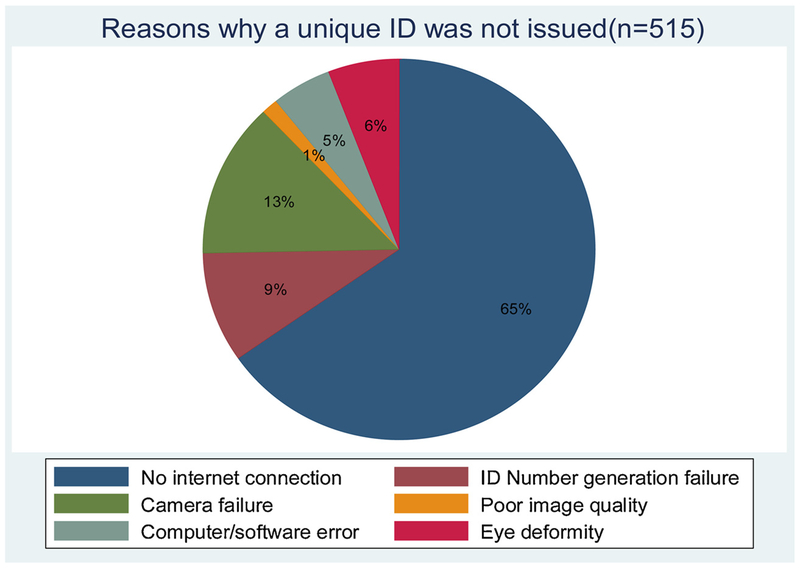
Issues responsible for failure of issuance of a unique ID are summarized in Fig. 4, with internet failure being the main one. Issuance of an already existent ID to a new patient (false match rate) was 0·5% while the failure of the system to recall an existent ID (false reject rate) was 4·8%. These identification errors improved with time as the software algorithm was improved to match the populations’ irises.
Fig. 4.
Reasons why a unique ID could not be issued.
4. Discussion
Iris biometric scanning was highly acceptable in both rural and urban settings in Kenya with 8794 unique patients accepting iris scanning at least once and 6663 repeat scans obtained over the study period. While high acceptability of biometric identification has been noted among HIV-infected populations, this has often been described in key populations and clinical research contexts [30,31]. Our high acceptability rate (98·9%) was remarkable since the project was integrated within routine programmatic service delivery of HIV testing and comprehensive care and treatment services. It was even higher than was observed in a HIV clinic in Los Angeles, California where upon survey, 72% of patients reported they would accept fingerprint biometrics for identification during their routine clinic visit [30,31]. Compared to fingerprints, iris scanning may be more acceptable in our setting because it is not used in any civil or government identification processes (e.g. Police records), thus eliminating fear of being arrested [32]. The high acceptability of multiple re-scans during clinic re-visits in our study shows that patients are willing to have their iris biometric data used for authentication during a clinic visit. To our knowledge, this is the first study in Africa demonstrating use of iris recognition in routine healthcare services. This is novel since previous use of biometrics for patient identification in SSA have been limited to clinical trials and research settings; highlighting that the HIV program in Kenya may be ready for biometrics use for unique patient identification to support case-based surveillance which remains a priority for PEPFAR [33].
Our low study participation refusal rate of 1·1% is comparable to other studies even in the US and Europe [34]. Among the few who declined to have an iris scan (9 out of 8794), the main reason cited was privacy and confidentiality concerns as data would be transmitted over the internet. This may have been exacerbated by the obvious WiFi dongle attached to the laptop during enrolment. Such dongles are commonly used for internet access locally and may have been associated with uses such as email or Facebook. For those who did not understand the technology or were afraid of the biometric camera, use of less intimidating gadgets (e.g. goggles or smartphones) could address their concerns.
Furthermore, our high biometric system sensitivity/accuracy (95%) is comparable to other studies using iris biometrics [34] and is higher than the sensitivity observed with fingerprint biometrics in Ghana (68·7%) and Uganda (75.5%) [35,36]. While the average system fail rate was low (5.3%), this inaccuracy was higher in the early study phase and declined over time. This was because in the initial software development stage, all identity was set at the lowest matching thresholds. However, participants in this study tended to have high image contrast between the sclera (white area) of the eye and the surrounding skin tone. This led to a high proportion of new identities generated for existing participants (false rejects), demonstrating a need for biometric tuning of the rejection and matching parameters. Tuning included adjustments to image quality rejection due to out of range contrast ratios, to limit new ID generation for re-scanning participants. By reviewing these early issues and adjusting the software, the accuracy was continuously improved throughout the study. This is one of the sentinel studies in Africa demonstrating such high levels of biometric identification system accuracy outside of a controlled trial context, showing that such systems can feasibly be optimized for use in ‘real world’ healthcare settings.
Despite the small scale of the project (4 sites), we demonstrate the utility of a unique identifier in improving program data inaccuracies. By identifying two re-testers posing as first-time testers, the biometric identification allowed them to be correctly classified as re-testers. In the absence of the iris identification system, the two patients who linked to different CCCs from their testing sites could as well have been classified as having failed to link to care. If used at-scale, this system allows for correct patient classification, improving the accuracy of programme data and could potentially support case-based HIV surveillance.
By the end of the study, in sites with a strong internet signal, the average time required for enrolment for a newly tested HIV-infected individual was 4·6 min, with the actual image capture and template generation taking about 20 s. This was even shorter during re-visits where only the biodata was collected. This is comparable to another study using fingerprint biometrics in Ghana that had an average of 7 min for a new enrolment [35]. Although ours was longer than the 2 min Corby et al observed in Brazil using iris scanning [34], the length of their questionnaire and other enrolment procedures asides from ID assignment are not described. Unlike fingerprint scanning which sometimes requires scanning of multiple fingers to improve sensitivity; the iris scanning is faster as it is typically done once. A short transaction time (time taken to issue an ID) is important as the additional waiting time introduced into the system by use of the iris recognition time is minimal. In our case, it could be enhanced by boosting the internet signal to ensure constant internet connectivity.
Some important limitations need to be considered. Though small, the false negative identification error rates (4·7%) could cause duplication of patient counts while the false positive errors could lead to patients sharing IDs. Security questions incorporating other text unique identifiers e.g. national ID number could be added to the data input system as options to facilitate confirmation of matching without compromising identity. ID transcription errors (from screen to electronic medical record system) could also be eliminated by incorporating automated ID transfer technologies, such as bluetooth transfer or onscreen scanning of data matrix from biometric software. Given that most of the system issues were attributable to lack of consistent internet connectivity particularly in rural areas, this could be improved by use of a system with offline functionality.
5. Conclusion
Our findings of high acceptability and system performance integrated within routine healthcare services demonstrate that use of iris biometrics for unique patient identification in routine HIV programmes is feasible.; supporting the UNAIDS position that “unique individual identifiers will strengthen fragmented health services in countries by linking data held within facilities and enabling the flow of information across the general health system and thereby also enhancing the quality, comprehensiveness and continuity of HIV-specific services” [27]. Our findings demonstrate potential for iris scanning to be scaled up as a unique patient identifier that can be effectively linked to electronic medical records and enhance individual tracking within the HIV care continuum, even in resource-limited settings.
Summary points.
As the world focuses on universal health coverage, there has been significant investment in electronic health information systems in Sub-Saharan. Data from such systems and repositories including PEPFAR’s Data for Accountability Transparency Impact Monitoring (DATIM) remain underutilized due to inaccuracies in patient identification and classification.
Unique patient identification within such systems is critical in addressing inaccuracies associated with patient misidentification thus improving the use of routine health programme data for surveillance.
This implementation study assessed the acceptability (uptake) of an iris recognition biometric system as well as its function in uniquely identifying patients within routine HIV testing and treatment programs in four large health facilities in Kenya.
About 99.9% of 8800 patients agreed to have an iris scan to obtain a unique ID, with 86% correctly re-identified during a repeat visit.
Considered alongside existing data, our findings imply that iris recognition biometric systems are widely acceptable and can be implemented within routine clinical care settings.
Our findings demonstrate potential for iris scanning to be scaled up as a unique patient identifier that can be effectively linked to electronic medical records to improve unique patient identification and tracking; enhancing disease surveillance activities in settings with enormous need and limited resources.
Acknowledgments
We would like to acknowledge all the patients and healthcare workers who participated in the study. Special thanks to Peter Muiruri (KNH), David Kariuki (Kiambu), Sam Otedo (Kisumu), who allowed us access to the health facilities and supported us to implement the study.
Funding
This study was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health grant number A1099974 S1.
Footnotes
Declaration of Competing Interest
The authors have no conflict of interest to declare.
References
- [1].Abraham P, et al. , Descriptive analysis of patient misidentification from incident report system data in a large academic hospital federation, J. Patient Saf 0 (00–00) (2018) 1–7, 10.1097/PTS.0000000000000478. [DOI] [PubMed] [Google Scholar]
- [2].Campbell K, et al. , Improving quality and safety through positive patient identification, Healthc. Q 18 (3) (2015) 56–60. [DOI] [PubMed] [Google Scholar]
- [3].Sandhu P, et al. , Effectiveness of laboratory practices to reducing patient misidentification due to specimen labeling errors at the time of specimen collection in healthcare settings: LMBP systematic review, J. Appl. Lab. Med 2 (2) (2017) 244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McMahon JH, Medland N, 90-90-90: how do we get there? Lancet HIV 1 (1) (2014) e10–1. [DOI] [PubMed] [Google Scholar]
- [5].McNairy ML, El-Sadr WM, The HIV care continuum: no partial credit given, Aids 26 (14) (2012) 1735–1738. [DOI] [PubMed] [Google Scholar]
- [6].Rosen S, Fox MP, Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review, PLoS Med. 8 (7) (2011) el001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mugglin C, et al. , Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis, Trop. Med. Int. Health 17 (12) (2012) 1509–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ojwang VO, et al. , Loss to follow-up among youth accessing outpatient HIV care and treatment services in Kisumu, Kenya, AIDS Care 28 (4) (2016) 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Braitstein P, et al. , Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya, Trop. Med. Int. Health 15 (7) (2010) 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keane J, et al. , Interventions to reduce loss to follow-up during all stages of the HIV care continuum in Sub-Saharan Africa: a systematic review, AIDS Behav. 21 (6) (2017) 1745–1754. [DOI] [PubMed] [Google Scholar]
- [11].Camlin CS, et al. , Gendered dimensions of population mobility associated with HIV across three epidemics in rural Eastern Africa, Health Place 57 (2019) 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buskin SE, et al. , Migration distorts surveillance estimates of engagement in care: results of public health investigations of persons who appear to be out of HIV care, Sex. Transm. Dis 41 (1) (2014) 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yiannoutsos CT, et al. , Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in Western Kenya, PLoS One 3 (12) (2008) e3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Geng EH, et al. , Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa, Trop. Med. Int. Health 15 (Suppl. 1) (2010) 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Geng EH, et al. , Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study, Lancet HIV 2 (3) (2015) e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McMahon JH, et al. , Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review, PLoS One 8 (2) (2013) e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bittle MJ, Charache P, Wassilchalk DM, Registration-associated patient misidentification in an academic medical center: causes and corrections, Comm. J. Qual. Patient Saf 33 (1) (2007) 25–33. [DOI] [PubMed] [Google Scholar]
- [18].Harichund C, Haripersad K, Ramjee R, Participant verification: prevention of co-enrolment in clinical trials in South Africa, S. Afr. Med. J 103 (7) (2013) 491–493. [DOI] [PubMed] [Google Scholar]
- [19].Grannis SJ, Overhage JM, McDonald C, Real world performance of approximate string comparators for use in patient matching, Stud. Health Technol. Inform 107 (Pt 1) (2004) 43–47. [PubMed] [Google Scholar]
- [20].Waruru A, et al. , Where No universal health care identifier exists: comparison and determination of the utility of score-based persons matching algorithms using demographic data, JMIR Public Health Surveill. 4 (4) (2018) e10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].W.W.H. Organization, Retention in HIV programmes: defining the challenges and identifying solutions, World Health Organization Meeting Report September 2011, WHO, Geneva, Switzerland, 2011. [Google Scholar]
- [22].Ferguson C, et al. , The wicked problem of patient misidentification: how could the technological revolution help address patient safety? J. Clin. Nurs 28 (13–14) (2019) 2365–2368, 10.1111/jocn.14848. [DOI] [PubMed] [Google Scholar]
- [23].Beck EJ, et al. , Developing and implementing national health identifiers in resource limited countries: why, what, who, when and how? Glob. Health Action 11 (1) (2018) 1440782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Services, T.D.o.H.a.H, Appavu SI, Analysis of Unique Patient Identifier Options: Final Report, 1997. November 24.
- [25].Mansfield-Devine S, Biometrics in developing countries, Biom. Technol. Today 2015 (4) (2015) 5–8. [Google Scholar]
- [26].Caldwell T, Biometrics in the developing world, Biom. Technol. Today 2013 (5) (2013) 5–8. [Google Scholar]
- [27].UNAIDS, Developing and Using Individual Identifiers for the Provision of Health Services Including HIV: Proceedings From a Workshop, (2009) 24–26 February: Montreux, Switzerland. [Google Scholar]
- [28].Cherutich P, et al. , Surveillance of HIV assisted partner services using routine health information systems in Kenya, BMC Med. Inform. Decis. Mak 16 (2016) 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Steiner A, et al. , Managing research and surveillance projects in real-time with a novel open-source eManagement tool designed for under-resourced countries, J. Am. Med. Inform. Assoc 23 (5) (2016) 916–923. [DOI] [PubMed] [Google Scholar]
- [30].Wall KM, et al. , Implementation of an electronic fingerprint-linked data collection system: a feasibility and acceptability study among Zambian female sex workers, Glob. Health 11 (2015) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cohen JK, et al. , Acceptability of Fingerprint Scanning for Personal Identification Among Patients Seeking HIV/STI-related Services, Los Angeles, 2011, J Acquir Immune Defic Syndr, 2012, pp. e59–60 United States. [DOI] [PubMed] [Google Scholar]
- [32].Verbeke F, Van Bastelaere S, Nyssen M, Patient identification and hospital information management systems in Sub-Saharan Africa: a prospective study in Rwanda and Burundi, Rwanda Medical Journal 69 (4) (2012) 7–12. [Google Scholar]
- [33].PEPFAR, U.S.D.o. State (Ed.), Strategy for Accelerating HIV/AIDS Epidemic Control (2017-2020), Office of the U.S. Global AIDS Coordinator and Health Diplomacy, U.S. Department of State, Washington D.C, 2017. [Google Scholar]
- [34].Corby PM, et al. , Using biometrics for participant identification in a research study: a case report, J. Am. Med. Inf. Assoc.: JAMIA 13 (2) (2006) 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Odei-Lartey EO, et al. , The application of a biometric identification technique for linking community and hospital data in rural Ghana, Glob. Health Action 9 (2016) 29854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].White EB, et al. , Feasibility, acceptability, and adoption of digital fingerprinting during contact investigation for tuberculosis in Kampala, Uganda: a parallel-convergent mixed-methods analysis, J. Med. Internet Res 20 (11) (2018) e11541. [DOI] [PMC free article] [PubMed] [Google Scholar]