Abstract
Background
Reports regarding the causative drugs of drug-induced cutaneous adverse reactions in China are indistinct, such that different regions have reported the spectrum of drugs differs substantially in different clinical conditions.
Objective
To explore the causative drugs that led to cutaneous reactions.
Methods
Adverse drug reaction reports from central China were collected and divided into cutaneous adverse reactions and severe cutaneous adverse reactions groups. Cases were reviewed retrospectively for causative drugs.
Results
The male:female ratio was equal in both cutaneous adverse reactions and severe cutaneous adverse reactions. In cutaneous adverse reactions (n = 482), the highest incidence happened between 51 and 60 years of age and the top three causative drugs were antibiotics (48%), Chinese medicine (16%), and allopurinol (9%). In severe cutaneous adverse reactions (n = 126), the highest incidence happened between 41 and 50 years of age and the top three causative drugs were sedative-hypnotics and antiepileptics (39%), antibiotics (22%), and allopurinol (15%). Carbamazepine was the most frequently used single-drug (16/18) in sedative-hypnotics and antiepileptics. β-lactams were the most frequently used antibiotics that induced both cutaneous adverse reactions and severe cutaneous adverse reactions.
Study limitations
The small sample size, retrospective design, collection of cutaneous adverse reactions and severe cutaneous adverse reactions at different time frames and locations, and exclusion of patients taking more than five medications are limitations of the study.
Conclusions
Gender does not affect cutaneous adverse reactions and severe cutaneous adverse reactions. The top three drugs to induce cutaneous adverse reactions are antibiotics, Chinese medicine, and allopurinol, while those that triggered severe cutaneous adverse reactions are sedative-hypnotics and antiepileptics, antibiotics, and allopurinol. Carbamazepine is the most frequent single drug that induces severe cutaneous adverse reactions. β-lactams are the most frequently used antibiotics that induce both cutaneous adverse reactions and severe cutaneous adverse reactions.
Keywords: Drug eruptions, Drug hypersensitivity, Drug-induced cutaneous adverse reactions, Drug-induced severe cutaneous adverse reactions, Pharmacovigilance
Introduction
Drug eruption is a symmetric cutaneous reaction that may occur when patients are taking medicines. It is known as drug-induced cutaneous adverse reactions (CARs), drug dermatitis, or dermatitis medicamentosa. Drug eruption is usually mild and may disappear when offending drugs are withdrawn; however, some severe cases may be fatal. According to reports, drug eruption affects more than 7% of the general population and an estimated 7000 deaths occur.1, 2 The incidence of severe cutaneous adverse reactions (SCARs) is generally very low. Taking Stevens-Johnsons syndrome (SJS) and toxic epidermal necrolysis (TEN) for example, around 5.76 cases occur out of one million people per year.3, 4, 5 Once such reactions occur, the total mortality could be as high as 30%,6 38%,7 or 32%,8 as stated by different reports.
Drugs known to induce severe drug eruption are mainly antibiotics, antiepileptic drugs, non-steroidal anti-inflammatory drugs (NSAIDs), and allopurinol. Data showed the main causative drugs were antibiotics (TEN, 40%; SJS, 40%) and pain relievers (TEN, 23%; SJS, 33%), and among antibiotics, sulfa and β-lactams were the most common causes of CARs.9, 10 The spectrum of drugs that induce CARs varied substantially in different clinical conditions. Identifying the causative drugs not only allows early cessation, but also improves the prognosis. Since the epidemiological data for CARs are limited in China at present, the authors conducted an analysis in hospitalized patients with drug eruption in central China to explore the leading drugs that cause CARs in Chinese population.
Methods
Patients
The study is a retrospective observational study. Four hundred and eighty two CARs patients admitted to Wuhan No. 1 Hospital from 2010 and 2011 and 126 SCARs patients admitted to Xiangya Hospital from 2009 to 2014 were assessed (Table 1). The patients’ general information, causative drugs, clinical manifestations, and outcomes were collected and analyzed. The diagnosis of drug eruption was made according to the patient's history of medication, clinical manifestations, and latent period by ruling out similar infectious skin diseases and rashes. Eruptions were diminished or relieved upon withdrawal of the sensitizing drug. The eruption manifestation was a typical symmetrical drug eruption, with bright red color and itch. If patients were taking multiple medicines, determining the sensitizing drugs may have been difficult. In this case, the suspected drug was determined by the doctors-in-charge. Only cases showing the definite causative drugs and taking no more than five total drugs were included. In CARs with probable or unknown drugs, the cases were excluded.
Table 1.
Patient information from two different hospitals.
| Hospital | Time (years) | Cases | Patient type |
|---|---|---|---|
| Wuhan No. 1 Hospital | 2010–2011 | 482 | Patients with drug eruptiona, b |
| Xiangya Hospital | 2009–2014 | 126 | Patients with severe drug eruptiona, b |
Unknown drugs that induce drug eruption were ruled out.
Patients intake ≤ 5 total drugs were analyzed.
Clinical manifestation classification
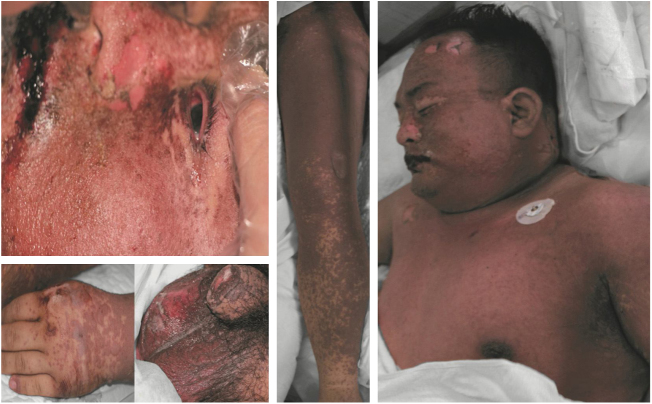
This study divided drug eruptions into two categories: (1) CARs: fixed drug eruption, urticarial drug eruption, measle-like or scarlatina-like drug eruption, eczematous drug eruption, purpura-like drug eruption, erythema multiforme, acneiform drug eruption, photosensitive drug eruption, etc. (2) SCARs: erythema multiforme major or SJS, drug rash with eosinophilia and systemic symptoms (DRESS), TEN, erythroderma, and exanthematous pustulosis.9, 11 One patient who presented TEN is shown in Fig. 1.
Fig. 1.
Dermatological manifestations of a patient with toxic epidermal necrolysis.
Drug classification
The most common drugs that induce drug eruption were divided into the following categories: (1) antibiotics; (2) analgesic-antipyretics, such as aspirin, acetaminophen, etc.; (3) sedative hypnotics and antiepileptic drugs, such as phenobarbitone, phenytoin sodium, carbamazepine, etc.; (4) serum preparations and vaccines, such as tetanus antitoxin, rabies vaccine, snake antivenoms, etc.; (5) biological agents. In addition, the anti-hyperuricemia drug allopurinol, antithyroids, and phenothiazines were also included.
Statistical analysis
All the numerical variables in this article were presented as mean ± standard error of the mean (SEM). Student's t-test was used on numerical variables of independent samples. The chi-squared test was used on categorical variables, i.e., enumerated group data. All data were analyzed by the SPSS v. 13.0 software package (SPSS Inc. – Chicago, IL, United States). A p-value < 0.05 was considered statistically significant.
Results
482 cases of CARs
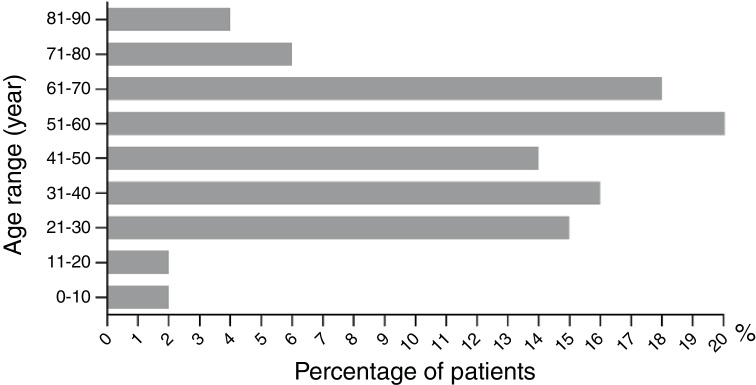
Among the 482 CARs cases in Wuhan No. 1 Hospital, the cases of male and female patients were equal, with no statistical difference (49%, 238/482 vs. 51%, 244/482) (p > 0.05). The age distribution between male and female were not statistically different (48.71 ± 1.90 vs. 46.24 ± 1.26 years old) (p > 0.05). The highest CARs incidence occurred between 51 and 60 years old, and the lowest happened between 71 and 80, 81 and 90, 0 and 10, and 11 and 20 years old (Fig. 2). Patients were divided into the single-medicine use group and the combined-medicine use group according to their causative drugs. The causative drugs were then subdivided into subcategories (Table 2). In the single-medicine use group, the most frequent drugs were antibiotics (48%, 201/420), followed by Chinese medicine (16%, 66/420) and allopurinol (9%, 38/420). These were followed by NSAIDs, sedative hypnotic antiepileptic drugs, nitroimidazole, antihypertensives and lipid-modifying agents, serums and vaccines, digestive system drugs, biological agents, antifungals, antithyroid preparations, and antiviral drugs. Other agents included anti-prostatic hyperplasia drugs, pesticides, mango, etc. In the combined-medicine use group, the most common combinations were antibiotics combined with other drugs. Antibiotics combined with antibiotics accounted for 34% (21/62). Antibiotics combined with other drugs, such as serum preparations, antipyretic analgesics, anticoagulants, antiviral drugs, and Chinese medicine accounted for 27% (17/62). The third place was antibiotics combined with nitroimidazole, accounting for 13% (8/62). Subsequently, it was Chinese medicine combined with another Chinese medicine, antiepileptic medicine combined with another antiepileptic medicine, and nitroimidazole combined with another nitroimidazole.
Fig. 2.
Age distribution of cutaneous adverse reactions (CARs). The chart illustrates the patient distribution at different age ranges (years old).
Table 2.
The categories of causative drugs in 482 cases of cutaneous adverse reactions.
| Group | Ranking | Causative drugs | ATC codes | Cases | % vs. subtotal | % vs. total |
|---|---|---|---|---|---|---|
| Single-medicine use | 1 | Antibiotics | A07AA, C05AB, D01AA, D06A, D06AX, G01AA, J02AA, J04AB, R02AB, R02AB | 201 | 48% | |
| 2 | Herbs and Chinese medicine | – | 66 | 16% | ||
| 3 | Allopurinol | M04AA01 | 38 | 9% | ||
| 4 | NSAIDs | M01A, M01AX, S01BC | 30 | 7% | ||
| 5 | Sedative-hypnotics and antiepileptics | N05C and N03 | 20 | 5% | ||
| 6 | Nitroimidazole | P01AB, P01CA | 12 | 3% | ||
| 7 | Antihypertensives and lipid modifying agents | C02 and C10 | 12 | 3% | ||
| 8 | Serum and vaccine | G03GA03, J06AA03, J06AA06 and J07 | 9 | 2% | ||
| 9 | Digestive system drugs | A09 | 5 | 1% | ||
| 10 | Biological agents | B05A, L03AB, L03AC, L04AB, etc. | 3 | 1% | ||
| 11 | Antifungals | D01 | 3 | 1% | ||
| 12 | Antithyroid preparations | H03B | 3 | 1% | ||
| 13 | Antivirals | S01AD | 1 | 0% | ||
| 14 | Othersa | – | 17 | 4% | ||
| Subtotal | 420 | 87% | ||||
| Combined-medicine use | 1 | Antibiotics + antibiotics | S01AA30 | 21 | 34% | |
| 2 | Antibiotics + others | S01AA20 | 17 | 27% | ||
| 3 | Antibiotics + nitroimidazole | – | 8 | 13% | ||
| 4 | Herb + herb | – | 3 | 5% | ||
| 5 | Antiepileptics + antiepileptics | – | 2 | 3% | ||
| 6 | Nitroimidazole + nitroimidazole | – | 1 | 2% | ||
| 7 | Othersb | – | 10 | 16% | ||
| Subtotal | 62 | 13% | ||||
| Total | 482 |
The Anatomical Therapeutic Chemical (ATC) coding system divides drugs into different groups according to the organ or system on which they act, or their therapeutic and chemical characteristics.
Anti-prostatic hyperplasia, pesticides, mango, etc.
Serum products, non-steroidal anti-inflammatory drugs (NSAIDs), anti-tumor medicine, herbs, etc.
Among those antibiotics (Table 3), β-lactams were the most frequently used (46%, 92/201), followed by furan (22%, 44/201), lincomycin (12%, 24/201), quinolone (12%, 24/201), macrolides (4%), and sulfonamides (3%). In β-lactams, amoxicillin was the most frequently used (43%, 40/92). In furan antibiotics, the most common was nifuratel (95%, 42/44).
Table 3.
The categories of causative antibiotics in 482 cases of cutaneous adverse reactions.
| Drugs | ATC codes | Cases | % |
|---|---|---|---|
| β-lactams | J01C, J01CR, J01D | 92 | 46% |
| Furan | D08AF, J01XE, P01CC | 44 | 22% |
| Lincomycin | J01FF02 | 24 | 12% |
| Quinolone | J01M, J01MA, J01MB, S01AE |
24 | 12% |
| Macrolides | J01FA | 8 | 4% |
| Sulfonamides | A07AB, C03BA, C03CA, D06BA, G01AE, S01AB | 7 | 3% |
| Others | – | 2 | 1% |
| Total | 201 | 100% |
126 cases of SCARs
Among the 126 SCARs cases in Xiangya Hospital, we found 43.50% (385/885) of patients were taking more than five drugs. To this end, patients taking more than five drugs were excluded due to it being overly complicated to determine the culprit drugs and/or to perform statistical analysis.
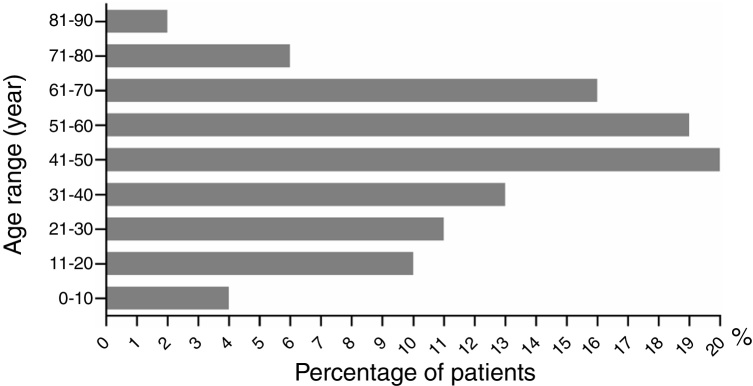
The ratio of males to females was 1 (43%, 54/126) to 1.33 (57%, 72/126) (p > 0.05). Male age to female age was 48.70 ± 2.65 vs. 43.79 ± 2.30 years old (p > 0.05). Age ranged from 2 to 86 years old. Patients aged under 10 and those between 71 and 90 had the lowest incidence rates, with five (4%, 5/126) and ten cases (8%, 10/126), respectively (Fig. 3). The highest incidence occurred at the age between 41 and 50 years old, with a proportion of 16% (20/126). The dermatological types of SCARs are listed in Table 4. Severe erythema multiforme was the most common type of SCARs, accounting for 38% (48/126). The second place was exanthematous pustulosis, accounting for 26% (33/126). TEN was rare, accounting for 2% (3/126).
Fig. 3.
Age distribution of severe cutaneous adverse reactions (SCARs). The chart illustrates the patient distribution at different age ranges (years old).
Table 4.
The dermatological types of 126 cases of severe cutaneous adverse reactions.
| SCARs types | Male | Female | Total | % |
|---|---|---|---|---|
| Severe erythema multiforme | 23 | 25 | 48 | 38% |
| Exanthematous pustulosis | 14 | 19 | 33 | 26% |
| Stevens-Johnsons syndrome | 0 | 4 | 4 | 3% |
| Toxic epidermal necrolysis | 2 | 1 | 3 | 2% |
| Erythroderma | 2 | 0 | 2 | 2% |
| Another severe drug eruption | 13 | 23 | 36 | 29% |
| Total | 54 | 72 | 126 | 100% |
The most common drugs in single-medicine use were sedative-hypnotics and antiepileptics, accounting for 39% (18/46) (Table 5). Antibiotics (22%, 10/46) and allopurinol (15%, 7/46) were the second and third categories that induce SCARs. They were followed by Chinese patent medicine or combined Chinese and drug medicine, nitroimidazole, dapsone, serum and vaccine, and digestive system medicines. Other agents such as fruits, vegetables, and diet pills were not included in the single-drug group, since they usually did not induce SCARs. Among sedative-hypnotics and antiepileptics, carbamazepine was the most frequently used single medicine (89%, 16/18). β-lactams were the most frequent antibiotics that induce SCARs.
Table 5.
The categories of causative drugs in 126 cases of severe cutaneous adverse reactions.
| Groups | Ranking | Causative drugs | ATC codes | Cases | % vs. subtotal | % vs. total |
|---|---|---|---|---|---|---|
| Single-medicine use | 1 | Sedative-hypnotics and antiepileptics | N05C and N03 | 18 | 39% | |
| 2 | Antibiotics | A07AA, C05AB, D01AA, D06A, D06AX, G01AA, J02AA, J04AB, R02AB, R02AB | 10 | 22% | ||
| 3 | Allopurinol | M04AA01 | 7 | 15% | ||
| 4 | Herbs and Chinese medicine | – | 5 | 11% | ||
| 5 | Nitroimidazole | P01AB, P01CA | 2 | 4% | ||
| 6 | Dapsone | J04BA02, D10AX05 | 2 | 4% | ||
| 7 | Serum and vaccine | G03GA03, J06AA03, J06AA06 and J07 | 1 | 2% | ||
| 8 | Digestive system medicines | A09 | 1 | 2% | ||
| Subtotal | 46 | 37% | ||||
| Combined- medicine use | 1 | Two drug combination | – | 34 | 43% | |
| 2 | Three drug combination | – | 23 | 29% | ||
| 3 | Four drug combination | – | 14 | 18% | ||
| 4 | Five drug combination | – | 9 | 11% | ||
| Subtotal | 80 | 63% | ||||
| Total | 126 |
In combined-medicine use, since drugs were randomly combined, we only analyzed the frequency of duplex, triple, quadruple, and quintuple drug combinations (Table 5). Duplex drug combination accounted for 43% (34/80), triple drugs accounted for 29% (23/80), quadruple drugs accounted for 18% (14/80), and quintuple drugs accounted for 11% (9/80). The medicine combination of SCARs was more complicated than that of CARs, e.g., some patients had antibiotics combined with antiviral drugs, serum preparations, and Chinese medicine.
Discussion
In this study, we analyzed 608 cases of drug eruption in Chinese population from central China. 482 cases of CARs suggested that men and women had equal chance to develop CARs, and those with age between 51 and 60 had the highest incidence of CARs. The suspected drugs were mainly in the following ten categories: (1) antibiotics: β-lactams were the most frequently used antibiotics; (2) Chinese medicine or Chinese patent medicine, or combined Chinese and drug medicine; (3) allopurinol; (4) NSAIDs; (5) sedative hypnotic antiepileptic drugs; (6) nitroimidazole; (7) antihypertensives and lipid-modifying agents; (8) serum and vaccine; (9) digestive system drugs; (10) biological agents. This study showed the top three CARs-inducing medicine categories were antibiotics, Chinese medicine, and allopurinol. In antibiotics, β-lactams, furan, and clindamycin were the top three antibiotic subgroups that induce CARs. Consistent with previous reports, β-lactams were the most common antibiotics to induce drug eruption.12, 13, 14, 15 Widely used in China, Chinese medicine, including herbs, Chinese patent medicine, combined Chinese and drug medicine, and Chinese medicine injections ranked in the second place to induce CARs in central China. Due to improper use of Chinese medicine, pharmaceutical problems, specific ingredients, complex components of Chinese medicine, or perhaps interaction with drugs, drug eruption induced by Chinese medicine was commonly seen. If the ingredients of Chinese medicine are not clear, use of Chinese medicine combined with other medicine should be more careful, especially when patients have allergy history and multiple drug usage. Furan was not a common cause of drug eruption and ranked the second place in antibiotics to induce CARs. This may due to the nifuratel vaginal suppository, which was extensively used in Wuhan city. It suggests that Wuhan city should pay a special attention to the adverse reactions of β-lactams such as amoxicillin and the gynecological suppository medicine nifuratel due to their high adverse reactions, and consider changing to another vendor.
There were 126 cases of SCARs, which showed that SCARs were most common between 40 and 70 years old of age. Women had more cases of SCARs than men, but without statistical significance. The top three causative drugs to induce SCARs were sedative-hypnotics and antiepileptics, antibiotics, and allopurinol. Among sedative-hypnotics and antiepileptics, carbamazepine was the most frequently used drug, making it the most frequent single-drug that induce SCARs. β-lactams were the most frequently used antibiotic that induce SCARs. Allopurinol ranked number three to induce SCARs, which is consistent with previous reports that allopurinol is a common drug to induce SCARs.16, 17, 18, 19
Meanwhile, it was found that most SCARs patients used a multiple-drug combination and had a complex medication history. They had triple, quadruple, and quintuple drug use. Compared with the combined-medicine use group in CARs, SCARs patients had more drug combinations. Despite primary diseases, SCARs patients usually had more drug intake, which greatly enhanced the incidence of SCARs. Avoiding interaction among different medications is important to lower the incidence of SCARs.
Despite the findings above, this study has limitations. The small sample size is one of them. This study only analyzed the patients who took no more than five drugs in SCARs, since more than five drug intake cases were too sophisticated for either determining the culprit drugs or doing statistical analysis. In this way, some meaningful data were eliminated for these severe cases. This estimation may underrepresent SCARs in Xiangya Hospital.
Conclusions
In central China, gender does not affect CARs and SCARs. The highest incidence of CARs occurs between 51 and 60 years old of age. The top three drugs to induce CARs are antibiotics, Chinese medicine, and allopurinol. Of antibiotics, β-lactams, nitrofurans, and lincomycin are the top three categories to induce CARs. However, SCARs are commonly seen between 41 and 50 years old of age. The top three drugs to induce SCARs in single-medicine use are sedative-hypnotics and antiepileptics, antibiotics, and allopurinol. Of the sedative-hypnotics and antiepileptics, carbamazepine is the most frequently used drug, making carbamazepine the most frequent single medicine to induce SCARs. β-lactams are the most frequently used antibiotics to induce SCARs. Despite these findings, this study has limitations. The small sample size, retrospective design, collection of the non-severe and severe CARs at different time frames and locations, and exclusion of patients taking more than five medications are considered the limitations of the study.
Financial support
This work was funded by grant from National Natural Science Foundation of China (No. 81603200).
Author's contribution
Jun Zhao, Lei Hu, Lihua Zhang, Maosong Zhou, and Lichen Gao: obtaining, analyzing and interpreting the data; effective participation in research orientation; intellectual participation in propaedeutic and therapeutic conduct of the cases studied; critical review of the literature.
Lin Cheng: conception and planning of the study; elaboration and writing of the manuscript; obtaining, analyzing and interpreting the data; effective participation in research orientation; intellectual participation in propaedeutic and therapeutic conduct of the cases studied; critical review and final approval of the manuscript.
Conflicts of interest
None declared.
Acknowledgments
The authors thank Dr. Ji Li from the Department of Dermatology, Xiangya Hospital, Central South University, Dr. Liming Tian from Wuhan No. 1 Hospital, Huazhong University of Science and Technology, and Prof. Hong-Hao Zhou from the Department of Clinical Pharmacology, Xiangya Hospital, Central South University for the help with collecting clinical cases.
Footnotes
How to cite this article: Zhao J, Hu L, Zhang L, Zhou M, Gao L, Cheng L. Causative drugs for drug-induced cutaneous reactions in central China: a 608-case analysis. An Bras Dermatol. 2019;94:664–70.
Study conducted at the Central South University, Changsha, China; Wenzhou Medical University, Wenzhou, China.
References
- 1.Gomes E.R., Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309–316. doi: 10.1097/01.all.0000173785.81024.33. [DOI] [PubMed] [Google Scholar]
- 2.Alomar M.J. Factors affecting the development of adverse drug reactions (Review article) Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey N., Jossi J., Bodmer M., Bircher A., Jick S.S., Meier C.R. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J Invest Dermatol. 2017;137:1240–1247. doi: 10.1016/j.jid.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Hsu D.Y., Brieva J., Silverberg N.B., Silverberg J.I. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136:1387–1397. doi: 10.1016/j.jid.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy. 2012;97:1–17. doi: 10.1159/000335612. [DOI] [PubMed] [Google Scholar]
- 6.Dodiuk-Gad R.P., Chung W.H., Valeyrie-Allanore L., Shear N.H. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015;16:475–493. doi: 10.1007/s40257-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 7.Weinand C., Xu W., Perbix W., Lefering R., Maegele M., Rathert M. 27 years of a single burn centre experience with Stevens-Johnson syndrome and toxic epidermal necrolysis: analysis of mortality risk for causative agents. Burns. 2013;39:1449–1455. doi: 10.1016/j.burns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Zajicek R., Pintar D., Broz L., Suca H., Königova R. Toxic epidermal necrolysis and Stevens-Johnson syndrome at the Prague Burn Centre 1998–2008. J Eur Acad Dermatol Venereol. 2012;26:639–643. doi: 10.1111/j.1468-3083.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Allergol Select. 2017;1:96–108. doi: 10.5414/ALX01508E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S.C., Hu S., Zhang S.Z., Huang J.W., Zhang J., Ji C. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in China. J Immunol Res. 2018;2018:4320195. doi: 10.1155/2018/4320195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf R., Orion E., Marcos B., Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–181. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Zambonino M.A., Corzo J.L., Muñoz C., Requena G., Ariza A., Mayorga C. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatr Allergy Immunol. 2014;25:80–87. doi: 10.1111/pai.12155. [DOI] [PubMed] [Google Scholar]
- 13.Shaw BG, Masic I, Gorgi N, Kalfayan N, Gilbert EM, Barr VO, et al. Appropriateness of β-lactam allergy record updates after an allergy service consult. J Pharm Pract. 2018 Sep 4:897190018797767.http://dx.doi.org/10.1177/0897190018797767. [Epub ahead of print]. [DOI] [PubMed]
- 14.Vardakas K.Z., Kalimeris G.D., Triarides N.A., Falagas M.E. An update on adverse drug reactions related to β-lactam antibiotics. Expert Opin Drug Saf. 2018;17:499–508. doi: 10.1080/14740338.2018.1462334. [DOI] [PubMed] [Google Scholar]
- 15.Zagursky R.J., Pichichero M.E. Cross-reactivity in β-lactam allergy. J Allergy Clin Immunol Pract. 2018;6:72–81. doi: 10.1016/j.jaip.2017.08.027. e1. [DOI] [PubMed] [Google Scholar]
- 16.Halevy S., Ghislain P.D., Mockenhaupt M., Fagot J.P., Bouwes Bavinck J.N., Sidoroff A. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58:25–32. doi: 10.1016/j.jaad.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Ding W.Y., Lee C.K., Choon S.E. Cutaneous adverse drug reactions seen in a tertiary hospital in Johor. Malaysia. Int J Dermatol. 2010;49:834–841. doi: 10.1111/j.1365-4632.2010.04481.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L., Xiong Y., Qin C.Z., Zhang W., Chen X.P., Li J. HLA-B*58:01 is strongly associated with allopurinol-induced severe cutaneous adverse reactions in Han Chinese patients: a multicentre retrospective case-control clinical study. Br J Dermatol. 2015;173:555–558. doi: 10.1111/bjd.13688. [DOI] [PubMed] [Google Scholar]
- 19.Stamp L.K., Day R.O., Yun J. Allopurinol hypersensitivity: investigating the cause and minimizing the risk. Nature reviews. Nat Rev Rheumatol. 2016;12:235–242. doi: 10.1038/nrrheum.2015.132. [DOI] [PubMed] [Google Scholar]