Abstract
Disruptive natural selection within populations exploiting different resources is considered to be a major driver of adaptive radiation and the production of biodiversity. Fitness functions, which describe the relationships between trait variation and fitness, can help to illuminate how this disruptive selection leads to population differentiation. However, a single fitness function represents only a particular selection regime over a single specified time period (often a single season or a year), and therefore might not capture longer-term dynamics. Here, we build a series of annual fitness functions that quantify the relationships between phenotype and apparent survival. These functions are based on a 9-year mark–recapture dataset of over 600 medium ground finches (Geospiza fortis) within a population bimodal for beak size. We then relate changes in the shape of these functions to climate variables. We find that disruptive selection between small and large beak morphotypes, as reported previously for 2 years, is present throughout the study period, but that the intensity of this selection varies in association with the harshness of environment. In particular, we find that disruptive selection was strongest when precipitation was high during the dry season of the previous year. Our results shed light on climatic factors associated with disruptive selection in Darwin's finches, and highlight the role of temporally varying fitness functions in modulating the extent of population differentiation.
Keywords: Darwin's finches, ecological speciation, fitness function, selection, Galápagos
1. Introduction
Adaptive radiation can be envisioned as occurring on phenotypic fitness functions or surfaces that have multiple high-fitness peaks separated by low-fitness valleys [1,2]. While there are numerous studies of natural populations that infer such peaks and valleys [3], few consider the effects of temporal variation [4]. In birds, for example, the classic representations of fitness functions for Darwin's finches [5], African seed crackers [6] and crossbills [7] do not provide estimates of disruptive selection across multiple years. Yet, temporal variation in fitness functions is very likely, which could facilitate or impede the process of adaptive radiation [8]. For instance, the presence of two discrete fitness peaks in one year might favour divergence; whereas a subsequent disappearance of those peaks might reverse any incipient divergence [9]. Temporal variation in fitness functions might be common given that (i) estimates of directional selection can vary widely through time [10], (ii) many populations show substantial phenotypic changes on short time scales [11] and (iii) several studies have documented speciation reversals, where formerly diverging species merge together again following environmental change [9,12]. However, direct assessments of temporal variation in fitness functions are generally lacking [4].
Temporal variation in fitness functions could be driven by many factors including intrinsic dynamics, such as density or frequency dependence [13], or extrinsic factors, such as biotic or abiotic environmental change [4]. Hinting at the importance of one particular factor, Siepielski et al. [10] found in a meta-analysis that 20–40% of temporal variation in directional selection could be explained by variation in precipitation. One context where variation in precipitation is expected to be particularly important is in neotropical environments with wet and dry seasons. These ecosystems can be subject to substantial interannual variation, most dramatically due to El Niño and La Niña events [14]. In such environments, many plants grow and reproduce predominantly during wet seasons; and so limited rainfall within those seasons—as well as severe dry seasons—can lead to extended droughts that limit primary productivity and cause high mortality for primary consumers [15].
Two alternative predictions can be advanced for how the variation in precipitation could influence selection between alternative fitness peaks. On the one hand, harsh conditions strengthen selection against maladapted individuals; hence increasing the strength of disruptive selection between alternative peaks. This prediction infers that niche differentiation between competing species should be greater when resources are more limited [16,17]. On the other hand, harsh conditions could increase competition among common phenotypes; hence lowering the heights of the fitness peaks—perhaps to the point that formerly discrete peaks are no longer separated by valleys. Indeed, many theoretical models [18,19] and some empirical studies [20] show that intense competition can reduce the heights of fitness peaks. To identify how variation in precipitation shapes evolutionary dynamics, we construct temporally variable fitness functions for a natural population of Darwin's finches.
(a). Study system
The adaptive radiation of Darwin's finches is thought to have been driven largely by the availability of different food types, which is in turn influenced by spatial and temporal variation in climatic conditions [21,22]. Accordingly, beak sizes of finches on different islands match the food types most readily available on those islands [5]. Moreover, closely related species show exaggerated beak size divergence when inhabiting the same island, suggesting that competition enhances divergence through character displacement [5,23]. Thus, different finch species are thought to have evolved phenotypes that correspond to different fitness peaks separated by fitness valleys that are jointly shaped by local resource distributions and local interspecific competition.
Several observations suggest that Darwin's finches’ fitness functions could be highly variable through time, potentially influencing their adaptive radiation. In particular, stochastic climatic events—especially El Niño and La Niña—that shape rainfall in the Galápagos are known to strongly modify plant reproduction, and hence, the abundance and distribution of seeds available for granivorous ground finches [24,25]. In drought years, with little rainfall during the wet season, the production of seeds is very low; whereas in wet years, with high rainfall during the wet season, seeds are usually produced in abundance [26,27]. Additionally, the dry seasons can vary from moderate amounts of rain to severe droughts with severe effects on the seed production. Inter-annual differences in seed production are known to have large effects on ground finches, which—during droughts—show high mortality [28], greater niche differentiation [25] and larger estimated directional coefficients [29,30]. Hence, estimating selection from fitness functions in multiple years could be used to explore how temporal environmental variation shapes disruptive selection and, thus, acts to drive or impede adaptive radiation.
Although natural selection in Darwin's finches surely influences multiple traits [21], much of the focus has been—for the seed-eating ground finches (Geospiza spp.)—on beak size and shape. Beak size (usually indexed as a linear combination of length, depth and width measurements) is highly heritable [21,30–33] and is clearly polygenic, including associations with several candidate genes [34,35]. Beak size functions in both food processing and species recognition [33,36], and hence represents a putative magic trait [19] that is under disruptive selection and influences reproductive isolation. Beak size correlates with diet [24], bite force [37], song features [38], mate choice [39] and selection [21]. Importantly, all of these effects and patterns are evident not only between species but also in the earliest stages of diversification within species [40].
Of particular interest are sympatric beak size morphotypes within the medium ground finch (Geospiza fortis), observed currently at El Garrapatero and formerly at Academy Bay on Santa Cruz Island [41]. The smaller morph is similar in size to G. fortis on many other islands [22], including Daphne Major [23]. The larger morph is not found in many other locations and, in fact, verges on the size of the large ground finch (Geospiza magnirostris) on at least some other islands; whereas G. magnirostris on Santa Cruz Island are larger still [22]. Overall, the differences between sympatric morphotypes of G. fortis on Santa Cruz mirror among-species differences in diet, bite force, song features, mate choice, allelic variants in candidate beak genes [35] and selection [23,36,41,42]. Thus, the two G. fortis beak size morphotypes provide an excellent system for studying how selection can shape the early stages of diversification.
We used a 9-year dataset to identify associations among beak size, fitness and climate. Our primary focus was on the putative selective disadvantage of birds with beak sizes that are intermediate between the small and large morphotypes in the well-studied bimodal population of G. fortis at El Garrapatero [16,22,23,42]. Our first objective was to identify whether observable disruptive selection between the small and large morphotypes was evident across the 9 years—as was the case in an earlier study [42] of a 2-year period in the same population. Confirming that disruptive selection is indeed present, we determined the intensity of disruptive selection across years. Finding that the pattern of selection was variable, we next asked whether temporal variation in rainfall in dry or wet seasons predicted temporal variation in disruptive selection. Finally, we used this analysis to evaluate alternative ideas for how variation in precipitation might influence fitness functions.
2. Material and methods
(a). Data collection and field study site
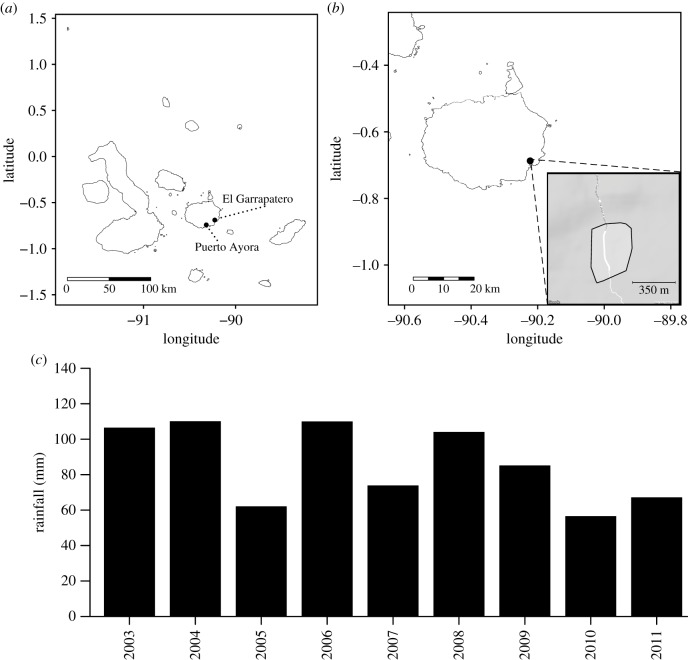
We captured individual birds of the medium ground finch (G. fortis) annually between 2003 and 2011 at El Garrapatero (0°41′22.9″ S, 90°13′19.7″ W), an arid zone site on Santa Cruz Island in Galápagos, Ecuador (figure 1a,b). Captures took place between January and April, which generally corresponds to the wet season—although the amount of rainfall during this season varied among years (dry season rainfall showing in figure 1c). The birds were captured in mist-nets placed across an area of about 20 hectares (figure 1b), with specific net locations determined by logistics and bird abundance. Captured birds were processed according to standard protocols (see electronic supplementary material; [23,40]). We then measured, with digital callipers (precision ± 0.02 mm), each bird's beak length, depth and width. Three separate measurements were taken for each dimension on each bird and repeatability from our data, estimated with a type II ANOVA, has a mean of 92.8% (trait repeatability is as follows: width 96.5%, length 89.5% and depth 92.4%) [44]. We further increased precision and accuracy by using the median of the three measurements for subsequent analyses [40].
Figure 1.
Map of the major Galápagos Islands. (a) Puerto Ayora, Santa Cruz Island and El Garrapatero (black dots). (b) Santa Cruz island (black dot is El Garrapatero). Inset: the polygon includes the sampling site at El Garrapatero. Maps from Google Maps (2017; Web Mercator projection, datum WGS84). (c) Climatic data from Puerto Ayora, located 10 km from El Garrapatero [43]. The y-axis corresponds to the cumulative dry season rainfall per year (June through December). Data from OpenStreetMap, http://osm2.carto.com/viz/feeaae54-9e89-11e4-ba3a-0e853d047bba/public_map (accessed 8 October 2019).
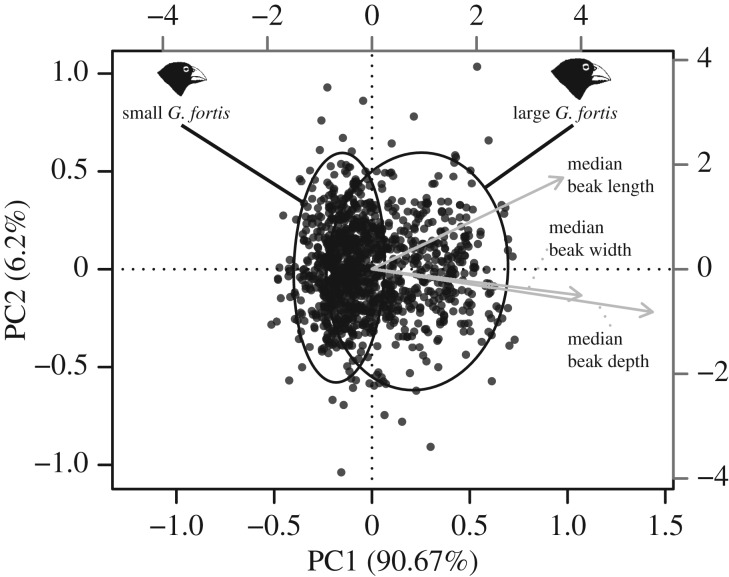
We first pooled G. fortis across all years (electronic supplementary material, table SI) for principal component analysis (PCA) of the three beak traits (length, depth and width; figure 2; electronic supplementary material, figure SA1) based on the covariance matrix because all beak traits were on the same scale (mm) and this ordination technique is consistent with previous work on Darwin's finches [23]. A correlation biplot is represented in figure 2. The first axis of variation (PC1) reflected overall beak size, as in previous work (see electronic supplementary material) [40,42]. Subsequent analyses focused on this axis as our research questions were specifically related to beak size (see Introduction).
Figure 2.
Correlation biplot of the PCA based on beak dimensions (length, width and depth) for G. fortis (B). The first axis of variation (PC1) represents variation in beak size (bigger beaks have higher scores) and PC2 represents variation in beak shape (pointer beaks have higher scores). The grey axes (top and right) are scaled for the trait vectors (light grey), whereas the black axes (bottom and left) are scaled for the points. The black ellipses refer to the 95% expectation-maximization algorithm for mixtures of univariate normal [45]. Illustrations of heads of finches are reproduced from [3].
We estimated annual dry-season rainfall (total amount of rain in millimetres from June through December; figure 1c) and wet-season rainfall (from January to May) at El Garrapatero using data from rain gauges at the town of Puerto Ayora 11 km to the southwest [43]. Rainfall was our focal climate variable because it is the main factor that affects plant reproduction and, hence, the abundance of food resources for ground finches [23]. Over the time frame of our study, the mean rainfall was 185.4 mm during the wet season (January through May) versus 96.37 mm during the dry season (June through December), with El Niño conditions present in 2004–2005, 2006–2007 and 2009–2010.
(b). Statistical model to estimate selection coefficients
We calculated apparent survival [46] for individual birds between pairs of years, assuming perfect detection [47]. We did not account for variation in recapture rates [47] because we did not want to overfit our models. Apparent survival between two specific years was specified as a bird being recaptured in the latter or any subsequent year. Thus, some of the birds inferred to have died might simply have remained uncaptured or might have emigrated [48,49]. Although mortality and emigration are different biological processes, emigrating birds are nevertheless permanent losses to the local population and, hence, have the same consequences for selection within a generation. As with most other such studies, we also assume that recapture probability is constant and not affected by the phenotypes in question, and that prior-capture history does not affect survival and recapture probabilities.
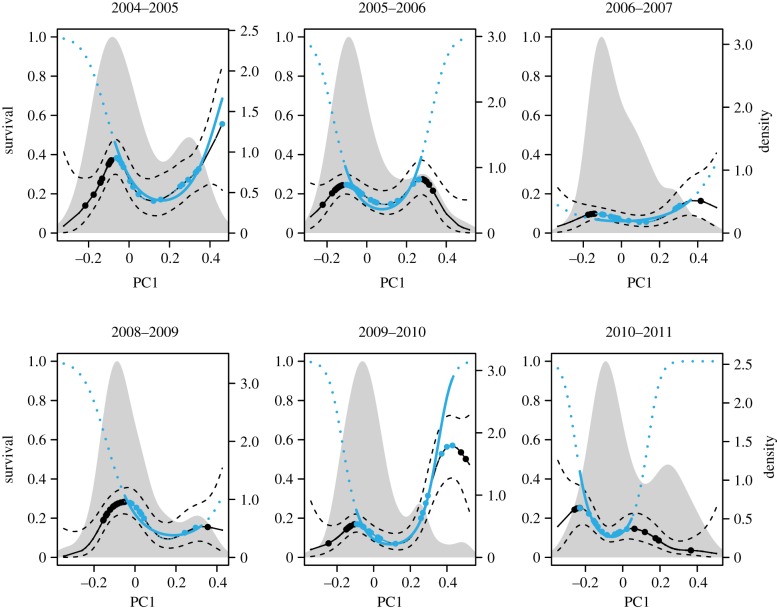
To describe the overall primary contributors to variation in fitness (apparent fitness, as above), we first calculated a generalized linear model (GLM) with apparent fitness as a function of the explanatory variables beak size (PC1) and dry season rainfall across all years. The model included both linear and quadratic terms for beak size (PC1), as well as an interaction between these terms and total rainfall in the previous year (electronic supplementary material, tables SII and SIII). To capture flexible shapes in the fitness function, we next characterized the fitness function in each year individually without imposing an a priori mode of selection. Hence, we used a generalized additive model (gam in the mgcv R package), which is a generalized linear model with the addition of smoothing functions of covariates (using a penalized smoothing of a thin plate regression spline; figure 3; [42,50–52]). The GAM is here intended as a heuristic (we do not focus on p-values) for inferring whether the fitness function has a convex shape consistent with disruptive selection against intermediate phenotypes. Details about the choice of smoothing parameter of the GAM can be found in the electronic supplementary material, figure SA2 and table SIV.
Figure 3.
Fitness functions for G. fortis at El Garrapatero across 6 years of data (larger-beaked birds have PC1 ≳ 0.2). Solid black lines show the generalized additive model (GAM) results (dashed black line represents 1 s.e.). Solid blue lines (and dotted blue lines, quadratic functions that open upward) show quadratic logistic models (GLM) computed between the fitness function's peaks. Black and blue dots represent individual birds. Solid grey shades (right y-axes) represent the density distribution of phenotypes. The blue dotted line is the GLM function beyond the data used to run the models. (Online version in colour.)
The fitness functions estimated from splines revealed at least two peaks in each year, consistent with an ongoing process of disruptive selection (figure 3). We next used a custom script (available on GitHub: https://github.com/beausoleilmo/temporal_fitness_landscape [53]) to extract the phenotypic values between the two maxima identified in each year. Using these between-peak trait values, we calculated nonlinear selection coefficients in order to estimate statistical significance specifically for the putative disruptive part of the fitness function (as in Hendry et al. [42]). Note that these selection estimates potentially include indirect selection caused by correlated traits; especially body size, given its high correlation with beak size (in our data, the Pearson correlation of mass (g) versus PC1 was r = 0.789). Fortunately, our hypotheses relate to total selection acting on beak size, which includes any indirect selection. The resulting estimates of disruptive selection were obtained via logistic regression (GLM) with a linear and a quadratic term to model both linear and nonlinear selection in each year [49,54]. In these logistic regressions, the subset (between-peak) of PC1 values used was first standardized to a mean of 0 and standard deviation of 1. The selection coefficient was standardized by dividing absolute fitness by mean fitness as in Janzen & Stern [54].
Because the logistic models represent only a subset of the phenotypic distribution, whereas the GAM represents a more comprehensive fitness function across morphospace, we kept only the logistic models that showed similar fits to the functions obtained from the spline models (this criterion led to the exclusion of the logistic coefficient for 2007). A Wald test was used to assess the significance of the raw logistic regression parameters. Given that we expected the quadratic term to be positive (meaning that the curve deflects upward, which is the pattern expected for disruptive selection), we halved the p-value to get a one-tailed test [42]. Our focal interest is the association between environmental variation and the effect size of the selection coefficient; these coefficients are generally weak and often non-significant for disruptive selection [55]. Quadratic coefficients from the logistic model were then converted to their linear equivalents according to Janzen & Stern [54] and were then doubled [56,57]. To assess logistic model fits, we report the pseudo-R2 (table 1) and the goodness of fit results from the Hosmer–Lemeshow tests [58].
Table 1.
Estimates from the logistic models and Hosmer–Lemeshow goodness-of-fit tests (HL GOF). The estimates correspond to the GLM models in figure 3. The numbers in square brackets are the confidence interval calculated for 1 s.d. The range data PC1 column refers to the interval of the PC1 scores between the two fitness peaks. Asterisk indicates significance at p < 0.05.
| HL GOF |
estimates |
deviance |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| year | χ2 | p-value | intercept | p-value | linear term (x) | p-value | nonlinear term (x2) | p-value | model deviance | null deviance | pseudo-R2 | range data PC1 |
| 2004–2005 | 8.17 | 0.42 | −1.59 [−2.39; −0.80] | <0.05* | −0.22 [−0.68; 0.23] | 0.42 | 0.64 [0.05; 1.24] | 0.04* | 80.39 | 83.76 | 0.04 | [0.19,1.95] |
| 2005–2006 | 3.83 | 0.87 | −1.83 [−2.48; −1.18] | <0.05* | −0.51 [−1.07; 0.04] | 0.13 | 0.44 [0.01; 0.87] | 0.04* | 110.15 | 113.27 | 0.03 | [0.06,1.33] |
| 2006–2007 | 16.44 | 0.04 | −2.74 [−3.42; −2.05] | <0.05* | 0.07 [−0.53; 0.67] | 0.85 | 0.15 [−0.29; 0.58] | 0.29 | 92.82 | 93.66 | 0.01 | [−0.04,1.62] |
| 2008–2009 | 11.81 | 0.16 | −1.83 [−2.82; −0.85] | <0.05* | −0.51 [−1.27; 0.25] | 0.27 | 0.29 [−0.44; 1.01] | 0.26 | 50.92 | 52.16 | 0.02 | [0.25,1.51] |
| 2009–2010 | 4.45 | 0.81 | −2.57 [−3.26; −1.88] | <0.05* | −0.42 [−1.00; 0.15] | 0.23 | 0.70 [0.28; 1.12] | <0.05* | 100.09 | 112.29 | 0.11 | [0.10,1.85] |
| 2010–2011 | 8.60 | 0.38 | −2.09 [−2.74; −1.45] | <0.05* | −0.23 [−0.66; 0.21] | 0.39 | 0.25 [−0.09; 0.59] | 0.11 | 78.13 | 80.38 | 0.03 | [−0.35,0.57] |
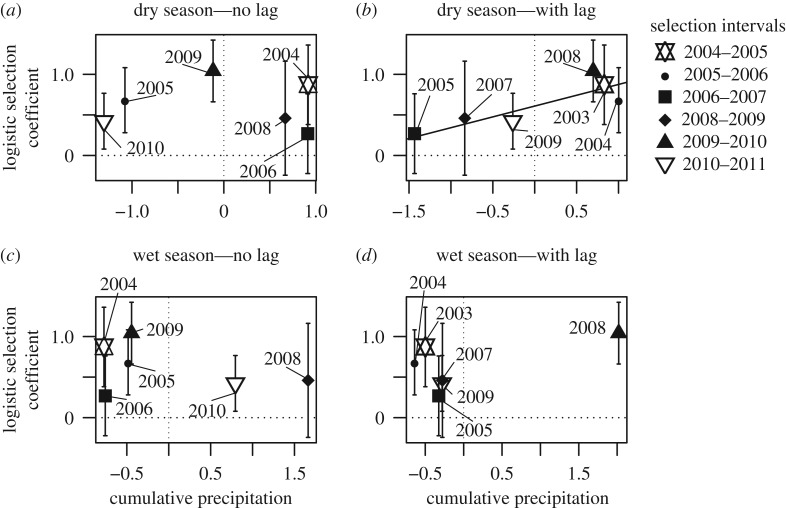
Finally, we related the between-peak quadratic coefficients to climate data, and thereby tested for correlations between environmental variation and disruptive selection between the peaks. Here, we modelled a weighted (1/s.e.2) linear regression between peak quadratic coefficients as predicted by the standardized mean = 0 and standard deviation = 1 across years. We identified three non-exclusive hypotheses regarding which period of rainfall would be most relevant to disruptive selection in a given year. First, wet season rainfall might be most important because low precipitation in the wet season would mean a protracted period of drought when combined with the dry season, thus potentially amplifying the strength of disruptive selection. Second, dry season rainfall might be most important if particularly severe dry seasons exacerbate fitness differences during the most strenuous time of year, again amplifying disruptive selection. Third, dry or wet season rainfall during the previous year might better predict the strength of selection than rainfall during the focal year, as rainfall in the previous year might influence the number of seeds in the seed bank during the subsequent year, as well as the number of birds competing for those seeds [30]. Hence, we performed four separate analyses, relating disruptive selection in a given year to wet season or dry season rainfall in that year (no lag) or in the previous year (1-year lag). We assessed the significance of each regression at α = 0.05; however, we emphasize that this climate association analysis is exploratory and hence our primary objective was to generate hypotheses for future formal testing as opposed to performing definitive tests of these hypotheses.
3. Results
We captured and measured 1073 G. fortis from 2003 to 2011 at El Garrapatero (electronic supplementary material, table SI). The first principal component (PC1) explained 90.67% of the total variation (figure 2) and represented variation in beak size—as in previous analyses [23,59]. A consistent feature of the fitness functions inferred from the splines was the presence of two fitness peaks for G. fortis beak size (figure 3; solid black lines)—a pattern strongly implying disruptive selection between the peaks. The fitness maxima generally corresponded to peaks of the phenotypic distributions of the two beak size morphotypes—a finding also consistent with a hypothesis of disruptive selection (figure 3 and tables 2 and 3). However, the strength of disruptive selection between the peaks varied considerably among years (figure 3). We therefore next focused on birds with beak sizes between the two fitness maxima in each year. One year (2007–2008) was excluded owing to a low sample size for these intermediate birds (n = 13, with only four surviving individuals) that also led to inconsistency between the splines and the logistic estimates. The Hosmer–Lemeshow goodness of fit tests indicated that the GLM model was a good fit for the data in years except 2006–2007 (table 1). The quadratic term from the GLM was strong and significant in three years (2004–2005, 2005–2006 and 2009–2010; tables 1 and 2), but weak and not significant in the other three years. Similar results were obtained based on classic logistic regression approaches (table 2).
Table 2.
Yearly between peak quadratic coefficients (GLM) estimated for birds between the two fitness peaks previously estimated by the GAM (figure 3). β and γ are the linear and quadratic standardized between peak quadratic coefficients. The 95% confidence interval (CI) is calculated for a one-tailed test (p-values were divided in two). N0 and N1 are the sample sizes for apparent mortality (0) and apparent survival (1). N is the total sample size. Asterisk indicates significance at p < 0.05.
| year | β average [95% CI] | β s.e. | β p-value | γ average [95% CI] | γ s.e. | γ p-value | N0 | N1 | Na |
|---|---|---|---|---|---|---|---|---|---|
| 2004–2005 | −0.15 [−0.46; 0.16] | 0.19 | 0.21 | 0.87 [0.07; 1.68] | 0.49 | 0.04* | 50 | 20 | 70 |
| 2005–2006 | −0.39 [−0.82; 0.03] | 0.26 | 0.07 | 0.68 [0.02; 1.34] | 0.40 | 0.05* | 88 | 23 | 111 |
| 2006–2007 | 0.06 [−0.49; 0.62] | 0.34 | 0.43 | 0.27 [−0.54; 1.08] | 0.49 | 0.29 | 172 | 13 | 185 |
| 2008–2009 | −0.41 [−1.02; 0.20] | 0.37 | 0.14 | 0.46 [−0.70; 1.62] | 0.70 | 0.26 | 44 | 11 | 55 |
| 2009–2010 | −0.32 [−0.75; 0.11] | 0.26 | 0.12 | 1.04 [0.42; 1.67] | 0.38 | 0.01* | 114 | 20 | 134 |
| 2010–2011 | −0.19 [−0.55; 0.17] | 0.22 | 0.20 | 0.42 [−0.14; 0.99] | 0.34 | 0.11 | 84 | 14 | 98 |
aSample size of individuals between the fitness peaks from figure 3 (blue points only).
Table 3.
Coefficients of linear regressions of the amount of rain and between peak quadratic coefficients for the same years as the between peak quadratic coefficients (2004, 2005, 2006, 2008, 2009 and 2010) and with a 1-year lag (2003, 2004, 2005, 2007, 2008 and 2009). The regressions are shown in figure 4. Asterisk indicates significance at p < 0.05.
| intercept [95% CI] | s.e. | p-value | quadratic coefficients [95% CI] |
s.e. | p-value | R2 | n | degrees of freedom | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| dry season | same year | 0.66 [0.39; 0.93] | 0.14 | 0.01* | γ | 0.04 [−0.25; 0.33] | 0.15 | 0.792 | 0.02 | 6 | 4 |
| 1-year lag | 0.61 [0.45; 0.77] | 0.08 | <0.05* | γ | 0.27 [0.09; 0.44] | 0.09 | 0.048* | 0.67 | |||
| wet season | same year | 0.64 [0.40; 0.88] | 0.12 | 0.01* | γ | −0.16 [−0.47; 0.15] | 0.16 | 0.370 | 0.20 | ||
| 1-year lag | 0.63 [0.43; 0.83] | 0.10 | <0.05* | γ | 0.19 [−0.01; 0.39] | 0.10 | 0.132 | 0.47 | |||
We detected an association between disruptive selection and climate (figure 4). A GLM including all years with sufficient data (2005, 2006, 2008, 2009, 2010, 2011) revealed a negative relationship between overall survival and cumulative dry-season rainfall the year before selection occurred (z-value = −1.97, p-value = 0.049; electronic supplementary material, table SIII). That is, greater dry season rainfall led to higher overall finch mortality. The weighted linear regression revealed a positive association between quadratic selection coefficients and cumulative rainfall over the previous dry season. Thus, we can infer that greater dry season rainfall generated stronger disruptive selection between the beak size morphotypes, although the association is weak and only marginally significant (slope = 0.27, R2 = 0.67, p-value = 0.048; figure 4b and table 3). No other periods of rainfall were predictive of disruptive selection (figure 4a,c,d).
Figure 4.
Between-peak quadratic coefficients as a function of cumulative rainfall. Valley quadratic coefficients obtained from fitness functions of the logistic regression. Rainfall is standardized across years to a mean = 0 and standard deviation = 1. (a,b) The weighted (1/s.e.2) linear relationship between dry season's precipitation and valley quadratic coefficients without a lag (a) and a 1-year lag (b), respectively. Weighted linear relationship between precipitation in the wet season and valley quadratic coefficients without a lag (c) and a 1-year lag (d), respectively. Each point is labelled with the year in which precipitation was calculated from, for a selection interval. The error bars are selection coefficient standard error in table 2. Line with a significant slope is shown.
4. Discussion
High variability in beak size for G. fortis on Santa Cruz Island has been repeatedly noted by researchers for nearly a century [22], with recent investigators specifically noting bimodal distributions of beak size measures [41]. A persistent question is how the two beak morphotypes have been maintained without having either fused back together or diverged into distinct species. Our approach here was to use a long-term mark–recapture dataset to investigate how changes in the consistency of disruptive selection might influence the degree of divergence at El Garrapatero, a relatively undisturbed site on Santa Cruz Island. We also explored one possible mechanism for changes in the strength of disruptive selection over time: variation in rainfall. Our analysis revealed temporally variable disruptive selection, with half of the years showing significant disruptive selection between fitness peaks (reduced fitness of birds with intermediate beak size) and half of the years showing non-significant disruptive selection. Additionally, inter-annual variation in selection was partly associated with the extent of rainfall in the previous year's dry season, with increased rainfall leading to greater overall finch mortality and stronger disruptive selection.
(a). Temporal variation and its causes
Many studies have reported inter-annual variation in selection, especially for directional selection [60]. By contrast, few have investigated temporal variation in disruptive selection between fitness peaks in natural populations (but see [61]). Variation in this form of selection is expected to be crucial in the early stages of adaptive divergence, ecological speciation and adaptive radiation [3,62]. To gain some additional insight into the importance of disruptive selection between fitness peaks, we calculated the lowest predicted fitness (apparent survival) between peaks in relation to the predicted fitness of birds on the two phenotypic optima. From this estimate, the depth of the fitness valley was greatest between years 2009–2010, where fitness in the valley bottom was 10.0% lower than fitness of the small-morph peak and 50.0% lower than fitness of the large-morph peak, as inferred from the splines in figure 3. This pattern differs most markedly from the fitness estimates for 2006–2007, where fitness in the valley bottom was 4.4% lower than fitness on the small-morph peak and 11.3% lower than fitness on the large-morph peak. These extreme alternatives for valley depth exceed the range of the few estimates that have been recorded for other sympatric morphotypes (or young species) in natural settings. For instance, comparative values for other systems are 6.6–30.7% for pupfish (fig. 2, durophage-generalist major axis in [63]) and 8.3–44.1% and for juvenile African seedcrackers (fig. 1, lower mandible length (mm) in [6]).
Our exploratory analysis suggests that climate may be a possible driver of the pronounced temporal variation in selection on G. fortis at El Garrapatero, with disruptive selection strongest in years with relatively high rainfall during the previous dry season. At first glance, this might seem to contradict previous work showing that selection on Darwin's finches is strongest during drought periods—as documented on Daphne Major [21,30,64]. However, the previous work focused on directional selection in a unimodal population, whereas we focused on disruptive selection in a bimodal population; hence, the different outcomes are not necessarily contradictory. Yet, two aspects of this outcome remain unanswered: (i) why does higher (rather than lower) rainfall generate strong disruptive selection, and (ii) why is this effect delayed by a 1-year lag? For the first question, we hypothesize that drier conditions could increase intraspecific competition among similar phenotypes, and hence more dramatically reduce the survival of common phenotypes at the phenotypic modes [65]. That is, drier conditions could shrink the peaks more than the valleys, hence generating weaker disruptive selection. For the second question, we hypothesize that higher dry season rainfall in a given year will lead to higher reproduction and higher offspring survival in that year, hence making competition more severe during the next year's dry season [30]. These are hypotheses emerging after analysis; yet, they suggest the value of reconsidering several standard assumptions of the factors that favour diversification in this classic system for studying adaptive radiation.
We recognize that our results are particular to the specific range of climate conditions that took place during our study, and thus might not translate to even more extreme conditions. For instance, the maximum dry season rainfall at El Garrapatero during our study was 110.1 mm (2004) and the minimum was 56.6 mm (2010). These levels are far from the extremes observed at other time periods or at other locations. Dry-season rainfall at our study site has previously been as high as 968.0 mm (1983) and as low as 23.8 mm (1980). Similarly, dry season rainfall is estimated to have been near zero during the key periods of strong selection on Daphne Major [21,30,64]. Additional years of sampling that span such extremes will be needed to see if our main result, that disruptive selection is stronger when conditions are less harsh in the previous year, holds (or is perhaps amplified) under even more extreme conditions.
(b). Consequences of temporal dynamics in disruptive selection
Disruptive selection is one mechanism that can maintain phenotypic and genetic variation [66–68]. Hence, persistent disruptive selection provides a reasonable hypothesis for the maintenance of divergent beak size in Santa Cruz G. fortis. Other mechanisms likely contributing to intraspecific variation for finches in general, and perhaps Santa Cruz G. fortis in particular, include hybridization between species and spatial variation in selection coupled with gene flow within species [69]. These processes have not been explicitly quantified for our study population but, overall, gene flow among islands is relatively low in relation to the size of resident populations [23,70] and movement within an island is also somewhat restricted. For instance, our long-term data include only one individual out of 8417 birds (0.01%) that moved between our two study sites separated by only 10 km. It has been argued that disruptive selection on a resource polymorphism is a key contributor to speciation and adaptive radiation [71]. It may be that El Garrapatero finches represents an ongoing analogue of this situation that likely drove the adaptive radiation of Darwin's finches in the first place [40].
What might be the influence of temporal variation in strength of disruptive selection? Generally, it has been argued that temporal variation in directional selection will maintain variation within populations [72]. However, the basic logic applied in studies of directional selection might not apply to temporal variation in disruptive selection. Instead, consistently strong disruptive selection would be expected to maintain variation more robustly than would a temporal mix of strong and weak disruptive selection, given that the latter would promote fusion. In the arid coastal zones of the Galápagos, the climate is typically harsh, with prolonged dry seasons resulting in strong selection. In an El Niño year, rain is expected to release disruptive selection in that year but perhaps, as shown here, enhance it in a subsequent year. Hence, temporal variation in selection could be a critical factor in the process of adaptive radiation.
We were interested not just in variation in selection but also in the degree to which it generates and maintains alternative morphs: reasonably discrete large and small beak size morphs with relatively few intermediates. To exemplify the difference in these two inferences, consider a comparison of two populations on Santa Cruz Island: the El Garrapatero G. fortis population that is the focus of this study, and the Academy Bay G. fortis population located approximately 10 km to the southwest. Both are highly variable in beak size [41], but only the former is currently bimodal, meaning that it is characterized by a statistically defensible dip in the beak size frequency distribution between large and small beak size morphs [41]. The reason for this difference between sites seems to be that recent human influences at Academy Bay have modified what finches feed on [73]—and thereby reduced or eliminated disruptive selection. Available data do not allow estimates of disruptive selection for Academy Bay, but the present study shows that temporal variation in disruptive selection can be strong even at relatively undisturbed sites. Perhaps this variation, especially the periods of weak disruptive selection, explains the fact that the two morphs do not appear to be progressing towards the status of separate species—even at the relatively undisturbed El Garrapatero site.
5. Whither now?
Few studies have quantified temporal variation in disruptive selection in natural populations, and we are not aware of any that have done so for bimodal populations diverging intra-specifically on the same axes as the adaptive radiation of which they are a part. A main finding of our study is that such selection is, in fact, variable in strength—leading to questions about its influence on adaptive radiation. An important next stage seems to be a theoretical one. We need to determine how the mean strength of disruptive selection, as well as the effect of temporal variation in that selection, should shape adaptive radiations. Consistently strong disruptive selection is likely to promote divergence [3], but the influence of temporal variation around that mean is uncertain. A first question might be the extent to which symmetrical variation around that mean should enhance or degrade divergence. In short, does a given high value contribute more or less to divergence than a correspondingly low value. Other important parameters might be the specific distribution of symmetrical variation around the mean (e.g. does a skewed distribution enhance or degrade divergence) and the nature of autocorrelation (do runs of similar deviations from the mean enhance or degrade divergence). Finally, asymmetry about the mean might be critical: that is, do extreme high levels of disruptive selection have a stronger effect on promoting divergence than similarly extreme low levels of disruptive selection on constraining divergence. Future studies that investigate temporal variation in fitness surfaces of multiple morphotypes or species will help to answer these questions, and thus improve our understanding of the potential for ecological speciation and adaptive radiation.
Supplementary Material
Acknowledgements
We thank the Charles Darwin Research Station, the Galápagos National Park Service, C. Leblond, K. Gotanda, S.C. Endara, N. Emery and data collectors. Also, J. Sakata, M. Morrissey and H. Stern kindly helped with statistics. Also, we want to thank the reviewers that made valuable comments on the manuscript.
Ethics
Fieldwork was conducted under the University of Massachusetts Amherst Animal Use Protocols for 2003, 2004, 2005, 2006: #23-10-09; 2007, 2008, 2009: #26-10-16; 2010, 2011: #2009-0063; and the University of Utah for 2008 and 2009 IACUC #07-08004. The Galápagos National Park Service permit are as follows: 2003, 2004, 2005, 2006, 2007: #PC-009-98; 2008: #PC-01-08; 2009: #PC-21-07, Ext. 01-09; 2010 and 2011: #PC-58-10.
Data accessibility
The script used for analysis can be found on GitHub: https://github.com/beausoleilmo/temporal_fitness_landscape/blob/master/scripts/Analysis_fit_land.R. Data available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.zcrjdfn6q [74].
Authors' contributions
M.-O.B., A.P.H. and R.D.H.B. conceived the study and wrote the paper. M.-O.B., J.P., A.P.H. and R.D.H.B. designed the research. M.-O.B., A.P.H., J.A.M.R., L.F.D.L., D.H.C., J.P. and R.D.H.B. coordinated the study. M.-O.B., S.A.K., L.F.D.L., S.K.H., J.A.C., J.A.M.R., D.H.C., J.A.H.K., J.P. and A.P.H. collected the data. M.-O.B., L.K.M. and L.O.F. conducted statistical analyses. All authors contributed to revisions and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research is supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants Program (R.D.H.B. and A.P.H.), a Canada Research Chair (R.D.H.B. and A.P.H.), NSERC Canada Graduate Scholarships, Fonds de recherche du Québec, Nature et technologies (M.-O.B.) and Secretaría Nacional de Ciencia, Tecnología e Innovación (L.F.D.L.). National Science Foundation supported S.A.K., S.K.H., J.A.H.K. and D.H.C.
References
- 1.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 2.Arnold SJ, Pfrender ME, Jones AG. 2001. The adaptive landscape as a conceptual bridge between micro- and macroevolution. Genetica 112, 9–32. ( 10.1023/A:1013373907708) [DOI] [PubMed] [Google Scholar]
- 3.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Calsbeek R, Gosden TP, Kuchta SR, Svensson EI. 2012. Fluctuating selection and dynamic adaptive landscapes. In The adaptive landscape in evolutionary biology (eds Svensson EI, Calsbeek R), pp. 89–109. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Schluter D, Grant PR. 1984. Determinants of morphological patterns in communities of Darwin's finches. Am. Nat. 123, 175–196. ( 10.1086/284196) [DOI] [Google Scholar]
- 6.Smith TB. 1993. Disruptive selection and the genetic basis of bill size polymorphism in the African finch Pyrenestes. Nature 363, 618–620. ( 10.1038/363618a0) [DOI] [Google Scholar]
- 7.Benkman CW. 2003. Divergent selection drives the adaptive radiation of crossbills. Evolution 57, 1176–1181. ( 10.1111/j.0014-3820.2003.tb00326.x) [DOI] [PubMed] [Google Scholar]
- 8.Gosden TP, Svensson EI. 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution 62, 845–856. ( 10.1111/j.1558-5646.2008.00323.x) [DOI] [PubMed] [Google Scholar]
- 9.Seehausen O, Takimoto G, Roy D, Jokela J. 2008. Speciation reversal and biodiversity dynamics with hybridization in changing environments. Mol. Ecol. 17, 30–44. ( 10.1111/j.1365-294X.2007.03529.x) [DOI] [PubMed] [Google Scholar]
- 10.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]
- 11.Hendry AP, Farrugia TJ, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. ( 10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 12.Kearns AM, et al. 2018. Genomic evidence of speciation reversal in ravens. Nat. Commun. 9, 1–13. ( 10.1038/s41467-018-03294-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svensson E, Sinervo B. 2000. Experimental excursions on adaptive landscapes: density-dependent selection on egg size. Evolution 54, 1396–1403. ( 10.1554/0014-3820(2000)054[1396:EEOALD]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 14.Palmer CE, Pyle RL. 1966. The climatological setting of the Galápagos. In The Galápagos (ed. Bowman RI.), pp. 93–99. Berkeley, CA: University of California Press. [Google Scholar]
- 15.Grant PR, Grant BR, Keller LF, Petren K. 2000. Effects of El Niño events on Darwin's finch productivity. Ecology 81, 2442–2457. ( 10.2307/177466) [DOI] [Google Scholar]
- 16.Grant PR, Grant BR, Smith JN, Abbott IJ, Abbott LK. 1976. Darwin's finches: population variation and natural selection. Proc. Natl Acad. Sci. USA 73, 257–261. ( 10.1073/pnas.73.1.257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JNM, Grant PR, Grant BR, Abbott IJ, Abbott LK. 1978. Seasonal variation in feeding habits of Darwin's ground finches. Ecology 59, 1137–1150. ( 10.2307/1938228) [DOI] [Google Scholar]
- 18.Dieckmann U, Doebeli M, Metz JAJ, Tautz D. 2004. Adaptive speciation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Gavrilets S. 2004. Fitness landscapes and the origin of species. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Svensson EI, Calsbeek R. 2012. The adaptive landscape in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Grant BR, Grant PR. 1993. Evolution of Darwin's finches caused by a rare climatic event. Proc. R. Soc. Lond. B 251, 111–117. ( 10.1098/rspb.1993.0016) [DOI] [Google Scholar]
- 22.Lack D. 1947. Darwin's finches. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Grant PR. 1999. Ecology and evolution of Darwin's finches. Princeton, NJ: Princeton University Press. [Google Scholar]
- 24.Abbott IJ, Abbott LK, Grant PR. 1977. Comparative ecology of Galápagos ground finches (Geospiza Gould): evaluation of the importance of floristic diversity and interspecific competition. Ecol. Monogr. 47, 151–184. ( 10.2307/1942615) [DOI] [Google Scholar]
- 25.De León LF, Podos J, Gardezi T, Herrel A, Hendry AP.. 2014. Darwin's finches and their diet niches: the sympatric coexistence of imperfect generalists. J. Evol. Biol. 27, 1093–1104. ( 10.1111/jeb.12383) [DOI] [PubMed] [Google Scholar]
- 26.Grant BR, Grant PR. 1981. Exploitation of Opuntia cactus by birds on the Galápagos. Oecologia 49, 179–187. ( 10.1007/BF00349186) [DOI] [PubMed] [Google Scholar]
- 27.Grant BR, Grant PR. 1989. Evolutionary dynamics of a natural population: the large cactus finch of the Galápagos. Chicago, IL: The University of Chicago Press. [DOI] [PubMed] [Google Scholar]
- 28.Grant PR, Grant BR. 2011. Causes of lifetime fitness of Darwin's finches in a fluctuating environment. Proc. Natl Acad. Sci. USA 108, 674–679. ( 10.1073/pnas.1018080108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant PR. 1985. Climatic fluctuations on the Galápagos Islands and their influence on Darwin's finches. Ornithol. Monogr. 36, 471–483. ( 10.2307/40168299) [DOI] [Google Scholar]
- 30.Grant PR, Grant BR. 2002. Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711. ( 10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 31.Boag PT. 1983. The heritability of external morphology in Darwin's ground finches (Geospiza) on Isla Daphne Major, Galápagos. Evolution 37, 877–894. ( 10.2307/2408404) [DOI] [PubMed] [Google Scholar]
- 32.Boag PT, Grant PR. 1978. Heritability of external morphology in Darwin's finches. Nature 274, 793–794. ( 10.1038/274793a0) [DOI] [Google Scholar]
- 33.Grant PR, Grant BR. 1997. Genetics and the origin of bird species. Proc. Natl Acad. Sci. USA 94, 7768–7775. ( 10.1073/pnas.94.15.7768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamichhaney S, Han F, Berglund J, Wang C. 2016. A beak size locus in Darwin's finches facilitated character displacement during a drought. Science 352, 470–474. ( 10.1126/science.aad8786) [DOI] [PubMed] [Google Scholar]
- 35.Chaves JA, Cooper EA, Hendry AP, Podos J, De León LF, Raeymaekers JAM, MacMillan WO, Uy JAC. 2016. Genomic variation at the tips of the adaptive radiation of Darwin's finches. Mol. Ecol. 25, 5282–5295. ( 10.1111/mec.13743) [DOI] [PubMed] [Google Scholar]
- 36.Huber SK, De León LF, Hendry AP, Bermingham E, Podos J.. 2007. Reproductive isolation of sympatric morphs in a population of Darwin's finches. Proc. R. Soc. B 274, 1709–1714. ( 10.1098/rspb.2007.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrel A, Podos J, Huber SK, Hendry AP. 2005. Evolution of bite force in Darwin's finches: a key role for head width. J. Evol. Biol. 18, 669–675. ( 10.1111/j.1420-9101.2004.00857.x) [DOI] [PubMed] [Google Scholar]
- 38.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188. ( 10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 39.Ratcliffe LM, Grant PR. 1983. Species recognition in Darwin's finches (Geospiza, Gould). II. Geographic-variation in mate preference. Anim. Behav. 31, 1154–1165. ( 10.1016/S0003-3472(83)80022-0) [DOI] [Google Scholar]
- 40.De León LF, Rolshausen G, Bermingham E, Podos J, Hendry AP.. 2012. Individual specialization and the seeds of adaptive radiation in Darwin's finches. Evol. Ecol. Res. 14, 365–380. [Google Scholar]
- 41.Hendry AP, Grant PR, Grant BR, Ford HA, Brewer MJ, Podos J. 2006. Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. B 273, 1887–1894. ( 10.1098/rspb.2006.3534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendry AP, Huber SK, De León LF, Herrel A, Podos J.. 2009. Disruptive selection in a bimodal population of Darwin's finches. Proc. R. Soc. B 276, 753–759. ( 10.1098/rspb.2008.1321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles Darwin Foundation. 2019. DataZone: climatology database Puerto Ayora. See https://www.darwinfoundation.org/en/datazone/climate/puerto-ayora (accessed 7 June 2016).
- 44.Sokal RR, Rohlf FJ. 1995. Biometry, 3rd edn New York, NY: WH Freeman and Company. [Google Scholar]
- 45.Benaglia T, Chauveau D, Hunter DR, Young D. 2009. MIXTOOLS: an R package for analyzing finite mixture models. J. Stat. Softw. 32, 1–29. ( 10.18637/jss.v032.i06) [DOI] [Google Scholar]
- 46.Gilroy JJ, Virzi T, Boulton RL, Lockwood JL. 2012. A new approach to the ‘apparent survival’ problem: estimating true survival rates from mark-recapture studies. Ecology 93, 1509–1516. ( 10.1890/12-0124.1) [DOI] [PubMed] [Google Scholar]
- 47.Kellner KF, Swihart RK. 2014. Accounting for imperfect detection in ecology: a quantitative review. PLoS ONE 9, 1–8. ( 10.1371/journal.pone.0111436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradel R, Hines JE, Lebreton J.-D, Nichols JD. 1997. Capture-recapture survival models taking account of transients. Auk 53, 60–72. ( 10.2307/2533097) [DOI] [Google Scholar]
- 49.Kingsolver JG, Smith SG. 1995. Estimating selection on quantitative traits using capture-recapture data. Evolution 49, 384–388. ( 10.1111/j.1558-5646.1995.tb02252.x) [DOI] [PubMed] [Google Scholar]
- 50.Wood SN. 2017. Generalized additive models: an introduction with R, 2nd edn Boca Raton, FL: Chapman & Hall/CRC Press. [Google Scholar]
- 51.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 52.Schluter D. 1988. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861. ( 10.1111/j.1558-5646.1988.tb02507.x) [DOI] [PubMed] [Google Scholar]
- 53.Beausoleil M-O, et al. 2019. Script available on GitHub: Temporally varying disruptive selection in the medium ground finch (Geospiza fortis). See https://github.com/beausoleilmo/temporal_fitness_landscape/blob/master/scripts/Analysis_fit_land.R. [DOI] [PMC free article] [PubMed]
- 54.Janzen FJ, Stern HS. 1998. Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571. ( 10.1111/j.1558-5646.1998.tb02237.x) [DOI] [PubMed] [Google Scholar]
- 55.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 56.Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. 2008. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440. ( 10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- 57.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 58.Hosmer DW Jr, Lemeshow S, Sturdivant RX. 2013. Applied logistic regression. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 59.Huber SK, Podos J. 2006. Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis). Biol. J. Linn. Soc. 88, 489–498. ( 10.1111/j.1095-8312.2006.00638.x) [DOI] [Google Scholar]
- 60.Siepielski AM, Dibattista JD, Carlson SM. 2009. It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol. Lett. 12, 1261–1276. ( 10.1111/j.1461-0248.2009.01381.x) [DOI] [PubMed] [Google Scholar]
- 61.Morrissey MB, Hadfield JD. 2011. Directional selection in temporally replicated studies is remarkably consistent. Evolution 66, 435–442. ( 10.1111/j.1558-5646.2011.01444.x) [DOI] [PubMed] [Google Scholar]
- 62.Nosil P. 2012. Ecological speciation. Oxford, UK: Oxford University Press. [Google Scholar]
- 63.Martin CH, Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339, 208–211. ( 10.1126/science.1227710) [DOI] [PubMed] [Google Scholar]
- 64.Grant PR, Grant BR. 2014. 40 years of evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 65.Bolnick DI. 2004. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution 58, 608–618. ( 10.1111/j.0014-3820.2004.tb01683.x) [DOI] [PubMed] [Google Scholar]
- 66.Rueffler C, Van Dooren TJM, Leimar O, Abrams PA.. 2006. Disruptive selection and then what? Trends Ecol. Evol. 21, 238–245. ( 10.1016/j.tree.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 67.Bolnick DI. 2004. Waiting for sympatric speciation. Evolution 58, 895–899. ( 10.1111/j.0014-3820.2004.tb00421.x) [DOI] [PubMed] [Google Scholar]
- 68.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357. ( 10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 69.Vagvolgyi J, Vagvolgyi MW. 1990. Hybridization and evolution in Darwin's finches of the Galápagos Islands. Accad. Naz. Linc. Atti Conv. Linc. 85, 749–772. [Google Scholar]
- 70.Lawson LP, John N, Kenneth P. 2019. Darwin's finches: a model of landscape effects on metacommunity dynamics in the Galápagos Archipelago. Ecography 42, 1636–1647. ( 10.1111/ecog.04511) [DOI] [Google Scholar]
- 71.Skúlason S, Smith TB. 1995. Resource polymorphisms in vertebrates. Trends Ecol. Evol. 10, 366–370. ( 10.1016/S0169-5347(00)89135-1) [DOI] [PubMed] [Google Scholar]
- 72.Sasaki A, Ellner S. 1997. Quantitative genetic variance maintained by fluctuating selection with overlapping generations: variance components and covariances. Evolution 51, 682–696. ( 10.1111/j.1558-5646.1997.tb03652.x) [DOI] [PubMed] [Google Scholar]
- 73.De León LF, Sharpe DMT, Gotanda KM, Raeymaekers JAM, Chaves JA, Hendry AP, Podos J. 2018. Urbanization erodes niche segregation in Darwin's finches. Evol. Appl. 47, 151–184. ( 10.1111/eva.12721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beausoleil M-O, et al. 2019. Data from: Temporally varying disruptive selection in the medium ground finch (Geospiza fortis) Dryad Digital Repository. ( 10.5061/dryad.zcrjdfn6q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Beausoleil M-O, et al. 2019. Data from: Temporally varying disruptive selection in the medium ground finch (Geospiza fortis) Dryad Digital Repository. ( 10.5061/dryad.zcrjdfn6q) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The script used for analysis can be found on GitHub: https://github.com/beausoleilmo/temporal_fitness_landscape/blob/master/scripts/Analysis_fit_land.R. Data available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.zcrjdfn6q [74].