Abstract
Despite significant progress in oncology, metastasis remains the leading cause of mortality of cancer patients. Understanding the foundations of this phenomenon could help contain or even prevent it. As suggested by many ecologists and cancer biologists, metastasis could be considered through the lens of biological dispersal: the movement of cancer cells from their birth site (the primary tumour) to other habitats where they resume proliferation (metastatic sites). However, whether this model can consistently be applied to the emergence and dynamics of metastasis remains unclear. Here, we provide a broad review of various aspects of the evolution of dispersal in ecosystems. We investigate whether similar ecological and evolutionary principles can be applied to metastasis, and how these processes may shape the spatio-temporal dynamics of disseminating cancer cells. We further discuss complementary hypotheses and propose experimental approaches to test the relevance of the evolutionary ecology of dispersal in studying metastasis.
Keywords: dispersal, metastasis, cancer, evolution
1. Introduction
Since the beginning of the ‘war on cancer’ [1], considerable advances have been made in understanding cancer progression and developing therapeutics to extend patient lifespan. Despite such progress, metastatic cancer remains extremely hard to cure, and is still the primary cause of death in cancer patients (e.g. for bone metastasis [2]). Our current knowledge of metastases thus still lacks essential data and information that could be used to target metastatic cells, or to exploit weaknesses of such cells (e.g. [3]). Many mechanisms, such as epithelial–mesenchymal transition and cell dedifferentiation, have been suggested and successfully tested to explain how cancer cells acquire the ability to leave the primary tumour and disseminate to other organs [3–6]. Yet, a fully satisfying explanation for the various causes of this process is clearly missing. As the tumour bulk expands and many cancer cells move away from the core, some of them may join the surrounding lymph and blood flow [7]. Following intravasation into blood microvessels, a small proportion of disseminating cells may survive long enough and succeed in extravasation to reach a habitat that is permissive enough to allow them to resume proliferation and invasion [8]. Although metastasis is a very inefficient process (only a minority of cancer cells disseminating away from the primary tumour actually manifest in clinically apparent metastases), ultimately these invading cells can lead to the demise of the organism.
Fortunately, the metastatic cascade can be understood through Darwinian evolutionary theories, as cancer cell populations undergo selection events during oncogenesis [9]. Most genotypic and phenotypic features of cancer cells are deemed as adaptations, and the same may apply to metastatic cells: the fact that they can and do metastasize may result from proliferative advantages to do so (e.g. [10,11]). Especially, a growing number of papers has considered dispersal ecology as a good framework to apprehend the dynamics of metastasis: in this case, biological dispersal consists of the movement of cancer cells from their birth site (the primary tumour) to other habitats where they resume proliferation (metastatic sites [12]). ‘Why do cancer cells metastasize?’ is conceptually equivalent to ‘why does one disperse?’. The few papers that have embraced this question propose that resource scarcity motivates the malignant cells to leave (e.g. [13–16]). Yet, from the literature on the evolution of dispersal, there are several other reasons why cancer cells could benefit from moving [17–19]. In this review, we address the current knowledge of metastasis in regard to the ecology and evolution of dispersal, and we investigate whether the characteristics of metastasis match the definition of dispersal. Moreover, cancer cells shift between habitats does not necessarily mean that they disperse, and we further discuss alternative hypotheses and propose possible approaches to disentangle the primary causes of metastasis.
2. The pros and cons of cancer cell dispersal
For dispersal to evolve in a population, the benefits of moving away must outweigh the cost of developing and maintaining dispersal traits, the risk of colonization failure and the spatial heterogeneity of the environment [20–23]. Organisms are more prone to disperse (i) when they initially live in low-quality environments, (ii) when they are clustered in groups of related individuals and (iii) when environmental quality is variable in time and desynchronized in space or when existing populations randomly go extinct [19]. More complex cases involving a combination of these factors (e.g. a high variability in environmental quality across time and space [24]) can lead to the evolution of polymorphic or alternative dispersal strategies, or even to cycles of dispersal evolution (e.g. [25]). The evolution of dispersal is also linked to the evolution of other important traits such as the ability to assess local conditions (e.g. local population density), local adaptation or survival during the dispersal episode.
(a). Costs of dispersal
Most of the time, the cost incurred by individuals leaving their birthplace is high, as they involve energetic, time, risk and opportunity expenses (see [23] for a review). None of these costs has been directly investigated in metastatic cancer cells, yet variation in the cost of dispersal can greatly influence its success [26–28]. Less than 0.1% of cells disseminating from the primary tumour manage to form metastases (a phenomenon referred to as ‘metastatic inefficiency’ [29]), which suggests that disseminating cancer cells do indeed incur at least some of these costs.
Before disseminating, organisms have to develop and maintain dispersal traits (figure 1a). This can be achieved through a phenotypic switch, for example, the dispersing locust morph Locusta migratoria grows larger wings prior to dispersal [30]. Organisms can also adapt to the migratory lifestyle and obligatorily express dispersal traits in a given proportion of offspring (e.g. the pappus parachutes of dandelion fruits [31]). Either way, in many cancers, disseminating cancer cells differentiate from non-disseminating ones, as they display mesenchymal rather than epithelial traits [3,32]. This switch clearly improves their disseminating abilities [3] and thus might be adaptive, yet seems not to be mandatory [33,34].
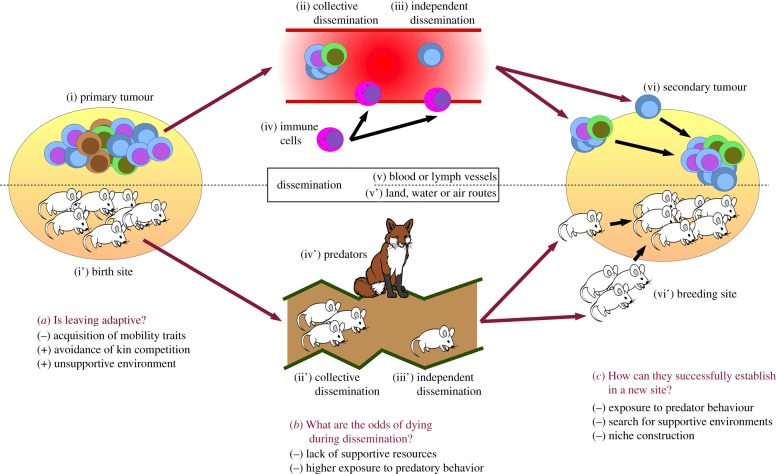
Figure 1.
The pros and cons of (cancer cell) dispersal. Dispersing individuals/cells have to leave (i) the primary tumour (respectively their birth sites) to disseminate (ii) collectively or (iii) independently. They may be exposed to (iv) predatory behaviour (from predators or immune cells) when joining (v) the lymph or blood flow (respectively land, water or air routes), before finally arriving to (vi) secondary tumours (respectively breeding sites). During all these steps, they are being exposed to beneficial or detrimental factors, which may or may not tip the balance towards dispersal. (Online version in colour.)
During dispersal, organisms are at high risk of being harmed, or even of dying (figure 1b). During any movement between habitats, they are much more exposed to dangers, such as starvation (e.g. [35]), accidents or predation (e.g. [36]). This latter risk seems to be strong enough so that many prey species avoid dispersal behaviour when predation risk is high (e.g. [37]). This risk seems moderate in the case of metastasis, as about 80% of disseminating cancer cells survive long enough in the lymph/blood flow to reach new organs [29], the rest dying from higher exposure to the immune system [38].
Having reached a new (micro)environment, the settlement might fail due to (i) the presence of predators [39], (ii) ending up in an unsuitable habitat [40] or (iii) the loss of facilitative interactions with other species (figure 1c) [41]. Though often ignored, dispersing organisms can also cope with the costs of habitat optimization, either by looking for supporting habitats [42] or by constructing their niches [43]. Yet, on the one hand, metastatic cells seem to be constrained by only two of these factors [29]: the loss of immunosuppression [44] and the loss of supporting interactions [45]. On the other hand, the primary tumour seems to bear the costs of habitat optimization by remotely initiating niche formation in certain organs [46,47].
(b). Causes of dispersal
In spite of all the above-listed costs, dispersal and metastasis are not rare phenomena, in wildlife and in cancer patients, respectively. Leaving their birth habitat to find a more suitable environment, to ‘hedge their bets’ against temporal fluctuations of the environment or to favour their kin, therefore appears to be mutually beneficial for individuals and cancer cells (figure 1a) [16,17].
Several factors can make a habitat less suitable for a species to thrive in, especially habitat decay: a sudden burst of proliferation in a community accelerates resource consumption and waste production, which in turn might lead to a depleted, toxic ecosystem. This phenomenon has been well documented in phosphorus- and nitrogen-polluted aquatic and marine ecosystems, among which many are at high risk of collapse due to recurrent algal blooms [48]. In the absence of higher-level consumers, pulse eutrophication (sporadic increases of resource influx) increases consumer population size beyond equilibrium and hence enhances competition for temporally heterogeneous resources (i.e. decreases per capita resource consumption rate), which in turn should favour facultative dispersal response to emigrate towards more suitable environments (i.e. eutrophic patches that are still devoid of competitors [17]). A similar phenomenon can actually be identified in tumours: the rapid proliferation of cancer cells quickly overwhelms the physiological turnover of oxygen and nutrients, and the emergence of glycolytic subclones favours the accumulation of lactic acid in the micro-environment. The resulting hypoxic, acidic micro-environment (the ‘cancer swamp’ [16,49]) is part of the tumour niche construction, but is hostile to the survival of acid-non-adapted cells [50]. The emergence of the cancer swamp could allegedly be a leading ultimate cause of dissemination and metastasis: not only could this degraded ecosystem theoretically not support a larger cell population [51], but many cancer cells might have better chances to thrive in other distant, undegraded habitats [16,52,53]. In agreement with this hypothesis, the emergence of hypoxia has been proximally linked to metastasis through pericyte depletion and the effect of hypoxia-induced transcription factors favouring intra- and extravasation of cancerous cells [54–56].
Whether cancer cells may evolve under kin selection remains debated [57]. However, cancer cells are involved in cooperative interactions (e.g. the collective production of growth factors [58]), which benefits could be mediated by their distance to related cells [59]. Indeed, limited dispersal favours spatial proximity between related individuals and enhances the likelihood of kin competition, which can cancel out the transmission of benefits among kin. Thus, on the one hand, many species should evolve long-distance dissemination specifically to avoid kin competition [18,60]. On the other hand, cooperation and aggregation can still favour the evolution of plastic negatively density-dependent long-range dispersal, when cooperators end up clumped together in a new habitat with weak competition [61]. In the absence of phenotypic plasticity of dispersal and in sexually reproducing populations, theoretical models predict that cooperation and dispersal tend to not co-evolve jointly, either through alternative evolutionary outcomes (i.e. evolution towards cooperation or dispersal, but not both [62]; but see [63] in the case of budding dispersal) or through the evolution of social polymorphisms with self-serving dispersers and sessile cooperators [64]. Circulating tumour cells (CTCs) have themselves been observed to disperse alone and in groups of up to around 100 cells (CT clusters [65]), and cancer cells reproduce asexually, so it is uncertain which of the aforementioned evolutionary outcomes is the most likely to explain a role of kin selection in metastasis.
3. Modes of cancer cell dispersal
Many cancer cells may be exposed to ecological factors that could drive them to metastasis, but only a few of them will disseminate and metastasize. This can be explained by a strong selection of adaptations to dissemination, with some cells being more prone than others to join dispersal (i.e. inter-cell heterogeneity). Identifying and targeting dispersal-prone cells might help the early containment of the metastatic process. Besides, several routes are likely to lead to metastasis [66], but why and how cells engage in one route rather than another remains to be determined.
(a). Which cells should disperse?
As argued above, the depreciation of the tumour micro-environment is likely to drive cancer cells to metastasis. Yet, should every cancer cell metastasize? The answer is obviously ‘no’, as a significant reduction in population density might be sufficient to lower the pressure on the remaining cells (e.g. [60]). But then, should dissemination intensify as the tumour grows (e.g. [67]), or should tumour size decrease or remain steady as disseminating cells flee the ship? Recent experimental evidence indicates that cancer cell dissemination may start as early as 1 year after initiation [68–71], and up to 80% of metastases result from early dissemination [69]. Metastasis thus coexists with other strategies that can lead cancer cells to thrive in the primary tumour micro-environment. Indeed, generally there is a trade-off between dispersal and local adaptation [72,73]: the cost of leaving can be higher than the cost of specializing to a particular habitat type, which in turn would discourage dispersal if habitat types keep constant in time. The fate of cancer cells towards metastasis could, therefore, be determined by the relative cost of local adaptation, which should vary a lot due to the spatial heterogeneity of primary tumours [74], and the comparative disadvantage of generalism versus specialization in local adaptation to environmental conditions. Moreover, since the permissivity to cancer proliferation varies with organs and over time, disseminating cancer cells could maximize the odds that cancer cells survive most micro-environmental changes.
Although metastasis could be an adaptation to the progressive transformation of the tumour into a cancer swamp [16,49], cancer cells can also fix their micro-environment by growing de novo blood vessels through the recruitment of endothelial cells (angiogenesis [56]) or by forming channel-like structures (vasculogenic mimicry [75]). This probably results in the spatial redistribution of glucose, oxygen and waste [74], and might determine the fate of cancer cell populations. In the areas of the tumour most distant to blood vessels, perturbations are temporally correlated (as little oxygen and metabolites diffuse till there), which might favour local adaptation as acid-tolerant, slow-growing subclones [74] rather than dispersal [22,24]. Closest to the blood vessels, the local concentrations of oxygen and nutrients are overall higher, but fluctuate even more in time [76,77]. One can thus expect that cancer cells surrounding blood vessels are the most prone to disperse (e.g. [75]), as they experience a highly fluctuating environment [22,24]. Yet, to differentiate this ultimate hypothesis from the proximal mechanism of a nearer access to blood flow, migration rates should be measured when resources are provided to cells with different schedules in in vitro cell culture models.
(b). To disperse alone or collectively?
Although CTCs have been known since the 1950s, collective routes to metastasis have not been in the spotlight of cancer research until recently [45,65]. The recent discovery of CT clusters goes hand in hand with their huge metastatic potential: they have been observed to be up to 50-fold more likely to establish metastases than independent CTCs and they are escorted by neutrophils [33,78–81]. Indeed, cells of CT clusters are clumped together by intercellular adhesion proteins [33,34], which protect them from anoikis during dissemination in blood vessels [82]. Moreover, despite being bigger than individual CTCs, CT clusters can actually join capillary microcirculation [83], where they are prone to be intercepted and form a microemboli (a small blot clot in the bloodstream) to the direct vicinity of an organ to metastasize [33]. Finally, as observed in xenografts [84], several distinct clones are necessary to form a tumour de novo. Contrary to independent CTCs, many CT clusters are oligo- or polyclonal [33,34,85], and thus could settle more easily by importing their collective niche in the new habitat [45,86].
Yet, despite the potential efficacy of CT clusters to produce metastases, they are found in only less than 20% of cancer patients, whereas independent CTCs are more widespread and abundant (greater than 60% of patients [87,88]) and emerge much earlier than CT clusters [87]. This apparent paradox could be compared to the competition–colonization trade-off [89,90]: for a given amount of resource allocated to dispersal, an individual can produce either few efficient propagules or many inefficient propagules, or any intermediate strategy between these two extremes. CTCs and CT clusters thus would be two distinct strategies achieving the same role with different levels of effectiveness. Independent CTCs might first colonize unoccupied sites, while the more efficient CT clusters might then outcompete the preexistent micrometastases in a few sites.
The kin selection might also play a role in the coexistence of both strategies. If the competition–colonization trade-off has been reported both in multicellular [91] and unicellular organisms [92], budding dispersal has mostly been reported to evolve under kin selection, notably in social arthropods (colony fission [93]; ballooning in arachnids [94]) and social microorganisms [95]. Dispersal in small groups can favour the evolutionary persistence of altruistic behaviours [95], and CT clusters might thus be the expression of preferential assortment (e.g. [96]). Moreover, Giuliano et al. [45] recently argued that the highly metastatic potential of CT clusters could be the result of the initiation of a pre-metastatic niche by independent CTCs—a phenomenon that was earlier reported for the primary tumours [46,47,97]. Independent CTC dispersal would thus also be the result of a complex, altruistic strategy driven by kin selection, independent CTCs paving the way for future CT cluster dispersal and ensuing metastasis.
4. The timing of cancer cell dispersal
Although metastasis is usually the final stage of cancer progression, there has been much debate on when it does really occur. The most recent estimates support early origins: cancer cell dissemination can occur before malignancy is histologically detectable [71,98]. Dissemination as early as 1 year after initiation can yield distant metastases [70]—more than 80% of them in the case of breast cancer [69,99]. Yet, if most micrometastases are formed early, it is unclear if CTCs and CT clusters found in the blood flow during clinical stages have evolved to disperse, and why metastases only become detectable in late cancer progression.
(a). The same causes through time?
A likely scenario is that the selective pressure driving cancer cell dispersal might shift through time, with late clinical-stage pressure strongly differing from selective pressure acting on cell dissemination abilities at the onset of the primary tumour development (figure 2 for a summary). Indeed, though competition for the colonization of new sites could be quite low during early dissemination, the relative benefits from dispersing should still make up for the risk of dying and/or settlement failure [100]. Early dissemination thus could be driven—at least in part—by the avoidance of kin competition: in early tumours, relatedness is expected to be very high among cancer cells and between cancer cells and the neighbouring tissues. Whether the degradation of the primary tumour is a significant enough pressure to drive metastasis is unclear at this stage. The potential benefits of dispersing towards different sites should gradually decrease as micrometastases are formed (and thus competitive pressure for the invasion of healthy organs increases), but selective pressures on dispersal could also increase throughout cancer progression due to modifications of the local environment. As the primary tumour grows, its degradation should worsen due to the growing accumulation of toxic waste. Moreover, the diversification of subclones across time [101] is likely to decrease the relatedness between cancer cells, which likely favours the emergence of cheaters and the collapse of cooperation networks [58]. Both micro-environmental decay and the lack of supportive interactions could make the tumour unbearable for many cancer cells, which would then have better chances of sustenance in other habitats, though they would be uncertain to settle in any of them. The relative decrease in relatedness among cancer cells in advanced tumours might also select negatively against dispersal [102,103], but the strength of the kin competition effect on dispersal is expected to decrease as population size increases anyway [104–106], so this effect might be very minor when compared with the disruption of cooperation networks mentioned above.
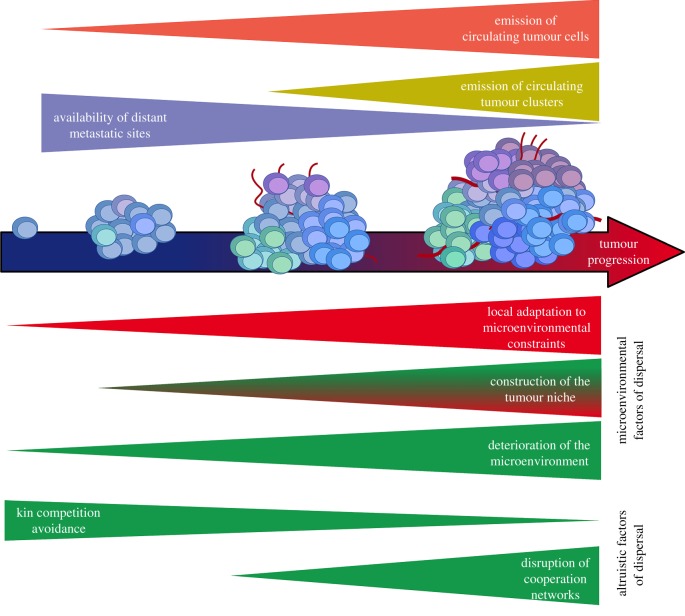
Figure 2.
Causes of cancer cell dispersal over time. Over cancer progression, more and more CTCs emerge from the primary tumour, soon to be replaced by CT clusters in the lymph and blood flow. The availability of metastatic sites thus decreases with the settlement of the first micrometastases. The drivers of cancer cell dispersal (displayed in green) involve micro-environmental factors and altruism between cancer cells. On the one hand, the deterioration of the primary tumour micro-environment and the increased access to vessels (due to the tumour niche construction) increase the likelihood that cancer cells join the lymph and blood flow, whereas niche construction and local adaptation decrease the benefits of dispersal. On the other hand, the diversification of subclones during the primary tumour growth decreases the pressure of kin competition avoidance, but increase the risk of disruption of cooperative networks. (Online version in colour.)
Opportunities of dispersal might even be stronger as cancer progresses. On the one hand, cancer cells have more time to evolve complex adaptations to dissemination and settlement, such as invasion and motility [13,32,107,108]. Moreover, therapeutical interventions and the immune system select cancer cells for treatment resistance [109], immune editing and immune regulation throughout cancer progression [110], which results in better chances of survival and settlement for late-disseminating cells [111]. On the other hand, as free metastatic sites become scarcer, the selection on metastatic abilities becomes stronger: either to successfully construct their niches in free sites or to outcompete previous settlers. This could explain the late emergence of CT clusters [112], in which metastatic potential is much higher than in CTCs [33].
(b). A kin selection perspective on metastatic dormancy
Though most micrometastases are formed early, the late occurrence of macrometastases has mostly been explained by dormancy [100,113–115]: once settled in a new habitat, metastatic cancer cells arrest cell division until reactivation. Thus, metastatic dormancy has sometimes been deemed imposed by immune control [116]. However, it could also be considered adaptive for metastatic cells themselves: dormant cells go under the radar of the immune system, and their stem-cell-like state allows them to survive during the time they adapt to their new habitat [117].
There is also some evidence that the primary tumour is able to inhibit metastatic proliferation [118,119], as undetected micrometastases frequently grow into macrometastases after the primary tumour is surgically resected. A similar phenomenon can be observed in many plant species, which force their seeds into dormancy so that they are reactivated only when environmental conditions are suited to germination [120]. But seed dormancy can also play a role in kin competition avoidance: dormancy of natal dispersers decreases the likelihood that two related individuals grow at the same time, and therefore compete for resources. Kin selection thus favours seed dormancy in species that rarely disperse or that disperse to short distances [121]. Early-disseminating cancer cells may likewise evolve the ability to enter dormancy if they receive benefits from related, neighbouring cells [122]. It is important to notice though that, alternatively, non-dispersing cells could simply evolve the ability to force other cells into dormancy in order to avoid competition altogether.
Conversely, metastatic dormancy could be the result of altruistic behaviour produced by the primary tumour and early dispersers to favour the settlement of late dispersers. Indeed, primary tumours have been observed to remotely initiate pre-metastatic niches in other organs, through the secretion of exosomes [46,47]. It is unclear whether this behaviour is adaptive or a non-adaptive by-product, but similar behaviours from early-disseminating CTCs have been hypothesized to explain the huge metastatic ability of late CT clusters [45]. The waste of early-disseminating cells favouring late dispersers could—at least partly—explain the late occurrence of macrometastases. If altruistic, these behaviours should evolve under kin selection; yet metastases could also behave as a stock of seeds that can support the primary tumour if needed [123].
5. Future directions
In this paper, we have discussed many aspects of the metastatic cascade and we have drawn similarities to the dispersal process—some of which remain untested and/or controversial. Here, we discuss a few alternative explanations for the metastatic process, and suggest a protocol to study the existence of dispersal dynamics in cancer.
(a). Complementary explanations
The fact that the dissemination of cancer cells is under selection is by no means a guarantee that the important selection pressures are the same as those found in dispersing organisms. There are actually other incentives for the primary tumour to spread some of its cells towards multiple other sites. For instance, cancer cell movement could evolve as an extremely altruistic behaviour, such as a lure-based defense mechanism against the immune system. Indeed, some CTCs could be a bait to lure the immune system out of the range of the primary tumour [124]: by scattering the attacks of the immune system, such a strategy could be adaptive for the primary tumour. Most of CTCs would be sacrificed but, by chance, a few of them could shelter in other tissues, and eventually form metastases. Under this hypothesis, one could expect not only that CTCs have a lower fitness than non-dispersers, but also that non-dispersers' fitness would drop if CTCs were selectively removed (e.g. with monoclonal antibodies) before being detected by the immune system. Another hypothesis would be that metastasis occurs as a result of group selection [125]. Given several cancer cell lineages simultaneously evolve in the body, primary tumours able to yield secondary tumours might be able to invade new environments, and thus to locally outcompete the tumours that do not disseminate. Under this hypothesis, metastasis would be a strategy allowing the primary and secondary tumours, as a whole, to outcompete every other lineage from the body.
(b). Putting the dispersal hypothesis to the test
If metastasis relies on the same causes as the evolution of dispersal, it could be triggered by the following causes: (H1) the deterioration of environmental conditions within the primary tumour and (H2) altruistic behaviours among kin cells. We suggest that a xenograft experiment might help investigate whether some of these factors can control the dynamics of metastasis, and ultimately whether the evolution of dispersal is a relevant paradigm to study metastasis. In the experiment described below, we propose (i) to isolate a few cancer lineages from in vivo tumours, (ii) to investigate their genotypes and phenotypes and (iii) to mix them into tumours à la carte. By xenografting these tumours into isogenic mice, by controlling for mice microbiota to avoid the onset of heterogeneous immunotherapy [126] and by following metastasis rates, one might be able to test all the hypotheses. It is important to note that these tumours could also be implanted in chemostats to follow dispersal rates in vitro, by counting cells acquiring disseminating traits.
To test the hypothesis (H1), we propose to use xenograft mice with unvascularized tumours composed of oxygen-dependent lineages. In the test groups, xenografts would contain a range of frequencies of fermentative, oxygen-independent lineages. As the fermentative cells should pollute their micro-environment more quickly than the respiratory ones, we predict that the number of metastases growing in mice would increase with the initial frequency of oxygen-independent lineages. To test the hypothesis (H2), we propose to use xenograft mice with unvascularized tumours composed of several genetically distinct clones. We predict that the number of metastases would be the highest for intermediate levels of intra-tumour heterogeneity: a certain level of genetic diversity is necessary for tumours to adapt (alternatively for metastases to settle), but cancer cells would have no incentive towards metastasis in the most heterogeneous tumours where virtually no other cell will be related to them.
It is important to note that we do not propose the hypotheses to be mutually exclusive. In fact, the metastatic process could be due to a combination of different phenomena in response to different selective pressures, so that the inefficiency of the metastatic process could be ‘only apparent’.
Glossary
- altruistic
what increases another individual's fitness while decreasing the carrier's
- circulating tumour cells (CTCs)
cells that have shed into the vasculature or lymphatics from a primary tumour
- dispersal
movement of organisms from their birth site to their breeding site
- hypoxia
condition in which a region of the body is deprived of adequate oxygen supply at the tissue level
- kin selection
evolutionary strategy that favours the reproductive success of an organism's relatives
- metastase
secondary tumour formed by cells derived from a primary site
- metastasis
spread of cancer cells from a primary site to a secondary site within the body
- niche construction
the process by which an organism alters its own local environment
- xenograft
tissue transplant from another species
Supplementary Material
Acknowledgements
The authors thank Athena Aktipis and the three anonymous reviewers for their helpful comments on the article.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
F.T. and B.U. are supported by an ANR TRANSCAN (ANR-18-CE35-0009), the Rotary Club Les Sables d'Olonne, and a CNRS ‘International Associated Laboratory Grant’.
References
- 1.Marshall E. 2011. Cancer research and the $90 billion metaphor. Science 331, 1540–1541. ( 10.1126/science.331.6024.1540-a) [DOI] [PubMed] [Google Scholar]
- 2.Svensson E, Christiansen CF, Ulrichsen SP, Rørth MR, Sørensen HT. 2017. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open 7, e016022 ( 10.1136/bmjopen-2017-016022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastushenko I, et al. 2018. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468. ( 10.1038/s41586-018-0040-3) [DOI] [PubMed] [Google Scholar]
- 4.Glinsky VV. 2006. Intravascular cell-to-cell adhesive interactions and bone metastasis. Cancer Metastasis Rev. 25, 531–540. ( 10.1007/s10555-006-9029-8) [DOI] [PubMed] [Google Scholar]
- 5.Sleeman JP, Nazarenko I, Thiele W. 2011. Do all roads lead to Rome? Routes to metastasis development. Int. J. Cancer 128, 2511–2526. ( 10.1002/ijc.26027) [DOI] [PubMed] [Google Scholar]
- 6.Brabletz T. 2012. To differentiate or not—routes towards metastasis. Nat. Rev. Cancer 12, 425–436. ( 10.1038/nrc3265) [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Zhang Y, Kriska A, Chen H. 2016. Epigenetic regulation in cancer metastasis. In Medical epigenetics (ed. Tollefsbol T.), pp. 499–514. Amsterdam, The Netherlands: Elsevier B.V. [Google Scholar]
- 8.Nguyen DX, Bos PD, Massagué J. 2009. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284. ( 10.1038/nrc2622) [DOI] [PubMed] [Google Scholar]
- 9.Ujvari B, Gatenby RA, Thomas F. 2017. Transmissible cancer: the evolution of interindividual metastasis. In Ecology and evolution of cancer (eds Ujvari B, Roche B, Thomas F), pp. 167–179. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 10.Sullivan R, Graham CH. 2007. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 26, 319–331. ( 10.1007/s10555-007-9062-2) [DOI] [PubMed] [Google Scholar]
- 11.Klein CA. 2013. Selection and adaptation during metastatic cancer progression. Nature 501, 365–372. ( 10.1038/nature12628) [DOI] [PubMed] [Google Scholar]
- 12.Chen K-W, Pienta KJ. 2011. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theor. Biol. Med. Model. 8, 36 ( 10.1186/1742-4682-8-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aktipis CA, Maley CC, Pepper JW. 2011. Dispersal evolution in neoplasms: the role of disregulated metabolism in the evolution of cell motility. Cancer Prev. Res. 5, 266–275. ( 10.1158/1940-6207.CAPR-11-0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Sprouffske K, Huang Q, Maley CC. 2011. Solving the puzzle of metastasis: the evolution of cell migration in neoplasms. PLoS ONE 6, e17933 ( 10.1371/journal.pone.0017933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pienta KJ, Robertson BA, Coffey DS, Taichman RS. 2013. The cancer diaspora: metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 19, 5849–5855. ( 10.1158/1078-0432.CCR-13-2158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amend SR, Roy S, Brown JS, Pienta KJ. 2016. Ecological paradigms to understand the dynamics of metastasis. Cancer Lett. 380, 237–242. ( 10.1016/j.canlet.2015.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 18.Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253. ( 10.1146/annurev.ecolsys.38.091206.095611) [DOI] [Google Scholar]
- 19.Duputié A, Massol F. 2013. An empiricist's guide to theoretical predictions on the evolution of dispersal. Interface Focus 3, 20130028 ( 10.1098/rsfs.2013.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkau BJ, Feldman MW. 1973. Selection for migration modification. Genetics 74, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastings A. 1983. Can spatial variation alone lead to selection for dispersal? Theor. Popul. Biol. 24, 244–251. ( 10.1016/0040-5809(83)90027-8) [DOI] [Google Scholar]
- 22.Travis JMJ. 2001. The color of noise and the evolution of dispersal. Ecol. Res. 16, 157–163. ( 10.1046/j.1440-1703.2001.00381.x) [DOI] [Google Scholar]
- 23.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 24.Massol F, Débarre F. 2015. Evolution of dispersal in spatially and temporally variable environments: the importance of life cycles. Evolution 69, 1925–1937. ( 10.1111/evo.12699) [DOI] [PubMed] [Google Scholar]
- 25.Doebeli M, Ruxton GD. 1997. Evolution of dispersal rates in metapopulation models: branching and cyclic dynamics in phenotype space. Evolution 51, 1730–1741. ( 10.1111/j.1558-5646.1997.tb05097.x) [DOI] [PubMed] [Google Scholar]
- 26.Roff DA. 1994. Habitat persistence and the evolution of wing dimorphism in insects. Am. Nat. 144, 772–798. ( 10.1086/285706) [DOI] [Google Scholar]
- 27.Hanski I, Alho J, Moilanen A. 2000. Estimating the parameters of survival and migration of individuals in metapopulations. Ecology 81, 239–251. ( 10.1890/0012-9658(2000)081[0239:ETPOSA]2.0.CO;2) [DOI] [Google Scholar]
- 28.Hanski I, Erälahti C, Kankare M, Ovaskainen O, Sirén H. 2004. Variation in migration propensity among individuals maintained by landscape structure. Ecol. Lett. 7, 958–966. ( 10.1111/j.1461-0248.2004.00654.x) [DOI] [Google Scholar]
- 29.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. 1998. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873. ( 10.1016/S0002-9440(10)65628-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zera AJ, Denno RF. 1997. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230. ( 10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]
- 31.Tackenberg O, Poschlod P, Kahmen S. 2003. Dandelion seed dispersal: the horizontal wind speed does not matter for long-distance dispersal—it is updraft! Plant Biol. 5, 451–454. ( 10.1055/s-2003-44789) [DOI] [Google Scholar]
- 32.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. 2009. Localized and reversible TGF-beta signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell Biol. 11, 1287–1296. ( 10.1038/ncb1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aceto N, et al. 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. ( 10.1016/j.cell.2014.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung KJ, et al. 2016. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863. ( 10.1073/pnas.1508541113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McWilliams SR, Guglielmo C, Pierce B, Klaassen M. 2004. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377–393. ( 10.1111/j.0908-8857.2004.03378.x) [DOI] [Google Scholar]
- 36.Bonnet X, Naulleau G, Shine R. 1999. The dangers of leaving home: dispersal and mortality in snakes. Biol. Conserv. 89, 39–50. ( 10.1016/S0006-3207(98)00140-2) [DOI] [Google Scholar]
- 37.Cote J, Fogarty S, Tymen B, Sih A, Brodin T. 2013. Personality-dependent dispersal cancelled under predation risk. Proc. R. Soc. B 280, 20132349 ( 10.1098/rspb.2013.2349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta GP, Massagué J. 2006. Cancer metastasis: building a framework. Cell 127, 679–695. ( 10.1016/j.cell.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 39.Blate GM, Peart DR, Leighton M. 1998. Post-dispersal predation on isolated seeds: a comparative study of 40 tree species in a Southeast Asian rainforest. Oikos 82, 522–538. ( 10.2307/3546373) [DOI] [Google Scholar]
- 40.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forero MG, Donazar JA, Hiraldo F. 2002. Causes and fitness consequences of natal dispersal in a population of black kites. Ecology 83, 858–872. ( 10.1890/0012-9658(2002)083[0858:CAFCON]2.0.CO;2) [DOI] [Google Scholar]
- 42.Hinsley SA. 2000. The costs of multiple patch use by birds. Landsc. Ecol. 15, 765–775. ( 10.1023/A:1008149403852) [DOI] [Google Scholar]
- 43.Huang D, Todd PA, Guest JR. 2007. Movement and aggregation in the fluted giant clam (Tridacna squamosa L.). J. Exp. Mar. Bio. Ecol. 342, 269–281. ( 10.1016/j.jembe.2006.10.051) [DOI] [Google Scholar]
- 44.Eyles J, et al. 2010. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039. ( 10.1172/JCI42002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giuliano M, Shaikh A, Lo HC, Arpino G, De Placido S, Zhang XH, Cristofanilli M, Schiff R, Trivedi MV. 2018. Perspective on circulating tumor cell clusters: why it takes a village to metastasize. Cancer Res. 78, 1–8. ( 10.1158/0008-5472.CAN-17-2748) [DOI] [PubMed] [Google Scholar]
- 46.Costa-Silva B, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. ( 10.1038/ncb3169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshino A, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. ( 10.1038/nature15756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heisler J, et al. 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. ( 10.1016/j.hal.2008.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amend SR, Pienta KJ. 2015. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget 6, 9669–9678. ( 10.18632/oncotarget.3430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatenby RA, Gillies RJ. 2004. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899. ( 10.1038/nrc1478) [DOI] [PubMed] [Google Scholar]
- 51.Deisboeck TS, Wang Z. 2007. Cancer dissemination: a consequence of limited carrying capacity? Med. Hypotheses 69, 173–177. ( 10.1016/j.mehy.2006.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aktipis A. 2016. Principles of cooperation across systems: from human sharing to multicellularity and cancer. Evol. Appl. 9, 17–36. ( 10.1111/eva.12303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiffman JD, White RM, Graham TA, Huang Q, Aktipis A. 2016. The Darwinian dynamics of motility and metastasis. In Frontiers in cancer research: evolutionary foundations, revolutionary directions (eds Maley CC, Greaves M), pp. 135–176. Berlin, Germany: Springer. [Google Scholar]
- 54.Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, Ellies L, Johnson RS. 2012. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell 21, 52–65. ( 10.1016/j.ccr.2011.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke VG, et al. 2012. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21, 66–81. ( 10.1016/j.ccr.2011.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanahan D, Coussens LM. 2012. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322. ( 10.1016/j.ccr.2012.02.022) [DOI] [PubMed] [Google Scholar]
- 57.Arnal A, et al. 2015. Evolutionary perspective of cancer: myth, metaphors and reality. Evol. Appl. 8, 541–544. ( 10.1111/eva.12265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Archetti M, Ferraro DA, Christofori G. 2015. Heterogeneity for IGF-II production maintained by public goods dynamics in neuroendocrine pancreatic cancer. Proc. Natl Acad. Sci. USA 112, 1833–1838. ( 10.1073/pnas.1414653112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton WD, May RM. 1977. Dispersal in stable habitats. Nature 269, 578–581. ( 10.1038/269578a0) [DOI] [Google Scholar]
- 60.Bitume EV, Bonte D, Ronce O, Bach F, Flaven E, Olivieri I, Nieberding CM. 2013. Density and genetic relatedness increase dispersal distance in a subsocial organism. Ecol. Lett. 16, 430–437. ( 10.1111/ele.12057) [DOI] [PubMed] [Google Scholar]
- 61.Jacob S, Wehi P, Clobert J, Legrand D, Schtickzelle N, Huet M, Chaine A. 2016. Cooperation-mediated plasticity in dispersal and colonization. Evolution (NY) 70, 2336–2345. ( 10.1111/evo.13028) [DOI] [PubMed] [Google Scholar]
- 62.Le Galliard J-F, Ferrière R, Dieckmann U. 2005. Adaptive evolution of social traits: origin, trajectories, and correlations of altruism and mobility. Am. Nat. 165, 206–224. ( 10.1086/427090) [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues AMM, Taylor TB. 2018. Ecological and demographic correlates of cooperation from individual to budding dispersal. J. Evol. Biol. 31, 1058–1070. ( 10.1111/jeb.13286) [DOI] [PubMed] [Google Scholar]
- 64.Mullon C, Keller L, Lehmann L. 2018. Social polymorphism is favoured by the co-evolution of dispersal with social behaviour. Nat. Ecol. Evol. 2, 132–140. ( 10.1038/s41559-017-0397-y) [DOI] [PubMed] [Google Scholar]
- 65.Cheung KJ, Ewald AJ. 2016. A collective route to metastasis: seeding by tumor cell clusters. Science 352, 167–169. ( 10.1126/science.aaf6546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katt ME, Wong AD, Searson PC. 2018. Dissemination from a solid tumor: examining the multiple parallel pathways. Trends Cancer 4, 20–37. ( 10.1016/j.trecan.2017.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronce O, Brachet S, Olivieri I, Gouyon P-H, Clobert J. 2005. Plastic changes in seed dispersal along ecological succession: theoretical predictions from an evolutionary model. J. Ecol. 93, 431–440. ( 10.1111/j.1365-2745.2005.00972.x) [DOI] [Google Scholar]
- 68.Zhao Z, Zhao B, Bai Y, Iamarino A, Gaffney SG, Schlessinger J. 2016. Early and multiple origins of metastatic lineages within primary tumors. Proc. Natl Acad. Sci. USA 113, 2140–2145. ( 10.1073/pnas.1525677113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosseini H, et al. 2016. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558. ( 10.1038/nature20785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lote H, et al. 2017. Carbon dating cancer: defining the chronology of metastatic progression in colorectal cancer. Ann. Oncol. 28, 1243–1249. ( 10.1093/annonc/mdx074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhim AD, et al. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361. ( 10.1016/j.cell.2011.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kisdi É. 2002. Dispersal: risk spreading versus local adaptation. Am. Nat. 159, 579–596. ( 10.1086/339989) [DOI] [PubMed] [Google Scholar]
- 73.Berdahl A, Torney CJ, Schertzer E, Levin SA. 2015. On the evolutionary interplay between dispersal and local adaptation in heterogeneous environments. Evolution (NY) 69, 1390–1405. ( 10.1111/evo.12664) [DOI] [PubMed] [Google Scholar]
- 74.Alfarouk KO, Ibrahim ME, Gatenby RA, Brown JS. 2013. Riparian ecosystems in human cancers. Evol. Appl. 6, 46–53. ( 10.1111/eva.12015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagenblast E, et al. 2015. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 520, 358–362. ( 10.1038/nature14403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cárdenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. 2008. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 68, 5812–5819. ( 10.1158/0008-5472.CAN-07-6387) [DOI] [PubMed] [Google Scholar]
- 77.Fang JS, Gillies RD, Gatenby RA. 2008. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin. Cancer Biol. 18, 330–337. ( 10.1016/j.semcancer.2008.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aceto N, Toner M, Maheswaran S, Haber DA. 2015. En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer 1, 44–52. ( 10.1016/j.trecan.2015.07.006) [DOI] [PubMed] [Google Scholar]
- 79.Fabisiewicz A, Grzybowska E. 2017. CTC clusters in cancer progression and metastasis. Med. Oncol. 34, 12 ( 10.1007/s12032-016-0875-0) [DOI] [PubMed] [Google Scholar]
- 80.Szczerba BM, et al. 2019. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557. ( 10.1038/s41586-019-0915-y) [DOI] [PubMed] [Google Scholar]
- 81.Gkountela S, et al. 2019. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112. ( 10.1016/j.cell.2018.11.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, Yu L-G. 2010. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154 ( 10.1186/1476-4598-9-154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Au SH, et al. 2015. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl Acad. Sci. USA 113, 4947–4952. ( 10.1073/pnas.1524448113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tabassum DP, Polyak K. 2015. Tumorigenesis: it takes a village. Nat. Rev. Cancer 15, 473–483. ( 10.1038/nrc3971) [DOI] [PubMed] [Google Scholar]
- 85.Maddipati R, Stanger BZ. 2015. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 5, 1086–1097. ( 10.1158/2159-8290.CD-15-0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Psaila B, Lyden D. 2009. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9, 285–293. ( 10.1038/nrc2621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hou J-M, et al. 2012. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532. ( 10.1200/JCO.2010.33.3716) [DOI] [PubMed] [Google Scholar]
- 88.Wang C, et al. 2017. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. 161, 83–94. ( 10.1007/s10549-016-4026-2) [DOI] [PubMed] [Google Scholar]
- 89.Hastings A. 1980. Disturbance, coexistence, history, and competition for space. Theor. Popul. Biol. 18, 363–373. ( 10.1016/0040-5809(80)90059-3) [DOI] [Google Scholar]
- 90.Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16. ( 10.2307/1939377) [DOI] [Google Scholar]
- 91.Turnbull LA, Coomes D, Hector A, Rees M. 2004. Seed mass and the competition/colonization trade-off: competitive interactions and spatial patterns in a guild of annual plants. J. Ecol. 92, 97–109. ( 10.1111/j.1365-2745.2004.00856.x) [DOI] [Google Scholar]
- 92.Livingston G, Matias M, Calcagno V, Barbera C, Combe M, Leibold MA, Mouquet N. 2012. Competition-colonization dynamics in experimental bacterial metacommunities. Nat. Commun. 3, 1234 ( 10.1038/ncomms2239) [DOI] [PubMed] [Google Scholar]
- 93.Cronin AL, Molet M, Doums C, Monnin T, Peeters C. 2013. Recurrent evolution of dependent colony foundation across eusocial insects. Annu. Rev. Entomol. 58, 37–55. ( 10.1146/annurev-ento-120811-153643) [DOI] [PubMed] [Google Scholar]
- 94.Clotuche G, Navajas M, Mailleux AC, Hance T. 2013. Reaching the ball or missing the flight? Collective dispersal in the two-spotted spider mite Tetranychus urticae. PLoS ONE 8, e77573 ( 10.1371/journal.pone.0077573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kümmerli R, Gardner A, West SA, Griffin AS. 2009. Limited dispersal, budding dispersal, and cooperation: an experimental study. Evolution (NY) 63, 939–949. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher JA, Doebeli M. 2009. A simple and general explanation for the evolution of altruism. Proc. R. Soc. B 276, 13–19. ( 10.1098/rspb.2008.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nielsen SR, et al. 2016. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat. Cell Biol. 18, 549–560. ( 10.1038/ncb3340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hüsemann Y, et al. 2008. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68. ( 10.1016/j.ccr.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 99.Harper KL, et al. 2016. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592. ( 10.1038/nature20609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, Wakatsuki T, Loupakis F, Lenz H-J. 2011. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin. Cancer Res. 18, 645–653. ( 10.1158/1078-0432.CCR-11-2186) [DOI] [PubMed] [Google Scholar]
- 101.Gerlinger M, et al. 2012. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892. ( 10.1056/NEJMoa1113205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank SA. 1986. Dispersal polymorphisms in subdivided populations. J. Theor. Biol. 122, 303–309. ( 10.1016/S0022-5193(86)80122-9) [DOI] [PubMed] [Google Scholar]
- 103.Taylor PD. 1988. An inclusive fitness model for dispersal of offspring. J. Theor. Biol. 130, 363–378. ( 10.1016/S0022-5193(88)80035-3) [DOI] [Google Scholar]
- 104.Leturque H, Rousset F. 2002. Dispersal, kin competition, and the ideal free distribution in a spatially heterogeneous population. Theor. Popul. Biol. 62, 169–180. ( 10.1006/tpbi.2002.1600) [DOI] [PubMed] [Google Scholar]
- 105.Ajar E. 2004. Analysis of disruptive selection in subdivided populations. BMC Evol. Biol. 3, 22 ( 10.1186/1471-2148-3-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Massol F, Duputié A, David P, Jarne P. 2010. Asymmetric patch size distribution leads to disruptive selection on dispersal. Evolution (NY) 65, 490–500. ( 10.1111/j.1558-5646.2010.01143.x) [DOI] [PubMed] [Google Scholar]
- 107.Gerlee P, Anderson ARA. 2009. Evolution of cell motility in an individual-based model of tumour growth. J. Theor. Biol. 259, 67–83. ( 10.1016/j.jtbi.2009.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee H-O, Silva AS, Concilio S, Li Y-S, Slifker M, Gatenby RA, Cheng JD. 2011. Evolution of tumor invasiveness: the adaptive tumor microenvironment landscape model. Cancer Res. 71, 6327–6337. ( 10.1158/0008-5472.CAN-11-0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soler A, Cayrefourcq L, Mazard T, Babayan A, Lamy PJ, Assou S, Assenat E, Pantel K, Alix-Panabières C. 2018. Autologous cell lines from circulating colon cancer cells captured from sequential liquid biopsies as model to study therapy-driven tumor changes. Sci. Rep. 8, 15931 ( 10.1038/s41598-018-34365-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franchina DG, He F, Brenner D. 2018. Survival of the fittest: cancer challenges T cell metabolism. Cancer Lett. 412, 216–223. ( 10.1016/j.canlet.2017.10.014) [DOI] [PubMed] [Google Scholar]
- 111.Romero I, Garrido F, Garcia-Lora AM. 2014. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res. 74, 6750–6757. ( 10.1158/0008-5472.CAN-14-2406) [DOI] [PubMed] [Google Scholar]
- 112.Hou JM, et al. 2011. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 178, 989–996. ( 10.1016/j.ajpath.2010.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pantel K, Alix-Panabières C. 2019. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424. ( 10.1038/s41571-019-0187-3) [DOI] [PubMed] [Google Scholar]
- 114.Townson JL, Chambers AF. 2006. Dormancy of solitary metastatic cells. Cell Cycle 5, 1744–1750. ( 10.4161/cc.5.16.2864) [DOI] [PubMed] [Google Scholar]
- 115.Friberg S, Nyström A. 2015. Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis 8, 43–49. ( 10.4137/CGM.S31244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Röcken M. 2010. Early tumor dissemination, but late metastasis: insights into tumor dormancy. J. Clin. Invest. 120, 1800–1803. ( 10.1172/JCI43424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giancotti FG. 2013. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764. ( 10.1016/j.cell.2013.10.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Demicheli R, Retsky MW, Hrushesky WJM, Baum M. 2007. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat. Clin. Pract. Oncol. 4, 699–710. ( 10.1038/ncponc0999) [DOI] [PubMed] [Google Scholar]
- 119.Peeters CFJM, de Waal RMW, Wobbes T, Ruers TJM. 2008. Metastatic dormancy imposed by the primary tumor: does it exist in humans? Ann. Surg. Oncol. 15, 3308–3315. ( 10.1245/s10434-008-0029-5) [DOI] [PubMed] [Google Scholar]
- 120.Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am. Nat. 131, 360–384. ( 10.1086/284795) [DOI] [Google Scholar]
- 121.Vitalis R, Rousset F, Kobayashi Y, Olivieri I, Gandon S. 2013. The joint evolution of dispersal and dormancy in a metapopulation with local extinctions and kin competition. Evolution (NY) 67, 1676–1691. ( 10.1111/evo.12069) [DOI] [PubMed] [Google Scholar]
- 122.Sprouffske K, Aktipis CA, Radich JP, Carroll M, Nedelcu AM, Maley CC. 2013. An evolutionary explanation for the presence of cancer nonstem cells in neoplasms. Evol. Appl. 6, 92–101. ( 10.1111/eva.12030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, Massagué J. 2009. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326. ( 10.1016/j.cell.2009.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mazel M, et al. 2015. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782. ( 10.1016/j.molonc.2015.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simon B, Fletcher JA, Doebeli M. 2013. Towards a general theory of group selection. Evolution (NY) 67, 1561–1572. ( 10.1111/j.1558-5646.2012.01835.x) [DOI] [PubMed] [Google Scholar]
- 126.Poutahidis T, Kleinewietfeld M, Erdman SE. 2014. Gut microbiota and the paradox of cancer immunotherapy. Front. Immunol. 5, 157 ( 10.3389/fimmu.2014.00157) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.