In their article, Juutilainen et al. [1]—henceforth JHLNH—presented sensible arguments explaining how magnetic fields (MF) could result in carcinogenesis. Briefly put, they combined a plausible interaction mechanism with a powerful hypothesis. The former—so-called, radical-pair mechanism (RPM)—states that MF can alter the ratio of singlet/triplet populations of radical pairs, thereby affecting the final yield of reactions that involve those populations. They proposed cryptochromes (CRYs) as the putative magnetosensitive molecules, which would work as sources of radical pairs induced by light and possibly also by light-independent processes. This choice, in term, paved the way for their hypothesis: given that CRYs are deeply involved in circadian regulation, affecting CRYs could disturb the normal physiology of cells and lead to carcinogenesis (e.g. by impairment of the DNA repair response). In other words, the physics of MF would be linked to the chemistry of CRYs through the RPM, while CRYs would be linked to the biology of carcinogenesis through their involvement in the circadian regulation of cellular processes. At the end of their article, JHLNH suggested a series of studies that could (and hopefully will) be undertaken to test the validity of their hypothesis.
Here, we find relevant to note that whereas, indeed, there are studies suggesting potentially detrimental effects of MF, there are also reports of a decrease in proliferation of cancer cells in vitro, and also of inhibition of tumour growth in mice [2]. Under the light of this apparent ambivalence regarding cancer development, it is worthwhile to ask whether the very same ideas presented by JHLNH could also encompass the possibility of using MF as therapeutic agents. In the context of this idea, it should be noted that deregulation of the cellular clock has been correlated to a loss of control of cell proliferation, metabolism, DNA replication and repair, senescence and apoptosis, as well as an increase of drug resistance in a number of cancers [3–5]. These facts are clearly in line with JHLNH's hypothesis: circadian disruption could certainly be carcinogenic. However, they also suggest the reciprocal mechanism: restoration of the circadian clock could turn out to be therapeutic. This is, indeed, the conclusion of Kiessling et al. [6], who reported that the reduction in melanoma tumour growth in mice treated with dexamethasone (DEX) was not caused by enhanced apoptosis, nor immune infiltration, but rather relied specifically on inducing circadian rhythms within the tumours. Could MF have an effect somehow similar to the one DEX had in Kiessling et al.'s study? The work by Manzella et al. [7] somehow suggests so. The authors showed that MF (0.1 mT 50 Hz for 1 h) modified the circadian rhythms of human dermal fibroblasts (HuDe). Specifically, they demonstrated a synchronizing effect of the MF: initially unsynchronized cells were entrained to the same phase in the expression of several clock genes, including Cry1. In our view, the study retrieves the notion of MF being able to work as a zeitgeber, an idea already proposed decades ago [8], which is worth exploring regardless of the details of the underlying physico-chemical interaction mechanism—which could indeed be the RPM, or some other such as alteration of free radical concentrations by metal ions under MF as in the Fenton reaction [9], the precession of magnetic moments [10], or direct action on intracellular magnetite particles [11].
At this point, based on the strong case made by JHLNH on the effect of MF on circadian rhythms, and having discussed the possibility of their being not only potentially harmful but also the contrary, it becomes of interest to ask which of the features of exposure should make the difference, and how. Here, let us introduce a distinction between three aspects of exposures: we will name them ‘positive content’, ‘intermittence’ and ‘cycling’—which will hereafter be used without the quotation marks. The first one refers to all the parameters of the MF while they are on, namely, DC amplitude, AC amplitude and frequency (or, in general, waveform), the angle between AC and DC, gradients, etc. By contrast, intermittence refers to the pattern (sequence and duration) of periods with the field ON or OFF (e.g. 1 min ON/5 min OFF), and also to the total duration of the exposure (e.g. 1 h). An analogy with light would be the following: if exposure consists of 12 h of light (L) and 12 h of darkness (D), the positive content of the exposure is characterized by the colour (spectrum) and intensity of the light, while the 12 L : 12 D h pattern characterizes the intermittence. Finally, the term cycling (sort of a ‘meta-intermittence’) refers to how often and for how long the exposures will be repeated (e.g. once a day for four weeks). We hypothesize here that the details of the positive content of MF are responsible for their coupling with molecular entities (i.e. atoms, ions, radicals, molecules), or the lack of such coupling. In other words, if the spatial and temporal configuration of the positive content is appropriate, then it will change the physico-chemical behaviour of the affected molecular entities. For instance, alteration of an enzymatic reaction rate or interference of protein–DNA interaction between transcription factors and their target genes' binding sites, such as in the circadian regulation of rhythmic gene expression. It is clear that the duration of each interval with the MF ON and that of the whole exposure must be key parameters, because too short durations could be innocuous (irrespective of the details of the positive content), and too long ones could trigger adaptation mechanisms leading to a saturation or even a complete compensation of the observed effect. An interesting example is found in the study by Cameron et al. [12], where the authors report a difference in tumour growth and tumour vascularization for exposures with the same MF parameters (10 mT half-rectified 120 Hz sine) but different durations. Another example is the generation of reactive oxygen species (ROS) in osteoblasts upon single or repeated exposures (7 min per day), where this latter led to complete adaptation after 5 days [13]. Sherrard et al. [14] also reported ROS generation upon pulsed electromagnetic fields (PEMF) and, remarkably, the effect depended on the presence of CRYs. The authors concluded that upon the appropriate ‘dose’ (quotation marks are ours), PEMF could have a clinical application, as a moderate ROS formation triggers protective mechanisms. However, they could also be harmful because it has been long known that an excess of ROS can trigger apoptosis [15]. Duration of exposure could be of paramount importance to carcinogenesis if MF were stressing enough to trigger apoptosis (e.g. by the interplay between them, CRYs and ROS generation—discussed in depth by JHLNH), but were turned off just afterwards. Indeed, Tang et al. [16], who coined the term ‘anastasis’, reported that provided a pro-apoptotic stimulus (they used ethanol) is removed after just the right exposure duration (long enough to trigger apoptosis, but shorter than the necessary for apoptosis to complete), cells can revert their fate and survive. Nevertheless, the surviving cells had a higher rate of mutations and chromosomal aberrations. In a follow-up study [17], the authors confirmed that anastatic cells underwent abnormal cell division and presented micronuclei, typical features of malignant cells.
Now, if more than one interval of positive content is used (i.e. MF are intermittent), the durations of the ‘silent’ intervals in between (i.e. MF OFF) should, at least a priori, be expected to have biological relevance. A critical study on this regard was reported by Litovitz et al. [18], who demonstrated the importance of intermittence parameters on the activity of ornithine decarboxylase (ODC) in L929 cells (they observed up to approx. 100% change of activity). It is noteworthy that ODC is a possible target in oncology, because it contributes to polyamine-induced cell proliferation and it is often overexpressed in breast, prostate, colorectal and endometrial cancers [19].
Intermittence can be either rhythmic (the duration of intervals of positive content and ‘silence’ is fixed, so a pattern repeats itself for the whole time of exposure), or arrhythmic, where interval durations are not fixed. Building on the notion of MF as a zeitgeber, we hypothesize that rhythmic exposures could lead to circadian restoration, thereby having a therapeutic effect, while arrhythmic ones could provoke circadian disruption and carcinogenicity (figure 1). However, a rhythmic intermittence could also work as a stressor, provided the rhythm happened to be sufficiently in conflict with intrinsic ultradian oscillations of the cells, which are known to span through several time-scales, from milliseconds to hours [20]. In this case, if the stressed cell was a malignant one the outcome could be beneficial (e.g. apoptosis), while if the cell was a normal one, the outcome could be harmful (e.g. by altered redox signalling and genetic instability).
Figure 1.
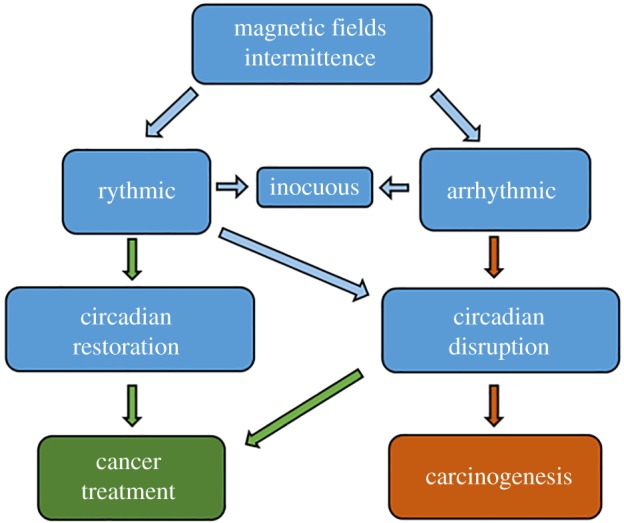
Hypotheses. The role of MF in cancer development—if any—depends on their rhythmicity, or lack of it. Rhythmic MF could lead to both, a carcinogenic or a therapeutic effect, depending on how the intrinsic oscillations of normal and malignant cells are affected. We suggest that it is unlikely that random MF would have therapeutic effects. (Online version in colour.)
In summary, we pose that it is worth exploring the possibility that MF can either induce or treat cancer through modification of circadian and ultradian rhythms. The outcome of an exposure (innocuous, detrimental or beneficial) might depend not only on the field parameters and duration of the positive content, but also on its intermittence and cycling patterns.
Supplementary Material
Data accessibility
This article does not contain any additional data.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by UNSL (grant no. PROICO 02-0518) and ANPCyT (grant no. PICT 2016-0332), Argentina. M.F.G. acknowledges a scholarship from the Instituto Nacional del Cáncer (Argentina).
References
- 1.Juutilainen J, Herrala M, Luukkonen J, Naarala J, Hore PJ. 2018. Magnetocarcinogenesis: is there a mechanism for carcinogenic effects of weak magnetic fields? Proc. R. Soc. B 285, 20180590 ( 10.1098/rspb.2018.0590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadalà M, Morales-Medina JC, Vallelunga A, Palmieri B, Laurino C, Iannitti T. 2016. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 5, 3128–3139. ( 10.1002/cam4.861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu L, Kettner NM. 2013. The circadian clock in cancer development and therapy. In Progress in molecular biology and translational science (ed. Gillette MU.), pp. 221–282. Amsterdam, The Netherlands: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padmanabhan K, Billaud M. 2017. Desynchronization of circadian clocks in cancer: a metabolic and epigenetic connection. Front. Endocrinol. 8, 136 ( 10.3389/fendo.2017.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shostak A. 2017. Circadian clock, cell division, and cancer: from molecules to organism. Int. J. Mol. Sci. 18, 873 ( 10.3390/ijms18040873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch K-F, Labrecque N, Cermakian N. 2017. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 15, 13 ( 10.1186/s12915-017-0349-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzella N, et al. 2015. Circadian gene expression and extremely low-frequency magnetic fields: an in vitro study: ELF-MF modulates clock gene expression. Bioelectromagnetics 36, 294–301. ( 10.1002/bem.21915) [DOI] [PubMed] [Google Scholar]
- 8.Cremer-Bartels G, Krause K, Mitoskas G, Brodersen D. 1984. Magnetic field of the earth as additional zeitgeber for endogenous rhythms? Naturwissenschaften 71, 567–574. ( 10.1007/BF01189180) [DOI] [PubMed] [Google Scholar]
- 9.Lai H. 2019. Exposure to static and extremely-low frequency electromagnetic fields and cellular free radicals. Electromagn. Biol. Med. 38, 231–248. ( 10.1080/15368378.2019.1656645) [DOI] [PubMed] [Google Scholar]
- 10.Binhi VN, Prato FS. 2018. Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Sci. Rep. 8, 13495 ( 10.1038/s41598-018-31847-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eder SHK, Cadiou H, Muhamad A, McNaughton PA, Kirschvink JL, Winklhofer M. 2012. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl Acad. Sci. USA 109, 12 022–12 027. ( 10.1073/pnas.1205653109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron IL, Markov MS, Hardman WE. 2014. Optimization of a therapeutic electromagnetic field (EMF) to retard breast cancer tumor growth and vascularity. Cancer Cell Int. 14, 125 ( 10.1186/s12935-014-0125-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehnert S, et al. 2017. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of •O2− and H2O2. Sci. Rep. 7, 14544 ( 10.1038/s41598-017-14983-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherrard RM, et al. 2018. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 16, e2006229 ( 10.1371/journal.pbio.2006229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon H-U, Haj-Yehia A, Levi-Shaffer F. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5, 415–418. ( 10.1023/a:1009616228304) [DOI] [PubMed] [Google Scholar]
- 16.Tang HL, et al. 2012. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 23, 2240–2252. ( 10.1091/mbc.e11-11-0926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang HL, Tang HM, Hardwick JM, Fung MC. 2015. Strategies for tracking anastasis, a cell survival phenomenon that reverses apoptosis. J. Vis. Exp. 16, e51964 ( 10.3791/51964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litovitz TA, Penafiel M, Krause D, Zhang D, Mullins JM. 1997. The role of temporal sensing in bioelectromagnetic effects. Bioelectromagnetics 18, 388–395. () [DOI] [PubMed] [Google Scholar]
- 19.Ye Z, Zeng Z, Shen Y, Yang Q, Chen D, Chen Z, Shen S. 2019. ODC1 promotes proliferation and mobility via the AKT/GSK3β/β-catenin pathway and modulation of acidotic microenvironment in human hepatocellular carcinoma. OncoTargets Ther. 12, 4081–4092. ( 10.2147/OTT.S198341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naarala J, Kolehmainen M, Juutilainen J. 2019. Electromagnetic fields, genomic instability and cancer: a systems biological view. Genes 10, 479 ( 10.3390/genes10060479) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not contain any additional data.


