Abstract
Bivalve biomineralization is a highly complex and organized process, involving several molecular components identified in adults and larval stages. However, information is still scarce on the ontogeny of the organic matrix before calcification occurs. In this work, first shell formation was investigated in the mussel Mytilus galloprovincialis. The time course of organic matrix and CaCO3 deposition were followed at close times post fertilization (24, 26, 29, 32, 48 h) by calcofluor and calcein staining, respectively. Both components showed an exponential trend in growth, with a delay between organic matrix and CaCO3 deposition. mRNA levels of genes involved in matrix deposition (chitin synthase; tyrosinase- TYR) and calcification (carbonic anhydrase; extrapallial protein) were quantified by qPCR at 24 and 48 hours post fertilization (hpf) with respect to eggs. All transcripts were upregulated across early development, with TYR showing highest mRNA levels from 24 hpf. TYR transcripts were closely associated with matrix deposition as shown by in situ hybridization. The involvement of tyrosinase activity was supported by data obtained with the enzyme inhibitor N-phenylthiourea. Our results underline the pivotal role of shell matrix in driving first CaCO3 deposition and the importance of tyrosinase in the formation of the first shell in M. galloprovincialis.
Keywords: Mytilus, early larval development, first shell formation, organic matrix, biomineralization, tyrosinase
1. Introduction
Biomineralization is a complex physiological process used by a wide range of metazoan species (from sponges to vertebrates, as well as calcifying algae) [1]. Within the phylum Mollusca, many species are characterized by the ability to build protective shells made of different CaCO3 polymorphs [2,3]. Although the physiology of early biomineralization in molluscs has been widely explored [3], less is known on the steps that precede first CaCO3 deposition.
Most studies on shell formation and composition in molluscs have been focused on bivalves, which are widespread in freshwater, estuarine and marine environments. In bivalve larvae, first shell formation occurs within 48 h of first development [3]. The process starts at the end of gastrulation with the formation of the shell field, the shell secreting embryonic tissue [3,4]. The shell field undergoes a transient invagination followed by evagination. As it spans over the larval body, the shell field secretes an organic matrix that provides a scaffold for mineral deposition during shell morphogenesis [3,4]. The organic matrix is mainly composed of chitin and acidic polysaccharides, proteins and glycoproteins, which have essential roles in different aspects of shell formation, such as CaCO3 nucleation, growth and choice of polymorphs [5]. Chitin is one of the major polysaccharides of larval and adult shells [6–8]. The first shell, or prodissoconch I, later growths into the prodissoconch II, discernible by the concentric growth lines [3]. The shell field ultimately differentiates into the mantle, the shell forming tissue in adults [3].
The molecular components involved in shell formation have been largely investigated in adults and larval stages of different bivalves (oysters, clams, mussels) [9–23]. Transcriptomics and proteomics data have identified several genes that play important roles in the biomineralization process, as well as a number of shell matrix proteins (SMPs). With regards to SMPs, although considerable differences have been found in adult and larval shells, some functional domains are shared by both SMP repertoires (von Willebrand factor type A, chitin-binding, carbonic anhydrase and acidic domains) [18]. However, the role of each component in the transition from the trocophora to the first shelled embryo, when the blueprint for calcification is first established, is not fully understood. In particular, despite data being available on shell calcification in early larval stages [20–23], and also in relation to ocean acidification [24,25], much less is known about the ontogeny of the organic matrix before calcification occurs [2]. Former studies underlined the role of chitin deposition [5–7]. More recently, data on oyster larvae suggested a role for tyrosinase in the initial phase of shell formation [26,27]. Sequencing of RNA identified changes in different calcification-related ion transporters and SMPs, including tyrosinase, in early larval stages of the Baltic mussel M. edulis (from 20 hours post fertilization—hpf) [23].
Here we investigate the first steps of shell formation from the trocophora (at 24 hpf) to the first D-veliger (at 48 hpf) in M. galloprovincialis, a species of ecological and commercial importance in the Mediterranean [28]. The time course of organic matrix and calcified shell deposition were monitored by calcofluor and calcein staining, respectively [25]. The approach involved quantifying the levels of mRNA transcripts of selected shell genes previously identified as targets for different chemicals that affect first shell formation [29–31] and tyrosinase, as a potential key step in early matrix development. The role of tyrosinase was further investigated by in situ hybridization (ISH) and treatment with the pharmacological inhibitor of tyrosinase activity N-phenylthiourea (PTU) [32,33].
2. Material and methods
(a). Animal handling and larval rearing
Sexually mature specimens of M. galloprovincialis (4–5 cm long), were collected in the Bay of Villefranche-sur-mer (43.682° N, 7.319° E—France) during the spawning season (January–March 2018). Animals were kept and maintained by the Centre de Ressources Biologiques Marines of the institute (CRBM) at the Institut de la Mer de Villefranche (IMEV), where they were acclimatized in flow-through vessels containing 0.2 µm filtered natural seawater Millipore filtered seawater (MFSW) (pH 8.0–8.2, 38 ppt salinity, 15°C). Spawning was induced by exposure at 28°C in MFSW in individual 200 ml containers. Fertilization and larval growth were carried out as previously described [29–31] (see electronic supplementary material). Larvae were grown at a density of 200 larvae ml−1, using 24-well plates for morphological analyses, six-well plates for qPCR and 50 ml cell culture flasks for ISH.
(b). Larval development and shell biogenesis
Shell biogenesis was followed at different hours post fertilization (24, 26, 29, 32 and 48 hpf), by evaluating the growth of both the organic and inorganic shell components using fluorescent dyes as previously described [25]. Calcein (Sigma Aldrich, Lyon, France), a calcium-dependent fluorophore, was used for CaCO3 staining of the calcified shell and added to the culture medium (final concentration 1 mM in 0.01% dimethyl sulfoxide; DMSO) before the addition of fertilized eggs. Calcofluor white Fluorescent Brightener 28 (Sigma-Aldrich, Lyon, France), a chitin-staining fluorophore, was employed to visualize the organic matrix, and directly added to the single wells on live larvae 5 min before each sampling time (final concentration 0.02 mM in 0.01% DMSO). At each sampling time, larvae were washed three times in MFSW to remove the excess of both dyes before fixation with 4% paraformaldehyde (PFA) in MFSW, and immediately imaged with a Leica TCS SP8 (Leica, France). Calcofluor (UV channel, Exc: 408 nm/Em: 450–490 nm) gave a blue signal for the organic matrix, while calcein (FITC channel, Exc: 488 nm/Em: 520–560 nm) visualized the calcified shell in green. Composite images were three-dimensionally rendered and rotated to measure the area (in µm2) of one valve in each larva stained by calcein and calcofluor by manual drawing using IMAGEJ software [24]. Measurements were performed on a total of at least 70 larvae per time-point obtained from five independent parental pairs (N ≥ 12 for each parental pair).
Experiments were also carried out in the presence of PTU, a well-known competitive inhibitor of diphenolase and phenoloxidase enzymes [32] that has been widely used to inhibit tyrosinase activities in marine invertebrates including bivalves [33]. Fertilized eggs were exposed to 10 µM PTU (Sigma Aldrich, Lyon, France) (final concentration 10 µM in 0.01% DMSO) and samples were observed from fertilization to 48 hpf. Experiments were performed in three independent parental pairs. Parallel samples were run in the presence of 0.01% DMSO to rule out possible solvent related effects (not shown).
(c). Mytilus galloprovincialis tyrosinase sequence analysis
The sequence of a M. galloprovincialis tyrosinase (Mg-TYR) was obtained by blasting the available sequence of tyrosinase-1 from M. coruscus (GenBank: KP757802.1) in M. galloprovincialis whole genome shotgun [34]. The sequence obtained (GenBank: KV583276.1) corresponded to a partial tyrosinase-like tyr-A3 protein (GenBank: OPL33388.1). The basic characteristics and conserved domains of the amino sequence of Mg-TYR were analysed through the SMART tools (http://smart.embl-heidelberg.de/) to confirm the functional activity. Mg-TYR cDNA open reading frame (ORF) and deduced amino acid sequence are shown in electronic supplementary material, figure S1A. The ORF is composed of 1879 base pairs (bps) and coding for 626 amino acids. The sequence showed a 90% query coverage with the ORF of Cg-Tyr1 (GenBank: AGZ15753.1), 92% with C. gigas putative tyrosinase like-protein tyr-3 (GenBank: EKC35330) and 52% with M. coruscus tyrosinase-like protein 1 (GenBank: KP757802), respectively. The catalytic activity of Mg-TYR is confirmed by the presence of the two copper binding domains and the six histidine residues included within [35]. The sequence also showed a chitin binding domain type 2. Multiple alignment of the two copper-binding domains (CuA and CuB) between Mg-TYR and tyrosinases of other bivalves M. coruscus (Mc-Tyr1) and C. gigas (Cgi-Tyr1) are reported in electronic supplementary material, figure S1B; conserved histidine residues are indicated in red.
(d). RNA extraction and qPCR
All procedures were carried out as previously described [29–31]. Unfertilized eggs (about 24 000 eggs ml−1) pooled from at least six females were collected by centrifugation at 400g for 10 min at 4°C, and the resulting pellet was frozen in liquid N2. After fertilization, larvae were grown in six well plates and collected at 24 and 48 hpf by a nylon mesh (20 µm pore-filter) and washed with artificial seawater (ASW) [29–31]. Three wells for each stage were pooled in order to obtain approximately 7000 embryos/replicate. The larval suspension was centrifuged at 800g, 10 min at 4°C. Larval pellets and unfertilized eggs were lysed in 1 ml of TRI Reagent (Sigma Aldrich, Milan, Italy). Total RNA was further extracted following the manufacturer's instructions (Sigma Aldrich, Milan, Italy). RNA concentration and quality were verified using the Qubit RNA assay (Thermo Fisher, Milan, Italy) and electrophoresis using a 1.5% agarose gel under denaturing conditions. First strand cDNA for each sample was synthesized from 1 µg total RNA [29]. Primers pairs employed for qPCR analysis are reported in electronic supplementary material, table S1. qPCR reactions were performed in triplicate in a final volume of 15 µl containing 7.5 µl iTaq universal master mix with ROX (BioRad Laboratories, Milan, Italy), 5 µl diluted cDNA and 0.3 µM specific primers. A control lacking cDNA template (no-template) was included in the qPCR analysis to determine the specificity of target cDNA amplification. Amplifications were performed in a StepOne real time PCR system apparatus using a standard ‘fast mode’ thermal protocol (sample ramp ± 2.2 °C s−1) (Thermo Fisher, Milan, Italy). For each target mRNA, melting curves were used to verify the specificity of the amplified products and the absence of artefacts. The amplification efficiency of each primer pair was calculated using a dilution series of cDNA (electronic supplementary material, table S1). HEL and EF-α1 were used as the best performing combination of reference gene products (EF1/HEL) for data normalization [29]. Analyses were performed on at least four independent mRNA samples. Calculations of relative expression of target mRNAs was performed by a comparative CT method [36] using the StepOne software tool (Thermo Fisher). Data, obtained from at least five independent mRNA samples, are reported as relative expression (log2-transformed fold changes) with respect to unfertilized eggs.
(e). In situ hybridization
Primer pairs of TYR and chitin synthase (CS) primer pairs for ISH are reported in electronic supplementary material, table S2, and were used to amplify one fragment of cDNA for each gene. PCR products (around 1.5 kilobase—kb) were cloned into a pGEM-T easy vector (Promega, Charbonnières-les-Bains, France). The selected recombinant plasmid was linearized (SpeI and NcoI restriction enzymes, New England BioLabs, Evry, France) and sequenced to check the orientation of the insert. Sense and antisense digoxigenin-labelled RNA probes were synthesized using the DIG RNA labelling mixture (Roche, Meylan, France) and T7/Sp6 RNA polymerase (Promega, Charbonnières-les-Bains, France). The probes were tested on larvae obtained from at least three independent parental pairs for a total of 150 individuals (N = 50 for each parental pair) imaged per time point.
The expression pattern of TYR and CS during shell biogenesis in M. galloprovincialis larvae at 24, 29, 32 and 48 hpf was investigated by ISH using an adaptation of the protocol already available for ascidian embryos ([37], see Methods in electronic supplementary material. The signals from the antisense/sense probes for TYR and CS in mussel larvae at 24 and 48 hpf are shown in electronic supplementary material, figure S2.
(f). Statistics
Morphometrical data were analysed by the non-parametric one-way Kruskal–Wallis test followed by the Tukey test (p < 0.05). Data of qPCR were analysed by Mann–Whitney U test. Statistical differences and regression equations were calculated using GraphPad Prism 5 software (GraphPad Inc.)
3. Results
(a). Identification of the main steps in early shell formation by double calcofluor/calcein staining
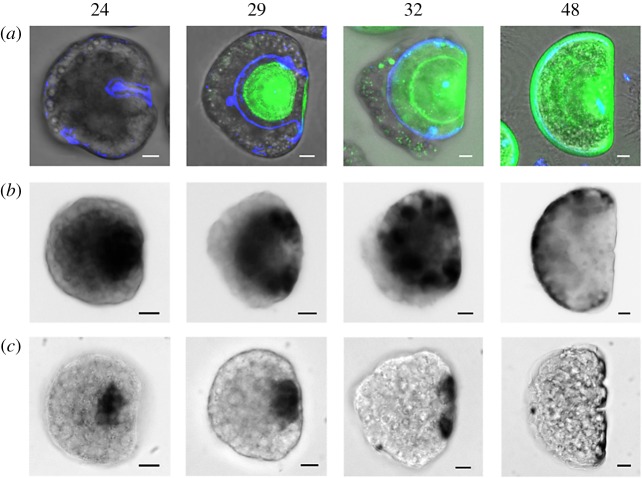
The double calcofluor/calcein staining [25] was employed to follow the progressive deposition of organic shell matrix and CaCO3, respectively, at close times post fertilization between the trochophora and D-veliger stages (24, 26, 29, 32 and 48 hpf). As shown in figure 1, at 24 hpf, the trochophora started to secrete the organic matrix; the calcofluor signal (blue) was mainly visible in a saddle shaped area corresponding to the shell field, which at this stage was still partially invaginated (figure 1b and electronic supplementary material, figure S3A, arrowheads). By this time, no calcein staining was observed, indicating the absence of stable CaCO3 deposition (figure 1c and electronic supplementary material, figure S3B). At 26 hpf, the shell field was expanded (electronic supplementary material, figure S3B) and calcification (green) started from the centre of the forming valve (figure 1b–d); the hinge region began to flatten following the progressive evagination of the shell field (electronic supplementary material, figure S3A, arrowheads). By 29 hpf, the calcified area occupied a large part of the growing shell, and the organic matrix could still be observed along the external margins of the valve (figure 1b,d and electronic supplementary material, figure S3B). However, no calcification was visible yet in the hinge region that, by this time, had completed the flattening (figure 1c,d and electronic supplementary material, figure S3B). At 32 hpf, the body organization changed dramatically, the larva taking the shape characteristic of the early veliger stage (figure 1a): the valve were largely calcified, showing the first accretion rings indicative of the progressive CaCO3 deposition (figure 1c). The calcified shell overlaid the organic matrix, except for a thin layer along the margins of the valve (figure 1d). By 48 hpf, the D-veliger stage was reached, with the calcified shell covering the whole body of the larva, and showing more evident concentric accretion rings (figure 1c,d).
Figure 1.
Confocal images showing the time course of early shell formation in M. galloprovincialis from the trochophora (24 hpf) to the D-veliger stage (48 hpf) (lateral view). (a) Brightfield image of the embryo; (b) calcofluor fluorescent signal (blue), corresponding to the organic matrix; (c) calcein fluorescence signal (green), corresponding to CaCO3 deposition; (d) merged calcofluor, calcein and brightfield images. Scale bars, 10 µm. The secretion of the organic matrix is visible from 24 hpf (blue) followed by calcification (green) at 26 hpf, with a progressive expansion of the organic matrix and deposition of the shell starting from the central part of each valve. At 29 hpf the calcified valves are well developed onto the organic matrix. By 32 hpf calcification reaches the external margins of the organic matrix and expands towards the hinge region. At 48 hpf the whole shell is calcified and completely encloses the larval body. (Online version in colour.)
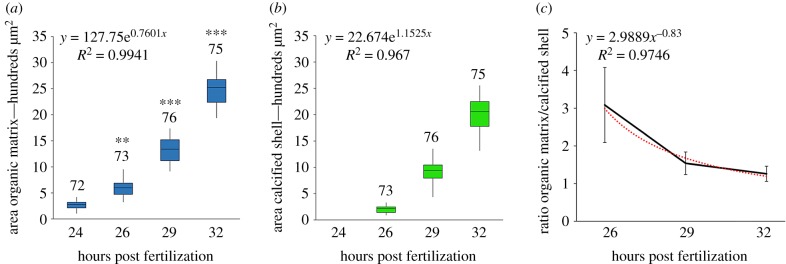
To better evaluate the time course of shell formation, the areas occupied by the matrix and the calcified shell, respectively, were measured in a single valve of each larva at different times post fertilization (figure 2a and 2b, respectively). The results show that the areas occupied by each shell component have a similar exponential trend in growth, with the areas of the organic matrix significantly higher than those of the calcified shell at all times post fertilization (figure 2a). Moreover, the ratio of organic matrix/calcified shell showed a decreasing exponential trend at different time points (figure 2c).
Figure 2.
Boxplots of data obtained from measurements of the areas (µm2) occupied by the shell matrix (a) and calcified shell (b) in mussel larvae from 24 to 32 hpf (single valve measurements per larva). The total number of individuals (from at least five parental pairs) analysed per time point is reported. The two structures show a similar exponential trend in growth, with the area of organic matrix statistically larger than that of the calcified shell at all times post fertilization. Statistical differences at each time post fertilization are reported in (a): **p ≤ 0.01; ***p ≤ 0.001. In (c) data are reported showing the exponential decrease in the ratio organic matrix/calcified shell at different times post fertilization. (Online version in colour.)
(b). Basal mRNA levels of genes involved in shell biogenesis
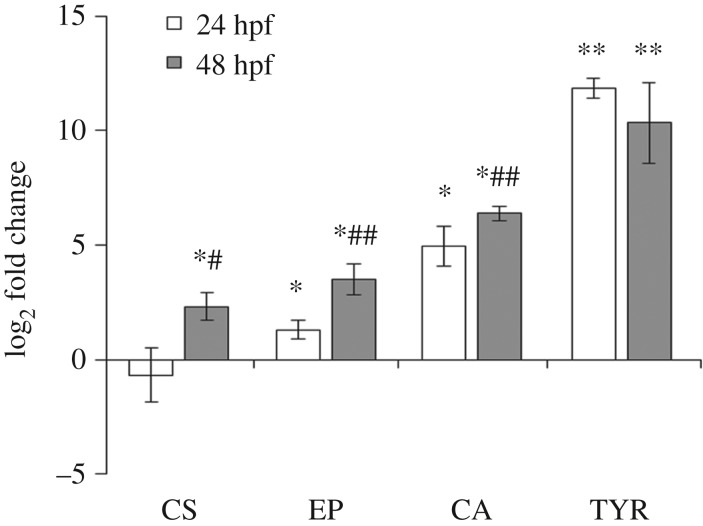
When basal transcription of each gene was first compared in unfertilized eggs (electronic supplementary material, table S3), extremely low mRNA levels were detected for carbonic anhydrase (CA) and extrapallial protein (EP), with those for TYR slightly higher. In contrast, higher expression was observed for CS, indicating a significant contribution of mRNA from maternal origin. Transcription of CS did not change at 24 hpf with respect to fertilized eggs; however, a significant increase was observed at 48 hpf (about fivefold) (figure 3). From 24 hpf EP mRNA levels were increased (2.5 folds with respect to eggs) and further upregulated in the transition from the trocophora to the D-veliger, showing up to an 11-fold increase at 48 hpf. At both 24 and 48 hpf CA was similarly but more strongly upregulated (about 30 and 80 folds with respect to eggs). Interestingly, the most upregulated gene was TYR, with a dramatic increase in transcription at 24 hpf with respect to eggs (about 3700-folds) without further increases at 48 hpf.
Figure 3.
Basal expression of genes involved in early shell formation in mussel larvae at trocophora (24 hpf) and D-veliger (48 hpf) stages: chitin synthase (CS), extrapallial protein precursor (EP), carbonic anhydrase (CA), tyrosinase (TYR). Data (mean ± s.d.), reported from the lowest to the highest level of expression, are shown as relative expression (log2-transformed fold changes) with respect to unfertilized eggs. *p < 0.05; **p < 0.01; 24 and 48 hpf versus eggs; #p < 0.05, ##p < 0.001 24 hpf versus 48 hpf.
(c). Expression patterns of genes involved in organic matrix synthesis
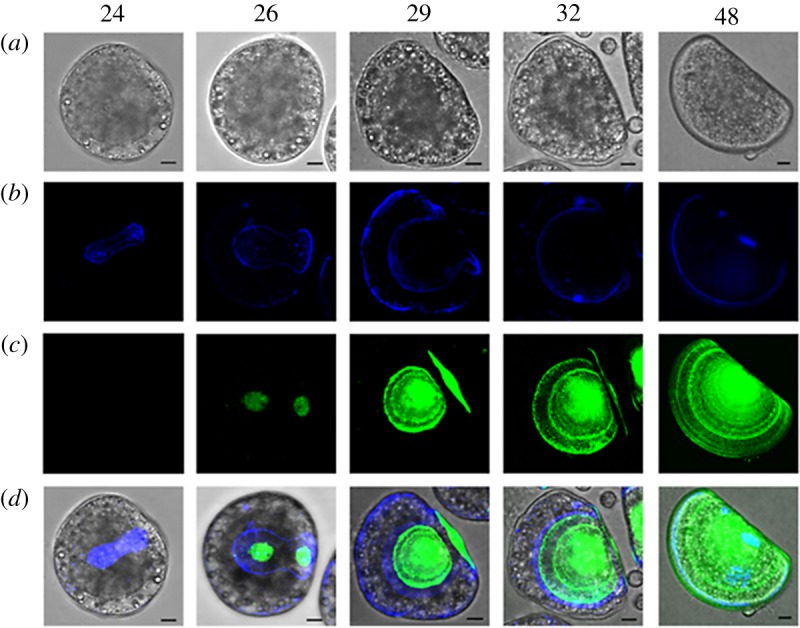
The expression pattern of TYR and CS was further investigated by ISH at 24, 29, 32, 48 hpf (figure 4), during the organic matrix and calcified shell deposition (figure 4a). Expression of TYR was detectable at 24 hpf in a wide area around the shell field (figure 4b), At 29 hpf a more distinct expression pattern was observed: although the TYR signal overlapped the growing shell, it was stronger on the margins of the matrix, and barely visible at the centre of calcifying valve (figure 4a,b). At 32 hpf, TYR expression was concentrated in rounded patches along the growing margin of the shell, and absent in the centre of the calcified areas. In the fully developed first D-veliger stage, at 48 hpf, expression was limited to the margins of the growing valve, and no signal was detectable in the calcified portion of the shell (figure 4).
Figure 4.
Expression pattern of TYR and CS in mussel larvae from 24 to 48 hpf evaluated by in situ hybridization—ISH. (a) Brightfield images and merged signals from calcofluor (blue), calcein (green) as in figure 1; (b) TYR; (c) CS. Both genes are involved in organic matrix synthesis but only TYR parallels the expansion of the shell, while CS is localized nearby the hinge region. Scale bar, 10 µm. (Online version in colour.)
A distinct pattern was observed for CS (figure 4c). At 24 hpf, expression was low and concentrated in a small area within the shell field. A similar, but stronger signal was detected at 29 hpf, in an area close to the hinge region. At 32 hpf, CS expression expanded along the hinge axis; finally, at 48 hpf, a weaker signal was observed in the same region but no signal on the shell margin was observed.
(d). Effect of tyrosinase inhibition on shell development
The role of tyrosinase was further investigated in larvae treated with PTU, a well-known synthetic inhibitor of diphenolase and phenoloxidase enzymes [32] (figure 5 and electronic supplementary material, figure S4, in comparison with control embryos). At 24 hpf, the calcofluor signal was concentrated only within the shell field (figure 5a). At 29 hpf, shell matrix deposition and growth were delayed, only a weak calcification was observed, and asymmetric growing valve were observed (figure 5b). Such an asymmetry was more evident at 32 hpf in both organic matrix and calcified shell; moreover, the pattern of calcification appeared inhomogeneous and the shell did not show the typical accretion rings (figure 5c). At 48 hpf, the larvae did not develop into D-veligers and the shell showed irregular patches of organic matrix and calcified areas (figure 5d). At this stage in particular, a variety of strong shell malformations were noticeable in comparison to control samples (electronic supplementary material, figure S4A): irregular calcification patterns (electronic supplementary material, figure S4B), valve with asymmetric growth of organic matrix and calcification (electronic supplementary material, figure S5C), absence of significant calcification and misshaped matrix (electronic supplementary material, figure S4D). The changes induced by PTU were reflected by significant decreases in the areas of both shell matrix and calcified shell in comparison to controls (−30% from 24 hpf and −50% from 29 hpf, respectively, p ≤ 0.001) that persisted at all later stages (not shown).
Figure 5.
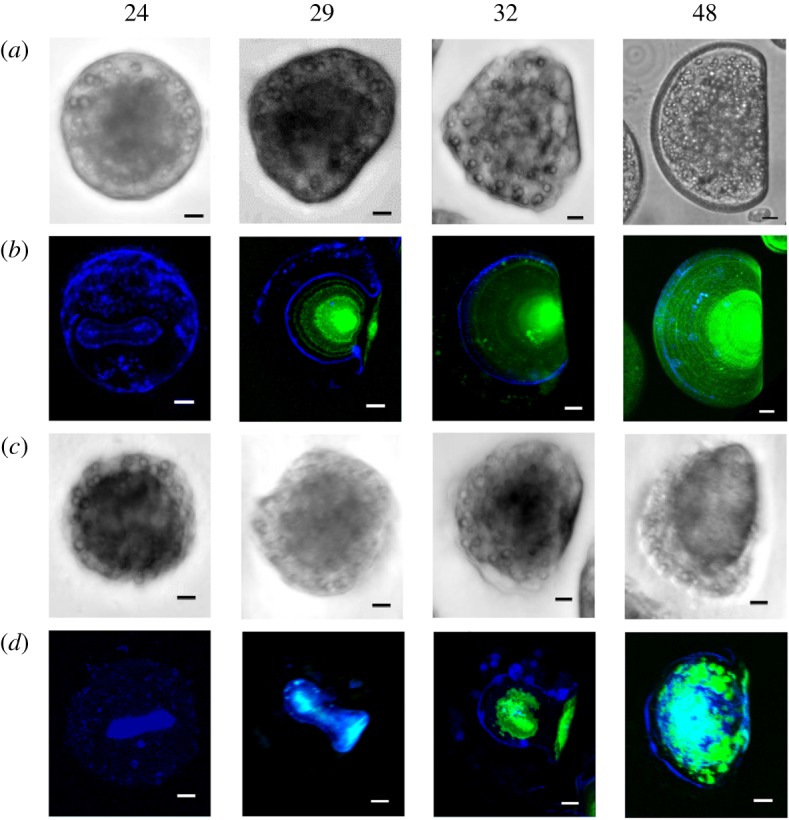
Effects of PTU (10 µM) on mussel early shell formation. Brightfield images and merged fluorescence signals of calcofluor and calcein are reported as in figure 1. Representative images show decrease in the area occupied by the organic matrix (blue) at 24 hpf, asymmetric valve and almost absent calcification at 29 hpf. Calcification is evident but asymmetric at 32 hpf, patched and dis-homogeneous at 48 hpf. Scale bar, 10 µm. N.B.: Since in PTU-exposed samples lower calcein and calcofluor signals were observed with respect to controls, images were recorded at higher laser voltage. (Online version in colour.)
4. Discussion
The results presented here provide a detailed quali- and quantitive description of both organic and inorganic components of the first shell formed in mussel larvae. These data underline the pivotal role of shell matrix in driving and organizing early CaCO3 deposition and shell growth, providing a first indication for a relationship between tyrosinase and organic matrix formation in mussels.
A clear time delay was observed between the secretion of the organic matrix and CaCO3 deposition: calcification followed the expansion of the organic matrix (indicated by the significantly smaller area at all times post fertilization). Moreover, the ratio organic matrix/calcified shell progressively decreased with the shell growth. These data clearly show that the organic matrix is the blueprint onto which calcification occurs from early steps of shell formation.
Expression of CS, CA and EP has been previously shown to be affected by exposure to different chemicals and associated with larval malformations in M. galloprovincialis [29–31]. TYR has been shown to play a role in shell formation of oyster larvae [26,27] and Baltic mussels [23]. When transcription was compared at 24 and 48 hpf with respect to eggs, the results show that all genes were generally upregulated, although to a different extent, across early development, with mRNA levels for CS < EP < CA < TYR.
Both CA and EP, whose mRNA levels were extremely low in eggs, were upregulated from 24 hpf, CA in particular, and further increases in transcription were observed at 48 hpf, supporting their role in calcification. As to those genes involved in matrix deposition, CSs are transmembrane glycosyltransferases responsible for the synthesis of chitin that represents a major constituent of larval shell matrix in M. galloprovincialis [6]. Among the four selected genes, CS showed the highest basal mRNA levels in eggs, and no upregulation was observed at 24 hpf; actually, at this stage the calcofluor signal, specific for β-glucans, and therefore to β-chitin, the most abundant chitin polymorph in both larvae and adults [6], was limited to the area corresponding to the shell field. A significant upregulation was observed at 48 hpf. In contrast, TYR, from a low expression in fertilized eggs, was the most upregulated gene, with a dramatic increase in transcription as early as 24 hpf. Tyrosinases (EC 1.14.18.1) are type 3 copper proteins, characterized by two copper-binding domains [38]. By oxidizing molecules containing phenol groups (such as tyrosine) into reactive o-quinones that then cause cross-links of substrate molecules, tyrosinases participate in various processes such as wound healing, pigment synthesis, host immunity and insect cuticle sclerotization [32,39]. The role of tyrosinases in shell formation has been investigated in adult and early larval stages of the oysters Crassostrea gigas and C. angulata, suggesting a close relationship between expression of a Cgi-tyr1 and a Ca-tyrA1, respectively, and early larval shell biogenesis [26,27]. Although several tyrosinase sequences have been described in Mytilus spp. [13,19,23], these are the first data on the mRNA level and expression pattern of tyrosinase in parallel with evaluation of shell matrix deposition. The results of ISH clearly show that TYR upregulation preceded and subsequently paralleled the growth of the organic matrix from 24 hpf.
In contrast, ISH of CS revealed a much lower and distinct expression pattern that was progressively concentrated along the hinge axis, and therefore did not correspond to the areas of the growing matrix. Similarly, preliminary data on ISH of EP and CA do not indicate specific transcription patterns related to initial shell morphogenesis (not shown). The results obtained by ISH for TYR and CS are not only in line with qPCR data, pointing at an earlier and stronger upregulation of TYR at 24 hpf, but also underline a distinct expression pattern of the two genes at 29, 32 and 48 hpf. Although these data do not allow us to understand the exact role of each gene in organic matrix deposition, for TYR the early increase in mRNA levels from 24 hpf and their localization suggest a role in early and progressive guiding matrix and shell growth. For CS, the later upregulation of mRNA transcripts and their localization at the hinge region may reflect a participation in the subsequent steps and, in particular, in the differentiation of the hinge.
Inhibition of tyrosinase activity clearly affected matrix deposition since 24 hpf. From 29 to 48 hpf, evident malformations and dramatic alterations of shell calcification and growth were also observed. As in other bivalves, in early mussel larvae tyrosinase activity might be related to some sort of maturation process of the organic matrix [27,39] that allows the next calcification step in terms of homogeneous and organized CaCO3 deposition [40]. The presence of a chitin binding domain in TYR further supports a physiological role for tyrosinase activity in correct chitin remodelling not only during organic matrix formation, but also in subsequent shell growth and calcification. Different proteins participate in initiating and controlling the nucleation, growth of inorganic crystals, as well as directing crystal growth through molecular recognitions [41,42]. Tyrosinases may be involved in shell matrix formation by cross-linking fibrous proteins rich in reactive quinones to form water insoluble, protease-resistant polymers [26,27,32]. Other enzymes with potential phenol oxidase activity should be investigated to obtain a comprehensive knowledge of the process of matrix deposition. Sequencing of mussel genome is revealing a very complex organization with high heterozygosity, abundance of repetitive sequences and extreme intraspecific sequence diversity among individuals, resulting in a large variety of transcripts for both immune-related [34,43] and biomineralization-related genes [19]. In this light, the changes in transcription of few gene sequences related to shell formation evaluated in the present study are only indicative of more heterogeneous and complex processes.
It has been recently reported that ocean acidification affects M. galloprovincialis larval soft-tissue development, independent from calcification [25]. Shell malformations induced by exposure to low pH, in particular shell hinge abnormalities, originate from an incorrect development and growth of the organic matrix, thus affecting the calcification blueprint [25]. Overall, the results obtained so far further support the hypothesis that shell calcification essentially takes on the shape of the secreted organic matrix. Knowledge on these processes may help better understanding of bivalve development in a global change scenario, in order to identify early signs of impact of different environmental stressors, from ocean acidification and warming to contaminant exposure. Although marine bivalves, living in complex environments such as coastal ecosystems, must have evolved mechanisms to maintain homeostasis for shell formation in response to natural environmental fluctuations, shell growth is a highly controlled and energy-limited process [24]. Understanding the homeostatic limits for larval shell development can provide clues about whether the magnitude and rate of environmental changes will exceed the buffering limits of embryo physiology, as well as predictive tools to identify potentially harmful compounds [44]. Different types of emerging contaminants have been shown to affect early shell formation and gene expression in M. galloprovicialis [29–31]. The results here obtained indicate that genes involved in shell matrix deposition, in particular tyrosinases, may represent significant targets for a number of environmental chemicals in early larval stages of mussels.
Supplementary Material
Acknowledgements
The authors would like to thank Laurent Gilletta, Alexandre Jean and Régis Lasbleiz and the Centre de Ressources Biologiques Marines of the CRBM-IMEV that is supported by EMBRC-France, whose French state funds are managed by the Agence Nationale de la Recherche (ANR) within the ‘Investissement d'Avenir’ program (ANR-10-INBS-02); the Imaging platform members: Mébarek Temagoult and Sameh Benaicha; the Ascidian BioCell group members: Alex McDougall, Janet Chenevert, Céline Hebras, Isa Gomez and Genia Gazo.
Ethics
Number of adult mussels used and duration of stress was minimized.
Data accessibility
This article has no additional data.
Authors' contributions
A.M. designed the research with input from R.D. and L.C. A.M. and T.B. performed research. R.D. contributed resources. L.C. wrote the paper with contributions from all authors.
Competing interests
We declare we have no competing interests.
Funding
This research was partly funded by EMBRC-France no. OOV-AAP 2018-2161 MERMAIDS. The experiments performed in R.D. laboratory were financed by an ANR grant (Marine-EmbryoTox project, no. ANR-14-OHRI-0009-01-1). A.M. was supported by the PhD in Marine Sciences, DISTAV, University of Genoa and by the Observatoire Oceanologique de Villefranche.
References
- 1.Knoll AH. 2003. Biomineralization and evolutionary history. Rev. Mineral. Geochem. 54, 329–356. ( 10.2113/0540329) [DOI] [Google Scholar]
- 2.Furuhashi T, Schwarzinger C, Miksik I, Smrz M, Beran A. 2009. Molluscan shell evolution with review of shell calcification hypothesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 154, 351–371. ( 10.1016/j.cbpb.2009.07.011) [DOI] [PubMed] [Google Scholar]
- 3.Marin F. 2012. The formation and mineralization of mollusk shell. Front. Biosci. S4, 1099–1125. ( 10.2741/s321) [DOI] [PubMed] [Google Scholar]
- 4.Kurita Y, Deguchi R, Wada H. 2009. Early development and cleavage pattern of the Japanese purple mussel, Septifer virgatus. Zoolog. Sci. 26, 814–820. ( 10.2108/zsj.26.814) [DOI] [PubMed] [Google Scholar]
- 5.Falini G, Fermani S. 2004. Chitin mineralization. Tissue Eng. 10, 1–6. ( 10.1089/107632704322791646) [DOI] [PubMed] [Google Scholar]
- 6.Weiss IM, Schönitzer V. 2006. The distribution of chitin in larval shells of the bivalve mollusk Mytilus galloprovincialis. J. Struct. Biol. 153, 264–277. ( 10.1016/j.jsb.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Sakuda S, Nagasawa H. 2007. Identification of chitin in the prismatic layer of the shell and a chitin synthase gene from the Japanese pearl oyster, Pinctada fucata. Biosci. Biotechnol. Biochem. 71, 1735–1744. ( 10.1271/bbb.70140) [DOI] [PubMed] [Google Scholar]
- 8.Yarra T, Gharbi K, Blaxter M, Peck LS, Clark MS. 2016. Characterization of the mantle transcriptome in bivalves: Pecten maximus, Mytilus edulis and Crassostrea gigas. Mar. Genomics 27, 9–15. ( 10.1016/j.margen.2016.04.003) [DOI] [PubMed] [Google Scholar]
- 9.Arivalagan J, Yarra T, Marie B, Sleight VA, Duvernois-Berthet E, Clark MS, Marie A, Berland S. 2016. Insights from the shell proteome: biomineralization to adaptation. Mol. Biol. Evol. 34, 66–77. ( 10.1093/molbev/msw219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marie B, et al. 2012. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl Acad. Sci. USA 109, 20 986–20 991. ( 10.1073/pnas.1210552109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Z, Bao L, Fan M, Gao P, Wang X, Qin C, Li X. 2015. In-depth proteomic analysis of nacre, prism, and myostracum of Mytilus shell. J. Proteomics 122, 26–40. ( 10.1016/j.jprot.2015.03.027) [DOI] [PubMed] [Google Scholar]
- 12.Gao P, Liao Z, Wang X, Bao L, Fan M, Li X, Wu C, Xia S. 2015. Layer-by-layer proteomic analysis of Mytilus galloprovincialis shell. PLoS ONE 10, e0133913 ( 10.1371/journal.pone.0133913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hüning AK, et al. 2016. A shell regeneration assay to identify biomineralization candidate genes in mytilid mussels. Mar. Genomics 27, 57–67. ( 10.1016/j.margen.2016.03.011) [DOI] [PubMed] [Google Scholar]
- 14.Marie B, Arivalagan J, Mathéron L, Bolbach G, Berland S, Marie A, Marin F. 2017. Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa. J. R. Soc. Interface 14, 20160846 ( 10.1098/rsif.2016.0846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song X, Liu Z, Wang L, Song L. 2019. Recent advances of shell matrix proteins and cellular orchestration in marine molluscan shell biomineralization. Front. Mar. Sci. 6, 41 ( 10.3389/fmars.2019.00041) [DOI] [Google Scholar]
- 16.Yin H, Ji B, Dobson PS, Mosbahi K, Glidle A, Gadegaard N, Freer A, Cooper JM, Cusack M. 2009. Screening of biomineralization using microfluidics. Anal. Chem. 81, 473–478. ( 10.1021/ac801980b) [DOI] [PubMed] [Google Scholar]
- 17.Freer A, Bridgett S, Jiang J, Cusack M. 2013. Biomineral proteins from Mytilus edulis mantle tissue transcriptome. Mar. Biotechnol. 16, 34–45. ( 10.1007/s10126-013-9516-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao R, et al. 2018. Dual gene repertoires for larval and adult shells reveal molecules essential for molluscan shell formation. Mol. Biol. Evol. 35, 2751–2761. ( 10.1093/molbev/msy172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malachowicz M, Wenne R. 2019. Mantle transcriptome sequencing of Mytilus spp. and identification of putative biomineralization genes. PeerJ 6, e6245 ( 10.7717/peerj.6245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medaković D. 2000. Carbonic anhydrase activity and biomineralization process in embryos, larvae and adult blue mussels Mytilus edulis L. Helgol. Mar. Res. 54, 1–6. ( 10.1007/s101520050030) [DOI] [Google Scholar]
- 21.Weiss IM, Tuross N, Addadi L, Weiner S. 2002. Mollusc larval shell formation: amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 293, 478–491. ( 10.1002/jez.90004) [DOI] [PubMed] [Google Scholar]
- 22.Ramesh K, Hu MY, Thomsen J, Bleich M, Melzner F. 2017. Mussel larvae modify calcifying fluid carbonate chemistry to promote calcification. Nat. Commun. 8, 1709 ( 10.1038/s41467-017-01806-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramesh K, Yarra T, Clark MS, John U, Melzner F. 2019. Expression of calcification-related ion transporters during blue mussel larval development. Ecol. Evol. 9, 7157–7172. ( 10.1002/ece3.5287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventura A, Schulz S, Dupont S. 2016. Maintained larval growth in mussel larvae exposed to acidified under-saturated seawater. Sci. Rep. 6, 23728 ( 10.1038/srep23728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapsenberg L, Miglioli A, Bitter MC, Tambutté E, Dumollard R, Gattuso JP. 2018. Ocean pH fluctuations affect mussel larvae at key developmental transitions. Proc. R. Soc. B 285, 20182381 ( 10.1098/rspb.2018.2381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huan P, Liu G, Wang H, Liu B. 2013. Identification of a tyrosinase gene potentially involved in early larval shell biogenesis of the Pacific oyster Crassostrea gigas. Dev. Genes Evol. 223, 389–394. ( 10.1007/s00427-013-0450-z) [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Pu F, Li L, You W, Ke C, Feng D. 2017. Functional analysis of a tyrosinase gene involved in early larval shell biogenesis in Crassostrea angulata and its response to ocean acidification. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 206, 8–15. ( 10.1016/j.cbpb.2017.01.006) [DOI] [PubMed] [Google Scholar]
- 28.FAO. 2004. Mytilus galloprovincialis. See www.fao.org/fishery/culturedspecies/Mytilusgalloprovincialis/en.
- 29.Balbi T, Franzellitti S, Fabbri R, Montagna M, Fabbri E, Canesi L. 2016. Impact of bisphenol A (BPA) on early embryo development in the marine mussel Mytilus galloprovincialis: effects on gene transcription. Environ. Pollut. 218, 996–1004. ( 10.1016/j.envpol.2016.08.050) [DOI] [PubMed] [Google Scholar]
- 30.Balbi T, Camisassi G, Montagna M, Fabbri R, Franzellitti S, Carbone C, Dawson K, Canesi L. 2017. Impact of cationic polystyrene nanoparticles (PS-NH2) on early embryo development of Mytilus galloprovincialis: effects on shell formation. Chemosphere 186, 1–9. ( 10.1016/j.chemosphere.2017.07.120) [DOI] [PubMed] [Google Scholar]
- 31.Balbi T, Montagna M, Fabbri R, Carbone C, Franzellitti S, Fabbri E, Canesi L. 2018. Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci. Total Environ. 642, 601–609. ( 10.1016/j.scitotenv.2018.06.125) [DOI] [PubMed] [Google Scholar]
- 32.Chang TS. 2009. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 10, 2440–2475. ( 10.3390/ijms10062440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luna-Acosta A, Thomas-Guyon H, Amari M, Rosenfeld E, Bustamante P, Fruitier-Arnaudin I. 2011. Differential tissue distribution and specificity of phenoloxidases from the Pacific oyster Crassostrea gigas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 159, 220–226. ( 10.1016/j.cbpb.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 34.Murgarella M, Puiu D, Novoa B, Figueras A, Posada D, Canchaya C. 2016. A first insight into the genome of the filter-feeder mussel Mytilus galloprovincialis. PLoS ONE 11, e0151561 ( 10.1371/journal.pone.0151561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguilera F, McDougall C, Degnan BM. 2014. Evolution of the tyrosinase gene family in bivalve molluscs: independent expansion of the mantle gene repertoire. Acta Biomater. 10, 3855–3865. ( 10.1016/j.actbio.2014.03.031) [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. ( 10.1038/nprot.2008.73) [DOI] [PubMed] [Google Scholar]
- 37.Paix A, Chenevert J, Sardet C. 2011. Localization and anchorage of maternal mRNAs to cortical structures of ascidian eggs and embryos using high resolution in situ hybridization. In Methods in molecular biology (ed. JM Walker), pp. 49–70. Basel, Switzerland: Springer; ( 10.1007/978-1-61779-005-8_4) [DOI] [PubMed] [Google Scholar]
- 38.Decker H, Tuczek F. 2000. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem. Sci. 25, 392–397. ( 10.1016/s0968-0004(00)01602-9) [DOI] [PubMed] [Google Scholar]
- 39.Andersen SO. 2010. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 40, 166–178. ( 10.1016/j.ibmb.2009.10.007) [DOI] [PubMed] [Google Scholar]
- 40.Sikes CS, Wheeler AP. 1986. The organic matrix from oyster shell as a regulator of calcification in vivo. Biol. Bull. 170, 494–505. ( 10.2307/1541857) [DOI] [Google Scholar]
- 41.Addadi L, Joester D, Nudelman F, Weiner S. 2006. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chemistry 12, 980–987. ( 10.1002/chem.200500980) [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Feng Y, Deveaux JG, Masoud MA, Chandra FS, Chen H, Zhang D, Feng L. 2019. Biomineralization forming process and bio-inspired nanomaterials for biomedical application: a review. Minerals 9, 68 ( 10.3390/min9020068) [DOI] [Google Scholar]
- 43.Figueras A, Moreira R, Sendra M, Novoa B. 2019. Genomics and immunity of the Mediterranean mussel Mytilus galloprovincialis in a changing environment. Fish Shellfish Immunol. 90, 440–445. ( 10.1016/j.fsi.2019.04.064) [DOI] [PubMed] [Google Scholar]
- 44.Hamdoun A, Epel D. 2007. Embryo stability and vulnerability in an always changing world. Proc. Natl Acad. Sci. USA 104, 1745–1750. ( 10.1073/pnas.0610108104) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.