Abstract
Trilobitomorphs are a species-rich Palaeozoic arthropod assemblage that unites trilobites with several other lineages that share similar appendage structure. Post-embryonic development of the exoskeleton is well documented for some trilobitomorphs, especially trilobites, but little is known of the ontogeny of their soft parts, limiting understanding of their autecology. Here, we document appendage structure of the Cambrian naraoiid trilobitomorph Naraoia spinosa by computed microtomography, resulting in three-dimensional reconstructions of appendages at both juvenile and adult stages. The adult has dense, strong spines on the protopods of post-antennal appendages, implying a predatory/scavenging behaviour. The absence of such gnathobasic structures, but instead tiny protopodal bristles and a number of endopodal setae, suggests a detritus-feeding strategy for the juvenile. Our data add strong morphological evidence for ecological niche shifting by Cambrian arthropods during their life cycles. A conserved number of appendages across the sampled developmental stages demonstrates that Naraoia ceased budding off new appendages by the mid-juvenile stage.
Keywords: Cambrian, Chengjiang biota, micro-CT, Naraoia spinosa, arthropod, gnathobase
1. Introduction
Naraoiids are an abundant group of non-biomineralized trilobitomorph arthropods, their exoskeleton organized as two lightly sclerotized shields jointed by a single articulation between the head and trunk [1–4]. First discovered in the Burgess Shale (Miaolingian Series, British Columbia), naraoiids range from Cambrian Series 2 to the Přidolian (Silurian) [5–7]. They are of broader interest for studies of early arthropods because of the exquisite detail in which their appendages are known. This information is mostly derived from specimens from the Chengjiang biota of south China (Cambrian Series 2) [2,3,8] and the Burgess Shale [1,4,9], allowing for the precise structure of the two limb rami, the morphology of the proximal part of the appendage, and the attachment of the appendages to the body to be resolved to an almost unrivaled degree within the trilobitomorphs.
Despite their non-biomineralized exoskeleton, naraoiids were classified as members of Trilobita, which are otherwise robustly calcified [1,10]. Hou & Bergström [8] instead proposed that trilobites and naraoiids are not each other's sister group within an assemblage of mostly non-biomineralized Palaeozoic arthropods named Artiopoda. The Artiopoda concept was underpinned by the serial similarity in the form of all post-antennal biramous limbs, and a distinctively trilobite-type appendage and monophyly in phylogenetic analyses have supported Artiopoda [11,12]. Several subsequent studies have recovered separate sister groups for both trilobites and naraoiids [13–15], though all of these taxa are consistently recovered within a subgroup of Artiopoda that corresponds to a monophyletic formulation of Trilobitomorpha (see fig. 6 of [11]).
Structure of the appendages has played a role in inferring the life habits of naraoiids. Whittington [1] and Caron & Jackson [16] considered naraoiids to be benthic from their interpretation of feeding strategies and relative abundance. Chen et al. [2] interpreted N. spinosa as being a benthic deposit feeder in view of a sediment-filled gut, laterally deflected antennae and preservational posture, though these were contested by subsequent studies which favoured a predatory/scavenging mode of life in light of digestive morphology [3,17]. A nekto-benthic mode of life for naraoiids has alternatively been proposed, viewing the appendages of Naraoia compacta as specialized for swimming [9]. However, no specialized swimming appendages have been discovered within other naraoiid species or genera.
In this study, we undertake high-resolution micro-computed tomography (CT) study of both the juvenile and adult specimens of N. spinosa. Micro-CT reconstructions reveal a full set of three-dimensional appendages with micrometre-scale details, as well as marked changes of appendicular structures between ontogenetic stages. These observations have allowed for a revised interpretation of naraoiid feeding and locomotory strategies.
2. Material and methods
(a). Materials
The specimens (YKLP 11408, 11409, 13941) were collected from the Haikou area of Kunming, China and are housed at Yunnan Key Laboratory for Palaeobiology, Yunnan University. They are preserved in the yellowish mudstones in the Yu'anshan Member of the Chiungchussu Formation, Cambrian Stage 3. The specimens are partially preserved as pyrite and/or iron oxide, as is common for Chengjiang fossils [18], which confers a density difference with the matrix that is conducive to X-ray microtomographic imaging.
(b). Fossil imaging
Microscopic photos were taken with a Leica DFC500 CCD camera attached to a Leica M205C stereomicroscope and a Leica M205FA fluorescence microscope, respectively. In order to image the buried structures, X-ray computed microtomography was applied. Specimens YKLP 11408 and YKLP 11409 (figures 1 and 2; electronic supplementary material, figure S1) were scanned with a GE Phoenix Nanotom m scanner. Scanning energy was set at 110 kV/100 µA and imaging pixel size ranged from 16.2 to 4.0 µm, with coarse resolution for large-field scanning and fine resolution for small-field scanning. Specimen YKLP 13941 (electronic supplementary material, figure S2) was scanned with a Zeiss Xradia 520 Versa X-ray Microscope, with scanning energy of 70 kV/86 µA and imaging pixel size of 9.0 µm. The resulting data, in the form of a suite of TIFF images (one to a few thousand in number), were processed with the software Drishti (v. 2.4 and v. 2.6.4) for three-dimensional observations of the fossils.
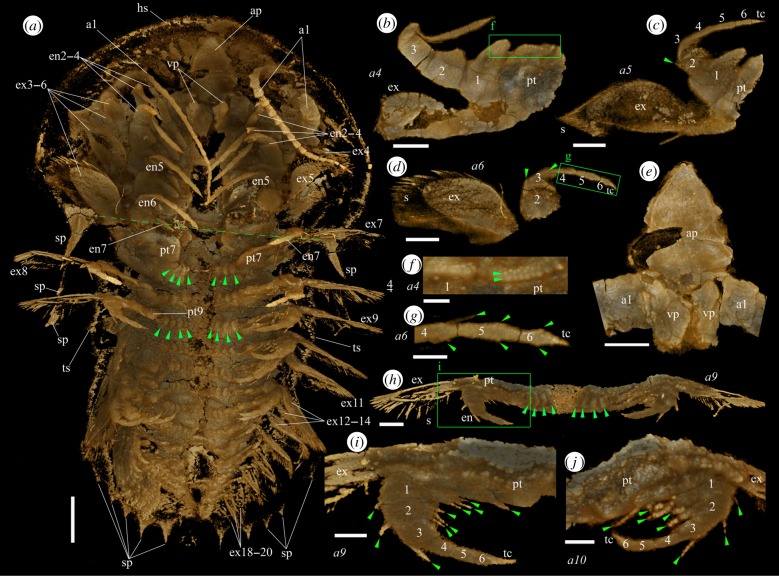
Figure 1.
Micro-CT images of a mid-stage juvenile specimen (YKLP 11408) of Naraoia spinosa Zhang & Hou (1985) from the Chengjiang Lagerstätte (Cambrian Series 2, Stage 3). (a) Ventral view of the animal. Green arrowheads indicate folds of arthrodial membrane proximal to the protopods of seventh and ninth appendages, respectively. Green dashed line shows position of posterior margin of head shield. (b) Right fourth appendage, posterior view. (c) Right fifth appendage, posterior view. (d) Right sixth appendage, posterior view. (e) Hypostomal complex and basal parts of first appendages (antennae), ventral view. (f) Enlargement of the area in the green rectangle in (b) but in ventral view. Green arrowheads indicate the two rows of bristles on protopod. (g) Enlargement of the area in the green rectangle of (d). Green arrowheads indicate endites at the sub-apical part of the endopodal podomeres. Note the seta on the endite of the fourth podomere. (h) Ninth appendages, ventral view. Green arrowheads indicate folds of arthrodial membrane proximal to the protopod (see also (a)). (i) Enlargement of the area in the green rectangle of (h). Green arrowheads indicate endopodal setae. (j) Part of left tenth appendage, oblique-ventral view. Green arrowheads indicate endopodal setae. Scale bars, 1 mm for (a), 500 µm for (b–e, h) and 200 µm for (f, g, i, j). ap, anterior plate of the hypostomal complex; an, the nth appendage; en, endopod; enx, endopod of xth appendage; exn, exopod of nth appendage; hs, head shield; ptn, protopod of nth appendage; s, seta; sp, spine; tc, terminal claw; ts, trunk shield; vp, ventral plate of the hypostomal complex. Numerals 1–6 indicate endopodal podomeres. (Online version in colour.)
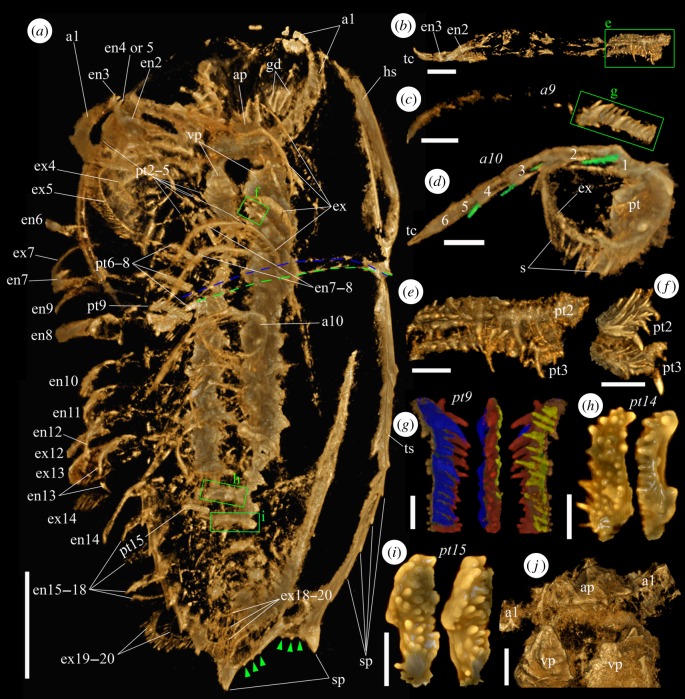
Figure 2.
Micro-CT images of an adult specimen (YKLP 11409) of Naraoia spinosa from the Chengjiang Lagerstätte. (a) Ventral view of the animal. Green dashed line marks the position of the posterior margin of the head shield, blue dashed line for the anterior margin of the trunk shield. Green arrowheads indicate tiny spines on the posterior margin of the trunk shield. (b) Right second and third appendages, exopods not preserved. (c) Right ninth appendage, exopod not preserved. (d) Left tenth appendage. Green coloration highlights apical setae on endopodal podomeres 1–4. (e) Protopods of right second and third appendages, interior view (green rectangle in (b)). (f) Protopods of left second and third appendages, interior view (one of the green rectangles in (a), viewing from the back side). (g) Protopod of right ninth appendage (green rectangles in (c)) in posterior, interior and anterior views. Blue, red and yellow colorations highlight different rows of spines. (h) Protopod of left 14th appendage in ventral and posteroventral views. (i) Protopod of left 15th appendage in ventral and anteroventral views. (j) Hypostomal complex and basal parts of first appendages (antennae), ventral view. Scale bars, 5 mm for (a), 1 mm for (b–d, j) and 500 µm for (e–i). Abbreviations as in figure 1. Numerals 1–6 indicate endopodal podomeres. (Online version in colour.)
3. External morphology
The specimens documented herein are identified as Naraoia spinosa Zhang & Hou (1985), one of two common naraoiids in the Chengjiang biota, based on their general outlines, appendage morphologies and the characteristic gut diverticula on the head shield (figures 1 and 2; electronic supplementary material, figures S1 and S2). The occurrence of marginal spines on the dorsal exoskeleton further indicates affinity to ‘morph A' [3]. We consider YKLP 11408 (length 11.37 mm, figure 1) as a mid-stage juvenile based on length-frequency data for this morph (fig. 17 in [3]). We refer to YKLP 11409 (length 30.74 mm, figure 2) as an adult based on its size, trunk shield that is significantly longer than the head shield, tiny spines along the posterior margin of the trunk shield (green arrowheads in figure 2a; cf. [2,3]), and appendage structures being more developed compared to those of the juvenile (see below), although the largest known specimens of the species have a body length of 5 cm [19].
The exoskeleton consists of a semicircular head shield and an elongate trunk shield that overlap each other only slightly (figures 1 and 2). Marginal spines are present on the trunk shield. In the adult, the last pair of these spines is much larger than the preceding ones (figure 2a).
The hypostomal complex consists of a triangular, anteriorly pointing plate and two subtriangular (though evidently broken), ventrally projecting plates (ap and vp, figures 1e, 2j and 3). The anterior plate of the juvenile is longer (length 1.44 mm) and is situated more anteriorly than that of the adult (length 1.20 mm). The multi-articulated antennae are inserted between the anterior plate and the ventral plates.
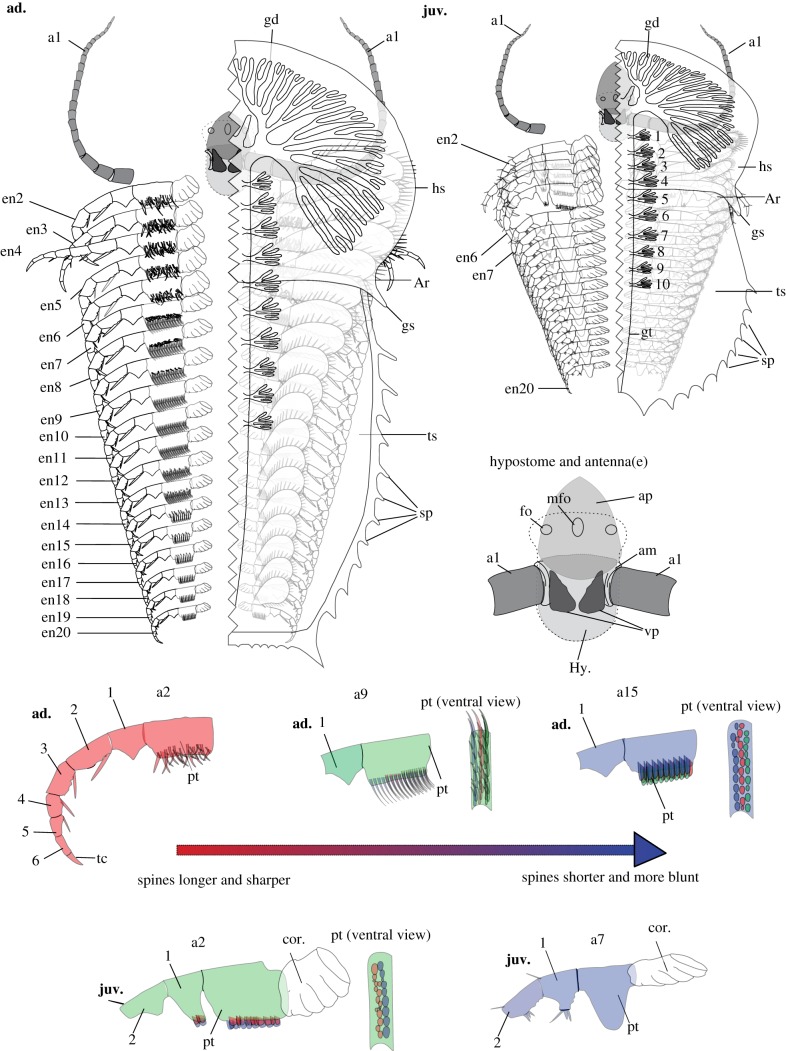
Figure 3.
Line drawings showing differentiation of the appendages of Naraoia spinosa and the difference between juvenile and adult. Left- hand upper: a detailed line drawing of N. spinosa, of early-adult stage of growth (ad.), based on YKLP 11409. Right- hand upper: a detailed line drawing of N. spinosa, of mid-juvenile stage of growth (juv.), based on YKLP 11408. The left-hand side of both the adult and juvenile specimens has been removed to better reveal the morphologies and arrangement of the appendages. The exopods on the left-hand side of both specimens, as well as the proximal-most portion of the left antenna, have also been removed to clearly display the in situ morphologies of the protopods. Morphology of posterior shield and gut morphology, within both the adult and the juvenile, based on fig. 20 of [3]. Right-hand centre: an enlarged line drawing of the hypostomal complex of N. spinosa based on both YKLP 11408 and 11409. General hypostome outline and positioning of frontal organs based on fig. 21 of [3]. Bottom of figure: detailed line drawings exhibiting the protopod morphologies of both mid juvenile (juv.) and early adult (ad.) specimens of N. spinosa based on YKLP 11408 and 11409. No. 1–10, numbered gut diverticula (main specimens) and numbered endopodal podomeres (dissected structures); ad., morphologies pertaining to adult specimens of N. spinosa; am, inferred presence of arthrodial membrane; an, the nth appendage; ap, anterior plate of the hypostomal complex; Ar, shield articulation; enx, the endopod of xth appendage; fo, the lateral frontal organs of the hypostome complex; gd, gut diverticula; gs, genal spine; gt, gut tract; hs, head shield; Hy., hypostome; juv., morphologies pertaining to juvenile specimens of N. spinosa; mfo, medial frontal organ of the hypostomal complex; pt, protopod; sp, spine; tc, terminal claw; ts, trunk shield; vp, ventral plate of the hypostomal complex. Numerals 1–6 indicate endopodal podomeres. (Online version in colour.)
Numbers of appendages (i.e. 20 pairs) are identical in the juvenile (figure 1a) and the adult (figure 2a). However, detailed morphology and the pattern of segmental differentiation of these appendages vary between them. In the juvenile, the post-antennal appendages are represented by two types based on protopodal armature, dividing the body tagmosis into two sections. In appendages 2–6, the enlarged, quadrate protopod carries two rows of variably tiny bristles on the inner edge and scattered bristles on the lateral surface (figures 1b,f and 3). Small bristles also occur on the prominent tooth-like endite of the first endopodal podomere, where they are less clearly patterned (figure 1f). A first endopodal podomere bearing a prominent endite is well known from other naraoiid species [1,3,8,9], but the detailed three-dimensional morphology of both the protopod and first endopodal podomere is clarified here. A similar endite, albeit being smaller, is present on the second endopodal podomere but not on subsequent ones. As observed previously (fig. 4m in [11]) the endopod is composed of six podomeres and a terminal claw. The distal endopodal podomeres are slender, each with a pair of tiny sub-apical endites that bear single setae (figures 1b–d,g and 3). The terminal claw is pointed and triangular. The exopod flap is connected to the protopod along a strong shaft, the morphology of which is clearly revealed (figure 1b,c). Appendage 6 lies under the trunk based on three-dimensional rotation of the specimen to trace the head-trunk boundary onto the ventral side, on which it is visible as a transverse line.
The more posterior trunk appendages differ from appendages 2–6 in several aspects. Numerous segments depict three or, mostly, four sub-equal lobes (figure 1a,h, green arrowheads) that are more likely folds of arthrodial membrane at the attachment of the protopod to the body than representing lobate endites of the protopod itself. These lobes (the ‘cormus' in fig. 45B of [8]) have a high fossilization potential in naraoiids, being documented in many specimens of Misszhouia longicaudata in particular [2,8], and their morphology and position are similar in our material and M. longicaudata. Whether these structures are present in the head appendages of YKLP 11408 is unknown, because the central part of the head shield of this specimen is missing (figure 1a), although their similar expression in head and trunk segments in M. longicaudata (fig. 9A in [2]) suggests they are likely to be present in the head of N. spinosa as well. The protopod of the posterior trunk appendages carries a bare, blunt lobe (figure 1i,j). Its size may have been taphonomically reduced in the ventrally compressed appendages 8–20 compared to the seventh appendage, where it is generally anteriorly positioned, with the size of protopod being similar to those of appendages 2–6 (figure 1a). Well-developed setae are present on the first three endopodal podomeres but not on the distal podomeres (figure 1i,j), and the endopodal podomeres generally become progressively more slender distally, rather than having the distal four podomeres uniformly slender as in appendages 2–6. The exopod shaft is relatively shorter than in appendages 2–6 and the flap is more slender, but the two are likewise separated by a distinct articulation. The distal paddle bears spiniform setae with rounded cross sections.
In comparison to the juvenile, the post-antennal appendages of the adult have generally spinose protopods, the spines being more pronounced on the anterior appendages (figures 2 and 3). In appendages 2 and 3 (figure 2b,e,f), the spine assemblage consists of one or two larger and about 30 more slender spines. The larger spines usually curve slightly proximally. In the middle appendages (e.g. appendage 9, figure 2c,g), spines are crudely arranged in three or four rows, mostly of similar size but slightly longer on the distal part of the protopod, and slightly curved towards the proximal of the appendage. In the more posterior appendages (e.g. appendages 14 and 15, figure 2h,i), these spines generally become shorter and blunter, their morphology resembling the protopodal bristles of the juvenile (figure 1f), although much larger. The endopod of the adult (as in the juvenile) consists of six podomeres and a pointed terminal claw. The endopod (e.g. appendage 10, figure 2d) has a robust seta articulated ventrodistally on several articles (figure 2d). Morphology of the exopods is generally similar to those of the juvenile, being flap-like and densely fringed with setae (figure 2a,d). However, a shaft is indistinctly defined, and the exopod flap itself is relatively larger, especially on the cephalic appendages.
Gnathobasic spines are also observed on several trunk segments in a late-stage juvenile (YKLP 13941; electronic supplementary material, figure S2, green arrowheads), which has a body length of 21 mm and has protopodal spines that are less pronounced than those in the adult (figure 2). Due to limits of preservation, however, the arrangement of these spines on the protopod and their presence in the head appendages are not clearly defined in this specimen.
4. Discussion
Our study presents unprecedently clear, three-dimensional morphologies of the entire appendage assemblage of the Cambrian arthropod Naraoia spinosa. We acknowledge that micro-CT can sometimes be biased by differential pyritisation of structures, as seen by the variable amount of detail preserved along the length of the body in single specimens. Accordingly, the effort has been taken to integrate our data with light photographic observations on Naraoia spinosa in previous studies [3], as well as other species of Naraoia and naraoiids more broadly. We infer, for example, that the tomographic data are often exposing only the marginal part of the protopods in the scanned adults (where spines are concentrated) because protopods observed in fully exposed specimens are prominently extended (as reconstructed in fig. 30 of [3]). As for specimen YKLP 11408, in view of its generally complete appearance and rich seta-scale details (figure 1), we believe micro-CT captures most of its external morphology.
It is especially noticeable that the protopods are modified into various forms along the body axis in different ontogenetic stages, presumably for food-processing purposes. The endopods and exopods are, by contrast, more conserved between body regions. In the adult, the densely distributed, strong protopodal spines arranged in multiple rows with long and short spines (figure 2) resemble those of extant arthropods such as horseshoe crabs, implying the capabilities of tearing hard food items, and therefore predatory and/or scavenging behaviour [20]. This supports the conclusion drawn from comparison of the digestive system of N. spinosa with Recent arthropods [17]. As well, the adult of N. spinosa depicts a morphological trend of shorter but more robust spines on the protopods of posterior appendages, consistent with feeding behaviour in horseshoe crabs and durophagous Cambrian arthropods such as Sidneyia, in which the posterior appendages break up prey that is passed forward to the more delicately spinose anterior appendages [21]. No shelly fragments are known from the gut of our scanned specimens or other material of N. spinosa, making durophagy less confident than is the case for Sidneyia, for example [22,23]. In the juvenile, the small bristles on the protopod of the head appendages (figure 1b,f) probably functioned for grinding smaller, softer food particles. The less-developed trunk appendages with (apparently) a bare protopod lobe and setulose endopods (figure 2i,j) may have been used to collect and transfer food particles, such as organic detritus and small plankton, to the head appendages for further processing. We suggest that the juvenile of N. spinosa had a different feeding strategy from the adult, relying on smaller prey or even being a plankton/detritus feeder. Such a finding echoes that by [24], who proposed a diet difference between the juvenile and adult of the Cambrian ‘great appendage' arthropod Leanchoilia illecebrosa based on ontogenetic differences in the deutocerebral great appendages. Moreover, we infer that the juvenile and adult of N. spinosa may also differ in their locomotory behaviour and respiratory modes. As shown in figures 1–3, exopods of the adult, especially from the posterior part of the body, are generally larger, and more densely fringed with setae compared with those of the juvenile. This may reflect differences in the animals' ability to swim and obtain dissolved oxygen from the ambient water.
The pattern of increasing spinosity of the gnathobases through ontogeny is also similar to many extant marine arthropods, as seen, for example, in Limulus [25]. This suggests that this trend may be a plesiomorphic character shared by other artiopodans, but few comparable data are available to test its distribution. In the case of agnostids, a clade that has long been the subject of debate as to whether or not they are allied to trilobites (reviewed by Moysiuk & Caron [26]), comparison of juveniles [27] and adults suggests limited ontogenetic change [26], although comparison is only possible between different genera, and the adult morphology is known in much coarser detail than is available for N. spinosa.
Artiopodans are generally thought to have limited differentiation of the post-antennal appendages. Our data for N. spinosa depict marked differentiation of the protopods along the body axis throughout ontogeny. In Trilobita, the spinosity, size of endites and orientation of the protopod have likewise been noted to vary along the anteroposterior axis in Eoredlichia intermedia and Triarthrus eatoni (reviewed in [28]), whereas Redlichia rex depicts differences in relative size of the lobes of the exopod between anterior and posterior appendages [28]. The recent finding that the first two post-antennal appendages of the xandarellid artiopodan Sinoburius lunaris are markedly differentiated from other cephalic and trunk appendages with respect to exopod morphology [29] suggests that appendages may be more tagmatized in this clade than previously known.
Our study also provides important information on the developmental mode of N. spinosa. It is found that the juvenile, which is about one-third the length of the studied adult (and just over one-fifth the length of the largest known specimens of the species), possesses the same number of appendages as the adult. This indicates that Naraoia attained the full appendage assemblage at an early ontogenetic stage, although the size, shape, segmentation pattern and chaetotaxy structures of various parts of the appendages continue to change afterwards. Such a pattern resembles that of Leanchoilia illecebrosa [30], for which a 2-mm-long juvenile already had nearly the same number of appendages as the adult (lengths up to 5 cm [19]). It is not clear, however, whether or not N. spinosa is strictly epimorphic, having already completed its segmentation in the embryo, but it contrasts with the overwhelming majority of trilobites, which have anamorphic development, adding body segments for a variable (and often lengthy) duration during postembryonic development before reaching the epimorphic phase. The ‘segmentation schedule' of trilobites [31] describes differences in timing between segment addition at the posterior growth zone and articulation between segmental sclerites. Unlike trilobites (and most other trilobitomorphs), naraoiids have a fused trunk shield and thus their segmentation schedule does not involve the process of articulation. Post-embryonic addition of segments (a hemianamorphic phase), which might have occurred at stages earlier than the larva studied here, would involve the trunk shield becoming relatively longer, assuming a typical pre-telson budding zone.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
We thank Hong Chen, Ting Zhao and Maoyin Zhang for photographing the specimens, and Shuyan Hou and Shaogang Zang for scanning the fossils.
Data accessibility
The computed tomography data are available on Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.jdfn2z372.
Authors' contributions
X.H. collected the fossils. D.Z. and Y.L. designed the research and processed micro-CT data with input from H.M. D.Z. and A.D.B. prepared the figures with input from co-authors. D.Z., G.D.E., Y.L. and A.D.B. wrote the first draft of the manuscript. All authors discussed and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study is supported by NSFC grant nos. 41861134032, 41472153, Yunnan Provincial grant nos. 2018FA025, 2018IA073, 2015HA021 and 2015HC029, and 1000 Talent Plan of China (Youth Program).
References
- 1.Whittington HB. 1977. The Middle Cambrian trilobite Naraoia, Burgess Shale, British Columbia. Phil. Trans. R. Soc. B 280, 409–443. ( 10.1098/rstb.1977.0117) [DOI] [Google Scholar]
- 2.Chen J-Y, Edgecombe GD, Ramsköld L. 1997. Morphological and ecological disparity in naraoiids (Arthropoda) from the Early Cambrian Chengjiang fauna, China. Rec. Aust. Mus. 49, 1–24. ( 10.3853/j.0067-1975.49.1997.249) [DOI] [Google Scholar]
- 3.Zhang X-L, Shu D-G, Erwin DH. 2007. Cambrian naraoiids (Arthropoda): morphology, ontogeny, systematics, and evolutionary relationships. J. Paleontol. 81, 1–52. ( 10.1666/06-082.1) [DOI] [Google Scholar]
- 4.Mayers B, Aria C, Caron J-B. 2018. Three new naraoiid species from the Burgess Shale, with a morphometric and phylogenetic reinvestigation of Naraoiidae. Palaeontology 2018, 1–32. ( 10.1111/pala.12383) [DOI] [Google Scholar]
- 5.Walcott CD. 1912. Cambrian geology and paleontology. II. Middle Cambrian Branchiopoda, Malacostraca, Trilobita and Merostomata. Smithson. Misc. Coll. 57, 145–228. [Google Scholar]
- 6.Zhang WT, Hou X-G. 1985. Preliminary notes on the occurrence of the unusual trilobite Naraoia in Asia. Acta Palaeontol. Sin. 24, 591–595. [Google Scholar]
- 7.Caron J-B, Rudkin DM, Milliken S. 2004. A new Late Silurian (Pridolian) naraoiid (Euarthropoda: Nektaspida) from the Bertie Formation of southern Ontario, Canada: delayed fallout from the Cambrian explosion. J. Paleontol. 78, 1138–1145. ( 10.1017/S0022336000043948) [DOI] [Google Scholar]
- 8.Hou X-G, Bergström J. 1997. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils Strata 45, 1–116. [Google Scholar]
- 9.Haug C, Haug JT. 2016. New insights into the appendage morphology of the Cambrian trilobite-like arthropod Naraoia compacta. Bull. Geosci. 91, 221–227. ( 10.3140/bull.geosci.1573) [DOI] [Google Scholar]
- 10.Fortey RA, Theron JN. 1994. A new Ordovician arthropod, Soomaspis, and the agnostid problem. Palaeontology 37, 841–861. [Google Scholar]
- 11.Ortega-Hernández J, Legg DA, Braddy SJ. 2013. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29, 15–45. ( 10.1111/j.1096-0031.2012.00413.x) [DOI] [PubMed] [Google Scholar]
- 12.Lerosey-Aubril R, Zhu X, Ortega-Hernández J. 2017. The Vicissicaudata revisited: insights from a new aglaspidid arthropod with caudal appendages from the Furongian of China. Sci. Rep. 7, 11117 ( 10.1038/s41598-017-11610-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgecombe GD, Ramsköld L. 1999. Relationships of Cambrian Arachnata and the systematic position of Trilobita. J. Paleontol. 73, 263–287. ( 10.1017/S0022336000027761) [DOI] [Google Scholar]
- 14.Cotton TJ, Braddy SJ. 2004. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edinb.: Earth Sci. 94, 169–193. ( 10.1017/S0263593300000596) [DOI] [Google Scholar]
- 15.Paterson JR, Edgecombe GD, García-Bellido DC, Jago JB, Gehling JG. 2010. Nektaspid arthropods from the Lower Cambrian Emu Bay Shale Lagerstätte, South Australia, with a reassessment of lamellipedian relationships. Palaeontology 53, 377–402. ( 10.1111/j.1475-4983.2010.00932.x) [DOI] [Google Scholar]
- 16.Caron J-B, Jackson DA. 2008. Palaeoecology of the Greater Phyllopod Bed community, Burgess Shale, Palaeogeogr. Palaeoclimatol. Palaeoecol. 258, 222–256. ( 10.1016/j.palaeo.2007.05.023) [DOI] [Google Scholar]
- 17.Vannier J, Chen J-Y. 2002. Digestive system and feeding mode in Cambrian naraoiid arthropods. Lethaia 35, 107–120. ( 10.1080/002411602320183971) [DOI] [Google Scholar]
- 18.Gabbott SE, Hou X-G, Norry MJ, Siveter DJ. 2004. Preservation of early Cambrian animals of the Chengjiang biota. Geology 32, 901–904. ( 10.1130/G20640.1) [DOI] [Google Scholar]
- 19.Hou X-G, Siveter DJ, Siveter DJ, Aldridge RJ, Cong P-Y, Gabbott SE, Ma X-Y, Purnell M, Williams M. 2017. The Cambrian fossils of Chengjiang, China: the flowering of early animal life, 2nd edn Oxford, UK: Wiley Blackwell. [Google Scholar]
- 20.Bicknell RDC, Ledogar JA, Wroe S, Gutzler BC, Watson WH, Paterson JR. 2018. Computational biomechanical analyses demonstrate similar shell-crushing abilities in modern and ancient arthropods. Proc. R. Soc. B 285, 20181935 ( 10.1098/rspb.2018.1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bicknell RDC, Paterson JR, Caron J-B, Skovsted CB. 2018. The gnathobasic spine microstructure of recent and Silurian chelicerates and the Cambrian artiopodan Sidneyia: functional and evolutionary implications. Arth. Struct. Dev. 47, 12–24. ( 10.1016/j.asd.2017.12.001) [DOI] [PubMed] [Google Scholar]
- 22.Zacaï A, Vannier J, Lerosey-Aubril R. 2015. Reconstructing the diet of a 505-million-year-old arthropod Sidneyia inexpectans from the Burgess Shale fauna. Arth. Struct. Dev. 45, 200–220. ( 10.1016/j.asd.2015.09.003) [DOI] [PubMed] [Google Scholar]
- 23.Bicknell RDC, Paterson JR. 2018. Reappraising the early evidence of durophagy and drilling predation in the fossil record: implications for escalation and the Cambrian explosion. Biol. Rev. 93, 754–784. ( 10.1111/brv.12365) [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Haug JT, Haug C, Briggs DEG, Hou XG. 2014. A 520 million-year-old chelicerate larva. Nat. Commun. 5, 4440 ( 10.1038/ncomms5440) [DOI] [PubMed] [Google Scholar]
- 25.Haug C, Roetzer MAIN. 2018. The ontogeny of Limulus polyphemus (Xiphosura s. str., Euchelicerata) revised: looking ‘under the skin’. Dev. Genes Evol. 228, 49–61. ( 10.1007/s00427-018-0603-1) [DOI] [PubMed] [Google Scholar]
- 26.Moysiuk J, Caron J-B. 2019. Burgess Shale fossils shed light on the agnostid problem. Proc. R. Soc. B 286, 20182314 ( 10.1098/rspb.2018.2314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller KJ, Walossek D. 1987. Morphology, ontogeny, and life habit of Agnostus pisiformis from the Upper Cambrian of Sweden. Fossils Strata 19, 1–124. [Google Scholar]
- 28.Holmes JD, Paterson JR, García-Bellido DC. In press The trilobite Redlichia from the lower Cambrian Emu Bay Shale Konservat-Lagerstätte of South Australia: systematics, ontogeny and soft-part anatomy . J. Syst. Palaeontol. ( 10.1080/14772019.2019.1605411) [DOI] [Google Scholar]
- 29.Chen X, Ortega-Hernández J, Wolfe JM, Zhai D, Hou X, Chen A, Mai H, Liu Y. 2019. The appendicular morphology of Sinoburius lunaris and the evolution of the artiopodan clade Xandarellida (Euarthropoda, early Cambrian) from South China. BMC Evol. Biol. 19, 165 ( 10.1186/s12862-019-1491-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Melzer RR, Haug JT, Haug C, Briggs DEG, Hörnig MK, He Y-Y, Hou XG. 2016. Three-dimensionally preserved minute larva of a great-appendage arthropod from the early Cambrian Chengjiang biota. Proc. Natl Acad. Sci. USA 113, 5542–5546. ( 10.1073/pnas.1522899113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes NC, Minelli A, Fusco G. 2006. The ontogeny of trilobite segmentation: a comparative approach. Paleobiology 32, 602–627. ( 10.1666/06017.1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The computed tomography data are available on Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.jdfn2z372.